Abstract
The lacrimal gland undergoes significant structural and functional deterioration with aging. Marked with increased inflammation and fibrosis, the aged lacrimal gland is unable to perform its protective function. As a result, the ocular surface becomes highly susceptible to various ocular surface pathologies, including corneal epitheliopathy. We and others have previously shown that mast cells mediate tissue inflammation by recruiting other immune cells. However, despite their well-known characteristics of secreting various inflammatory mediators, whether mast cells contribute to the immune cell aggregation and activation, and acinar dystrophy of the aged lacrimal gland has not been investigated. Here, we demonstrate the role of mast cells in age-related lacrimal gland pathophysiology using mast cell-deficient (cKitw-sh) mice. Our data demonstrated a significant increase in mast cell frequencies and immune cell infiltration in the lacrimal gland of aged mice. Interestingly, mast cell deficiency resulted in a substantial reduction in inflammation and preservation of lacrimal gland structure, suggesting that mast cells mediate the aging process of the lacrimal gland.
Subject terms: Ageing, Corneal diseases
Introduction
Aging is a process defined by the conglomeration of physiological changes that lead to the deterioration of functional properties at the cellular, tissue, and organ level1. Often associated with the dysregulation of the immune system, aging increases the risk of many disorders2,3. During aging, lacrimal glands undergo significant structural alterations resulting in ductal obstruction and subsequent acinar atrophy and fibrosis4. In conjunction with structural changes, aged lacrimal glands show a marked increase in lymphocytic infiltration5. Lacrimal gland, a major component of the lacrimal functional unit, plays a critical role in maintaining tear film homeostasis1,6. Accumulation of structural damage and immune dysregulation hampers lacrimal gland function and results in various ocular pathologies, including dry eye disease and corneal epitheliopathy.
Mast cells, tissue-resident immune cells, are distributed throughout most organs in the body, including glandular tissues such as the lacrimal gland7,8. Beyond their well-characterized role in allergic immune responses, mast cells are now recognized as multifunctional effector immune cells9–11 and have been associated with various pathological conditions, including fibrotic disease12,13 and chronic inflammation14,15. By releasing preformed and de novo synthesized inflammatory mediators, mast cells contribute to the acute inflammatory response and the progression of chronic diseases11. Recently, several studies have shown a significant increase in plasma levels of inflammatory mediators, cytokines, and acute-phase proteins in aged patients16,17. Organ senescence, in essence, results from chronic stimulation of both innate and adaptive immune systems18,19. Despite the unique characteristics of mast cells in secreting various inflammatory mediators and cytokines, few studies have explored the contribution of mast cells in aging.
In this study, we utilized mast cell-deficient cKitw-sh mice to assess the contribution of mast cells in age-related lacrimal gland deterioration. Our data show that the frequency and activation of mast cells are significantly upregulated in the lacrimal gland of aged mice in conjunction with inflammation and acinar atrophy. However, aged cKitw-sh mice did not display age-related deterioration of glandular acini nor significant immune cell infiltration. In addition, significantly less age-related corneal epitheliopathy was observed in mast cell-deficient mice, suggesting that mast cells orchestrate inflammation-mediated lacrimal gland dystrophy and subsequent functional dysregulation observed during aging.
Results
Lacrimal glands of aged mice show increased mast cell frequencies
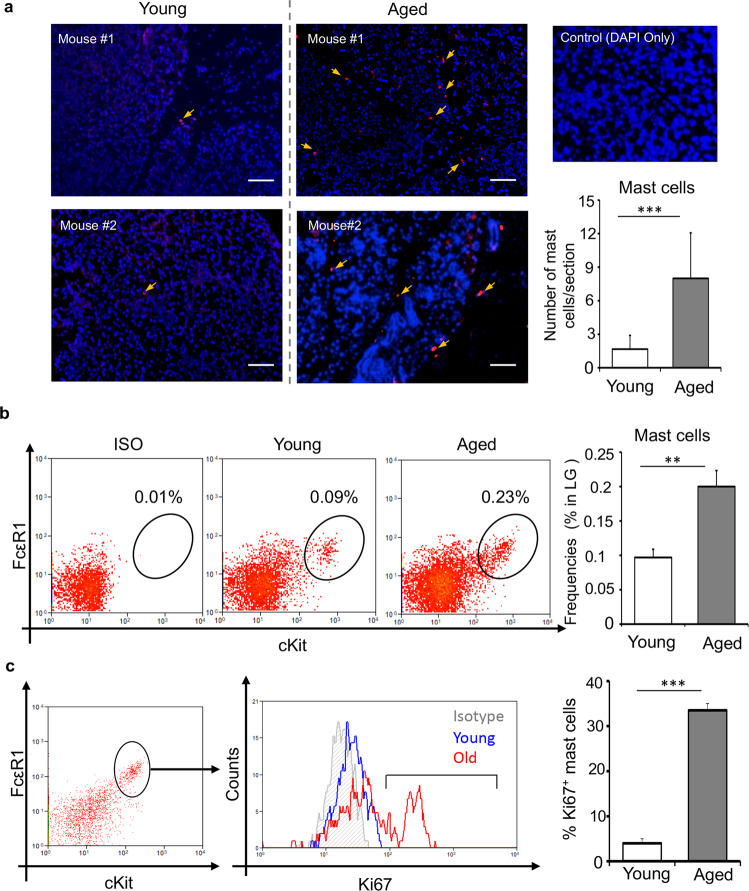
To study the infiltration and distribution of mast cells in the lacrimal gland, young and aged C57BL/6 mice were euthanized to harvest the lacrimal glands. The lacrimal glands were immunostained with mast cell-specific avidin (TexasRed)20. Lacrimal glands of young mice showed a fewer number of mast cells; however, a significant increase in mast cell number was observed predominantly in the interlobular space of the lacrimal glands of the aged mice (p < 0.001) (Fig. 1a). To further confirm our observation, we studied the frequencies of mast cell infiltration into the lacrimal gland of aged mice using flow cytometry. Single-cell suspensions were prepared from the lacrimal gland of young and aged mice and immunostained with FcεR1 and cKit antibodies. Consistent with our immunohistochemistry data, flow cytometry analysis demonstrated a significant increase in FcεR1+cKit+ mast cell frequencies in the lacrimal gland of aged mice, relative to that of young mice (p < 0.01) (Fig. 1b). Interestingly, a higher percentage of mast cells expressed Ki67 in the lacrimal gland of aged mice, compared to young controls (p < 0.001), suggesting that the increase in mast cell frequencies is due to higher proliferation (Fig. 1c). Taken together, our data indicate that frequency of mast cells increases in the lacrimal gland with age and that mast cells are located mainly in the interlobular space.
