Abstract
Although both the 2014 and 2020 World Health Organization (WHO) criteria require unequivocal glandular and squamous differentiation for a diagnosis of cervical adenosquamous carcinoma (ASC), in practice, ASC diagnoses are often made in tumors that lack unequivocal squamous and/or glandular differentiation. Considering the ambiguous etiologic, morphological, and clinical features and outcomes associated with ASCs, we sought to redefine these tumors.
We reviewed slides from 59 initially diagnosed ASCs (including glassy cell carcinoma and related lesions) to confirm an ASC diagnosis only in the presence of unequivocal malignant glandular and squamous differentiation. Select cases underwent immunohistochemical profiling as well as human papillomavirus (HPV) testing by in situ hybridization.
Of the 59 cases originally classified as ASCs, 34 retained their ASC diagnosis, 9 were reclassified as pure invasive stratified mucin-producing carcinomas (iSMCs), 10 as iSMCs with other components (such as HPV-associated mucinous, usual-type, or adenosquamous carcinomas), and 4 as HPV-associated usual or mucinous adenocarcinomas with benign-appearing squamous metaplasia. Two glassy adenocarcinomas were reclassified as poorly differentiated HPV-associated carcinomas based on morphology and immunophenotype. There were no significant immunophenotypic differences between ASCs and pure iSMCs with regard to HPV and other markers including p16 expression. Although limited by a small sample size, survival outcomes seemed to be similar between all groups.
ASCs should be diagnosed only in the presence of unequivocal malignant glandular and squamous differentiation. The two putative glassy cell carcinomas studied did not meet our criteria for ASC and categorizing them as such should be reconsidered.
Keywords: cervical adenosquamous carcinoma, glassy cell carcinoma, invasive SMC, invasive stratified mucin-producing intraepithelial lesions, HPV
Introduction
Invasive cervical adenosquamous carcinoma (ASC) is a relatively uncommon histologic subtype of cervical malignant neoplasms classified by the previous and current World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs as a separate entity distinct from both squamous and glandular malignant tumors of the cervix1,2. ASC was first described as a “mixed carcinoma” by Glucksmann and Cherry in 1956, and later as “adenosquamous adenocarcinoma” by Greene in 19633,4. The WHO, since 2014, defines ASC as a malignant epithelial tumor composed of a mixture of invasive adenocarcinoma and squamous cell carcinoma1,2. Historically, both glassy cell carcinoma3,5,6 and “mucoepidermoid carcinoma”7,8 have been considered malignancies lying within the spectrum of ASC. The 2014 WHO classification system considered glassy cell carcinoma a subtype of ASC, but 2020 WHO classification does not include this tumor type1,2. The term “mucoepidermoid carcinoma” should only be used for the extremely rare lesions identical to those occurring in the salivary glands, particularly those that contain squamous (epidermoid), intermediate, and mucin-producing cells1.
Tumors historically diagnosed as ASC in practice appear to represent a spectrum of lesions, some of which do not exhibit definitive malignant squamous and/or glandular differentiation. This degree of heterogeneity has led to variable reported estimates of ASC prevalence (2% to 50% of all invasive cervical carcinomas)9,10. The principal rationale for classifying ASCs separately from both squamous cell carcinomas and adenocarcinomas relates not only to morphology but also to reported differences in associated rates of human papillomavirus (HPV)11–16 and clinical outcomes10,12,17,18, with some of the older literature emphasizing the clinically aggressive nature of ASC. Given the enigmatic etiologic, morphological and clinical features of ASCs, we sought to better define these tumors by examining a large series of cases originally diagnosed as ASC.
Material and methods
Case selection
Slides from 462 endocervical adenocarcinomas and adenosquamous carcinomas (ASCs and glassy cell carcinomas) were collected and reviewed by an international panel of pathologists from 8 institutions (USA: Memorial Sloan Kettering Cancer Center (MSK), New York, and Massachusetts General Hospital, Boston, MA; Romania: University of Medicine and Pharmacy of Targu Mures and Regional Institute of Oncology, Iasi; Japan: Jikei University School of Medicine, Tokyo; Mexico: Hospital de Oncología Mexico City, Mexico City; Israel: Sheba Medical Center, Tel-Hashomer, Ramat Gan; and Italy: Ospedale Sacro Cuore Don Calabria, Negrar). Only invasive tumors with at least 6 months follow-up were included. The following tumors were excluded: in situ carcinomas; squamous carcinomas; tumors with a neuroendocrine component; carcinosarcomas; any tumor demonstrating clinical, macroscopic, or microscopic features suggesting a lower uterine segment, uterine corpus, or adnexal primary; tumors represented by only biopsies and curettings; excisions lacking lymph node assessment; and specimens from patients treated with neoadjuvant chemotherapy and/or radiation therapy. Specimens from 70 loop electrosurgical excision procedures (LEEPs), 8 trachelectomies, 41 conizations, and 343 hysterectomies were collected. Fifty-three of the 462 total cases were excluded due to failure to meet entry criteria, missing blocks, or concern that the available slides were not representative of the lesion, leaving 409 cases for study. Institutional review board approval was obtained.
