Abstract
Objective:
To determine the threshold annualized esophagectomy volume that is associated with improved survival, oncologic resection, and post-operative outcomes.
Summary Background Data:
Esophagectomy at high-volume centers is associated with improved outcomes; however, the definition of high-volume remains debated.
Methods:
The 2004–2016 National Cancer Database (NCDB) was queried for patients with clinical stage I-III esophageal cancer undergoing esophagectomy. Center esophagectomy volume was modeled as a continuous variable using restricted cubic splines. Maximally selected ranks were used to identify an inflection point of center volume and survival. Survival was compared using multivariable Cox Proportional Hazards methods. Multivariable logistic regression was used to examine secondary outcomes.
Results:
Overall, 13,493 patients met study criteria. Median center esophagectomy volume was 8.2 (IQR 3.2–17.2) cases/year. On restricted cubic splines, inflection points were identified at 9 and 30 cases/year. A multivariable Cox model was constructed modeling annualized center surgical volume as a continuous variable using three linear splines and inflection points at 9 and 30 cases/year. On multivariable analysis, increasing center volume up to 9 cases/year was associated with a substantial survival benefit (HR 0.97, 95% CI 0.95–0.98; p=<0.001). On multivariable logistic regression, factors associated with undergoing surgery at a high-volume center (greater than 9 cases/year) included private insurance, care at an academic center, completion of high school education, and greater travel distance.
Conclusions:
This NCDB study utilizing multivariable analysis and restricted cubic splines suggests the threshold definition of a high-volume esophagectomy center as one that performs at least 10 operations a year.
Mini-Abstract
Undergoing esophagectomy at “high-volume” centers is associated with better short- and long-term outcomes; however, the definition of “high-volume” remains unclear. Herein, we utilized rigorous statistical methods and identified a threshold of at least 10 cases per year for improved outcomes among patients with clinical stage I-III esophageal cancer.
Introduction
Esophagectomy is the cornerstone of treatment with curative intent for clinical stage I-III esophageal cancer. Despite advances in surgical technique and technology, as well as increased specialization and regionalization of complex cancer care, esophagectomy remains a highly morbid procedure.1–5 As with other complex surgical procedures, a beneficial volume-outcomes relationship has been well described for both perioperative outcomes and long-term survival following esophagectomy.5–8 However, literature regarding the threshold center esophagectomy volume at which these outcomes improve is inconsistent and represents a knowledge gap.
First established in 2000, the Leapfrog Group is a non-profit organization that makes recommendations on the minimum hospital- and surgeon-volume for complex surgical procedures with recognized volume-outcomes relationships.9 Early reports defined the minimum annual hospital esophagectomy volume to be 13 cases per year, with the 2021 report recommending a minimum 20 cases per year at the hospital level and 7 cases per year at the individual surgeon level.9–11 Alternatively, the Agency for Healthcare Research and Quality (AHRQ) has previously proposed a benchmark of at least six cases per year as a hospital quality indicator.12 Additional retrospective analyses have studied volumes ranging from 6–15 cases per year, while others critique the sole use of volume to define “Centers of Excellence”.7, 13–15
There is no doubt that patients undergoing esophagectomy at high-volume centers benefit in both the perioperative and long-term settings; however, the methods by which high-volume is defined are poorly understood. Herein, we aimed to utilize rigorous statistical methods to determine the threshold annualized esophagectomy volume that is associated with improved survival, oncologic resection, and post-operative outcomes in a national database.
Methods
Data Source
The National Cancer Database (NCDB) was used for retrospective analysis. The NCDB is a national clinical oncology registry maintained through a collaboration between the American College of Surgeons and American Cancer Society. Trained cancer registrars enter patient- and hospital-level data from over 1,500 Commission on Cancer (CoC) accredited facilities across the United States using a well-defined data dictionary.16 This data includes relevant information on cancer patients’ demographics, diagnosis, tumor characteristics, treatment, and outcomes. Altogether, the NCDB is estimated to capture 70–80% of cancer diagnoses in the United States, making it a robust data source in which to ask such questions.17, 18 All NCDB patient data is deidentified; therefore, this retrospective study was deemed exempt by the Duke University Medical Center Institutional Review Board.
Patient Selection
The NCDB was queried for patients with clinical stage I-III esophageal cancer who underwent esophagectomy at the reporting CoC-accredited facilities between 2004–2016. Esophagectomy was defined as those with curative intent and included both partial and total esophagectomy with or without gastrectomy. Patients with both adenocarcinoma and squamous histology were included. For patients with stage III disease, only those who received neoadjuvant therapy were included to mitigate the effect of guideline-discordant treatment on postoperative outcomes. Patients who underwent surgical resection more than 180 days from diagnosis or had missing survival status were excluded.
Statistical Analysis
Annualized esophagectomy volume was calculated for each reporting CoC-accredited facility over the thirteen-year study period. Patients were stratified into quartiles by center annualized esophagectomy volume. Baseline demographics for the four groups were calculated and compared using the Pearson’s chi-squared and Kruskal-Wallis tests for categorical and continuous variables, respectively. Overall survival was compared among patients in each volume quartile using Kaplan-Meier survival analysis and the log-rank test.
The primary objective was to determine the threshold center esophagectomy volume at which a survival benefit exists. To evaluate this, center annualized esophagectomy volume was modeled as a continuous variable using restricted cubic splines. Maximally selected ranks were used to identify an inflection point of center volume and survival (R package “maxstat”).19 Survival was compared using multivariable Cox Proportional Hazards methods. After defining the threshold for a high-volume center, multivariable logistic regression was used to determine factors associated with undergoing esophagectomy at such centers. Separate multivariable logistic regression models were created to examine secondary outcomes of 30- and 90-day post-operative mortality, pathologic upstaging, guideline-concordant nodal harvest, and margin positivity. All adjusted models incorporated known covariates that were designated a priori. Two-way interactions between annualized center volume and other covariates, including age, year of diagnosis, histology, stage, surgery at an academic center, travel distance, and different center for diagnosis and treatment were tested. The only significant two-way interaction was between annualized center volume and clinical stage (analysis of variance [ANOVA] p<0.05). Missing data were handled with complete case analysis in regression. All statistical analyses were performed using R version 4.1.1 for Mac (Vienna, Austria) with a designated significance threshold of 0.05 or less.
