Abstract
Aim and background
Identifying risk factors for cancer initiation and progression is the cornerstone of the preventive approach to cancer management and control (EPMA J. 4(1):6, 2013). Tobacco smoking is a well-recognized risk factor for initiation and spread of several cancers. The predictive, preventive, and personalized medicine (PPPM) approach to cancer management and control focuses on smoking cessation as an essential cancer prevention strategy. Towards this end, this study examines the temporal patterns of cancer burden due to tobacco smoking in the last three decades at global, regional, and national levels.
Data and methods
The data pertaining to the burden of 16 cancers attributable to tobacco smoking at global, regional, and national levels were procured from the Global Burden of Disease 2019 Study. Two main indicators, deaths and disability-adjusted life years (DALYs), were used to describe the burden of cancers attributable to tobacco smoking. The socio-economic development of countries was measured using the socio-demographic index (SDI).
Results
Globally, deaths due to neoplasms caused by tobacco smoking increased from 1.5 million in 1990 to 2.5 million in 2019, whereas the age-standardized mortality rate (ASMR) decreased from 39.8/100,000 to 30.6/100,000 and the age-standardized DALY rate (ASDALR) decreased from 948.9/100,000 to 677.3/100,000 between 1990 and 2019. Males accounted for approximately 80% of global deaths and DALYs in 2019. Populous regions of Asia and a few regions of Europe account for the largest absolute burden, whereas countries in Europe and America have the highest age-standardized rates of cancers due to tobacco smoking. In 8 out of 21 regions, there were more than 100,000 deaths due to cancers attributable to tobacco smoking led by East Asia, followed by Western Europe in 2019. The regions of Sub-Saharan Africa (except southern region) had one of the lowest absolute counts of deaths, DALYs, and age-standardized rates. In 2019, tracheal, bronchus, and lung (TBL), esophageal, stomach, colorectal, and pancreatic cancer were the top 5 neoplasms attributable to tobacco smoking, with different burdens in regions as per their development status. The ASMR and ASDALR of neoplasms due to tobacco smoking were positively correlated with SDI, with pairwise correlation coefficient of 0.55 and 0.52, respectively.
Conclusion
As a preventive tool, tobacco smoking cessation has the biggest potential among all risk factors for preventing millions of cancer deaths every year. Cancer burden due to tobacco smoking is found to be higher in males and is positively associated with socio-economic development of countries. As tobacco smoking begins mostly at younger ages and the epidemic is unfolding in several parts of the world, more accelerated efforts are required towards tobacco cessation and preventing youth from entering this addiction. The PPPM approach to medicine suggests that not only personalized and precision medicine must be provided to cancer patients afflicted by tobacco smoking but personalized and targeted preventive solutions must be provided to prevent initiation and progression of smoking.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13167-022-00308-y.
Keywords: Tobacco smoking, Deaths, Disability-adjusted life years, Predictive preventive personalized medicine, Targeted prevention, Preventive medicine
Introduction
Tobacco smoking is a global epidemic with an estimated 1.14 billion tobacco smokers worldwide and tobacco smoking accounting for 7.7 million deaths and 20 million disability-adjusted life years (DALYs) worldwide in 2019 [1]. The total economic cost of tobacco smoking (including healthcare cost and productivity loss) was estimated to be PPP $1852 billion (US $1436 billion) in 2012 [2]. As of now, tobacco smoking has been recognized as the biggest risk factor for several diseases, including cancers. Tobacco smoking and its role in carcinogenesis was highlighted by the landmark studies by Wynder and Graham [3] and Doll and Hill [4], and the ill effects of smoking were recognized publicly after the United States surgeon general’s report on tobacco smoking [5]. In the beginning, tobacco smoking was recognized as a risk factor for lung carcinoma, but later epidemiological studies found an association of tobacco smoking with several different cancers. The International Agency for Research on Cancer lists tobacco smoking as a risk factor with sufficient evidence for cancers on several sites, including pharynx, esophagus, stomach, colorectum, pancreas, larynx, oral cavity, lung, ovary, kidney, urinary bladder, and few leukemia types, among others [6].
