1. Introduction
Headache and facial myofascial pain are complex, often overlapping, painful disorders. Biopsychosocial characteristics associated with each disorder may account for the overlap in some individuals.[15,30] In others, headache may occur as a disorder secondary to a temporomandibular disorder (TMD), as defined by the International Classification of Headache Disorders – third edition (ICHD-3),[20]. The distinction among these pain conditions, however, can be difficult due to any of location overlap, common neural pathways, common associated features, and mutual associations with other characteristics. Consequently, clinical decision-making can be challenging.
Concepts and terminology regarding headache attributed to TMD have evolved to now include putatively causal criteria based on the timing of their onset and emphasis for the primary disorder given to painful TMDs[20] and not temporomandibular joint (TMJ) pathology (designated in the prior ICHD-2).[21] However, this shift has been accompanied by uncertainty as to whether headache secondary to TMD is separate from the primary TMD.[5,6,35] Similarly, the close relationship between headache and painful TMD[16] creates uncertainty as to whether this headache secondary to TMD is separate from primary headaches.
In approaching this conundrum, it is useful to distinguish between two types of comorbidity: homotypic comorbidity, which refers to disorders within a diagnostic class, and heterotypic comorbidity, which refers to disorder across different classes.[1] While TMD and primary headaches clearly belong to different disease classes (i.e., reflective of heterotypic comorbidity), it is unclear whether headache attributed to TMD is homotypic or heterotypic to primary headaches. In other words, we questioned whether headache attributed to TMD is simply a rose (i.e., an existing primary headache) by another name? We reasoned that, if headache putatively attributed to TMD shares a majority of attributes with primary headaches, then the putative secondary headache is likely homotypic with the primary headaches, not a truly separate disorder. Conversely, if the attributes are not shared, the putative secondary headache is more likely to be heterotypic to the primary headaches and thereby a separate headache disorder.
This study investigates occurrence of headache and TMD in a community-based sample of U.S. adults. The first aim was to describe the frequency of headache attributed to TMD and of headache that is comorbid (i.e., non-causally associated) with TMD. The second aim investigated whether biopsychosocial risk factors for chronic pain distinguish headache attributed to TMD from headache that is comorbid with TMD. A third reference group of headache without TMD was investigated. We reasoned that if headache attributed to TMD was meaningfully different from headache that is comorbid with TMD, biopsychosocial risk factors for the two conditions should satisfy two criteria: (a) the risk factor should differ appreciably between people with headache attributed to TMD and people with headache that is comorbid with TMD; and (b) the risk factor should differ appreciably between people with headache that is comorbid with TMD and people who have headache in the absence of TMD.
2. Methods
2.1. Study Design, Setting, and Participants.
This cross-sectional study used data collected between December 2014 and May 2016 in the second phase of the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA-II) study.[33] Details on recruitment, data collection, and subsequent follow-up are described in prior publications.[2,32] In brief, recruitment in OPPERA-I of a convenience sample of volunteers was targeted at a population of community dwellers, aged 18 to 44 years, in and around four US academic health centers: University at Buffalo, Buffalo, New York; University of Florida, Gainesville, Florida; University of Maryland, Baltimore, Maryland; and University of North Carolina, Chapel Hill, North Carolina. The second phase included follow-up of 543 OPPERA-1 participants and new enrollment of 127 adults aged 18-74 with recent-onset painful TMD. Nearly all of the participants with recent-onset painful TMD reported prior episodes of painful TMD, with chronicity >6 months.
2.2. Headache and TMD classification.
A pain condition questionnaire ascertained information about headache that aligned with the International Classification of Headache Disorders (ICHD-3) diagnostic criteria.[20] For further assessment of types of headaches in the prior 3 months, the ID-Migraine questionnaire[22] was used to classify migraine while questions based on the ICHD-3 beta[19] were used to classify tension-type headache (TTH). In summary, participants were classified as having migraine when they reported headache(s) on 1 or more day per month with at least two of three symptoms accompanying the headache: nausea, sensitivity to light, or being kept from daily activities. Participants were classified as having TTH if they met criteria for either infrequent, frequent, or chronic TTH but not for probable TTH. For the analyses, headache classification was present or absent, as based on at least one headache meeting criteria for any of these types: migraine alone, TTH alone, or both migraine and TTH.
Classification of TMD was based on the DC/TMD and associated clinical examination.[23,24] Classification of painful TMD (henceforth, “TMD”) required the following five criteria for either myalgia or arthralgia: (1) examiner verification of pain from history in the masseter, temporalis, submandibular, or TMJ areas or headache from history in the temporalis area; (2) pain or headache on 5 or more of the 30 days preceding examination; (3) evoked pain or headache in the same muscles and/or TMJ(s) following palpation of those structures or jaw mobility testing; (4) reported familiarity of evoked pain or headache experienced in the prior 30 days; and (5) pain was modified by jaw function according to history.
Headache attributed to TMD was classified using the DC/TMD, the available standard at the time of data collection, which specifies that all five of the following criteria must be fulfilled:[29] (1) headache location by history included the temple region; (2) temple location of headache confirmed on examination; (3) familiar headache pain was provoked by palpation or jaw mobility testing; (4) headache was modified by function; and (5) presence of myalgia or arthralgia as defined by the DC/TMD.
Based on those classifications, three groups were created to address the study aims: a) headache without TMD, which comprised individuals with any type of primary headache but not painful TMD; b) headache comorbid with TMD, which comprised individuals with headache and a painful TMD but not headache attributed to TMD; and c) headache attributed to TMD, which comprised individuals with a primary headache as well as headache attributed to TMD. The latter two groups are referred to collectively as “combined headache groups”.
2.3. Competing classifications for headache.
The pain condition questionnaire and the medical history questionnaire asked about other headache characteristics permitting the following additional classifications. Cervicogenic headache was classified for individuals who reported at least one of three temporal relation criteria: initial headache disorder onset close to the time when neck or shoulder pain first began, headaches worsen when neck or shoulder pain worsens, and headaches get better when neck or shoulder pain gets better. Rhinosinusitis headache was classified for individuals who met three criteria; a) headache that occurred at the same time as pain or tenderness in the face; b) any sinus symptom of post-nasal drip, sore throat, or cough reported with frequency of at least “often” (within 5-option scale of “never or rarely” to “always”); and c) at least one of three temporal relation criteria (initial headache disorder onset close to the time when any sinus symptom first began, headaches worsen when any sinus symptom worsens, and headaches get better when any sinus symptom gets better). Post-traumatic headache was classified for individuals who reported development or worsening of any type of headache near the time of a head injury.