Results
Baseline Characteristics
Overall, 13,493 patients met study criteria (Figure 1). Median center esophagectomy volume was 8.2 cases per year (IQR 3.2–17.2) (Supplemental Figure 1). Patients were stratified into volume quartiles accordingly (Q1 ≤ 3.2, Q2 3.2–8.2, Q3 8.2–17.2, Q4 ≥ 17.2 cases per year). Baseline demographics, tumor, and treatment characteristics are shown in Supplemental Table 1. Patients undergoing esophagectomy at centers in the highest volume quartile were more likely to have insurance (98.6% in Q4 vs. 97%, in Q1, p<0.001), reside in states with Medicaid expansion (41.9% in Q4 vs. 35.1% in Q1, p<0.001), and areas with higher baseline education levels. These patients were also more likely to be diagnosed and treated at different facilities (86.2% in Q4 vs. 43.3% in Q1, p<0.001), travel farther distances for surgery (median 58.1 miles in Q4 vs 9.7 miles in Q1, p<0.001), and undergo surgery at an academic center (100% in Q4 vs. 20.6% in Q1, p<0.001). Patients undergoing esophagectomy at high-volume centers were more likely to have clinical stage III disease (38.8% in Q4 vs. 29.4% in Q1, p<0.001) with high grade tumors (48.2% in Q4 vs. 43.6% in Q1, p<0.001). There were greater lymph node harvests (median 16 nodes in Q4 vs. 9 nodes in Q1, p<0.001) and lower rates of margin-positivity (3.8% in Q4 vs. 7.1% in Q1, p<0.001) among patients undergoing esophagectomy at high-volume centers. There was decreasing 30- and 90-day post-operative mortality among the increasing volume quartiles.
Figure 1.
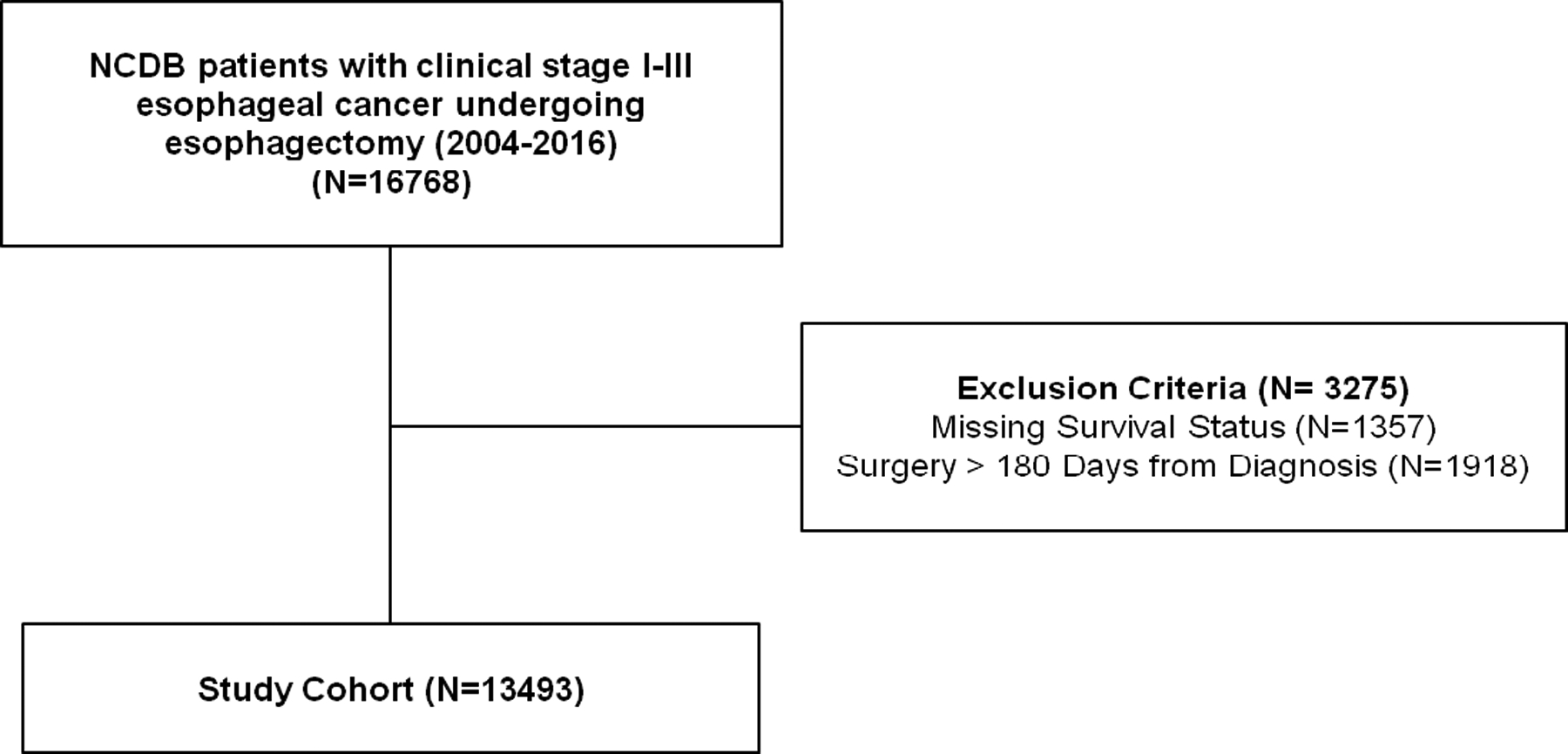
Study Consort Diagram.
Survival
Median survival in the overall cohort was 3.42 years (95% CI 3.28–3.57). There was a significant difference in the Kaplan-Meier survival curves among the center esophagectomy volume quartiles (log-rank p<0.001) (Figure 2). Patients receiving esophagectomy in the highest volume centers had a significant survival benefit compared to those undergoing resection at low-volume centers with a stepwise increase in median survival among volume quartiles (Figure 2).
Figure 2.