Predictive, preventive, and personalized medicine (PPPM) provides a new approach to medicine by applying a proactive approach to cancer management and control [7]. Under this approach, recognition of major risk factors responsible for cancer initiation and progression is an essential component of cancer management and control [8]. Cigarette smoke is made up of more than 7000 chemicals; out of which, more than 70 have been found to be carcinogenic [9]. Nicotine-derived N-nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is the most lethal carcinogen in tobacco smoke and is linked with lung carcinogenesis in humans [10]. A recent study has found that insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), possibly via N6-methyladenosine (m6a) modification, results in NNK-induced malignant changes [11]. Besides the direct effects of carcinogenic compounds, tobacco smoking also plays a role in carcinogenesis through heightened inflammation, which can ultimately culminate in lung cancer [12]. Several studies have discussed the developments in the PPPM approach of cancer management and control [12–18]. The relative success of cancer prevention policies in the past and devising prevention policies suiting the socio-economic setup of different countries and regions for the future requires an assessment of the temporal patterns of cancer burden attributable to tobacco smoking at the global, regional, and national levels.
Our study seeks to provide a comprehensive and holistic examination of the burden of neoplasms due to tobacco smoking in 21 regions and 204 countries using data from the Global Burden of Diseases, Injuries, and Risk Factors 2019 Study [19–21]. Specifically, this study has three objectives. First, we describe the risk-attributable burden of neoplasms due to tobacco smoking using data on country, age, and sex-wise deaths and DALYs from 1990 to 2019. Apart from deaths and incidence, DALYs has become an important metric to evaluate the burden of a disease, as it incorporates the effects of both death and disability caused by the disease. The second objective of this study is to examine the temporal patterns of mortality and DALYs of 16 neoplasms attributable to tobacco smoking in 21 regions. We also examine how the burden of neoplasms varied as per sex in different regions. The third objective of this study is to examine the bivariate association between smoking-attributable cancer burden and socio-demographic index (SDI)—an indicator of the development status of countries.
The results of our study can help countries devise targeted prevention strategies suiting their socio-economic and demographic landscape, which again signifies the importance of the personalized and targeted solutions advocated by the PPPM approach rather than focusing on the “one-size-fits-all” kind of solutions.
Data and methods
The estimates of deaths, DALYs, and age-standardized rates of all cancers attributable to tobacco smoking in 204 countries/territories and 21 regions for the period 1990–2019 were procured from the Global Burden of Disease (GBD) results tool [22]. The attributable burden of a risk factor involves estimating disease burden (measured by deaths and DALYs) and then estimating the population attributable fraction (PAF) of a risk factor. The GBD uses all information available from cancer registries, vital statistics, and verbal autopsies to arrive at the estimates of incidence, mortality, and DALYs. The estimates of mortality are generated using spatiotemporal Gaussian process regression, mortality-to-incidence ratio, and cause of death ensemble model in multiple steps detailed elsewhere [19, 20, 23]. The years of life lost are calculated as the difference between standard life expectancy and age at each premature death due to the disease. The DALYs are calculated as the sum total of years lost and years lived with disability due to the disease. The age-standardized rates are calculated using GBD standard population.
The GBD estimates the burden of diseases due to 87 risk factors, out of which, we extracted estimates of tobacco smoking-attributable deaths and DALYs due to 16 neoplasms. The sixteen neoplasms which have been linked with tobacco smoking as per epidemiological studies and are included in GBD estimation framework are: bladder, breast, cervical, colorectum, esophageal, kidney, larynx, leukemia, lip and oral cavity (LOC), liver, nasopharynx, other pharynx, pancreatic, prostate, stomach, and tracheal, bronchus, and lung (TBL) cancer. The GBD risk factor methodology is detailed elsewhere [21]; here, we briefly outline the steps involved in the estimation of risk-attributable burden. The GBD risk factor estimation begins with the identification of risk-outcome pairs; only those risk-outcome pairs are chosen which have plausible/convincing evidence as per World Cancer Research Fund criteria. The relative risk (RR) is calculated using meta-analysis as a function of risk exposure. Thereafter, the PAF and attributable burden are calculated as a function of RR and theoretical minimum risk exposure level (TMREL)—the level of exposure of a risk factor with minimum risk. For harmful risk factors with monotonically increasing risk functions such as smoking, TMREL is taken to be zero.