2.4. Demographic and Comorbidity Characteristics.
Individuals were characterized by age (years), gender (female or male), and race (White or non-Whites). Classification of comorbid pain conditions relied on information from the pain condition questionnaire. Irritable Bowel Syndrome (IBS) was classified based on presence of abdominal discomfort or pain over the 90 days coupled with positive responses to two additional criteria, according to the Rome-III diagnostic criteria.[8] Fibromyalgia was classified based on presence of aches or pains anywhere in the body that lasted for 1 or more days in the prior 90 days and findings of tenderness from tender point examination in opposing quadrants and the spine.[37] Low back pain was classified when regional pain was experienced in the last 90 days in anatomical areas depicted with a drawing, and positive responses to two additional criteria.[7] Further details are available elsewhere.[24]
2.5. Biopsychosocial Risk Factors.
Variables were selected based on content validity, informativeness from prior OPPERA publications where full information is presented regarding each of the variables, [15,17,24,26,30,33] and representativeness of the variables within the respective following domains: germane to TMD, health, pain, psychosocial and behavioral, and quantitative sensory testing (QST); refer to Appendix Table for the complete list. Note that variable selection was not based on any specific statistical criteria.
2.6. Statistical Methods.
Study participants were cross-classified according to pain classification to create a Euler diagram, where the number of individuals within the respective shapes is proportional to the surface area. Contingency tables compared the distribution of study groups according to demographic and comorbid characteristics, and frequencies were compared statistically using binary logistic regression (for binary variables) or multinomial logistic regression (for headache). Post-hoc contrasts tested for differences between: headache alone vs the combined headache groups (comorbid with TMD, and headache attributed to TMD); and headache comorbid with TMD vs headache attributed to TMD. Descriptive statistics were computed for each predictor variable, by group, and effect size was calculated using Cohen’s d which was classified as small (≥0.20 to <0.50), medium (≥0.50 to <0.80), and large (≥0.80).[4]
Continuous predictor variables were transformed to z-scores and differences between the three study groups were analyzed using one-way analysis of variance with GLM procedure in SAS version 9.4. Post-hoc estimate statements calculated mean differences and their 95% confidence limits (CL) between the referent group, headache without TMD, and each of headache comorbid with TMD and headache attributed to TMD groups. Planned post-hoc contrasts were used to estimate and test for differences between mean differences that were corrected for multiple testing (64 variables) using Bonferroni correction estimations which set a critical p-value threshold of 0.05/64=0.0008. These tests evaluated the two premises described for the study’s second aim: (a) the risk factor should differ appreciably between people with headache that is comorbid with TMD and people who have headache in the absence of TMD; and (b) the risk factor should differ appreciably between people with headache attributed to TMD and people with headache that is comorbid with TMD.
3. Results
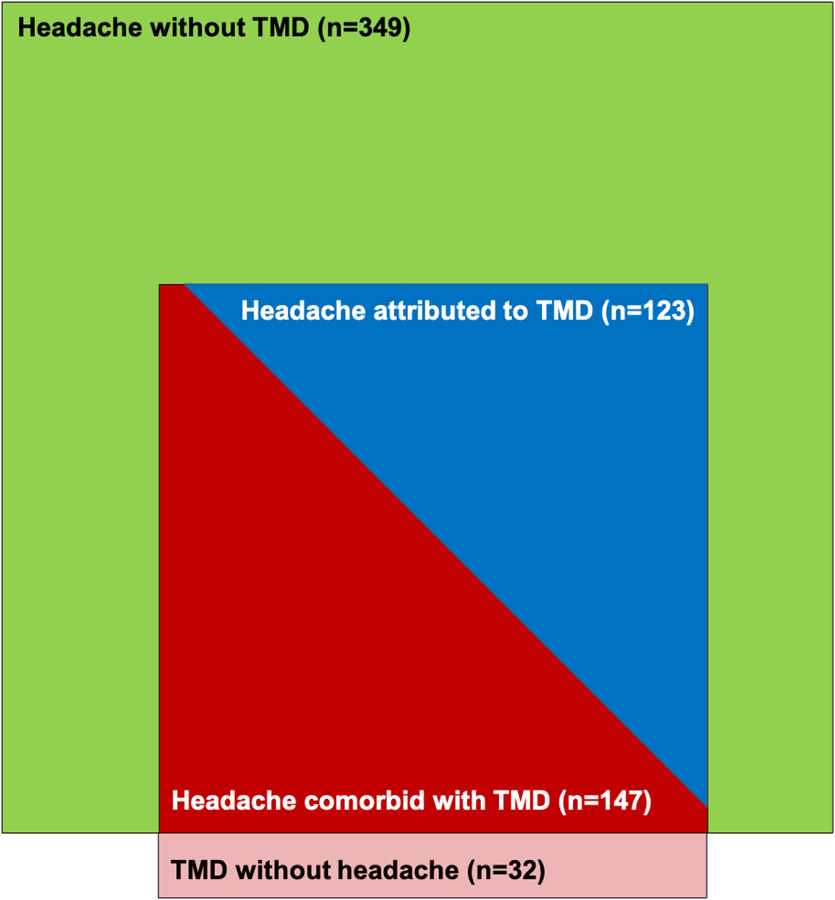
The Euler diagram (Figure 1) depicting the frequencies of study group classification highlights that among headaches of interest, headache comorbid with TMD and headache attributed to TMD are more restrictive headache types compared to headache alone.
Figure 1.
Mutually exclusive groups created for this study. Euler diagram depicts all participants with a headache and all participants with a painful TMD. The groups of headache comorbid with TMD and headache attributed to TMD are situated within the headache without TMD group, in order to illustrate the study sample of interest. The TMD without headache group is not included in the present analyses.