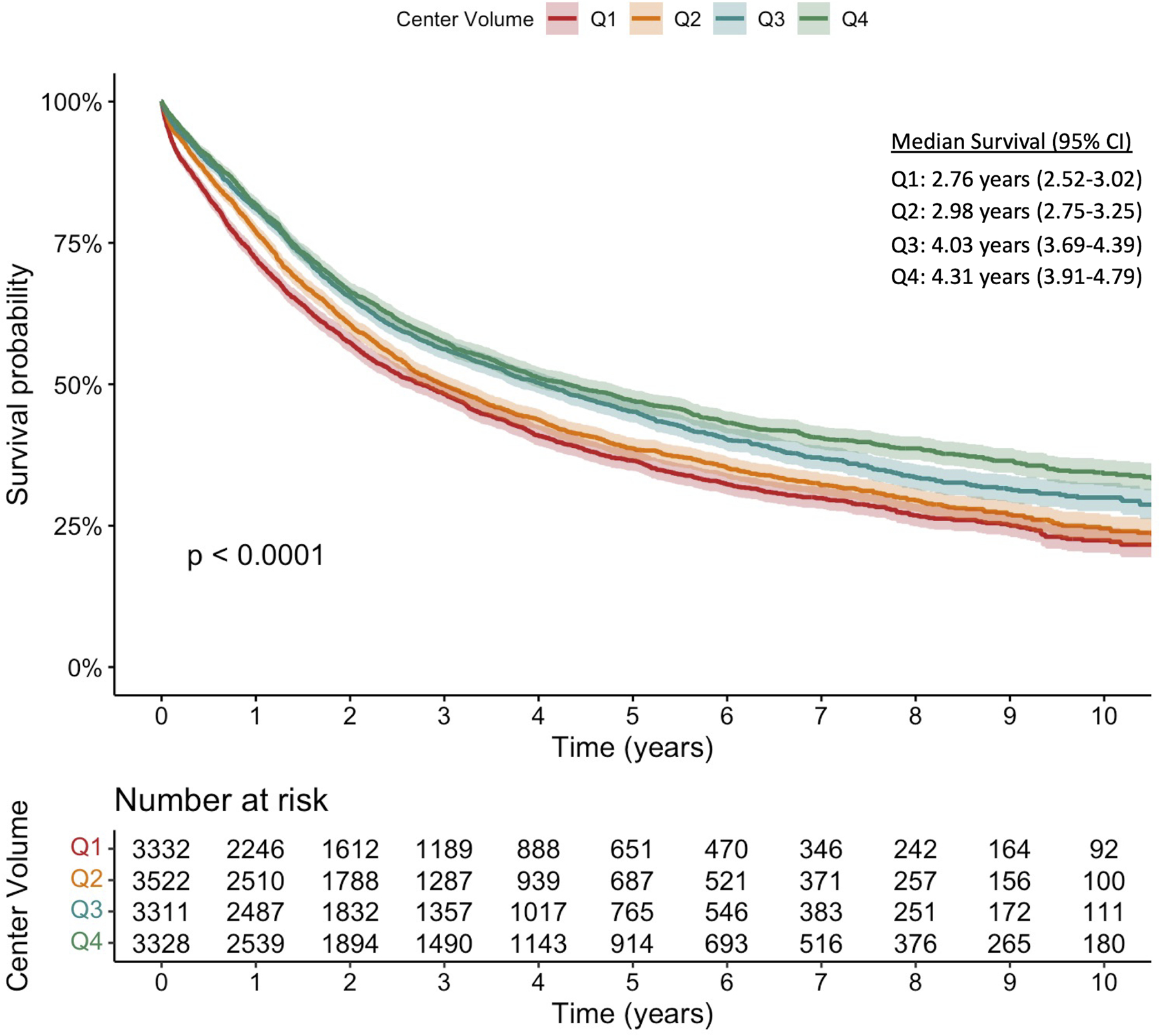
Kaplan-Meier survival curves for patients with clinical stage I-III esophageal cancer stratified by center esophagectomy volume as grouped in quartiles (Q1–4). The p-value represents the result of the log-rank test.
Annualized center esophagectomy volume was modeled with restricted cubic splines in an unadjusted Cox regression; inflection points in overall survival were identified at 9 and 30 cases per year (Figure 3). Maximally selected rank statistics confirmed a significant cutpoint of 8.85, rounded up to 9 (Suppl Figure 2). Conditional landmark analysis excluding patients with 90-day post-operative mortality was performed with consistent findings (Suppl Figures 3 and 4). Accordingly, a multivariable Cox proportional hazards model was constructed modeling annualized center esophagectomy volume as a continuous variable using three linear splines and inflection points at 9 and 30 cases per year (Figure 4A) (Table 1). On multivariable analysis, increasing annualized center volume up to 9 cases per year was associated with a substantial survival benefit (HR 0.97 per case/year, 95% CI 0.95–0.98; p=<0.001); beyond 9 cases per year, there was a small survival benefit associated with increased center surgical volume up to 30 cases per year and greater (HR 0.99, 95% CI 0.98–1.00, p=0.001; HR 0.99, 95% CI 0.98–1.00, p=0.003; respectively). There was a significant interaction between clinical stage and center esophagectomy volume (p<0.001) (Figure 4B). Subgroup analysis by stage revealed that for patients with both stage I and III esophageal cancer, there was no survival benefit for increasing case volume beyond 9 cases per year (Supplemental Table 2). Alternatively, in stage II disease, there was no survival benefit for increasing case volume beyond 30 cases per year (Supplemental Table 2). Additional covariates including age, year of diagnosis, histology, surgery at an academic center, travel distance, and different center for diagnosis and treatment were all tested for two-way interaction with center esophagectomy volume and were not significant.
Figure 3.
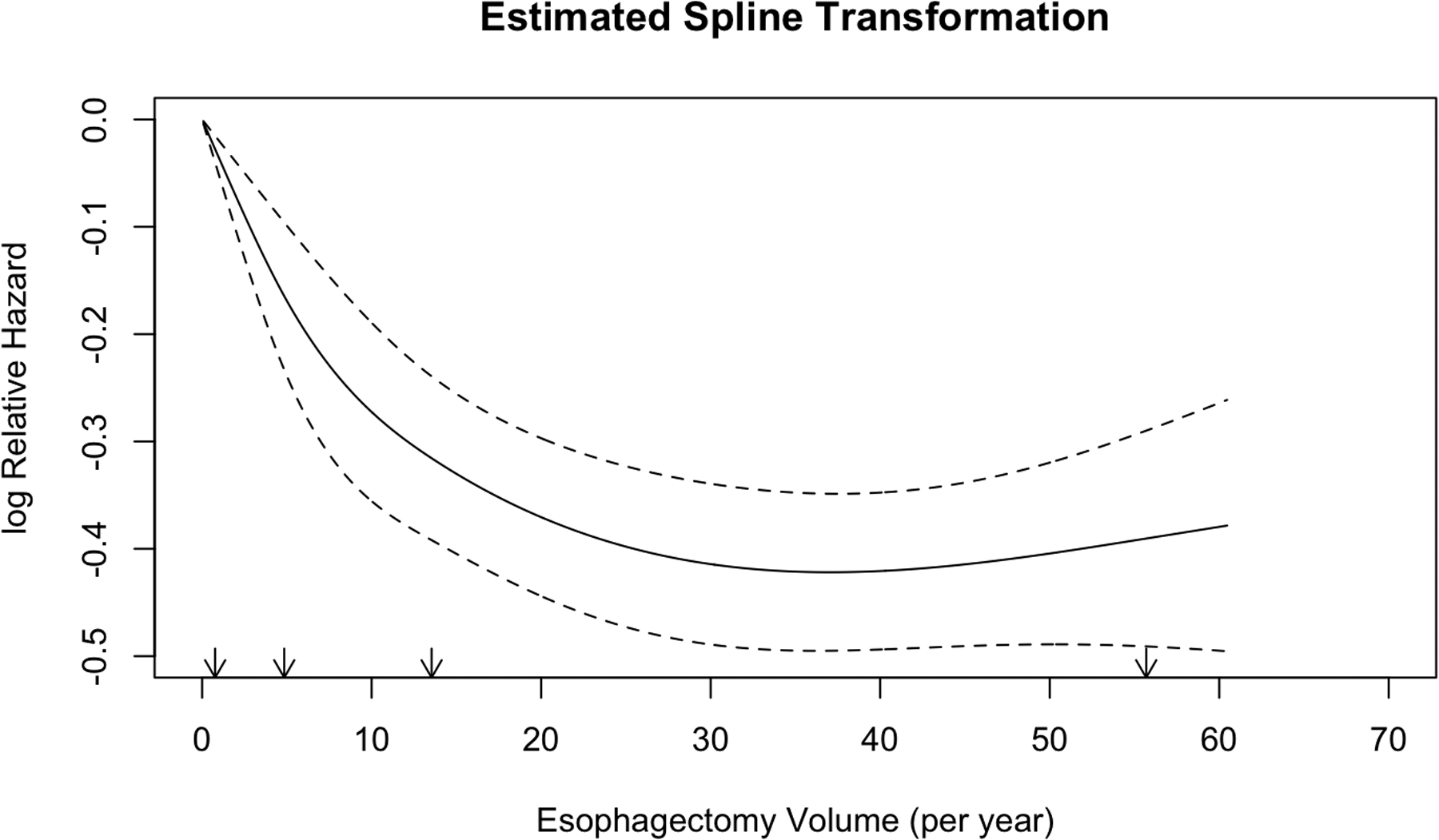
Unadjusted restricted cubic spline transformation of annualized center esophagectomy volume. Arrows denote prespecified knots selected in unadjusted analysis. The y-axis demonstrated unadjusted log hazard of mortality and the x-axis shows annualized center esophagectomy volume (cases per year). The dotted lines reflect bounds of the 95% confidence interval.