Morphological assessment
All microscopic subtypes of endocervical adenocarcinomas and ASCs were included in the study. We required examination of all hematoxylin and eosin (H&E) slides containing tumor (average of 12 slides per case). A consensus diagnosis was reached in every case, with at least 2, and as many as 4, study pathologists reviewing slides at a multi-head microscope. The 409 study cases were classified according to the new endocervical adenocarcinoma classification system (International Endocervical Adenocarcinoma Criteria and Classification [IECC])19, which formed the basis for the 2020 WHO classification1.
Cases were classified as ASC only when unequivocal invasive malignant glandular and squamous differentiation was present, each component representing at least 10% of the tumor. Cases were classified as pure glassy cell carcinoma when all tumor cells had sharp cytoplasmic margins, “ground glass” eosinophilic cytoplasm, and large round or ovoid nuclei with prominent nucleoli. The IECC and 2020 WHO recognize invasive stratified mucin-producing carcinomas (iSMCs) as part of the spectrum of HPV-associated endocervical adenocarcinomas (HPVA), while noting histologic similarity to lesions historically diagnosed as ASC1,19,20. For the purposes of this study, pure iSMCs were classified separately from HPV-associated usual type, mucinous, or adenosquamous carcinomas with >10% but <90% iSMC components (iSMC with components). HPVAs with a benign squamous component resembling squamous metaplasia were not considered ASCs. ASCs were classified as high grade when either the glandular or squamous component was high grade. Otherwise, ASCs were considered low grade. Solid architecture (>50%) or diffusely distributed high nuclear grade was considered “high grade” for the glandular component, while high nuclear to cytoplasmic ratios without keratinization was considered “high grade” for the squamous component.
Clinical information on type of surgical treatment, tumor size, stage, follow-up, lymph node metastasis, distant metastasis, and recurrence and survival status was collected.
Tissue microarray construction and immunohistochemical reactions
Tissue microarrays (TMAs) were constructed using previously described methods21,22. These included 25 putative ASC cases from New York, Boston, Mexico, Japan, Italy and Romania for analysis of p16, p53, progesterone receptor (PR), androgen receptor (AR), Vimentin, HER2, HIK1083, MUC6, CAIX, SATB2, HNF-1beta, PAX8, CK7, CDX2, GATA3, p63, and p40 expression (Table 1). Each of the tumors from the New York, Mexican, and Romanian centers were represented by three 0.6-mm cores; those from Japan were represented by single 3-mm cores. Stains were scored by consensus among 2 study pathologists (RAS and SS). Disagreements were extremely rare (approximately 2–3%) and were adjudicated by re-reviewing the stated criteria for positivity, as described below. p16 was interpreted as positive if diffuse block-like staining was found in all cores, and negative if there was no or patchy staining. p53 was interpreted as positive if ≥75% of tumor cell nuclei were strongly positive or if no staining was present in the background of an intact internal control. PR, AR, PAX8, CK7, and HNF-1beta were interpreted as positive if >25% (Score 3 or 4) of tumor cell nuclei or cytoplasm were stained. Scoring was as follows: Score 0: <5%; Score 1+: 5–10%; Score 2+: 11–25%; Score 3+: 26–75%; and Score 4+: >75%. Vimentin was interpreted as positive if ≥50% of tumor cells showed membranous/cytoplasmic staining. HER2 was scored using the College of American Pathologists (CAP) guidelines for gastric carcinoma: 3+ membranous positive23. HIK1083, MUC6, CAIX, SATB2, GATA3, p63, p40, and CDX2 were considered positive if any nuclear staining was noted in >5% of tumor cells. HIK1083 is currently not available in the United States.
Table 1:
Immunohistochemical Antibodies
| Antibody | CLONE | VENDOR | Instrument (dilution) |
|---|---|---|---|
| Vimentin | V9 | Roche | Roche Discovery XT |
| p53 | D07 | Roche | Roche Benchmark Ultra |
| p16 | E6H4 | Roche | Roche Benchmark Ultra |
| PAX8 | Poly | Protein Tech | Roche Benchmark Ultra |
| AR | Poly | Santa Cruz Biotechnology | Roche Discovery XT |
| PR | 1E2 | Roche | Roche Discovery XT |
| HER2 | 4B5 | Roche | Roche Discovery XT |
| HIK1083 | HIK1083 | Kanto | Manual (1/20) |
| MUC 6 | CLH5 | Novocastra | Manual (1/200) |
| CA IX | Poly | Novus | Roche Benchmark Ultra |
| SATB2 | EP281 | Cell Marque | Roche Benchmark Ultra |
| HNF1beta | CLO374 | Sigma | Leica Bond III |
| CK7 | OV-TV12/30 | DAKO | Roche Benchmark Ultra |
| CDX2 | CDX2–88 | Biogenex | Roche Benchmark Ultra |
| p63 | 4A4 | Roche | Roche Benchmark Ultra |
| p40 | BC28 | Biocare | Roche Benchmark Ultra |
HPV detection
HPV detection for high-risk HPV subtypes was performed on ASCs in the TMA that had sufficient tissue to score and had not been improperly fixed or stored (n=23). HPV in situ hybridization with a chromogen was performed using the Advanced Cell Diagnostics (ACD) (Hayward, CA) RNAscope® system (catalogue no.312598). The RNAscope® Probe “HPV HR18” contains probes targeting E6 and E7 mRNA for the following high-risk subtypes: HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82. The methodology and interpretation were discussed in detail in a previous paper24. Positive and negative control cases were used for optimal results. A full range of cytoplasmic and nuclear signals were encountered. Cases were interpreted to be HPV-positive if any brown signal appeared (nuclear, cytoplasmic) in the presence of a negative control.