The three study groups are compared in Table 1 for demographic, other comorbid characteristics, and headache type. Age did not distinguish the three headache groups. In contrast, all of the remaining variables did distinguish the groups with headache comorbid with TMD and headache attributable to TMD from the group with headache without TMD, exhibiting greater proportions of females, white race, each overlapping pain condition (IBS, low back pain, and fibromyalgia), each of the competing types of secondary headache (cervicogenic, rhinosinusitis, and post-traumatic), and migraine alone or in combination with tension-type headache. Higher proportions of females, combination of migraine and tension-type headache, and cervicogenic headache were found in individuals with headache attributed to TMD vs those with headache comorbid with TMD.
Table 1.
Demographics and other comorbidity characteristics of three study groups
| (a) Headache without TMD (n=349) |
(b) Headache comorbid with TMD (n=147) |
(c) Headache attributed to TMD (n=123) |
p-values | ||
|---|---|---|---|---|---|
| Avg of c,b vs. a |
c vs. b | ||||
| Demographic characteristic | |||||
| Age: mean (IQR) years | 38.3 (30–46) | 38.5 (28–47) | 37.4 (28–46) | 0.380 | 0.690 |
| Gender: % female | 68.2 | 69.4 | 84.6 | 0.001 | 0.004 |
| Race: % white | 62.2 | 68.7 | 74.8 | 0.010 | 0.270 |
| Overlapping pain conditions (%) | |||||
| Irritable Bowel Syndrome | 20.8 | 39.3 | 39.8 | 0.000 | 0.930 |
| Low back pain | 19.0 | 37.2 | 34.8 | 0.001 | 0.680 |
| Fibromyalgia | 2.6 | 17.2 | 26.3 | 0.000 | 0.080 |
| Competing classifications for headache (%) * | |||||
| Cervicogenic headache | 22.5 | 47.6 | 64.4 | 0.000 | 0.007 |
| Rhinosinusitis headache | 5.0 | 16.7 | 17.8 | 0.000 | 0.810 |
| Post-traumatic headache | 4.4 | 11.1 | 15.3 | 0.000 | 0.320 |
| Primary headache type (%) ** | |||||
| Migraine without TTH | 10.3 | 15.7 | 21.1 | 0.000 | 0.070 |
| TTH without migraine | 68.5 | 53.1 | 39.0 | ||
| Both migraine and TTH | 21.2 | 31.3 | 39.8 | 0.000 | 0.050 |
Not mutually exclusive
Mutually exclusive; χ2 (4 df) = 34.2, p<0.0001; TTH: tension-type headache
3.1. Group comparisons.
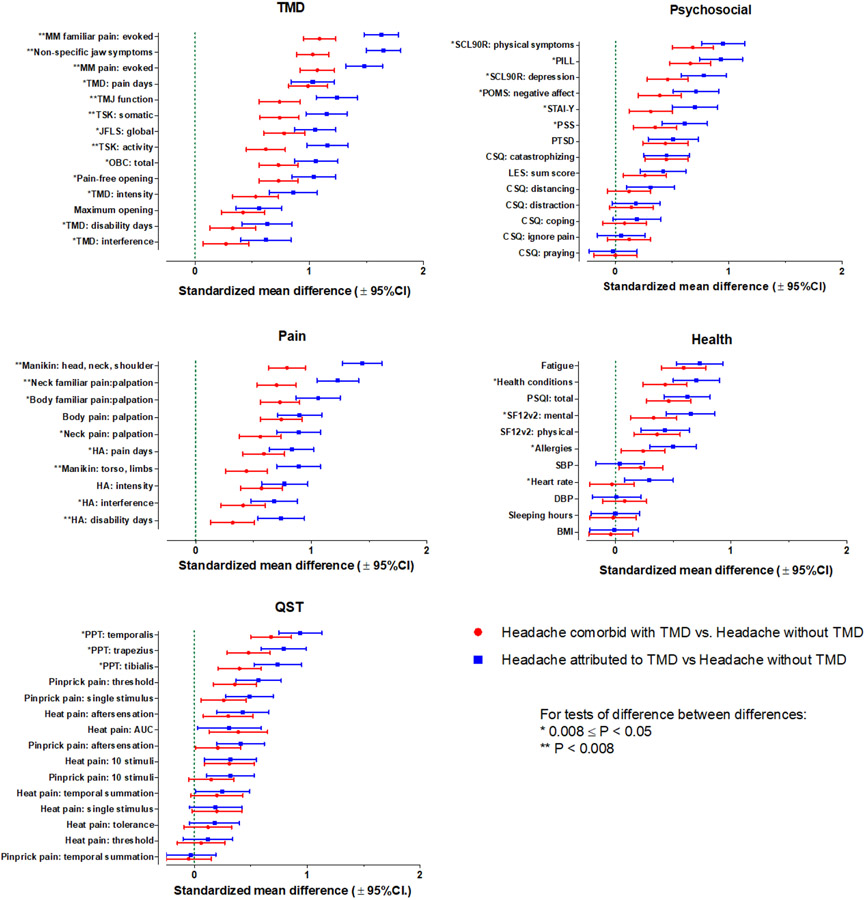
Descriptive statistics (mean, s.e.) for each variable by study group are listed in Table 2. Point estimates and confidence limits of the study predictor variables contrasting the three groups are shown in Figure 2. Figure 2 also includes Bonferroni-corrected p-values for the pairwise contrasts of differences between differences between study groups. All of the selected pain-relevant predictors were significantly greater in both headache comorbid with TMD and headache attributed to TMD, compared to headache without TMD. The largest group differences for pain-related markers emerged for all of the body variables: upper quarter manikin, neck familiar pain from palpation, body familiar pain from palpation, and body pain from palpation. The increase in pain-related markers was the smallest for headache disability days and headache interference. For all markers, the point estimate for headache attributed to TMD exhibited a larger value, compared to that for headache comorbid with TMD, and the point estimates were significantly greater for all markers except measures of body palpation pain and headache intensity. Collectively, these findings indicate that, relative to the group with primary headache alone, multiple markers related to headache were greater in the group headache comorbid with TMD, and the differences were greater still in the group with headache attributed to TMD.