Figure 4.
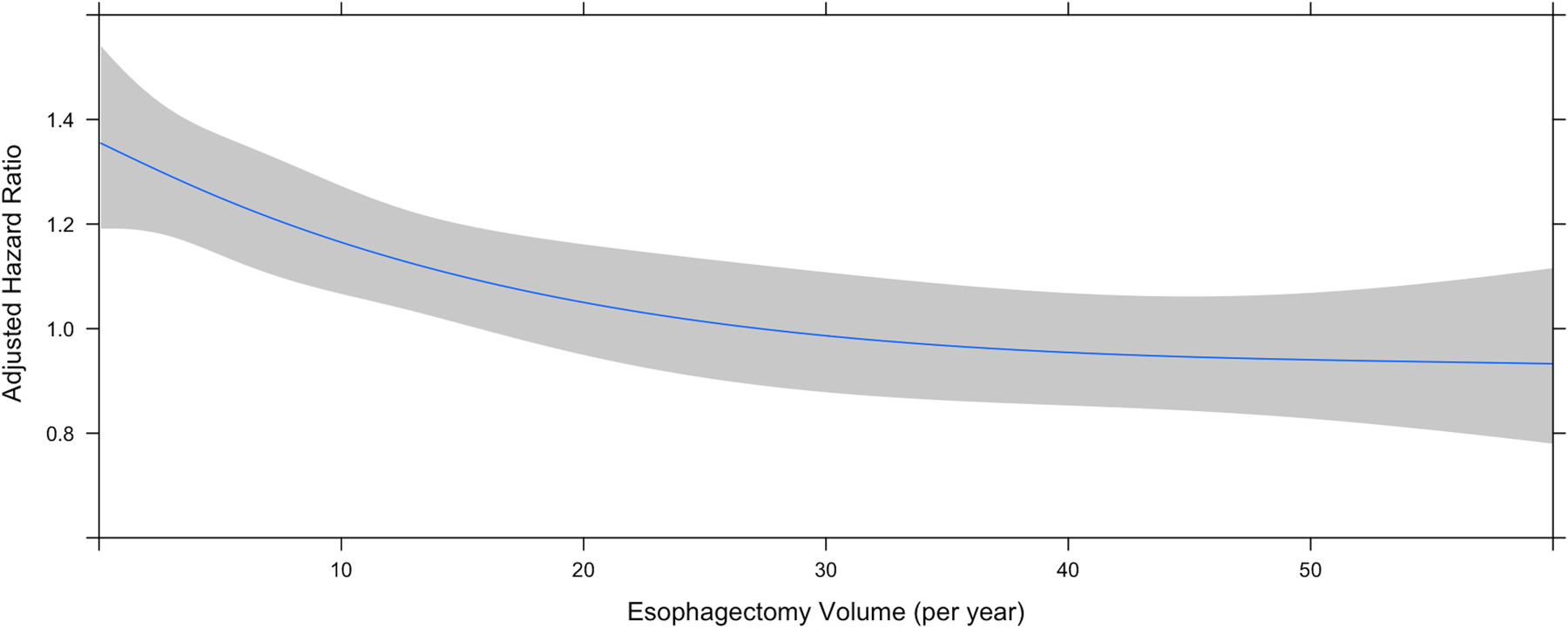
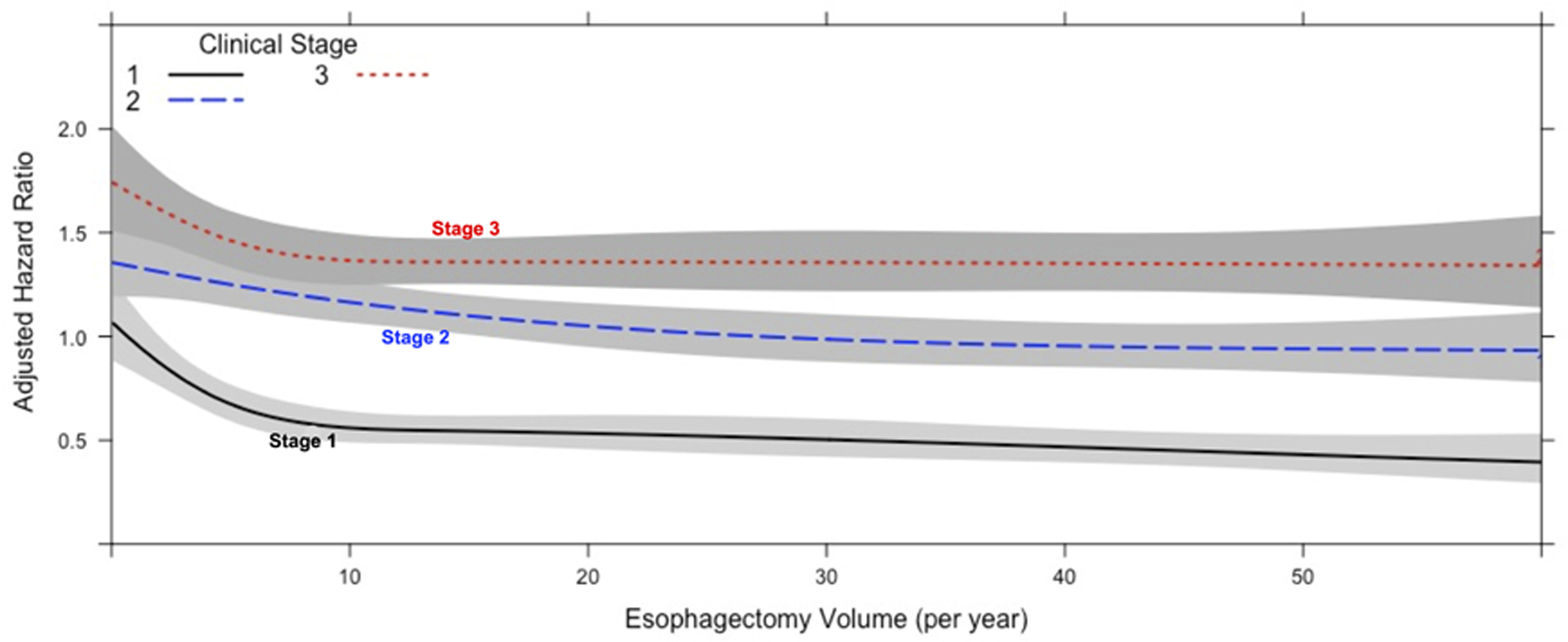
Center annualized esophagectomy volume modeled with restricted cubic splines. A) Graph demonstrating center esophagectomy volume as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term among volume and stage. The lines depict the survival curve modelled with restricted cubic splines. The shaded area represents the bounds of the 95% confidence interval. B) Graph demonstrating the interaction between center esophagectomy volume and clinical stage as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term among volume and stage.