For continuous risk , the PAF is calculated by the following equation [21].
In above equation, is the risk factor, is the cause, for age group , sex , location , and time (year), and is defined as a function of the exposure level; is the distribution of exposure. The attributable burden of tobacco smoking (i.e., deaths and DALYs) is calculated by multiplying deaths and DALYs due to cancer with the PAF calculated for the smoking-cancer pair.
The development status of countries was measured using SDI—an indicator of development proposed and developed by the GBD as an alternative to human development index. It comprises three components: per capita income, educational attainment, and total fertility rate in a country. Based on SDI values in 2019, the countries were categorized into five quintiles: low SDI, lower-middle SDI, middle SDI, upper-middle SDI, and high SDI. Lastly, the data on adult tobacco smoking prevalence in 164 countries were procured from the WHO global health observatory for 2005 and 2019 [24]. All the data analysis and visualization were done using statistical software Stata 13.0, R 4.1.1, and Python 3.8.
Results
Global burden of neoplasms due to tobacco smoking
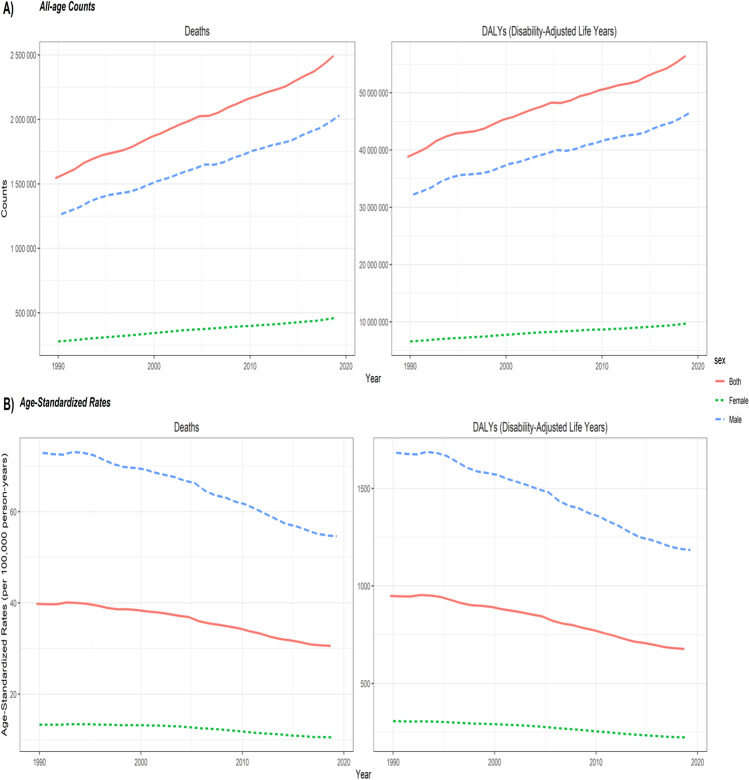
Globally, deaths due to neoplasms caused by tobacco smoking for both sexes combined increased from 1.5 million in 1990 to 2.5 million in 2019, whereas the age-standardized mortality rate (ASMR) decreased from 39.8/100,000 in 1990 to 30.6/100,000. Number of DALYs increased from 38.8 million in 1990 to 56.4 million in 2019, and the age-standardized DALY rate (ASDALR) decreased from 948.9/100,000 in 1990 to 677.3/100,000 in 2019. Out of 2.5 million deaths and 56.4 million DALYs in 2019, males accounted for 2.0 million deaths (80%) and 46.7 million DALYs (82.8%). Notably, deaths and DALYs increased between 1990 and 2019 in both males and females, whereas the age-standardized rates decreased between 1990 and 2019 (Fig. 1).
Fig. 1.