Table 2:
Descriptive statistics and effect sizes. Variable order within domain is according to the magnitude of the contrasts between co-occurring headache and TMD, vs headache alone.
| a) HA without TMD |
b) Headache comorbid with TMD |
c) Headache attributed to TMD |
Contrasts: | |||||
|---|---|---|---|---|---|---|---|---|
| mean | s.e. | Mean | s.e. | mean | s.e. | Avg of c,b vs. a |
c vs. b | |
|
|
|
|
|
|||||
| Pain domain | ||||||||
| Manikin: head, neck, shoulder | 0.7 | (0.0) | 2.3 | (0.1) | 3.6 | (0.2) | 1.12 | 0.66 |
| Neck familiar pain: palpation | 1.1 | (0.0) | 2.6 | (0.1) | 3.8 | (0.1) | 0.97 | 0.53 |
| Body familiar pain: palpation | 0.9 | (0.0) | 3.0 | (0.2) | 4.0 | (0.3) | 0.89 | 0.33 |
| Body pain: palpation | 3.5 | (0.1) | 6.2 | (0.2) | 6.8 | (0.3) | 0.82 | 0.16 |
| Neck pain: palpation | 3.7 | (0.1) | 4.7 | (0.1) | 5.3 | (0.0) | 0.72 | 0.33 |
| HA: pain days | 3.4 | (0.2) | 7.6 | (0.6) | 9.3 | (0.7) | 0.71 | 0.24 |
| Manikin: torso, limbs | 2.9 | (0.1) | 4.9 | (0.3) | 6.9 | (0.6) | 0.67 | 0.46 |
| HA: intensity | 34.5 | (0.9) | 44.8 | (1.4) | 48.4 | (1.5) | 0.67 | 0.20 |
| HA: interference | 18.1 | (1.1) | 28.1 | (1.9) | 34.5 | (2.4) | 0.54 | 0.27 |
| HA: disability days | 1.5 | (0.2) | 5.3 | (1.0) | 10.2 | (1.8) | 0.53 | 0.42 |
| TMD domain | ||||||||
| MM familiar pain: evoked | 1.7 | (0.2) | 11.0 | (0.6) | 15.6 | (0.8) | 1.36 | 0.54 |
| Non-specific jaw symptoms | 0.8 | (0.0) | 2.9 | (0.1) | 4.1 | (0.1) | 1.34 | 0.62 |
| MM pain: evoked | 4.5 | (0.2) | 13.5 | (0.5) | 17.0 | (0.7) | 1.27 | 0.41 |
| TMD: pain days | 4.2 | (0.6) | 22.1 | (1.7) | 22.8 | (1.5) | 1.01 | 0.04 |
| TMJ function | 1.0 | (0.0) | 2.3 | (0.1) | 3.2 | (0.1) | 0.99 | 0.50 |
| TSK: somatic | 6.2 | (0.1) | 8.7 | (0.2) | 10.0 | (0.3) | 0.94 | 0.41 |
| JFLS: global | 0.4 | (0.0) | 1.5 | (0.1) | 1.8 | (0.1) | 0.91 | 0.27 |
| TSK: activity | 9.3 | (0.1) | 12.0 | (0.3) | 14.4 | (0.4) | 0.89 | 0.55 |
| OBC: total | 21.2 | (0.5) | 29.7 | (0.9) | 33.5 | (1.0) | 0.89 | 0.33 |
| Pain-free opening | 44.8 | (0.4) | 36.9 | (0.9) | 33.6 | (0.9) | 0.89 | 0.30 |
| TMD: intensity | 32.0 | (1.1) | 42.3 | (1.6) | 49.0 | (1.7) | 0.70 | 0.34 |
| Maximum opening | 50.6 | (0.3) | 47.1 | (0.7) | 45.9 | (0.7) | 0.49 | 0.14 |
| TMD: disability days | 1.1 | (0.2) | 6.2 | (1.5) | 10.9 | (2.1) | 0.48 | 0.30 |
| TMD: interference | 14.2 | (1.3) | 20.4 | (1.9) | 28.7 | (2.3) | 0.45 | 0.35 |
| QST domain | ||||||||
| PPT: temporalis | 191.7 | (3.8) | 144.7 | (4.5) | 127.0 | (4.2) | 0.81 | 0.26 |
| PPT: trapezius | 336.1 | (7.9) | 267.1 | (10.8) | 224.0 | (9.7) | 0.63 | 0.30 |
| PPT: tibialis | 429.6 | (7.9) | 369.7 | (11.9) | 319.6 | (13.0) | 0.57 | 0.34 |
| Pinprick pain: threshold | 261.0 | (8.7) | 204.6 | (12.0) | 171.0 | (12.6) | 0.46 | 0.21 |
| Pinprick pain: single stimulus | 16.0 | (0.9) | 20.8 | (1.7) | 25.3 | (2.0) | 0.38 | 0.24 |
| Heat pain: aftersensation | 9.7 | (0.9) | 15.0 | (1.6) | 17.2 | (2.1) | 0.36 | 0.12 |
| Heat pain: AUC | 418.3 | (16.7) | 509.0 | (23.9) | 488.9 | (27.3) | 0.35 | −0.09 |
| Pinprick pain: aftersensation | 7.9 | (0.7) | 10.8 | (1.2) | 13.7 | (1.4) | 0.31 | 0.21 |
| Heat pain: 10 stimuli | 67.3 | (1.8) | 76.4 | (2.3) | 76.7 | (2.6) | 0.31 | 0.01 |
| Pinprick pain: 10 stimuli | 38.0 | (1.4) | 42.1 | (2.3) | 46.8 | (2.5) | 0.24 | 0.17 |
| Heat pain: temporal summation | 23.6 | (1.6) | 29.1 | (2.6) | 30.2 | (2.6) | 0.23 | 0.04 |
| Heat pain: single stimulus | 42.3 | (1.7) | 48.3 | (2.8) | 48.2 | (3.2) | 0.19 | −0.00 |
| Heat pain: tolerance | 46.1 | (0.1) | 45.8 | (0.2) | 45.6 | (0.2) | 0.15 | 0.06 |
| Heat pain: threshold | 41.9 | (0.1) | 41.7 | (0.3) | 41.5 | (0.3) | 0.09 | 0.06 |
| Pinprick pain: temporal summation | 22.1 | (1.0) | 21.3 | (1.4) | 21.5 | (1.4) | −0.04 | 0.01 |
| Psychosocial domain | ||||||||
| SCL90R: physical symptoms | 0.3 | (0.0) | 0.7 | (0.0) | 0.8 | (0.0) | 0.82 | 0.26 |
| PILL | 94.7 | (1.2) | 113.7 | (2.4) | 121.8 | (2.7) | 0.79 | 0.28 |
| SCL90R: depression | 0.4 | (0.0) | 0.7 | (0.0) | 0.8 | (0.0) | 0.62 | 0.32 |
| POMS: negative affect | 53.3 | (0.8) | 60.4 | (1.4) | 66.0 | (1.7) | 0.55 | 0.31 |
| STAI-Y | 36.3 | (0.5) | 39.7 | (0.8) | 44.1 | (1.1) | 0.50 | 0.39 |
| PSS | 19.7 | (0.4) | 22.9 | (0.7) | 25.3 | (0.9) | 0.48 | 0.26 |
| PTSD | 10.4 | (0.9) | 19.5 | (1.9) | 21.0 | (2.5) | 0.47 | 0.07 |
| CSQ: catastrophizing | 0.8 | (0.0) | 1.3 | (0.0) | 1.3 | (0.1) | 0.45 | 0.00 |
| LES: sum score | 8.9 | (0.4) | 11.3 | (0.8) | 12.7 | (0.9) | 0.34 | 0.16 |
| CSQ: distancing | 1.0 | (0.0) | 1.2 | (0.1) | 1.4 | (0.1) | 0.21 | 0.19 |
| CSQ: distraction | 2.3 | (0.0) | 2.5 | (0.1) | 2.5 | (0.1) | 0.16 | 0.04 |