Fig. 1. Increased mast cell frequencies in the lacrimal glands of aged mice.
a Representative immunohistochemistry micrographs (left) and cumulative bar chart (right) showing mast cell distribution per lacrimal gland cross-section (Scale bar, 100 µm). Lacrimal gland of C57BL/6 mice were immunostained with fluorescent-conjugated avidin (Texas Red) showing mast cells (yellow arrows). b Representative flow cytometry dot plots (left) and cumulative bar chart (right) showing the frequencies of FcεR1+cKit+ mast cells, gated on CD45+ cells (Gating strategy in Supplementary Fig. S1), in the lacrimal gland of young and aged C57BL/6 mice, compared to isotype-stained control. c Representative flow cytometry histogram (left) and cumulative bar chart (right) showing frequencies of Ki67+ mast cells in the lacrimal gland of young and aged mice, compared to isotype-stained control. Cumulative data (mean ± SD) from three independent experiments are shown, with each experiment consisting of n of 4 to 6 mice/group. **p < 0.01, ***p < 0.001.
Aged mice show increased activation of mast cells at the lacrimal gland and the ocular surface
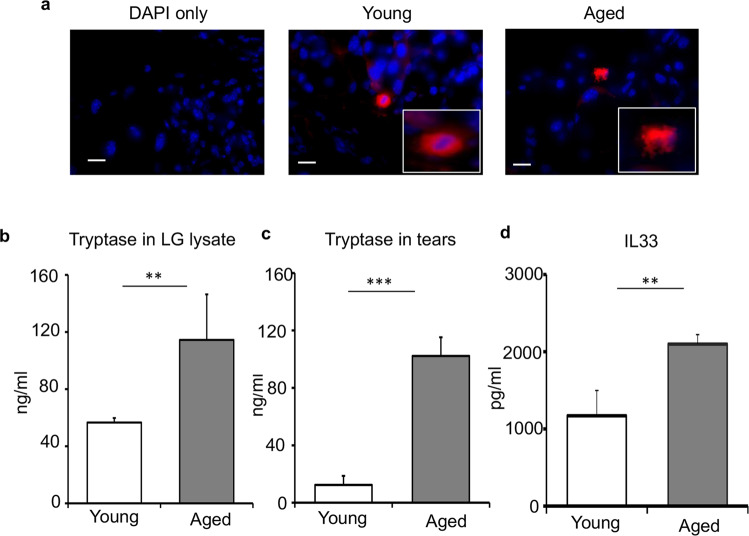
Having observed increased frequencies of mast cells in the lacrimal gland of aged mice, we next sought to determine the effect of aging on mast cell function. Avidin-stained cross-sections of lacrimal glands, harvested from aged mice, showed mast cells undergoing degranulation, as visualized by the release of avidin-stained heparin into the extracellular space (Fig. 2a)20. However, this pattern of degranulation was not observed in the lacrimal gland of young mice (Fig. 2a). To further evaluate mast cell activation, total tryptase levels in the lacrimal gland lysates were quantified. Lacrimal glands of aged mice showed a significant 2.5-fold higher level of tryptase as compared to that of young mice (p < 0.01) (Fig. 2b). Consistent with increased mast cell activation in the lacrimal gland, we observed a significant 4-fold higher secretion of tryptase in the tear wash collected from the ocular surface of aged mice relative to young mice (p < 0.001) (Fig. 2c). Furthermore, we observed an increase in the expression of interleukin 33 (IL33), a potent activator of mast cells9, in the lacrimal gland of aged mice, compared to young controls (p < 0.01) (Fig. 2d). Taken together, these data suggest that aging results in higher and persistent activation of mast cells.