Statistical analysis
Fisher-Exact test was used to compare the immunohistochemical profiles between pure invasive stratified mucin-producing carcinomas and adenosquamous carcinomas. OS (overall survival) and PFS (progression free survival) were calculated from the date of diagnosis. Deaths were considered events in OS. Both progression and deaths were considered events in PFS. Median survival and survival rate at 5 years were estimated using Kaplan Meier method. Log-rank test or permutation Log-Rank test25 if a variable contains <3 events at certain level applied when comparing survival between groups. All the analyses were performed using R.4.1.1 (https://www.R-project.org/). p<0.05 was considered statistically significant.
Results
Composition of study group and pathologic findings
Of the 409 cases, 57 were originally diagnosed as ASC and 2 as glassy cell carcinomas. After review for this study, 34 of 57 cases retained their diagnosis as pure ASC (Figures 1 and 2). The other cases were reclassified as pure iSMC (n=9) (Figure 3) or iSMC with components such as HPV-associated, usual-type adenocarcinoma (n=4) (Figure 4), ASC (n=3), or mucinous adenocarcinoma, not otherwise specified (NOS) (n=3). There were also 4 mimickers of ASC—3 HPVA usual-type and 1 mucinous adenocarcinoma with benign-appearing squamous metaplasia (Figure 5). The 2 glassy cell carcinomas (Figure 6) were reinterpreted as HPV-associated poorly differentiated carcinoma, NOS after morphological evaluation (no overt squamous or glandular components) and after immunohistochemical results showed p63 and p40 negativity.
Figure 1.

Distribution of endocervical adenocarcinomas, adenosquamous carcinomas, and mimickers after microscopic evaluation. (ECA, endocervical adenocarcinoma; ASC, cervical adenosquamous carcinoma; GCC, glassy cell carcinoma; NOS, not otherwise specified; iSMC, invasive stratified mucin-producing carcinoma)
Figure 2.
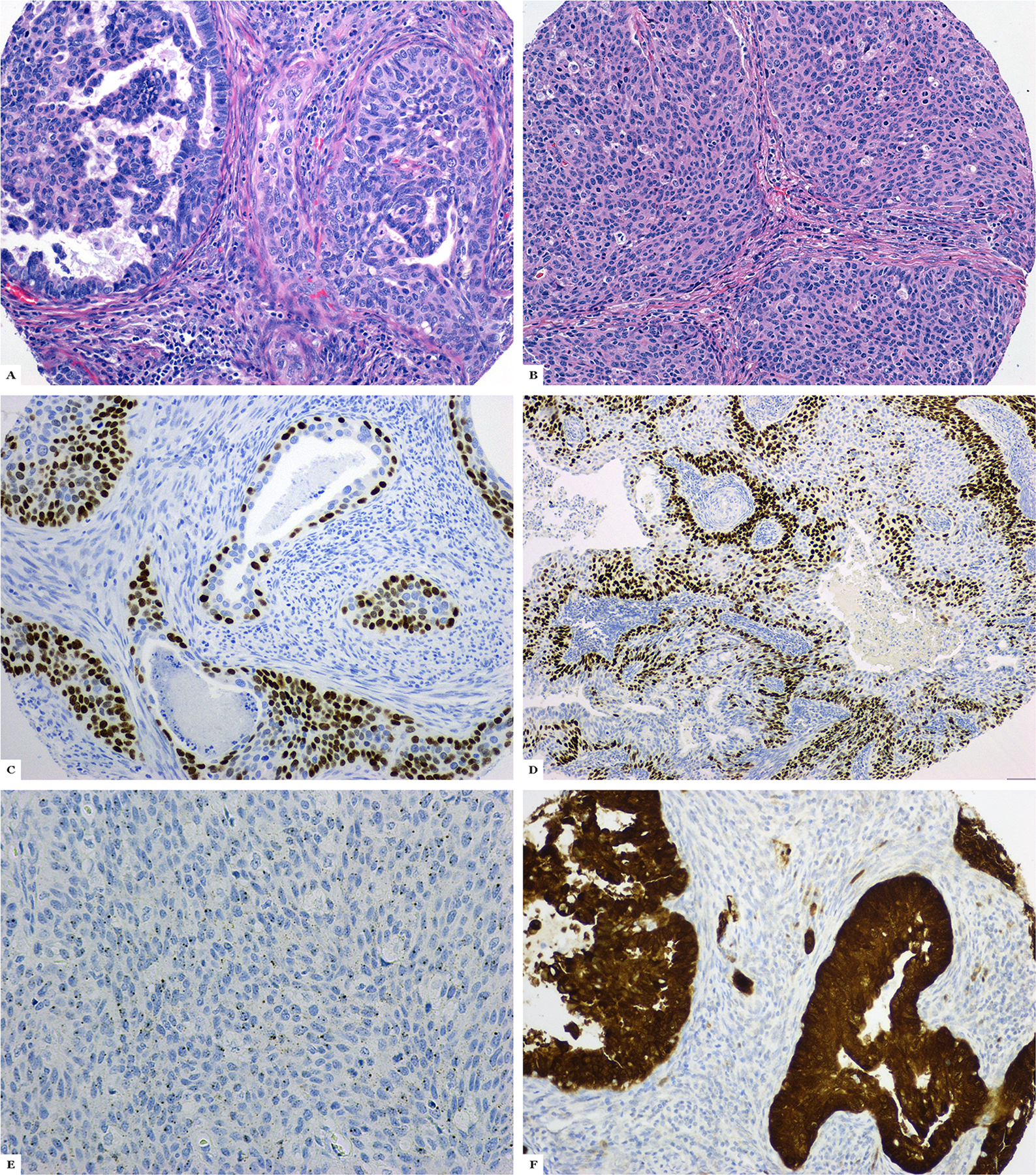
Adenosquamous carcinoma. A: Malignant glandular and squamous differentiation is present; B: Poorly differentiated squamous component; C: p63 expression; D: p40 expression; E: Positive high-risk human papillomavirus in situ hybridization; F: Block-like p16 expression