| CSQ: coping | 3.6 | (0.0) | 3.8 | (0.1) | 3.9 | (0.1) | 0.13 | 0.11 |
| CSQ: ignore Pain | 2.6 | (0.0) | 2.8 | (0.1) | 2.7 | (0.1) | 0.09 | −0.07 |
| CSQ: praying | 2.3 | (0.1) | 2.3 | (0.1) | 2.2 | (0.1) | −0.01 | −0.02 |
| Health domain | ||||||||
| Fatigue score | 1.4 | (0.0) | 1.8 | (0.0) | 1.9 | (0.0) | 0.66 | 0.14 |
| Health conditions | 1.0 | (0.0) | 1.7 | (0.1) | 2.1 | (0.1) | 0.57 | 0.27 |
| PSQI: total | 5.4 | (0.1) | 7.1 | (0.3) | 7.7 | (0.3) | 0.54 | 0.16 |
| SF12: mental | 51.2 | (0.5) | 47.8 | (0.8) | 44.3 | (1.1) | 0.49 | 0.33 |
| SF12: physical | 51.1 | (0.4) | 47.3 | (0.9) | 46.7 | (1.1) | 0.39 | 0.06 |
| Allergies | 1.2 | (0.0) | 1.6 | (0.1) | 2.0 | (0.1) | 0.37 | 0.26 |
| SBP | 118.3 | (0.7) | 121.7 | (1.4) | 119.0 | (1.3) | 0.13 | −0.18 |
| Heart rate | 64.6 | (0.5) | 64.2 | (0.9) | 67.9 | (0.9) | 0.13 | 0.33 |
| DBP | 69.5 | (0.5) | 70.4 | (1.0) | 69.6 | (0.9) | 0.05 | −0.08 |
| Sleeping hours | 6.9 | (0.0) | 6.9 | (0.1) | 6.9 | (0.1) | −0.01 | 0.03 |
| BMI | 28.9 | (0.4) | 28.6 | (0.5) | 28.9 | (0.6) | −0.03 | 0.03 |
Means and standard errors (s.e.) are z-score standardizations of original scales. Contrasts are standardized mean differences, color coded according to categories for Cohen's D effect sizes, namely Small = 0.2 to <0.5, Medium = 0.5 to <0.8; or Large = ≥0.8
Figure 2.
Association of biopsychosocial predictor variables with the three headache groups, organized by predictor domain. Standardized mean differences and 95% CI are shown for the two groups that exhibit headache co-occurring with TMD, in comparison to the reference group, headache without TMD, which is indicated as the dashed green line for the null relationship of 0.
Fourteen variables germane to TMD were evaluated, and all were significantly greater in both headache comorbid with TMD and headache attributed to TMD, compared to headache without TMD. For all markers, the point estimate for headache attributed to TMD exhibited a larger value, compared to that for headache comorbid with TMD, and for 13 of the 14 variables, the point estimates were significantly greater. The exception was maximal unassisted jaw opening where there was no significant difference between headache comorbid with TMD versus headache attributed to TMD. Collectively, these findings indicate that, relative to the group with primary headache alone, multiple markers related specifically to TMD were substantially greater in the group with comorbid painful TMD, and the differences in these TMD-specific markers were greater still in the group with headache attributed to TMD.
Fifteen QST variables were evaluated, and 9 were significantly greater in both headache comorbid with TMD and headache attributed to TMD, compared to headache without TMD. These included pressure pain threshold from each of 3 body sites; pinprick pain threshold, single stimulus, and aftersensation; and ratings of heat pain from 10 stimuli, aftersensation, and overall area under the curve (AUC). For most of the markers, the point estimate for headache attributed to TMD exhibited an equal or larger value, compared to that for headache comorbid with TMD. However, the difference in point estimates was statistically significant for only the 3 PPT measures. Collectively, these findings indicate that, relative to the group with primary headache alone, multiple markers related to somatic pain processing were substantially greater in the group with headache comorbid with painful TMD, while other QST markers were no different, and the differences in these QST markers were yet modestly greater in the group with headache attributed to TMD.
Fourteen psychosocial variables were evaluated, and 9 were significantly greater in both headache comorbid with TMD and headache attributed to TMD, compared to headache without TMD. These included measures related to somatic focus; depression and negative affect; anxiety, catastrophizing, stress, and PTSD; and life events. For most of the markers, the point estimate for headache attributed to TMD was equal to or larger than headache comorbid with TMD, although the difference in point estimates was statistically significant for only six variables: physical symptoms, somatic focus, depression, negative affect, anxiety, and stress. Collectively, these findings indicate that, relative to the group with primary headache alone, some markers related to the psychosocial domain were substantially greater in the group with headache comorbid with painful TMD, and the difference in these psychosocial markers were yet modestly greater in the group with headache attributed to TMD.