Fig. 2. Increased mast cell activation in the lacrimal gland and at the ocular surface of aged mice.
a Representative immunohistochemistry micrographs of young and aged C57BL/6 mice lacrimal glands stained with fluorescent-conjugated avidin (Texas Red), capturing degranulating mast cell in the lacrimal gland of aged mice (scale bar, 10 µm). b Bar chart depicting tryptase levels in the lacrimal gland (LG) lysates of young and aged mice. c Bar chart quantifying levels of tryptase in ocular surface tear wash. Ocular surface tear wash of young and aged mice was collected, and mast cell activation was assessed by measuring the levels of tryptase. d Bar chart depicting levels of IL33 in the lacrimal gland lysates of young and aged mice. Cumulative data (mean ± SD) from three independent experiments are shown, with each experiment consisting of n of 4 to 6 mice/group. **p < 0.01, ***p < 0.001.
Aged mice exhibit inflammatory lacrimal gland and corneal epitheliopathy
Upon mast cell activation, mast cells release a plethora of preformed inflammatory mediators that cause tissue damage21. Given our observation of increased activation of mast cells and previous publications showing increased inflammation in aged lacrimal glands22, we sought to investigate whether age-mediated changes in the inflammatory milieu result in structural and functional changes. H&E analysis of lacrimal gland cross-sections showed an increase in immune cell foci in aged mice compared to young mice. In addition, substantial tissue fibrosis and acinar atrophy were observed in the lacrimal gland of aged mice (Fig. 3a). Exacerbation of inflammation in aged mice was further confirmed by the elevated levels of the proinflammatory cytokine interleukin 1β (IL1β) in lacrimal gland lysates, as evaluated by ELISA analysis. Indeed, a 3-fold increase in IL1β levels in lysates was observed in the aged lacrimal glands (p < 0.001) (Fig. 3b), relative to young controls. These data show that aging exacerbates inflammation in the lacrimal gland and disrupts the glandular structure.
Fig. 3. Aged mice showing increased immune cell infiltration in the lacrimal gland and corneal epitheliopathy.
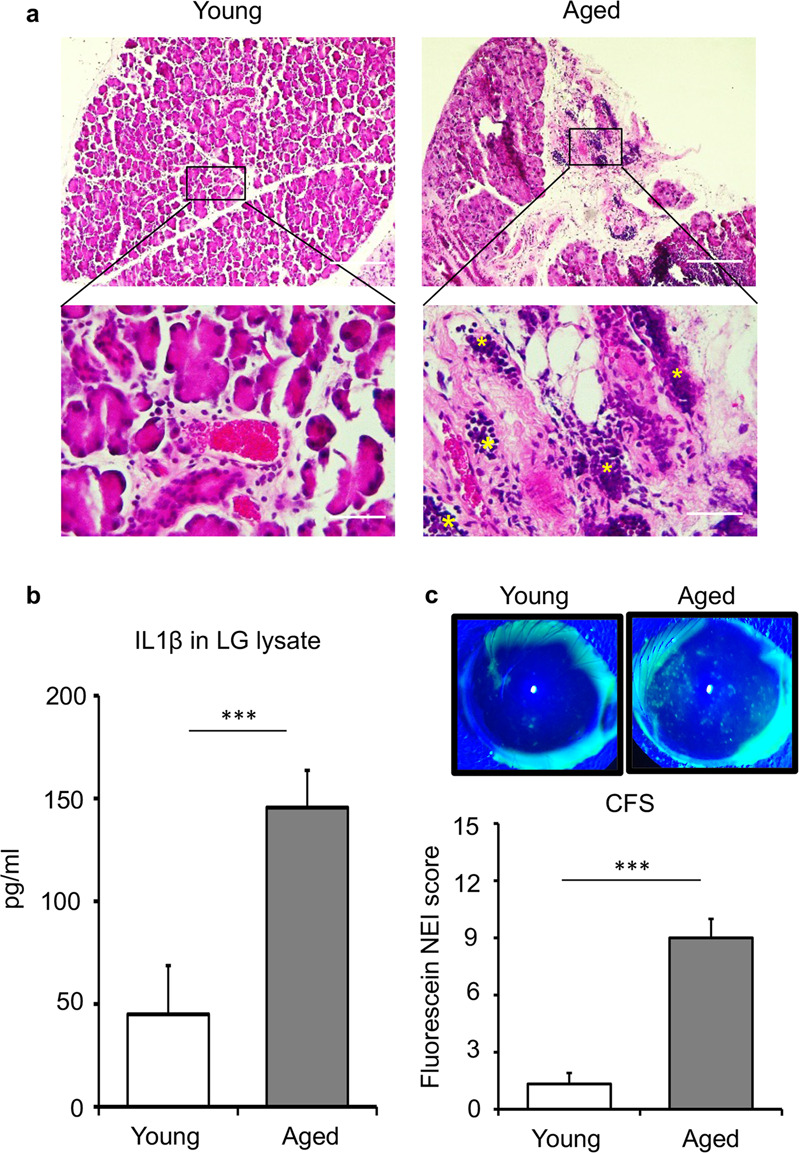
a Cross-sections of young and aged C57BL/6 mice lacrimal glands were stained with hematoxylin and eosin to visualize immune cell foci (yellow stars) and acinar atrophy (scale bar, 100 µm (upper); 20 µm (lower)). b Bar chart depicting protein levels of IL1β in the lacrimal gland lysates of young and aged mice, using ELISA analysis. c Representative slit-lamp images (upper panel) and cumulative bar chart (lower panel) measuring corneal fluorescein-staining (CFS) of young and aged mice. Cumulative data (mean ± SD) from three independent experiments are shown, with each experiment consisting of n of 4 to 6 mice/group. ***p < 0.001.