Figure 3.

Invasive stratified mucin-producing carcinoma (iSMC). A: Invasive nests of cells with stratified mucinous cells surrounded by a palisade; B: p63 expression in the peripheral palisade; C: Block-like p16 expression
Figure 4.

Invasive stratified mucin-producing carcinoma (iSMC) combined with usual-type adenocarcinoma
Figure 5.

Usual-type adenocarcinoma with massive benign-appearing squamous differentiation
Figure 6.

Glassy cell carcinoma (A) with positive high-risk human papillomavirus in situ hybridization (B)
None of the 34 patients with ASC was pregnant or had a recent history of pregnancy. Mean and median patient ages were 46 and 44 years, respectively (range, 24–68 years). FIGO stage distribution was as follows: stage I, 76%; stage II, 21%; and stage III, 3.4%. Regional lymph nodes were assessed in all 34 ASCs, with over 380 lymph nodes examined. Twelve ASCs had metastatic lymph node involvement (35%); two of the 10 had 5 involved lymph nodes. Pelvic metastasis occurred in 1 patient (to the ovary).
71% of the ASCs were high grade. 38% of the 34 ASCs had precursor lesions—high-grade squamous intraepithelial lesions, adenocarcinoma in situ, or stratified mucin-producing intraepithelial lesion (SMILE). Lympho-vascular invasion was present in 26 ASCs (76%). The immunohistochemical profiles of the ASCs and pure iSMCs, the most common mimicker of ASC, are shown in Table 2. ASCs were block-like positive for p16 in 72% of cases and positive for HPV in 86% of cases. The pure iSMCs were positive for p16 in 50% of the cases, similar to the rate in ASCs, while 100% of them were positive for HPV. Significant differences in immunophenotype between the ASCs and pure iSMCs were not observed in any of the markers performed: HPV (p=0.56), p16 (p=0.39), PAX8 (p= 0.66; more in ASC), p40 (p=0.21; more in ASC), p63 (p= 0.99; more in ASC), MUC6 (p=0.15; less in ASC), HNF1beta (p=0.63), vimentin (p= 0.26), p53 (p= 0.22), SATB2 (>0.99), HER2 (p=0.46), GATA3 (p=0.43), HIK1083 (>0.99), CA9 (p=0.64), PR (p>0.99), CK7 (p> 0.99) expression (Table 2).