Eleven health status variables were evaluated, and 6 were significantly greater in both headache comorbid with TMD and headache attributed to TMD, compared to headache without TMD. These included fatigue, number of health conditions, sleep quality, mental and physical health, and allergies. For most of the markers, the point estimate for headache attributed to TMD was equal to or larger than the point estimate for headache comorbid with TMD, although the difference in point estimates was statistically significant for only four variables: number of health conditions, mental health, and allergies were the point estimates significantly greater in the headache attributed to TMD group compared to headache comorbid with TMD. Collectively, these findings indicate that, relative to the group with primary headache alone, some markers from the health domain were substantially greater in the group headache comorbid with TMD, and the differences in these health markers were yet modestly greater in the group with headache attributed to TMD.
3.2. Effect sizes.
Effect sizes related to all of the above pair-wise differences are listed in Table 2. For the pain domain and the TMD domain variables, large effect size differences were observed when comparing either of the combined headache groups against the headache alone group, while small or medium effect sizes characterized the contrasts between headache comorbid with TMD and headache attributed to TMD groups. In contrast, for QST, psychosocial, and health status domains, small or very small effect sizes were seen for all comparisons.
4. Discussion
In this study of individuals with a primary headache and painful TMD in a convenience sample of community-dwelling adults, nearly all individuals with a painful TMD had headache, while only 44% of individuals with headache had a TMD. The asymmetry suggests possible differential mechanisms, which would be consistent with heterotypic comorbidity of headache attributed to TMD and primary headache. Based on the two criteria proposed in this study’s aim, evidence in favor of differential mechanisms was observed for variables in each of the five domains studied: (a) significant differences in 34 characteristics (across all 5 domains of clinical pain, masticatory system, psychosocial status, QST, and health status) emerged between headache attributed to TMD and headache comorbid with TMD; and (b) significant differences in 48 characteristics distinguished individuals with headache coexisting with a painful TMD from individuals who have headache in the absence of a TMD. In summary, with 75% of the total 64 characteristics found to be substantially greater in both the headache comorbid with the TMD and the headache attributed to TMD groups, our findings attest to the similarities of both the combined headache groups at a qualitative level. However, with the effect sizes of more than 50% of the 64 characteristics found to be large for headache comorbid with TMD and larger again for headache attributed to TMD, our results also attest to the separability of the combined headache groups in quantitative terms. Furthermore, the strongest evidence was seen for variables in the TMD domain. The more severe the masticatory system characteristics associated with a painful TMD, the more likely the painful TMD contributed to a secondary headache, vs non-specific comorbidity of primary headache and TMD. The dominant effect of measures relevant to the masticatory system was likewise apparent when inferences were based on either Bonferroni-adjusted P-values or effect sizes. From a disease classification perspective, the evidence overall supports headache attributed to TMD as heterotypic to primary headache and thereby consistent with its inclusion as a form of secondary headache in the ICHD-3.
While it was not surprising to observe a large degree of overlap between headache and painful TMD, the nature of the overlap was striking. Among individuals with coexisting headache and TMD, 44% had headache attributed to TMD, leaving a bare majority (56%) with non-specific comorbidity of headache and TMD. This is consistent with the literature regarding chronic overlapping pain conditions more generally.[15,17,24,30,33] In tertiary headache centers, perhaps 70% of patients also have a painful TMD, and that proportion increases when primary headache types are co-occurring.[3] The comorbidity of TMD with headache is notably lower in our community sample compared to a tertiary headache clinic. Individuals referred to such clinics generally have more severe headaches, and we have separately shown that a severity spectrum exists when two or more overlapping pain conditions co-occur.[24] In comparison to TMD alone, the dominant overlap in prevalence of painful TMD and headache has been explained by topographical attributes[28] and referred pain mechanisms.[11,12] Moreover, headache attributed to TMD is substantially more likely to coexist with myofascial TMD pain with referral than with local myalgia.[25] The headache severity spectrum may also be explained by differential mechanisms as well as other processes. Nociceptive and pain processing mechanisms include extent of referred pain, peripheral and central sensitization co-occurring in closely adjacent body regions, and impairment of the descending modulatory system.[5] In addition, overlap of headache with masticatory muscle pain worsens sleep quality, catastrophizing, and hyperalgesia in the masseter and temporalis areas,[6] of which only the hyperalgesia occurred in this sample. Such factors that contribute to the overlap and are noted to be more significant in females than in males[13] may explain the high proportion of females in the headache attributed to TMD group in our sample. Taking all of our findings together, the present evidence supports the clinical application of a dual diagnostic approach that (1) focuses on a continuum perspective for understanding severity of headache and face pain, and (2) incorporates perception and coping as part of identifying treatment options.[27] To ignore the co-existence of a painful TMD with primary headache, and the possible presence also of headache attributed to TMD, is to sidestep comorbidity critical to understanding the nature, impact, and treatment needs of the individual with a primary headache.
The cross-sectional design of this study precludes causal inference between change in proportions of headache types and presence of headache attributed to TMD. And, it may be that the two headache disorders have reciprocal causal mechanisms. For example, other findings from this OPPERA cohort suggest that systemic dysregulation across time increases the probability that pain episodes will occur and, with increasing dysregulation, those episodes can become a disorder.[31,34] Moreover, increased pain intensity and frequency of headache are more predictive of worsening psychological status rather than diagnosis,[27] and a worsening of psychological status is associated with painful TMD onset,[14] supporting a complex dynamic system of causation. Overall, individuals with a painful TMD and headache attributed to TMD, compared to individuals with TMD only, report greater pain chronicity, pain intensity, pain-related disability, depressive symptoms, and physical symptoms, and are more likely to have persistent pain such as more days of pain.[25] Based on these findings, one possibility is that increased primary headache prevalence is coextensive with worsening of a comorbid painful TMD, and headache attributed to TMD is a next step in that evolution of comorbid pain disorders.