The lacrimal gland is a key organ in maintaining ocular surface homeostasis1,6; thus, we sought to investigate the effect of the aged lacrimal gland on the ocular surface. To evaluate corneal epitheliopathy, fluorescein staining was performed, and images were analyzed using a standardized National Eye Institute (NEI) fluorescein staining score23. Our data demonstrate a significant increase in the punctated fluorescein staining in the corneas of aged mice relative to that of young mice (p < 0.001) (Fig. 3c). These data suggest that the increase in immune foci and acinar atrophy in the lacrimal gland of aged mice is associated with an increase in corneal epitheliopathy.
Aged mast cell-deficient mice exhibit less lacrimal gland immune cell infiltration
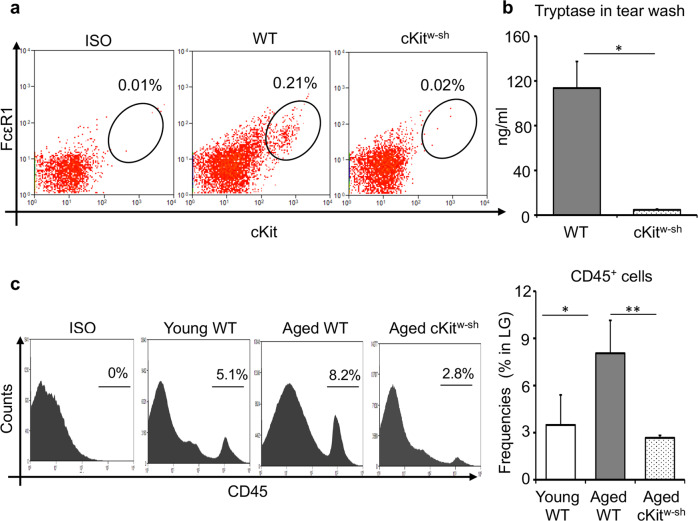
Having demonstrated that mast cells increase in frequency and activation in the lacrimal gland of aged mice, we next sought to determine whether mast cells contribute to the age-related high immune cell infiltration of the lacrimal gland by utilizing mast cell-deficient cKitw-sh mice. Our flow cytometry analysis confirmed the deficiency of FcεR1+cKit+ mast cells in the lacrimal gland of cKitw-sh mice (Fig. 4a). Consistently, negligible levels of tryptase were observed in the tears of cKitw-sh mice, relative to age-matched wild-type C57BL/6 mice (Fig. 4b). To evaluate the total number of leukocytes in the lacrimal gland of aged cKitw-sh mice, single-cell suspensions of the lacrimal gland were stained with anti-CD45 antibody for flow cytometry analysis. A significant increase in the total CD45+ immune cell frequencies was observed in the lacrimal gland of aged wild-type mice compared to young wild-type controls (p < 0.05). However, this increase was not observed in the lacrimal gland of mast cell-deficient mice, as demonstrated by the significant reduction in the frequencies of CD45+ immune cells (Fig. 4c). These data demonstrate that mast cell deficiency results in a significant reduction in the total immune cell infiltration into the lacrimal gland of aged mice.
Fig. 4. Reduced infiltration of immune cells in the lacrimal gland of aged mast-deficient mice.
a Representative flow cytometry dot plots confirming the deficiency of FcεR1+cKit+ mast cells, gated on CD45+ cells (Gating strategy in Supplementary Fig. S1), in the lacrimal glands of cKitw-sh mice, compared to wildtype C57BL/6 control (WT). b Bar chart depicting tryptase levels in tear wash collected from the ocular surface of WT and cKitw-sh mice. c Representative flow cytometry histogram (left) and bar chart depicting frequencies of total CD45+ leukocyte in the lacrimal gland of young and aged WT C57BL/6, and aged cKitw-sh mice (Gating strategy in Supplementary Fig. S1). Cumulative data (mean ± SD) from three independent experiments are shown, with each experiment consisting of n of 4 to 6 mice/group. *p < 0.05, **p < 0.01.