Table 2:
The immunohistochemical profile of pure invasive stratified mucin-producing carcinomas in comparison with adenosquamous carcinomas
| Characteristic | Pure iSMC, N = 91 | Pure ASC, N = 341 | p-value2,3 |
|---|---|---|---|
| HPV | 0.56 | ||
| Pos | 7(100%) | 19(86%) | |
| Neg | 0(0%) | 3(14%) | |
| Missing | 2 | 12 | |
| p16 | 0.39 | ||
| Pos | 4(50%) | 18(72%) | |
| Neg | 4(50%) | 7(28%) | |
| Missing | 1 | 9 | |
| PAX8 | 0.66 | ||
| Pos | 2(29%) | 10(45%) | |
| Neg | 5(71%) | 12(55%) | |
| Missing | 2 | 12 | |
| p40 | 0.21 | ||
| Pos | 2(29%) | 13(59%) | |
| Neg | 5(71%) | 9(41%) | |
| Missing | 2 | 12 | |
| p63 | >0.99 | ||
| Pos | 4(57%) | 15(62%) | |
| Neg | 3(43%) | 9(38%) | |
| Missing | 2 | 10 | |
| PR | >0.99 | ||
| Pos | 2(25%) | 6(26%) | |
| Neg | 6(75%) | 17(74%) | |
| Missing | 1 | 11 | |
| AR | |||
| Pos | 0(0%) | 0(0%) | |
| Neg | 8(100%) | 24(100%) | |
| Missing | 1 | 10 | |
| CA9 | 0.64 | ||
| Pos | 4(57%) | 16(73%) | |
| Neg | 3(43%) | 6(27%) | |
| Missing | 2 | 12 | |
| MUC6 | 0.15 | ||
| Pos | 4(57%) | 5(22%) | |
| Neg | 3(43%) | 18(78%) | |
| Missing | 2 | 11 | |
| HIK1083 | >0.99 | ||
| Pos | 0(0%) | 1(4.5%) | |
| Neg | 7(100%) | 21(95%) | |
| Missing | 2 | 12 | |
| HNF1beta | 0.63 | ||
| Pos | 1(14%) | 7(32%) | |
| Neg | 6(86%) | 15(68%) | |
| Missing | 2 | 12 | |
| GATA3 | 0.43 | ||
| Pos | 1(14%) | 1(4.5%) | |
| Neg | 6(86%) | 21(95%) | |
| Missing | 2 | 12 | |
| VIM | 0.26 | ||
| Pos | 1(12%) | 0(0%) | |
| Neg | 7(88%) | 23(100%) | |
| Missing | 1 | 11 | |
| HER2 | 0.46 | ||
| Pos | 1(12%) | 1(4.3%) | |
| Neg | 7(88%) | 22(96%) | |
| Missing | 1 | 11 | |
| SATB2 | >0.99 | ||
| Pos | 0(0%) | 0(0%) | |
| Neg | 7(100%) | 22(100%) | |
| Missing | 2 | 12 | |
| CDX2 | |||
| Pos | 0(0%) | 0(0%) | |
| Neg | 7(100%) | 22(100%) | |
| Missing | 2 | 12 | |
| p53 | 0.22 | ||
| Pos | 2(29%) | 2(8.7%) | |
| Neg | 5(71%) | 21(91%) | |
| Missing | 2 | 11 | |
| CK7 | >0.99 | ||
| Pos | 7(100%) | 20(87%) | |
| Neg | 0(0%) | 3(13%) | |
| Missing | 2 | 11 |
n(%)
Fisher’s exact test
No p value provided if ‘POS’ level had 0 counts and p value for ‘POS’ level count<3 (i.e. HIK1083, GATA3, vimentin, HER2) are highly untrustful due to super small sample size
Clinical findings and survival results
Follow-up data were available for 21 of the patients with ASC. Median time to follow-up was 90.9 months (range, 6.1–189.5 months). Only 1 patient with ASC died of disease. She was 47 years old and had a FIGO stage II, grade 3 ASC treated with radical hysterectomy, bilateral salpingo-oophorectomy and lymph node dissection followed by chemotherapy. She died 24 months after initial diagnosis with lung metastases. Two patients, both of whom presented with FIGO stage II disease, are alive with disease. One had a histologically confirmed para-aortic nodal recurrence 14 months from diagnosis, and the other developed 2 pulmonary nodules, interpreted as metastases, 57 months after the initial diagnosis. Both patients were originally treated with radical hysterectomy and lymph node dissection followed by chemotherapy and radiation therapy.
Regarding survival, we compared 34 cases of adenosquamous carcinomas with 285 cases of HPVAs (usual and mucinous type including NOS, intestinal and signet-ring only) as well as with 19 cases of iSMC (9 cases of pure iSMC and 10 cases of iSMC with comonents). There was no difference in OS and PFS between different groups. The OS and PFS for HPVAs versus pure bona fide ASCs was HR=1.97(95%CI: 0.26– 14.66), p= 0.464 and HR= 1.11(95%CI: 0.34–3.59), p= 0.865, respectively; OS and PFS for iSMCs (pure and with components) versus pure bona fide ASCs was HR= 2.46(95%CI: 0.22–27.15), p= 0.35 and HR= 1.36(95%CI: 0.27–6.76), p= 0.706, respectively; OS and PFS for iSMCs with components versus pure iSMCs was HR=0.45(95%CI: 0.03–7.18), p=0.502 and HR=0.33(95%CI: 0.03–3.78), p=0.481, respectively (Figures 7 and 8).
Figure 7.

Analysis of overall survival (OS) between between cervical adenosquamous carcinomas (ASCs) versus Human papillomavirus (HPV)-associated ECAs (HPVAs); ASCs versus invasive stratified mucin-producing carcinomas (iSMCs) (pure and with components); iSMCs pure versus iSMCs with components.
Figure 8.

Analysis of progression-free survival (PFS) between cervical adenosquamous carcinomas (ASCs) versus Human papillomavirus (HPV)-associated ECAs (HPVAs); ASCs versus invasive stratified mucin-producing carcinomas (iSMCs) (pure and with components); iSMCs pure versus iSMCs with components.