Determining whether headache attributed to TMD is homotypic or heterotypic with other potentially similar headaches cannot rely on a single study or single research design. We highlight findings from diverse studies. Migraine appears to be more strongly associated with the progression of painful TMDs, and headache attributed to TMD is associated with a transformation of headache type with migraine dominating.[36] Sensitization occurs with both painful TMD and headache attributed to TMD,[18] and the present findings suggest the likelihood of even greater sensitization with the secondary headache. With increasing severity of the pain dimension occupied by painful TMD, headache attributed to TMD, and primary headache, the balance between continuous nociceptive drive and critical thresholds for perception regarding headache presence may reflect ongoing alterations, potentially mediated by differential activation of endogenous pain modulatory pathways.[9] Collectively, the available evidence suggests that regardless of causal mechanisms for any individual pain disorder, their joint occurrence creates potentially new interactive patterns of causation.
Research regarding taxonomic structure for secondary conditions often confronts circularity in either design or reasoning as a potential threat to validity. For example, a high overlap in location of TMD myalgia and headache attributed to that TMD was the unfortunate consequence of confounding due to the selection criteria,[10] a common problem in observational studies. Those study findings may be unresolved, however, in that the authors tested the DC/TMD criteria for headache attributed to TMD, which require that the headache include the temporalis muscle region;[29] in contrast, the ICHD-3 criteria do not restrict the location for this particular secondary headache. The advantages of the ICHD-3 criteria are, clinically, a more inclusive framework for headache attributed to TMD and, for research, a reduction in potential problems of circularity (or confounding) when examining possible comorbidity. To that end, this study also relied on the DC/TMD criteria for the secondary headache classification, and consequently the impact and relevance of these findings should only be enhanced when considering the more inclusive secondary headache currently defined by the ICHD-3. For example, among individuals with both primary headache and a painful TMD, we would expect that the majority would also have headache attributed to TMD.
Two limitations in this study should be noted. The first is the absence of a comparison group comprised of individuals with a painful TMD and without any headache; such a group would provide the needed reference for an equally compelling question, whether the variables of interest in the present study distinguish headache attributed to TMD from the primary condition, painful TMD; that is, whether secondary headache is heterotypic or homotypic to the painful TMD cannot be determined. As we describe above, however, the overall value of such a comparison may be less than seems apparent, and efforts to recruit such a sample may not represent the most efficient use of research resources. Moreover, we suspect that such a group would more likely be very similar to the group with only primary headache. As previously demonstrated in this sample, individuals with headache alone or painful TMD alone reported similar pain intensity, pain interference, number of impact days, and proportion with high-impact pain.[24] The second limitation revolves around the complexity of primary headache classification and that the potential co-relationship between both primary and secondary headaches in the group defined by presence of headache attributed to TMD could be further explored. Consequently, one interpretation from the present study is that the greater severity of the significant variables within the group of 64 may be not because the secondary headache is a separate headache type but rather due to, perhaps, reciprocal causation between primary and secondary headaches in the individuals within that group.
In summary, the collective evidence from published literature and this study shows that the presence of overlapping pain conditions reflects substantial worsening of a large majority of characteristics associated with those conditions, and this includes secondary headache as yet another potential overlapping pain condition in an individual. The symptomatic dominance of a condition such as headache can easily overshadow the equal importance of identifying other comorbid primary conditions, such as a painful TMD. A headache secondary to the TMD and which is a separate condition from any co-existing primary headache will be associated with yet greater suffering and impact. Headache attributed to TMDs is qualitatively perceived by the person as “headache” and is distinguished from “pain”, warrants evaluation equal to that of primary headaches, and points to differential management that will likely augment efficacy of treatments focused specifically on the primary headache. For example, for combined headache comorbid with TMD and in particular for headache attributed to TMD, primary treatments for painful TMD should be added at the outset of the usual standard treatments for headache, whether TTH or migraine.
Supplementary Material
Acknowledgements and Disclosures
This work was supported by National Institutes of Health grants U01DE017018 (NIDCR). The OPPERA program also acknowledges resources provided for this project by the participating institutions: University at Buffalo; University of Florida; University of Maryland; and University of North Carolina at Chapel Hill.
Footnotes
Disclosures: The authors have nothing else to disclose.
Contributor Information
Sonia Sharma, Neuro Pain Management Center, Department of Neurosurgery, University of Rochester Medical Center, Rochester, NY..
Gary D. Slade, Division of Pediatric and Population Health, UNC Adams School of Dentistry, Chapel Hill, NC.; Department of Epidemiology University of North Carolina Gillings School of Global Public Health, Chapel Hill, NC..
Roger B. Fillingim, Pain Research and Intervention Center of Excellence (PRICE), University of Florida, Gainesville, Florida, USA..
Richard Ohrbach, Department of Oral Diagnostic Sciences. University at Buffalo School of Dental Medicine, Buffalo, NY.