Table 1.
Multivariable Cox Proportional Hazards model of factors independently associated with overall survival in patients with clinical stage I-III esophageal cancer undergoing esophagectomy.
| Variable | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age (per year) | 1.02 | 1.02–1.02 | <0.001 |
| Female Sex (reference: male) | 0.87 | 0.81–0.93 | <0.001 |
| Race (reference: caucasian) | |||
| Black | 1.05 | 0.93–1.18 | 0.46 |
| Other | 0.78 | 0.63–0.96 | 0.02 |
| Year of diagnosis (per year) | 0.99 | 0.98–1.00 | 0.06 |
| CDCC Score (reference: 0) | |||
| 1 | 1.12 | 1.06–1.19 | <0.001 |
| ≥2 | 1.21 | 1.10–1.33 | <0.001 |
| Insurance (reference: none) | |||
| Private | 0.95 | 0.80–1.13 | 0.58 |
| Government | 1.03 | 0.87–1.23 | 0.71 |
| Treatment location (reference: metro) | |||
| Urban | 1.13 | 1.06–1.20 | <0.001 |
| Rural | 1.02 | 0.87–1.21 | 0.77 |
| Facility location (reference: New England (CT,MA,ME,NH,RI,VT)) | |||
| Middle Atlantic (NJ, NY, PA) | 0.93 | 0.83–1.05 | 0.25 |
| South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV) | 1.09 | 0.98–1.22 | 0.12 |
| East North Central (IL, IN, MI, OH, WI) | 1.09 | 0.97–1.22 | 0.15 |
| East South Central (AL, KY, MS, TN) | 1.16 | 1.01–1.33 | 0.03 |
| West North Central (IA, KS, MN, MO, ND, NE, SD) | 0.95 | 0.84–1.08 | 0.46 |
| West South Central (AR, LA, OK, TX) | 0.97 | 0.84–1.13 | 0.73 |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 0.95 | 0.81–1.11 | 0.49 |
| Pacific (AK, CA, HI, OR, WA) | 0.85 | 0.74–0.97 | 0.02 |
| Diagnosis and Treatment at Different Facilities (reference: same facility) | 0.94 | 0.89–0.99 | 0.03 |
| Distance Traveled to Facility (per mile) | 1.00 | 1.00–1.00 | 0.94 |
| Academic center (reference: nonacademic) | 1.01 | 0.94–1.08 | 0.84 |
| Clinical Stage (reference: stage I) | |||
| Stage II | 1.65 | 1.49–1.83 | <0.001 |
| Stage III | 1.96 | 1.75–2.20 | <0.001 |
| Squamous Cell Histology (reference: adenocarcinoma) | 1.13 | 1.05–1.20 | <0.001 |
| Neoadjuvant Chemotherapy | 1.07 | 0.96–1.20 | 0.20 |
| Neoadjuvant Radiation | 1.05 | 0.95–1.16 | 0.32 |
| Center Esophagectomy Volume (increasing cases per year) | |||
| <9 | 0.97 | 0.95–0.98 | <0.001 |
| >9 and <30 | 0.99 | 0.98–1.00 | 0.001 |
| >30 | 0.99 | 0.98–1.00 | 0.003 |
| Interaction of Clinical Stage and Center Esophagectomy Volume | <0.001 |
Secondary Analyses
In a multivariable logistic regression model, there were many patient- and center-specific factors associated with undergoing esophagectomy at a high-volume center (≥9 cases per year). These included private insurance (compared to no insurance), increasing travel distance (per mile), surgery at an academic center, diagnosis and treatment at different facilities, and increasing education levels (Table 2). Factors associated with undergoing esophagectomy at a low-volume center included Black race, and urban or rural residence (compared to metropolitan) (Table 2).
Table 2.
Multivariable logistic regression of factors independently associated with undergoing esophagectomy at a high-volume center, defined as ≥9 per year, among patients with clinical stage I-III esophageal cancer.