Discussion
Historically diagnosed ASCs appear to be a heterogeneous group of tumors, which is in accordance with our series and the published literature1,2,6–8,17,26–28. They have included cases of infiltrating tumors with distinct neoplastic squamous and glandular differentiation (i.e., pure ASCs), HPVAs with benign squamous differentiation, HPVAs with iSMC components, ASCs with iSMC components, pure iSMCs, and glassy cell carcinomas. In our study, only 34 of 59 cases originally diagnosed as pure ASC retained that diagnosis on review, while the 2 glassy cell carcinomas studied could not be supported as ASC variants on morphological or immunohistochemical evaluation. The remaining cases were pure iSMCs (n=9), iSMCs with components (in association with usual-type, HPVA mucinous, or ASC [n=10]) and benign squamous metaplasia in association with usual-type and HPVA mucinous endocervical adenocarcinomas (n=4).
Tumor categorization used for this study overlaps significantly with that of WHO 2020, which was inspired by the IECC and was specifically developed to enable practitioners to be able to recognize HPV-associated and –independent variants of endocervical adenocarcinomas. In the original IECC paper, 95% of tumors demonstrating HPV-associated morphology were positive by HR-HPV mRNA and 90% showed block-like p16 staining, indicating better performance characteristic for the in-situ hybridization assay19. In the current study, wherein only rare tumor types were studied, we report that 86% of ASCs were HPV positive by in situ hybridization, compared to 72% with block-like p16 staining; 100% of iSMCs were HPV-positive, while only 50% showed block-like p16 staining. The reasons underlying these discrepancies and the existence of cases without detectable HPV are discussed in detail in a related manuscript19. The use of an in-situ hybridization probe targeting E6 and E7 mRNA of 18 different HR-HPV types, instead of the more common DNA-based probes, underlies the superior performance of the former assay.
iSMC is a newly recognized subtype of endocervical adenocarcinoma that differs from stratified mucin-producing intraepithelial lesion (SMILE)29. iSMC was first described by Lastra et al20 in 2016 as an invasive adenocarcinoma containing nests of stratified columnar epithelium with round to ovoid hyperchromatic nuclei, intracytoplasmic mucin in the form of large mucin droplets, or more delicate and collapsing vacuoles that created spacing between adjacent nuclei and peripheral palisading. In the Lastra series, the amount of mucin among the cases (of which 7 were pure iSMCS and 1 was usual-type endocervical adenocarcinoma with an iSMC component) varied from abundant to scarce. In a subsequent publication describing 3 additional cases of iSMC, these HPV-related tumors were reported to show stratified mucinous epithelium that mimicked the appearance of immature squamous metaplasia, presumably in mucin-poor examples30. In a recent study, our group has demonstrated that iSMC can display architectural diversity and variable cytologic appearance31. This likely accounts for the historical misclassification of iSMCs as ASCs. iSMCs were recently reported to show some notable immunohistochemical differences from other HPV-associated endocervical adenocarcinoma subtypes, such as a higher prevalence of p40 and p63 expression and a lower prevalence of PAX8 expression, with possibly more frequent aberrant p53 staining32. These data suggest that iSMCs diverge from other mucinous HPVAs and could be categorized separately. HPVAs with benign squamous differentiation, HPVAs with iSMC components, and pure iSMCs should not be regarded as ASCs because they do not contain a malignant squamous component.
There are, apparently, no differences in clinical outcomes between these categories; however, iSMCs are morphologically distinct and have some immunophenotypic differences with ASCs. Most iSMCs and ASCs are positive for p16 and HPV and no statistically significant differences were found in the expression of HPV, p40, p63, PAX8, HNF1beta, vimentin, p53, CK7, PR, AR, CA9, HIK1083, GATA3, HER2, SATB2, CDX2, p53 and MUC6 when studied as binary, non-continuous variables. iSMCs have attenuated p40 and p63 expression, compared to ASCs. These two stains often show patchy positivity in the peripheral palisade of tumor cell nests, unlike the typically more diffuse staining of the solid/squamous components of ASC. Moreover, the scant p63 and p40 expression in pure iSMCs in the palisade around invasive nests and the relative lack of PAX8 suggest that these tumors may be of reserve cell origin, as has been suggested29, compared with pure ASCs.
The older literature suggested that ASC is a relatively aggressive disease type, especially in advanced stages, occurring more frequently in pregnant and younger patients than either squamous carcinomas or endocervical adenocarcinomas17,33,34. In our study, ASCs were associated with clinical outcomes similar to those of HPVAs, including mucinous endocervical adenocarcinomas, as reported previously9,35,36, whether or not the iSMC present was pure or with components. This is probably due to the limited number of iSMC cases included since more recent data showed that iSMC is more aggressive that other HPVAs due to propensity for LNM and local/distant recurrences37.