References
- [1].Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry 1999;40(1):57–87. [PubMed] [Google Scholar]
- [2].Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Smith S, Maixner W, Gonzalez Y, Gordon SM, Lim P-F, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study protocol, sample characteristics and loss-to-follow-up: the OPPERA prospective cohort study. Journal of Pain 2013;14(12, supplement 2):T2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia 2008;28(8):832–841. [DOI] [PubMed] [Google Scholar]
- [4].Cohen J A power primer. Psychol Bull 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- [5].Conti P, Costa Y, Gonçalves D, Svensson P. Headaches and myofascial temporomandibular disorders: overlapping entities, separate managements? J Oral Rehabil 2016;43(9):702–715. [DOI] [PubMed] [Google Scholar]
- [6].Costa YM, Alves da Costa DR, de Lima Ferreira AP, Porporatti AL, Svensson P, Rodrigues Conti PC, Bonjardim LR. Headache exacerbates pain characteristics in temporomandibular disorders. J Oral Facial Pain Headache 2017;31(4):339–345. [DOI] [PubMed] [Google Scholar]
- [7].Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, Hartvigsen J, Leino-Arjas P, Latza U, Reis S, Gil Del Real MT, Kovacs FM, Oberg B, Cedraschi C, Bouter LM, Koes BW, Picavet HS, van Tulder MW, Burton K, Foster NE, Macfarlane GJ, Thomas E, Underwood M, Waddell G, Shekelle P, Volinn E, Von Korff M. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine 2008;33(1):95–103. [DOI] [PubMed] [Google Scholar]
- [8].Drossman D, Corazziari E, Delvaux M, Spiller R, Tailley N, Thompson W, Whitehead WE. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates, Inc., 2006. [Google Scholar]
- [9].Exposto FG, Bendixen KH, Ernberg M, Bach FW, Svensson P. Assessment of pain modulatory and somatosensory profiles in chronic tension-type headache patients. Pain Medicine 2021;22(10):2356–2365. [DOI] [PubMed] [Google Scholar]
- [10].Exposto FG, Renner N, Bendixen KH, Svensson P. Pain in the temple? Headache, muscle pain or both: A retrospective analysis. Cephalalgia 2021;41(14):1486–1491. [DOI] [PubMed] [Google Scholar]
- [11].Fernández-De-Las-Peñas C, Fernández-Mayoralas DM, Ortega-Santiago R, Ambite-Quesada S, Palacios-Cena D, Pareja JA. Referred pain from myofascial trigger points in head and neck–shoulder muscles reproduces head pain features in children with chronic tension type headache. The journal of headache and pain 2011;12(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fernández-de-Las-Peñas C, Ge H-Y, Arendt-Nielsen L, Cuadrado ML, Pareja JA. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. The Clinical journal of pain 2007;23(9):786–792. [DOI] [PubMed] [Google Scholar]
- [13].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley III JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychosocial factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 2013;14(12, supplement 2):T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fillingim RB, Ohrbach R, Greenspan JD, Sanders AE, Rathnayaka N, Maixner W, Slade GD. Associations of psychological factors with multiple chronic overlapping pain conditions. J Oral Facial Pain Headache 2020;34(0):s85–s100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gonçalves DA, Bigal ME, Jales LC, Camparis CM, Speciali JG. Headache and symptoms of temporomandibular disorder: an epidemiological study. Headache: the journal of head and face pain 2010;50(2):231–241. [DOI] [PubMed] [Google Scholar]
- [17].Greenspan JD, Slade GD, Rathnayaka N, Fillingim RB, Ohrbach R, Maixner W. Experimental pain sensitivity in subjects with TMD and multiple other chronic pain conditions: the OPPERA Prospective Cohort Study. Journal of Oral & Facial Pain & Headache 2020;34(0):s43–s56b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hara K, Shinozaki T, Okada-Ogawa A, Matsukawa Y, Dezawa K, Nakaya Y, Chen J-Y, Noma N, Oka S, Iwata K, Imamura Y. Headache attributed to temporomandibular disorders and masticatory myofascial pain. Journal of Oral Science 2016;58(2):195–204. [DOI] [PubMed] [Google Scholar]
- [19].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- [20].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- [21].Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia 2004;24 supplement 1:1–160. [DOI] [PubMed] [Google Scholar]
- [22].Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, Harrison W. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology 2003;61(3):375–382. [DOI] [PubMed] [Google Scholar]
- [23].Ohrbach R, Gonzalez Y, List T, Michelotti A, Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Clinical Examination Protocol. International RDC/TMD Consortium Network, 2014. [Google Scholar]
- [24].Ohrbach R, Sharma S, Fillingim RB, Greenspan JD, Rosen JD, Slade G. Clinical characteristics of pain among five chronic overlapping pain conditions. J Oral Facial Pain Headache 2020;34(0):s29–s42ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reiter S, Emodi-Perlman A, Kasiel H, Abboud W, Friedman-Rubin P, Arias OW, Manor Y. Headache Attributed to Temporomandibular Disorders: Axis I and II Findings According to the Diagnostic Criteria for Temporomandibular Disorders. J Oral Facial Pain Headache 2021;35(2):119–128. [DOI] [PubMed] [Google Scholar]
- [26].Sanders A, Greenspan JD, Fillingim RB, Rathnayaka N, Ohrbach R, Slade G. Associations of sleep disturbance, atopy, and other health measures with chronic overlapping pain conditions. Journal of Oral & Facial Pain & Headache 2020;34(0):s73–s84b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scharff L, Turk D, Marcus D. Psychosocial and behavioral characteristics in chronic headache patients: support for a continuum and dual-diagnostic approach. Cephalalgia 1995;15(3):216–223. [DOI] [PubMed] [Google Scholar]
- [28].Schiffman E, Ohrbach R, List T, Anderson G, Jensen R, John MT, Nixdorf D, Goulet J-P, Kang W, Truelove E, Clavel A, Fricton J, Look J. Diagnostic criteria for headache attributed to temporomandibular disorders. Cephalalgia 2012;32(9):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J-P, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg L, Haythornthwaite J, Hollender L, Jensen R, John MT, deLaat A, deLeeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sharma S, Slade GD, Fillingim RB, Greenspan JD, Rathnayaka N, Ohrbach R. Attributes germane to temporomandibular disorders and their associations with five chronic overlapping pain conditions. J Oral Facial Pain Headache 2020;34(0):s57–s72e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Slade G, Sanders A, Bair E, Brownstein NC, Dampier D, Knott C, Fillingim RB, Maixner W, Smith S, Greenspan JD, Dubner R, Ohrbach R. Preclinical episodes of orofacial pain symptoms and their association with healthcare behaviors in the OPPERA prospective cohort study. Pain 2013;154(3):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Slade GD, Bair E, By K, Mulkey F, Baraian C, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim P-F, Maixner W, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. Journal of Pain 2011;12(11, supplement 3):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Slade GD, Greenspan JD, Fillingim RB, Maixner W, Sharma S, Ohrbach R. Overlap of five chronic pain conditions: temporomandibular disorders, headache, back pain, irritable bowel syndrome, and fibromyalgia. J Oral Facial Pain Headache 2020;34(0):s15–s28g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J Dent Res 2016;95(10):1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Svensson P Muscle pain in the head: overlap between temporomandibular disorders and tension-type headache. Current Opinion in Neurology 2007;20:320–325. [DOI] [PubMed] [Google Scholar]
- [36].Tchivileva IE, Ohrbach R, Fillingim RB, Greenspan JD, Maixner W, Slade GD. Temporal change in headache and its contribution to risk of developing first-onset TMD in the OPPERA study. Pain 2017;158:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.