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age (per year) | 1.00 | 1.00–1.01 | 0.12 |
| Sex (reference: male) | 1.12 | 0.98–1.28 | 0.09 |
| Race (reference: Caucasian) | |||
| Black | 0.75 | 0.59–0.96 | 0.02 |
| Other | 0.78 | 0.56–1.09 | 0.14 |
| Year of diagnosis (per year) | 0.99 | 0.97–1.00 | 0.06 |
| CDCC Score (reference: 0) | |||
| 1 | 0.97 | 0.87–1.09 | 0.63 |
| ≥2 | 1.09 | 0.90–1.33 | 0.37 |
| Insurance (reference: none) | |||
| Private | 1.64 | 1.16–2.31 | 0.005 |
| Government | 1.36 | 0.96–1.93 | 0.09 |
| Distance Traveled to Facility (per mile) | 1.01 | 1.01–1.01 | <0.001 |
| Patient Residence (reference: metro) | |||
| Urban | 0.80 | 0.69–0.92 | 0.002 |
| Rural | 0.49 | 0.34–0.70 | <0.001 |
| Academic center (reference: nonacademic) | 19.21 | 16.95–21.79 | <0.001 |
| Diagnosis and Treatment at Different Facilities (reference: same facility) | 2.17 | 1.96–2.41 | <0.001 |
| Education quartile (reference: Q1 >21% not completed) | |||
| Q2 (13–20.9%) | 1.22 | 1.02–1.45 | 0.03 |
| Q3 (7–12.9%) | 1.59 | 1.31–1.92 | <0.001 |
| Q4 (<7%) | 2.04 | 1.64–2.54 | <0.001 |
| Income quartile (reference: Q1 <$38,000) | |||
| Q2 ($38,000–47,999) | 0.94 | 0.79–1.12 | 0.48 |
| Q3 ($48,000–62,999) | 0.84 | 0.70–1.01 | 0.06 |
| Q4 (>$63,000) | 0.64 | 0.52–0.79 | <0.001 |
| Facility location (reference: New England (CT,MA,ME,NH,RI,VT)) | |||
| Middle Atlantic (NJ, NY, PA) | 1.57 | 1.27–1.95 | <0.001 |
| South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV) | 0.87 | 0.70–1.08 | 0.22 |
| East North Central (IL, IN, MI, OH, WI) | 1.43 | 1.16–1.77 | 0.001 |
| East South Central (AL, KY, MS, TN) | 1.47 | 1.11–1.94 | 0.007 |
| West North Central (IA, KS, MN, MO, ND, NE, SD) | 1.34 | 1.06–1.69 | 0.01 |
| West South Central (AR, LA, OK, TX) | 0.33 | 0.25–0.44 | <0.001 |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 0.31 | 0.22–0.43 | <0.001 |
| Pacific (AK, CA, HI, OR, WA) | 0.91 | 0.71–1.18 | 0.49 |
| Squamous Histology (reference: adenocarcinoma) | 0.90 | 0.79–1.03 | 0.14 |
| Clinical Stage (reference: stage I) | |||
| Stage II | 0.76 | 0.65–0.88 | <0.001 |
| Stage III | 1.04 | 0.87–1.25 | 0.66 |
| Neoadjuvant Chemotherapy (reference: none) | 0.97 | 0.78–1.21 | 0.81 |
| Neoadjuvant Radiation (reference: none) | 0.93 | 0.76–1.14 | 0.48 |
Separate multivariable logistic regression models to evaluate secondary outcomes were performed. As in prior analyses, annualized center esophagectomy volume was modeled as a continuous variable using three linear splines and inflection points at 9 and 30 cases per year (Table 3). Increasing annualized center volume up to 9 cases per year was independently associated with reduced odds of 30- and 90-day post-operative mortality (OR 0.92, 95% CI 0.88–0.97, p<0.001; OR 0.94, 95% CI 0.91–0.97, p<0.001; respectively). Increasing annualized center volume beyond 9 cases per year had no further associated benefit for 30-day post-operative mortality; however, increasing volume beyond 30 cases per year was associated with improved 90-day post-operative mortality (OR 0.98, 05% CI 0.96–0.99, p=0.01). Increasing annualized center volume between 9 and 30 cases per year was independently associated with pathologic upstaging (OR 1.02, 95% CI 1.00–1.03, p=0.01). Additionally, increasing annualized center volume up to 9 cases per year was independently associated with higher odds of harvesting ≥15 lymph nodes (OR 1.07, 95% CI 1.06–1.09, p<0.001). Increasing center volume beyond 30 cases per year was associated with greater odds of 30-day readmission (OR 1.02, 95% CI 1.01–1.04, p=0.002). On adjusted analysis, there was no longer a statistically significant association between margin positivity and annualized center volume.
Table 3.
Results of separate multivariable logistic regression models for secondary outcomes of post-operative mortality, oncologic resection, and short-term outcomes with annualized center esophagectomy volume modeled as a continuous variable using three linear splines and inflection points at 9 and 30 cases per year.
| Multivariable Logistic Regression Model | <9 cases per year | 9–30 cases per year | >30 cases per year | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| 30-Day Mortality | 0.92 (0.88–0.97) | <0.001 | 1.00 (0.97–1.03) | 0.97 | 0.98 (0.96–1.01) | 0.26 |
| 90-Day Mortality | 0.94 (0.91–0.97) | <0.001 | 1.01 (0.99–1.03) | 0.17 | 0.98 (0.96–0.99) | 0.009 |
| Nodal Harvest (>= 15) | 1.07 (1.06–1.09) | <0.001 | 1.06 (1.05–1.07) | <0.001 | 0.98 (0.97–0.98) | <0.001 |
| Positive Margins | 0.99 (0.95–1.02) | 0.47 | 0.98 (0.96–1.00) | 0.14 | 0.98 (0.96–1.00) | 0.12 |
| Pathologic Upstaging | 0.99 (0.96–1.02) | 0.63 | 1.02 (1.00–1.03) | 0.009 | 0.99 (0.98–1.01) | 0.34 |
| 30-Day Readmission | 1.02 (0.99–1.06) | 0.22 | 0.99 (0.97–1.01) | 0.32 | 1.02 (1.01–1.04) | 0.002 |
Discussion
In this retrospective analysis of the NCDB, we confirm prior findings of a volume-outcomes relationship in patients with clinical stage I-III esophageal cancer undergoing resection. Even though this relationship is well recognized by many, the threshold center esophagectomy volume associated with improved outcomes and survival is heavily debated. This study is the first, to our knowledge, to utilize restricted cubic splines to identify such a threshold. Our findings suggest that increasing center esophagectomy volume to a threshold of 9 cases per year was associated with a significant survival benefit and reduced 30- and 90-day post-operative mortality. Additionally, we identify many patient- and center-specific factors that are associated with undergoing esophagectomy at facilities meeting this volume criteria. While outcomes after complex operations such as esophagectomy are likely multifactorial, volume certainly plays a role.
Similar to most natural relationships, the one between center esophagectomy volume and survival is nonlinear. Traditional regression modelling may miss or underestimate the effect sizes of such nonlinear relationships. One solution to this challenge is restricted cubic splines which, estimate such relationships with cubic polynomial functions (or curves) rather than linear ones.20 While prior studies have arbitrarily defined “high volume” centers or provided recommendations with vague methodology, we provide a novel approach using a methodologically rigorous, risk-adjusted definition.7, 9 The proposed volume threshold of greater than 9 esophagectomy cases per year is markedly less than the current Leapfrog group recommendations of 20 cases per year, however, slightly higher than that suggested in recent retrospective analyses and prior recommendations by the AHRQ.7, 9, 12 Performing at least 10 cases per year translates to roughly one esophagectomy every 5–6 weeks. This benchmark number is similar to that recommended for other complex and morbid surgical procedures, such as open abdominal aortic aneurysm repair.21, 22 Similar questions have been posed in pancreas cancer, with a study utilizing logistic regression identifying threshold pancreaticoduodenectomy (Whipple procedure) volumes of 9 and 36 cases per year associated with significant reductions in 90-day mortality.23 In this context, performing at least 10 esophagectomy cases per year is clinically feasible and consistent with proposed center volumes of similarly complex and morbid operations.