Ultrastructural studies that pointed to the presence of glandular and squamous differentiation38,39 originally supported the idea that glassy cell carcinomas were a type of ASC. Although evidence of glandular differentiation is reportedly rather obvious using electron microscopy, only focal concentrations of tonofilaments have been construed as evidence of squamous differentiation. On examination of H&E slides, however, these tumors display a uniform population of neoplastic cells, unlike ASC, although there are rare reports of glassy cell carcinomas with subtle intracytoplasmic mucin or squamous differentiation. Glassy cell carcinoma has been thought of as an aggressive subtype of cervical carcinoma40,41, but a recent publication reported the successful treatment of 5 glassy cell carcinoma patients with advanced stage disease42. Based on studying only 2 such cases, neither expressing markers associated with squamous differentiation, we conclude that glassy cell carcinomas are either not adenosquamous carcinomas or are so poorly differentiated that they can only be classified as such with difficulty. Further studies addressing this issue are therefore required for further elucidation of this entity.
In summary, ASCs can be diagnosed in the presence of unequivocal evidence of malignant glandular and squamous differentiation. Mimics such as iSMC, iSMC with components, and HPVAs with benign-appearing squamous metaplasia should not be diagnosed as ASCs based on distinguishing morphological features and some immunohistochemical differences, despite the fact that clinical outcomes appear similar in this study. Since the two putative glassy cell carcinomas studied did not meet our criteria for ASC and lacked evidence of squamous differentiation with immunohistochemistry, one should reconsider whether these tumors should be categorized as ASCs.
Funding:
This study was funded in part through the NIH/NCI MSK Cancer Center Support Grant P30 CA008748 (Dr. Soslow and Dr. Park).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Availability of data and material: All data can be provided by the corresponding author (Simona Stolnicu)
Ethics approval: This study was approved by the institutional review boards of each participating centre. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Consent for publication: All authors agreed to publish the results of the present study
References
- 1.Herrington CS, Kim KR, Kong C, et al. Tumours of the uterine cervix. W. C. o. T. E. B. F. g. tumours. Lyon (France), International Agency for Research on Cancer; 2020: 336–389. [Google Scholar]
- 2.Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs, 4th ed. Lyon, France: IARC Press, 2014. [Google Scholar]
- 3.Cherry CP, Glucksmann A. Incidence, histology, and response to radiation of mixed carcinomas (adenoacanthomas) of the uterine cervix. Cancer 1956; 9:971–99. [DOI] [PubMed] [Google Scholar]
- 4.Green LS, Muirhead W. Improvement in results of treatment of carcinoma of the cervix. J Can Assoc Radiol 1963; 14:191–199. [PubMed] [Google Scholar]
- 5.Costa MJ, Kenny MB, Hewan-Lowe K, et al. Glassy cell features in adenosquamous carcinoma of the uterine cervix. Histologic, ultrastructural, immunohistochemical, and clinical findings. Am J Clin Pathol 1991; 96:520–528. [DOI] [PubMed] [Google Scholar]
- 6.Young RH, Clement PB. Endocervical adenocarcinoma and its variants: their morphology and differential diagnosis. Histopathology 2002; 41:185–207. [DOI] [PubMed] [Google Scholar]
- 7.Lennerz JK, Perry A, Mills JC, et al. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol 2009; 33:835–843. [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG. New developments in endocervical glandular lesions. Histopathology 2013; 62:138–160. [DOI] [PubMed] [Google Scholar]
- 9.Shingleton HM, Gore H, Bradley DH, et al. Adenocarcinoma of the cervix. I. Clinical evaluation and pathologic features. Am J Obstet Gynecol 1981;139:799–814. [DOI] [PubMed] [Google Scholar]
- 10.Tavassoli FA, Deville P. Tumours of the breast and female genital organs Lyon, France: IARC Press, 2003. [Google Scholar]
- 11.An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol 2005; 18:528–534. [DOI] [PubMed] [Google Scholar]
- 12.Kato N, Katayama Y, Kaimori M, et al. Glassy cell carcinoma of the uterine cervix: histochemical, immunohistochemical, and molecular genetic observations. Int J Gynecol Pathol 2002; 21:134–140. [DOI] [PubMed] [Google Scholar]
- 13.Kurman RJ, Ronnett BM, Sherman ME, et al. AFIP Atlas of Tumor Pathology, Series 3, Fascicle 4 Washington, DC: Armed Forces Institute of Pathology, 1992. [Google Scholar]
- 14.Tase T, Okagaki T, Clark BA, et al. Human papillomavirus types and localization in adenocarcinoma and adenosquamous carcinoma of the uterine cervix: a study by in situ DNA hybridization. Cancer Res 1988; 48:993–998. [PubMed] [Google Scholar]