Global temporal patterns of cancer burden attributable to tobacco smoking, 1990–2019. Deaths: all-age deaths; DALYs: disability-adjusted life years. The age-standardized rates of deaths and DALYs are expressed per 100,000 population. Data source: Global Burden of Disease 2019 Study
Table 1 shows the burden of 16 neoplasms attributable to tobacco smoking in 1990 and 2019, and Supplementary Fig. 1 shows the temporal patterns of number of deaths and ASMR due to 16 neoplasms between 1990 and 2019. Out of 16 cancers, tobacco smoking caused the highest number of deaths due to TBL cancer (1.3 million), esophageal cancer (203,328), and stomach cancer (171,920) in 2019 (Table 1). Tobacco smoking accounted for 28.6 million DALYs due to TBL cancer, 4.7 million due to esophageal cancer, and 3.8 million due to stomach cancer in 2019. Between 1990 and 2019, the absolute count of deaths and DALYs due to tobacco smoking increased for all 16 cancers (Table 1). Among 16 cancers, the absolute count of deaths and DALYs increased most rapidly as well as the lowest reduction in age-standardized rates occurred for the cancers of the kidney, pancreatic, and other pharynx (Table 1). For esophageal cancer, the absolute count of deaths remained almost same in 2005 and 2019, whereas, in the case of stomach cancer, deaths due to tobacco smoking decreased after 2005 (Supplementary Fig. 1). As a result, stomach cancer had more deaths, DALYs, and age-standardized rates than esophageal cancer in 1990; however, by 2019, esophageal cancer replaced stomach cancer as the second-ranked cancer due to tobacco smoking.
Table 1.
Burden of all neoplasms due to tobacco smoking in 1990 and 2019
| 1990 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer | Deaths | ASMR | DALYs | ASDALR | Deaths | ASMR | DALYs | ASDALR |
| Bladder cancer | 49,869 | 1.38 | 1,100,126 | 28.12 | 77,521 | 0.98 | 1,615,088 | 19.67 |
| Breast cancer | 16,295 | 0.42 | 476,106 | 11.46 | 18,958 | 0.23 | 513,437 | 6.13 |
| Cervical cancer | 27,422 | 0.67 | 863,495 | 20.08 | 30,137 | 0.36 | 893,735 | 10.62 |
| Colon and rectum cancer | 78,784 | 2.11 | 1,856,999 | 46.45 | 142,931 | 1.77 | 3,226,829 | 38.87 |
| Esophageal cancer | 134,682 | 3.42 | 3,475,304 | 84.38 | 203,328 | 2.48 | 4,746,524 | 56.71 |
| Kidney cancer | 14,980 | 0.39 | 370,849 | 9.13 | 30,126 | 0.37 | 687,231 | 8.25 |
| Larynx cancer | 62,626 | 1.57 | 1,741,178 | 41.81 | 78,269 | 0.95 | 2,023,841 | 24.05 |
| Leukemia | 43,064 | 1.12 | 1,131,917 | 27.43 | 64,585 | 0.80 | 1,529,056 | 18.46 |
| Lip and oral cavity cancer | 37,407 | 0.94 | 1,040,593 | 25.01 | 63,434 | 0.77 | 1,656,260 | 19.69 |
| Liver cancer | 66,456 | 1.64 | 1,871,099 | 44.53 | 85,884 | 1.04 | 2,125,827 | 25.33 |
| Nasopharynx cancer | 12,848 | 0.31 | 393,963 | 9.24 | 17,918 | 0.21 | 526,563 | 6.24 |
| Other pharynx cancer | 27,028 | 0.66 | 779,977 | 18.54 | 53,612 | 0.64 | 1,442,986 | 17.07 |
| Pancreatic cancer | 53,204 | 1.42 | 1,217,116 | 30.43 | 113,384 | 1.40 | 2,443,356 | 29.42 |
| Prostate cancer | 19,316 | 0.55 | 384,707 | 10.23 | 29,298 | 0.37 | 571,587 | 7.04 |
| Stomach cancer | 152,082 | 3.94 | 3,705,929 | 91.16 | 171,920 | 2.12 | 3,812,627 | 45.82 |