Mast cell deficiency prevents age-associated acinar atrophy and corneal epitheliopathy
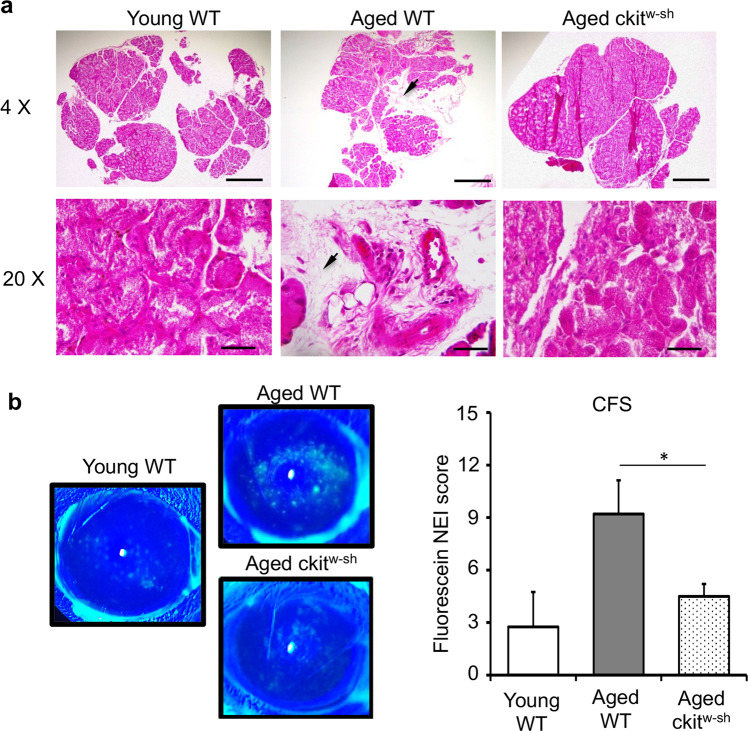
Finally, to confirm the contribution of mast cells in promoting age-related lacrimal gland atrophy and subsequent corneal epitheliopathy, we first evaluated the lacrimal gland tissue structure in mast cell-deficient mice. Unlike the significant loss of tissue architecture and excessive immune cell infiltration observed in the lacrimal gland of aged wildtype mice, lacrimal gland cross-sections of age-matched cKitw-sh mice, stained with H&E, showed preserved gland acini and a lack of immune cells (Fig. 5a). Finally, we investigated whether mast cell deficiency affects age-related corneal epitheliopathy using corneal fluorescein-staining. A significantly smaller area of damaged epithelium was observed in the mast cell-deficient mice, compared to age-matched controls (p < 0.05) (Fig. 5b). Collectively, our data demonstrate that mast cell deficiency results in the abrogation of the age-related lacrimal gland atrophy and subsequent corneal epitheliopathy.
Fig. 5. Decreased acinar atrophy and corneal epitheliopathy in aged mast-cell deficient mice.
a Cross-sections of lacrimal glands of young and aged wildtype C57BL/6 (WT), and aged cKitW-sh mice stained with hematoxylin and eosin to visualize tissue atrophy (black arrow) and immune cell infiltration (scale bar, 500 µm (upper); 50 µm (lower)). b Representative slit-lamp images (left) and cumulative bar chart (right) quantifying corneal fluorescein staining (CFS) of the indicated mice. Cumulative data (mean ± SD) from three independent experiments are shown, with each experiment consisting of n of 4 to 6 mice/group. *p < 0.05.
Discussion
Aging is a complex natural process that involves every molecule, cell, and organ in the body24. Behavioral changes and exposure to environmental risk factors further contribute to the aging process25. The ocular surface is particularly prone to external environmental exposure as it constantly interfaces the environment. In the lacrimal gland, aging results in an increase in tissue inflammation, which subsequently leads to acinar atrophy and fibrosis1. Aged lacrimal gland ridden with dilated and tortuous secretory ducts have pronounced ductal obstruction4,5. We have previously reported that ocular surface mast cells are the first responders to initiate the onset of acute inflammation9,26. In the current study, we observed that the age-related increase in lacrimal gland inflammation is associated with an increase in mast cell frequency. Furthermore, our data show the degranulation of activated mast cells in the lacrimal gland of aged mice, as evidenced by an increase in the tryptase levels in the lacrimal gland and at the ocular surface. Moreover, mast cell deficiency resulted in reduced age-related acinar atrophy and corneal epitheliopathy.
Mast cells, renowned for their role in the allergic immune response, release a plethora of preformed and newly synthesized cytokines and chemokines that recruit a variety of immune cells to the site of allergen exposure27,28. Recently, using models of non-allergic inflammation, we and others have reported various ocular pathologies result from non-IgE-mediated activation of mast cells9,29,30. As long-lived immune cells, mast cells proliferate in its resident microenvironment and have been shown to increase with aging in the skin31. Given the inflammatory function of ocular surface mast cells in mediating tissue damage, our current study investigated the role of mast cells in aged-related pathological deterioration of the lacrimal gland. We observed that mast cells increase in frequency in the lacrimal gland of aged mice and are localized mainly in the interlobular space. Of note, Ki67 nuclear antigen positivity of mast cells in aged lacrimal gland suggests the increased frequencies are primarily due to their active proliferation in the lacrimal gland.
Exposure to environmental stressors have been shown to activate stress-sensing neural pathways to release neuromediators, such as Substance P and calcitonin gene-related peptide (CGRP), which promote ocular inflammation32–34. Interestingly, we observed an increase in the expression of IL33, a danger-associated molecular pattern (DAMP)9, in the lacrimal gland of aged mice. Recent reports have shown that DAMPs are associated with sterile inflammation caused by aging35–37. Our observation of high levels of IL33 in the lacrimal gland and unique barrier function of ocular surface, suggest the external stressors contribute to the high activation of mast cells in aged lacrimal gland.