- 15.Tase T, Okagaki T, Clark BA, et al. Human papillomavirus DNA in adenocarcinoma in situ, microinvasive adenocarcinoma of the uterine cervix, and coexisting cervical squamous intraepithelial neoplasia. Int J Gynecol Pathol 1989; 8:8–17. [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa Y, Forslund O, Teshima H, et al. Human papillomavirus DNA in adenocarcinoma and adenosquamous carcinoma of the uterine cervix detected by polymerase chain reaction (PCR). Gynecol Oncol 1994; 53:190–195. [DOI] [PubMed] [Google Scholar]
- 17.Farley JH, Hickey KW, Carlson JW, et al. Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer 2003; 97:2196–2202. [DOI] [PubMed] [Google Scholar]
- 18.Shingleton HM, Bell MC, Fremgen A, et al. Is there really a difference in survival of women with squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma of the cervix? Cancer 1995; 76:1948–1955. [DOI] [PubMed] [Google Scholar]
- 19.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol 2018; 42:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMC): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol 2016; 40:262–269. [DOI] [PubMed] [Google Scholar]
- 21.Hedvat CV, Hegde A, Chaganti RS, et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol 2002; 33:968–974. [DOI] [PubMed] [Google Scholar]
- 22.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4:844–847. [DOI] [PubMed] [Google Scholar]
- 23.Bartley AN, Washington MK, Colasacco C, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017; 35:446–464. [DOI] [PubMed] [Google Scholar]
- 24.Stolnicu S, Hoang L, Chiu D et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am J Surg Pathol 2019; 43(4): 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller G, Venkatraman ES. Resampling procedures to compare two survival distributions in the presence of right censored data. Biometrics 1996; 52:1204–1213. [Google Scholar]
- 26.Kurman RJ, Ronnett BM, Sherman ME, et al. AFIP Atlas of Tumor Pathology Washington, DC: Armed Forces Institute of Pathology, 2010. [Google Scholar]
- 27.Johnston GA Jr, Azizi F, Reale F, et al. Glassy cell carcinoma of the cervix: report of three cases. J Natl Med Assoc 1982; 74:361–363. [PMC free article] [PubMed] [Google Scholar]
- 28.Littman P, Clement PB, Henriksen B, et al. Glassy cell carcinoma of the cervix. Cancer 1976; 37:2238–2246. [DOI] [PubMed] [Google Scholar]
- 29.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol 2000; 24:1414–1419. [DOI] [PubMed] [Google Scholar]
- 30.Onishi J, Sato Y, Sawaguchi A, et al. Stratified mucin-producing intraepithelial lesion with invasive carcinoma: 12 cases with immunohistochemical and ultrastructural findings. Hum Pathol 2016; 55:174–181. [DOI] [PubMed] [Google Scholar]
- 31.Stolnicu S, Segura S, Parra-Herran C et al. : Invasive stratified mucin-producing carcinoma (ISMC) of the cervix. A study on morphologic diversity. Am J Surg Pathol 2020; 44(7): 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolnicu S, Barsan I, Hoang L, et al. Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol 2018; 42(8):989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bethwaite P, Yeong ML, Holloway L, et al. The prognosis of adenosquamous carcinomas of the uterine cervix. Br J Obstet Gynaecol 1992; 99:745–750. [DOI] [PubMed] [Google Scholar]
- 34.Pekin T, Kavak Z, Yildizhan B, et al. Prognosis and treatment of primary adenocarcinoma and adenosquamous cell carcinoma of the uterine cervix. Eur J Gynaecol Oncol 2001; 22:160–163. [PubMed] [Google Scholar]
- 35.Korhonen M, Stenback F. Adenocarcinoma metastatic to the uterine cervix. Gynecol Obstet Invest 1984; 17:57–65. [DOI] [PubMed] [Google Scholar]
- 36.Randall ME, Constable WC, Hahn SS, et al. Results of the radiotherapeutic management of carcinoma of the cervix with emphasis on the influence of histologic classification. Cancer 1988; 62:48–53. [DOI] [PubMed] [Google Scholar]
- 37.Stolnicu S, Boros M, Segura S et al. : Invasive stratified mucinous carcinoma (iSMC) of the cervix often presents with high-risk features that are determinants of poor outcome: An international multicentric study. Am J Surg Pathol 2020; 44(10): 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulbright TM, Gersell DJ. Glassy cell carcinoma of the uterine cervix. A light and electron microscopic study of five cases. Cancer 1983; 51:2255–2263. [DOI] [PubMed] [Google Scholar]
- 39.Zaino RJ, Nahhas WA, Mortel R. Glassy cell carcinoma of the uterine cervix. An ultrastructural study and review. Arch Pathol Lab Med 1982;106:250–254. [PubMed] [Google Scholar]
- 40.Pak HY, Yokota SB, Paladugu RR, et al. Glassy cell carcinoma of the cervix. Cytologic and clinicopathologic analysis. Cancer 1983; 52:307–312. [DOI] [PubMed] [Google Scholar]
- 41.Tamimi HK, Ek M, Hesla J, et al. Glassy cell carcinoma of the cervix redefined. Obstet Gynecol 1988; 71(6 Pt 1):837–841. [PubMed] [Google Scholar]
- 42.Yoon N, Kim JY, Kim HS. Clinical outcomes of advanced-stage glassy cell carcinoma of the uterine cervix: a need for reappraisal. Oncotarget 2016; 7:78448–78454. [DOI] [PMC free article] [PubMed] [Google Scholar]