The factors underlying the volume-outcomes association for complex cancer operations are likely multifactorial. Certainly, an individual surgeon’s technical skill and expertise must be considered. Unfortunately, given the nature of the NCDB we were not able to evaluate outcomes on the surgeon-level. However, individual surgeon volumes contribute to overall facility volume, which we were able to measure. In the context of increasing centralization of complex cancer care, patients are more often travelling farther for their care. Surgeons and institutions with high esophagectomy volume are often associated with strong multidisciplinary teams including medical and radiation oncologists as well as intensive care teams.24 Indeed, we found that centers with increasing volume were associated with guideline-concordant lymph node harvest and pathologic upstaging, indicating a more oncologically sound resection. This, in turn, provides the most accurate information that will ultimately be used to guide the need for adjuvant therapies. We also describe a strong association between academic research centers and high-volume esophagectomy centers, which may also afford patients the opportunity to participate in clinical trials and receive novel therapies that could potentially prolong survival.
Surgeons and teams at high-volume esophagectomy centers not only have more technical repetitions in the operating room, but also have the requisite familiarity with postoperative care and common complications. In the case of esophagectomy, these can include, but are not limited to, surgical site infections, pneumonia, acute kidney injury, venous thromboembolism, chylothorax, and anastomotic leak.25, 26 High-volume centers often have superior support systems that extend beyond the surgeon, including nursing and ancillary staff, diagnostic and interventional colleagues, as well as high-quality intensive care units.23 Alternatively, low-volume centers often lack familiarity with the subtleties of post-operative care nor have the magnitude of resources to recognize and manage such complications. Subsequently, these low-volume facilities have been observed to have higher rates of “failure to rescue.”26, 27 The lower odds of 30- and 90-day mortality as center volume approached 9 cases per year likely reflects the reduction in “failure to rescue” rates as center volume increases. “Failure to rescue” is not limited to the index hospitalization as post-operative recovery from esophagectomy is lengthy, with high rates of failure to thrive, and can require months to regain preoperative functional status.28 We found that the highest volume centers, those with increasing annual volume beyond 30 cases per year, had higher rates of 30-day readmission. Given that this higher threshold is associated with a survival benefit as well as reduced 90-day post-operative mortality, this finding likely represents the recognition and rescue of outpatient complications that low-volume centers may not catch. Increased center volume beyond 30 cases per year, which yields increased familiarity with the intricacies of perioperative care, both inpatient and outpatient, may reduce rates of failure to rescue in this high-risk population.
This study has several limitations. As with all retrospective studies, there is a level of selection bias for which we cannot fully adjust. For instance, we cannot account for referral patterns or patient preferences in where they undergo surgery. Additionally, the NCDB does not provide the requisite granularity to analyze the impact of individual surgeon-volume on patient outcomes. Certainly, centers may meet our proposed volume threshold; however, their individual surgeon case volumes may remain low. Institutional data should be reviewed for individual surgeons to best evaluate the impact of their case volumes on outcomes. Further, the NCDB does not capture important post-operative morbidities such as anastomotic leak or need for reoperation to fully describe how “failure to rescue” may play into this important relationship. Potentially confounding variables like preoperative pulmonary function, nutrition, and frailty are also not explicitly coded in the database. The NCDB also only reports overall survival, so we are unable to characterize disease-specific survival.
During the study period, there have been increasing shifts towards minimally invasive techniques (i.e., robotics, video-assisted thoracoscopic surgery) for esophagectomy, although, this has only been documented in the NCDB since 2010. The associated learning curve with these approaches may result in different volume-outcomes thresholds; however, this study lacks the sample size, particularly for robotic cases, to answer such questions. As more data becomes available, future studies should undoubtedly include surgical approach as a potential confounder and investigate differences in volume-outcomes thresholds.
Despite these limitations, our study expands upon the existing literature by utilizing rigorous statistical methods to define high-volume centers. Volume-outcomes relationships have been examined using these methods in other specialties.29 Additionally, our group has previously utilized restricted cubic splines in regression analysis to study volume-outcomes in endoscopic resection, threshold tumor sizes for treatment options in non-small cell lung cancer, lymph node ratios for adjuvant therapy decision-making, and optimal time to surgery in esophageal cancer.30–34 Restricted cubic splines can be a powerful tool for analysis of continuous predictor variables on outcomes, particularly when a complex non-linear relationship exists.20, 35 Simple categorization of such variables into quartiles or quintiles can miss important relationships.20 Therefore, the strength and novelty of our study is in the methodologic approach and the robust visualization of the center esophagectomy volume-outcomes relationship that it provides.
Conclusions
This retrospective analysis of the NCDB contributes to the mounting literature on the volume-outcomes relationship for patients undergoing esophagectomy. We corroborate prior findings in support of improved short- and long-term outcomes, in addition to identifying a candidate threshold annualized center esophagectomy volume at which this occurs. While there are certainly many contributing factors to this complex relationship that cannot be fully accounted for, multivariable analysis and restricted cubic splines suggest the threshold definition of a high-volume esophagectomy center as one that performs at least 10 operations a year; however, increasing center volume beyond 30 cases per year may have greater implications on reducing failure to rescue.
Supplementary Material
Acknowledgements
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Funding:
K. Rhodin is supported by NIH 5T32CA093245–15 in surgical oncology.
Footnotes
Conference Presentation: Oral presentation on May 17th, 2022, at the American Association of Thoracic Surgeons (AATS) 102nd Annual Meeting, Boston, MA
References
- 1.Sakamoto T, Fujiogi M, Matsui H, et al. Comparing Perioperative Mortality and Morbidity of Minimally Invasive Esophagectomy Versus Open Esophagectomy for Esophageal Cancer: A Nationwide Retrospective Analysis. Ann Surg Aug 1 2021;274(2):324–330. doi: 10.1097/sla.0000000000003500 [DOI] [PubMed] [Google Scholar]
- 2.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol Nov 2010;17(11):2824–31. doi: 10.1245/s10434-010-1159-0 [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Goodney PP, Orringer MB, Birkmeyer JD. Specialty Training and Mortality After Esophageal Cancer Resection. Ann Thorac Surg 2005/07/01/ 2005;80(1):282–286. doi: 10.1016/j.athoracsur.2005.01.044 [DOI] [PubMed] [Google Scholar]
- 4.Learn PA, Bach PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care Dec 2010;48(12):1041–9. doi: 10.1097/MLR.0b013e3181f37d5f [DOI] [PubMed] [Google Scholar]
- 5.Wouters MWJM, Karim-Kos HE, le Cessie S, et al. Centralization of Esophageal Cancer Surgery: Does It Improve Clinical Outcome? Ann Surg Oncol 2009/07/01 2009;16(7):1789–1798. doi: 10.1245/s10434-009-0458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med Apr 11 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337 [DOI] [PubMed] [Google Scholar]
- 7.Patel DC, Jeffrey Yang C-F, He H, et al. Influence of facility volume on long-term survival of patients undergoing esophagectomy for esophageal cancer. J Thorac Cardiovasc Surg doi: 10.1016/j.jtcvs.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 8.Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg May 2011;253(5):912–7. doi: 10.1097/SLA.0b013e318213862f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leapfrog Group. Complex Adult and Pediatric Surgery. Accessed January 31, 2022. https://ratings.leapfroggroup.org/measure/hospital/complex-adult-and-pediatric-surgery
- 10.Varghese TK Jr, Wood DE, Farjah F, et al. Variation in esophagectomy outcomes in hospitals meeting Leapfrog volume outcome standards. Ann Thorac Surg Apr 2011;91(4):1003–9; discussion 1009–10. doi: 10.1016/j.athoracsur.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surg Jun 2004;135(6):569–75. doi: 10.1016/j.surg.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Guide to inpatient quality indicators. Accessed February 3, 2022. https://qualityindicators.ahrq.gov/Downloads/Modules/IQI/V21/iqi_guide_rev4.pdf
- 13.Meguid RA, Weiss ES, Chang DC, et al. The effect of volume on esophageal cancer resections: what constitutes acceptable resection volumes for centers of excellence? J Thorac Cardiovasc Surg Jan 2009;137(1):23–9. doi: 10.1016/j.jtcvs.2008.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice TW, Blackstone EH. Esophagectomy volume threshold as a criterion for centers of excellence: causation or cause, strategy or strategem? J Thorac Cardiovasc Surg Jan 2009;137(1):10–2. doi: 10.1016/j.jtcvs.2008.06.041 [DOI] [PubMed] [Google Scholar]
- 15.Rodgers M, Jobe BA, O’Rourke RW, et al. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg Sep 2007;142(9):829–39. doi: 10.1001/archsurg.142.9.829 [DOI] [PubMed] [Google Scholar]
- 16.Winchester DP, Stewart AK, Phillips JL, Ward EE. The National Cancer Data Base: Past, Present, and Future. Ann Surg Oncol 2010/01/01 2010;17(1):4–7. doi: 10.1245/s10434-009-0771-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol Mar 2008;15(3):683–90. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol Jun 15 2009;99(8):488–90. doi: 10.1002/jso.21173 [DOI] [PubMed] [Google Scholar]
- 19.Hothorn T Package ‘maxstat’. Accessed February 14, 2022. https://cran.r-project.org/web/packages/maxstat/maxstat.pdf
- 20.Croxford R Restricted Cubic Spline Regression: A Brief Introduction. Accessed February 9, 2022. https://support.sas.com/resources/papers/proceedings16/5621-2016.pdf
- 21.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67(1):2–77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 22.Scali ST, Beck AW, Sedrakyan A, et al. Hospital Volume Association With Abdominal Aortic Aneurysm Repair Mortality. Circulation. 2019;140(15):1285–1287. doi:doi: 10.1161/CIRCULATIONAHA.119.042504 [DOI] [PubMed] [Google Scholar]
- 23.Panni RZ, Panni UY, Liu J, et al. Re-defining a high volume center for pancreaticoduodenectomy. HPB (Oxford). May 2021;23(5):733–738. doi: 10.1016/j.hpb.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Ihse I The Volume-Outcome Relationship in Cancer Surgery: A Hard Sell. Ann Surg 2003;238(6):777–781. doi: 10.1097/01.sla.0000098616.19622.af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimminger PP, Goense L, Gockel I, et al. Diagnosis, assessment, and management of surgical complications following esophagectomy. Ann N Y Acad Sci Dec 2018;1434(1):254–273. doi: 10.1111/nyas.13920 [DOI] [PubMed] [Google Scholar]
- 26.Abdelsattar ZM, Habermann E, Borah BJ, Moriarty JP, Rojas RL, Blackmon SH. Understanding Failure to Rescue After Esophagectomy in the United States. Ann Thorac Surg Mar 2020;109(3):865–871. doi: 10.1016/j.athoracsur.2019.09.044 [DOI] [PubMed] [Google Scholar]
- 27.Liou DZ, Serna-Gallegos D, Mirocha J, Bairamian V, Alban RF, Soukiasian HJ. Predictors of Failure to Rescue After Esophagectomy. Ann Thorac Surg Mar 2018;105(3):871–878. doi: 10.1016/j.athoracsur.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 28.Rhodin KE, Raman V, Jawitz OK, Tong BC, Harpole DH, D’Amico TA. The Effect of Timing of Adjuvant Therapy on Survival After Esophagectomy. Ann Thorac Surg Sep 2020;110(3):1023–1029. doi: 10.1016/j.athoracsur.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi B, Jenkinson R, Austin PC, et al. Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study. BMJ : British Medical Journal. 2014;348:g3284. doi: 10.1136/bmj.g3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jawitz NG, Raman V, Jawitz OK, et al. Utilization Trends and Volume-Outcomes Relationship of Endoscopic Resection for Early Stage Esophageal Cancer. Ann Surg Mar 3 2021;doi: 10.1097/sla.0000000000004834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raman V, Jawitz OK, Cerullo M, et al. Tumor Size, Histology, and Survival after Stereotactic Ablative Radiotherapy and Sublobar Resection in Node-negative Non-small Cell Lung Cancer. Ann Surg Jan 7 2021;Publish Ahead of Printdoi: 10.1097/sla.0000000000004730 [DOI] [PubMed] [Google Scholar]
- 32.Raman V, Jawitz OK, Farrow NE, et al. The Relationship Between Lymph Node Ratio and Survival Benefit With Adjuvant Chemotherapy in Node-positive Esophageal Adenocarcinoma. Ann Surg Mar 1 2022;275(3):e562–e567. doi: 10.1097/sla.0000000000004150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raman V, Jawitz OK, Voigt SL, et al. The Effect of Tumor Size and Histologic Findings on Outcomes After Segmentectomy vs Lobectomy for Clinically Node-Negative Non-Small Cell Lung Cancer. Chest Jan 2021;159(1):390–400. doi: 10.1016/j.chest.2020.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman V, Jawitz OK, Voigt SL, et al. Effect of time to surgery on outcomes in stage I esophageal adenocarcinoma. J Thorac Cardiovasc Surg Apr 2020;159(4):1626–1635.e1. doi: 10.1016/j.jtcvs.2019.09.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier J, Wu QV, Gooley TA. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplantation. 2020/04/01 2020;55(4):675–680. doi: 10.1038/s41409-019-0679-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