The lacrimal glands, along with the ocular surface, constitute the lacrimal functional unit to maintain tear film homeostasis and corneal integrity38,39. Thus, the dysfunction of the lacrimal gland results in various epitheliopathic disorders, including dry eye disease38,40. In the clinic, the population of patients suffering from dry eye disease substantially increases with age, suggesting that the aged lacrimal gland, marked with fibrosis, significantly impairs ocular surface homeostasis41,42. Consistent with previous reports1,22, we observed various morphological changes in the structure of the lacrimal gland of aged mice, such as periductal fibrosis and acinar atrophy. Interestingly, structural damage observed in the aged lacrimal gland was accompanied by excessive infiltration of immune cells. Given the increase in mast cell activation with aging, our data collectively suggest that mast cells contribute to the inflammation-induced structural damage of aged lacrimal gland.
To confirm the contribution of mast cells in age-related alterations of lacrimal gland structure and the loss of corneal integrity, we utilized aged mast cell-deficient cKitw-sh mice43 and studied the morphological alteration and inflammation in the lacrimal gland. Mast cell deficiency resulted in a significant reduction in the age-related infiltration of immune cells into the lacrimal glands5. Specifically, we observed a dramatic downregulation of total CD45+ immune cell infiltration in the lacrimal gland of aged cKitw-sh mice. Moreover, no age-related acinar atrophy and glandular fibrosis were observed in cKitw-sh mice compared to age-matched wild-type controls. Our experiments using mast cell-deficient mice strongly indicate that mast cells are crucial for immune cell recruitment to the aged lacrimal gland and subsequent acinar atrophy. With the preservation of lacrimal gland structure, mast cell-deficient mice did not display the aged-mediated loss of corneal integrity, as evidenced by the absence of corneal epitheliopathy.
In summary, our findings provide insights into the role of mast cells in age-associated chronic inflammation and tissue dystrophy. Aged lacrimal glands, displaying structural atrophy and fibrosis, exhibit increased mast cell frequencies and activation. Furthermore, abrogation of age-related inflammation in the lacrimal glands of mast cell-deficient mice suggests mast cells are major mediators of chronic inflammation in aged lacrimal glands.
Methods
Animals
Six-to 8-week-old (young) and 8-to-12 month-old (aged) C57BL/6 wild-type and age-matched mast cell-deficient cKitw-sh mice, congenic to C57BL/6 mice, (Stock No: 012861) were purchased from the Jackson Laboratory (Bar Harbor, ME) for the described experiments. cKitw-sh mice were confirmed for their deficiency in mast cells at the ocular surface and the lacrimal gland using flow cytometry. cKitw-sh mice, unlike the other mast cell-deficient strain (cKitw-v), have a comparable generation of CD45+ immune cells in the bone marrow9,43. The mice were housed in the Schepens Eye Research Institute animal vivarium. All experiments were reviewed and approved by the Schepens Eye Research Institute Animal Care and Use Committee. The mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Lacrimal gland tissue harvesting
Carbon dioxide (CO2) inhalation was used to euthanize the mice to harvest the lacrimal glands. The main extraorbital lacrimal gland was accessed through an incision made between the lateral commissure of the eye and ear. By using forceps, the gland was separated from the parotid gland and exteriorized and harvested.
Lacrimal gland tissue digestion
Single-cell suspensions were prepared from lacrimal glands as previously described44. The lacrimal glands were digested in collagenase digestion solution (DMEM media containing 464 U/ml of Collagenase II, 8 U/mL of DNAse I, 2 mM L-glutamine, 1% non-essential amino acids and 1% penicillin/streptomycin) (Lonza, Walkersville, MD, USA) for 50 minutes at 37 °C and 100 RPM. During the digestion, the tissue was broken up by triturating every 15 minutes with increasingly smaller pipette tips. The digested tissue was filtered through a 100 µm mesh and washed two times with 2 mM EDTA in Ca2+ and Mg2+ -free phosphate-buffered saline (PBS) to stop collagenase digestion. Next, digested tissue was incubated with TrypLE Express (Invitrogen, Carlsbad, CA) for 2 minutes at 37 °C to prepare single cells. Thereafter, TrypLE Express was diluted with 1 U/mL DNAse I in DMEM for 3 min followed by washing with 10% fetal bovine serum (FBS) in DMEM to inactivate any residual trypsin activity.
Flow cytometry
Single-cell suspensions were stained with fluorochrome-conjugated anti-CD45 (1:100; Cat# 103105), -cKit (1:100; Cat# 105811), -FcεR1 (1:100; Cat# 103105) or -Ki67 (1:100; Cat# 151211) antibodies. Isotypes used were PE Rat IgG2b, κ Isotype Ctrl (Cat# 400607), APC Rat IgG2b, κ Isotype Ctrl (Cat# 400611), Pacific Blue Armenian Hamster IgG Isotype Ctrl (Cat# 400925), and FITC Rat IgG2b, κ Isotype Ctrl (Cat# 400633)45,46. Antibodies and respective isotype controls were purchased from BioLegend (San Diego, CA, USA). LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and Summit software (Dako Colorado, Inc., Fort Collins, CO, USA) were used to acquire and analyze the cells.
Tryptase assays
Mast Cell Degranulation Assay Kit (Sigma–Aldrich) were used to quantify levels of tryptase47. The kit detects the chromophore p-nitroaniline (pNA) cleaved from the labeled substrate tosyl-gly-pro-lys-pNA. In brief, the lacrimal gland lysates and the ocular surface tear wash were incubated with 0.1 mg/mL tosyl-gly-pro-lys-pNA (substrate) for 2 hours at 37 °C. A SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Jose, CA, USA) was used to quantify free pNA at 405 nm.
Enzyme-linked immunosorbent assay (ELISA)
The lacrimal glands harvested from young and aged mice were lysed in PBS by three complete freeze-thaw cycles (−80 °C for 10 minutes, 37 oC water bath for 5 minutes). Levels of IL1β and IL33 in the lacrimal gland lysates were quantified using commercially available ELISA kits (BioLegend, San Diego, CA, USA), as per the manufacturer’s instructions.
Immunohistochemistry
The lacrimal glands were harvested and formalin-fixed paraffin-embedded (FFPE) sections (4 µm) were prepared and blocked with 2% BSA and anti-FcR antibodies (catalog #14-0161-86, Affymetrix eBioscience)48,49. Sections were stained with avidin-TexasRed (ThermoFisher) overnight at 4 °C. Slides were then mounted using DAPI-containing VECTASHIELD® mounting medium (Vector Laboratories) and examined under a fluorescence microscope (Nikon Eclipse E800; Nikon Instruments, Melville, NY, USA).
Histology
Cross-sections were prepared from formalin-fixed lacrimal glands harvested from young and aged mice, as previously described22. Briefly, the harvested lacrimal gland were fixed in 10% formalin and cross-sectioned in 4 µm thickness. The sections were stained with hematoxylin and eosin to visualize tissue morphology. Corneal tissue structure was analyzed under a brightfield microscope (Nikon Eclipse E800; Nikon Instruments, Melville, NY, USA).
Slit-lamp microscopy and corneal epitheliopathy scoring
Corneal epitheliopathy was examined by applying 1 μL of 2.5% sodium fluorescein (vital staining) on the ocular surface using a micropipette. After 3 minutes, the ocular surface was visualized using a slit-lamp biomicroscope under cobalt blue light and digital images of corneal epithelial defects were taken. The degree of corneal epitheliopathy was scored according to the National Eye Institute (NEI) grading scale. In brief, the NEI grading scale consists of a grid that divides the corneal area into five sections, each of which is assigned a score between 0 and 3 depending on the amount and distribution of the corneal fluorescein staining (CFS); the total CFS score ranges from 0/15 (absence of corneal epitheliopathy) to 15/15 (severe epitheliopathy).
Statistical analysis
Unpaired two-tailed Student t-test was used to compare means between two groups. The significance level was set at p < 0.05. Data are presented as means ± standard deviations of three independent experiments. Samples sizes were estimated on the basis of previous experimental studies using mast cell deficient cKitw-sh mice and sterile inflammation9,45,47.
Supplementary information
Acknowledgements
This work was supported by the National Institutes of Health grants R01EY029727 and P30EY003790.
Author contributions
E.E. contributed the underlying hypothesis, designed and performed the experiments, analyzed the data and wrote the manuscript. W.C. assisted in writing and revising manuscript. S.K.M. assisted in designing the experiment, data analysis and manuscript writing. A.S. assisted in data analysis. D.Z. assisted in writing and reviewing the manuscript. S.K.C. contributed the underlying hypothesis, designed the study, analyzed data, and wrote the manuscript.
Data availability
Included in article: The data that support the findings of this study are available in the methods of this article. Detailed data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41514-023-00099-0.
References
- 1.Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul. Surf. 2008;6:162. doi: 10.1016/S1542-0124(12)70177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid. Redox Signal. 2011;14:1551. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller L, di Benedetto S, Pawelec G. The immune system and its dysregulation with aging. Subcell Biochem. 2019;91:21–43. doi: 10.1007/978-981-13-3681-2_2. [DOI] [PubMed] [Google Scholar]
- 4.Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology. 1995;102:678–686. doi: 10.1016/S0161-6420(95)30971-2. [DOI] [PubMed] [Google Scholar]
- 5.Damato B, Allan D, Murray S, Lee W. Senile atrophy of the human lacrimal gland: the contribution of chronic inflammatory disease. Br. J. Ophthalmol. 1984;68:674–680. doi: 10.1136/bjo.68.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dartt D, Willcox M. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013;117:1. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribatti D, Crivellato E. The role of mast cell in tissue morphogenesis. Thymus, duodenum, and mammary gland as examples. Exp. Cell Res. 2016;341:105–109. doi: 10.1016/j.yexcr.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Williams RM, Singh J, Sharkey KA. Innervation and mast cells of the rat lacrimal gland: The effects of age. Adv. Exp. Med. Biol. 1994;350:67–74. doi: 10.1007/978-1-4615-2417-5_12. [DOI] [PubMed] [Google Scholar]
- 9.Elbasiony E, Mittal SK, Foulsham W, Cho WK, Chauhan SK. Epithelium-derived IL-33 activates mast cells to initiate neutrophil recruitment following corneal injury. Ocul. Surf. 2020;18:633–640. doi: 10.1016/j.jtos.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, et al. Mast cells contribute to the induction of ocular mucosal alloimmunity. Am. J. Transplant. 2019;19:662–673. doi: 10.1111/ajt.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front. Immunol. 2016;0:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overed-Sayer C, Rapley L, Mustelin T, Clarke DL. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2014;0:174. doi: 10.3389/fphar.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veerappan A, et al. Mast cells: a pivotal role in pulmonary fibrosis. DNA Cell Biol. 2013;32:206. doi: 10.1089/dna.2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz M, et al. Mast cells in the promotion and limitation of chronic inflammation. Immunol. Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 15.Graziottin A, Skaper S, Fusco M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol. Endocrinol. 2014;30:472–477. doi: 10.3109/09513590.2014.911280. [DOI] [PubMed] [Google Scholar]
- 16.Marcos-Pérez D, et al. Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front Immunol. 2018;0:1056. doi: 10.3389/fimmu.2018.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krabbe, K. S., Pedersen, M. & Bruunsgaard, H. Inflammatory mediators in the elderly. 10.1016/j.exger.2004.01.009. [DOI] [PubMed]
- 18.Fulop T, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2018;0:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawelec G. Hallmarks of human ‘immunosenescence’: adaptation or dysregulation? Immun. Ageing. 2012;9:15. doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tharp MD, Seelig LL, Tigelaar RE, Bergstresser PR. Conjugated avidin binds to mast cell Granules1. J. Histochem. Cytochem. Inc. 1985;33:22–32. doi: 10.1177/33.1.2578142. [DOI] [PubMed] [Google Scholar]
- 21.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ríos JD, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp. Eye Res. 2005;80:477. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemp M. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 24.Harman D. Aging: overview. Ann. N Y Acad. Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 25.de Magalhães Rios JL, et al. Symptoms prevalence among office workers of a sealed versus a non-sealed building: associations to indoor air quality. Environ. Int. 2009;35:1136–1141. doi: 10.1016/j.envint.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Sahu SK, et al. Mast cells initiate the recruitment of neutrophils following ocular surface injury. Invest. Ophthalmol. Vis. Sci. 2018;59:1732–1740. doi: 10.1167/iovs.17-23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and acquired immunity. Eur. J. Immunol. 2010;40:1843. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galli S, et al. Mast cells as ‘tunable’ effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 29.Cho WK, Mittal SK, Elbasiony E, Chauhan SK. Activation of ocular surface mast cells promotes corneal neovascularization. Ocul. Surf. 2020;18:857–864. doi: 10.1016/j.jtos.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Blokhuis B, Garssen J, Redegeld F. Non-IgE mediated mast cell activation. Eur. J. Pharmacol. 2016;778:33–43. doi: 10.1016/j.ejphar.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Pilkington SM, Barron MJ, Watson REB, Griffiths CEM, Bulfone‐Paus S. Aged human skin accumulates mast cells with altered functionality that localize to macrophages and vasoactive intestinal peptide‐positive nerve fibres. Br. J. Dermatol. 2019;180:849. doi: 10.1111/bjd.17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Miguel A, et al. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. Am. J. Ophthalmol. 2014;157:788–798.e2. doi: 10.1016/j.ajo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Singh RB, et al. Modulating the tachykinin: role of substance P and neurokinin receptor expression in ocular surface disorders. Ocul. Surf. 2022;25:142–153. doi: 10.1016/j.jtos.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantelli F, Micera A, Sacchetti M, Bonini S. Neurogenic inflammation of the ocular surface. Curr. Opin. Allergy Clin. Immunol. 2010;10:498–504. doi: 10.1097/ACI.0b013e32833e16cc. [DOI] [PubMed] [Google Scholar]
- 35.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Béraud D, et al. α-Synuclein alters toll-like receptor expression. Front. Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Nagelhout T, Gamache D, Roberts L, Brady M, Yanni J. Preservation of tear film integrity and inhibition of corneal injury by dexamethasone in a rabbit model of lacrimal gland inflammation-induced dry eye. J. Ocul. Pharmacol. Ther. 2005;21:139–148. doi: 10.1089/jop.2005.21.139. [DOI] [PubMed] [Google Scholar]
- 40.Stern M, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Moss S, Klein R, Klein B. Incidence of dry eye in an older population. Arch. Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Hindman HB. Aging: a predisposition to dry eyes. J. Ophthalmol. 2014;2014:781683. doi: 10.1155/2014/781683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimbaldeston MA, et al. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley D, et al. RNA-Seq and CyTOF immuno-profiling of regenerating lacrimal glands identifies a novel subset of cells expressing muscle-related proteins. PLoS One. 2017;12:e0179385. doi: 10.1371/journal.pone.0179385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho WK, Mittal SK, Elbasiony E, Chauhan SK. Ocular surface mast cells promote inflammatory lymphangiogenesis. Microvasc Res. 2022;141:104320. doi: 10.1016/j.mvr.2022.104320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J. Immunol. 2010;185:4691–4697. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho WK, Mittal SK, Elbasiony E, Chauhan SK. Spatial distribution of mast cells regulates asymmetrical angiogenesis at the ocular surface. Am. J. Pathol. 2021;191:1108–1117. doi: 10.1016/j.ajpath.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omoto M, et al. Hepatocyte growth factor suppresses inflammation and promotes epithelium repair in corneal injury. Mol. Ther. 2017;25:1881–1888. doi: 10.1016/j.ymthe.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014;192:817–823. doi: 10.4049/jimmunol.1203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Included in article: The data that support the findings of this study are available in the methods of this article. Detailed data that support the findings of this study are available from the corresponding author upon reasonable request.