| Tracheal, bronchus, and lung cancer | 747,908 | 19.22 | 18,391,550 | 450.92 | 1,311,721 | 16.13 | 28,631,972 | 343.97 |
| Total | 1,543,971 | 39.8 | 38,800,908 | 948.9 | 2,493,026 | 30.6 | 56,446,919 | 677.3 |
Data source: Global Burden of Disease 2019 Study
Deaths all-age deaths, DALYs disability-adjusted life years, ASMR age-standardized mortality rate, ASDALR age-standardized DALY rate
Region-wise burden of neoplasms due to tobacco smoking in 2019
In terms of absolute counts, East Asia was the leading region in 2019, with 924,474 deaths and 21.0 million DALYs. Western Europe, with 332,759 deaths and 6.8 million DALYs, was the second leading region, followed by high-income North America with 256,115 deaths and 5.3 million DALYs in 2019 (Supplementary Table 1). The age-standardized rates of smoking-attributable cancers were the highest in Central Europe, with ASMR of 50.4/100,000 and ASDALR of 1261.1/100,000 in 2019. East Asia was the second-ranked region with ASMR of 45.0/100,000 and ASDALR of 972.6/100,000, followed by high-income North America (ASMR: 40.0/100000; ASDALR: 874.8/100,000). Western Sub-Saharan Africa (SSA) recorded the lowest ASMR of 6.7/100,000 and ASDALR of 152.2/100,000 in 2019. The age-standardized rates were the lowest in the SSA regions, with 3/5 regions with the lowest age-standardized rates belonging to SSA. Among SSA regions, only southern SSA had intermediate levels of ASMR (22.8/100,000) and ASDALR (543.5/100,000) in 2019 (Supplementary Table 1).
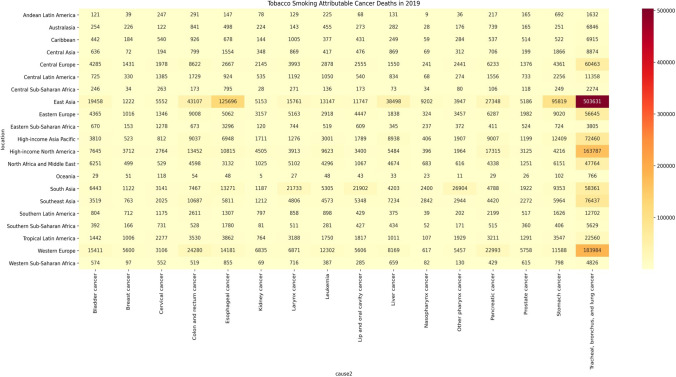
Figure 2 shows the deaths due to 16 neoplasms caused by tobacco smoking in 21 regions in 2019. In most regions, TBL cancer was the leading neoplasm caused by tobacco smoking, with death count being the highest in East Asia (503,631), followed by Western Europe (183,984) and high-income North America (163,787). East Asia was the leading region in terms of deaths caused by tobacco smoking for ten cancers, followed by South Asia leading in three cancers (larynx, LOC, and other pharynx) and Western Europe leading in three cancers (breast, kidney, and prostate). Besides TBL cancer, tobacco smoking caused more than 100,000 deaths due to esophageal cancer in East Asia (125,696). To understand the relative burden of different neoplasms in different regions, we also presented the ASMR of 16 neoplasms in 21 regions in 2019 (Supplementary Fig. 2). Across regions, TBL cancer had the highest ASMR due to tobacco smoking. Even for TBL cancer, the ASMR due to tobacco smoking varied from the low of 2.88/100,000 in Western SSA and 3.01/100,000 in Andean Latin America to 28.25/100,000 in Central Europe and 25.49/100,000 in high-income North America. The rank of different cancers, other than TBL cancer, due to tobacco smoking was different in regions as per continent as well as development levels of the regions (Supplementary Fig. 2).
Fig. 2.
Deaths due to 16 cancers attributable to tobacco smoking in 2019. The numbers in the cells represent absolute count of deaths due to a cancer in a given region. Data source: Global Burden of Disease 2019 Study
Country-wise burden of neoplasms due to tobacco smoking in 2019
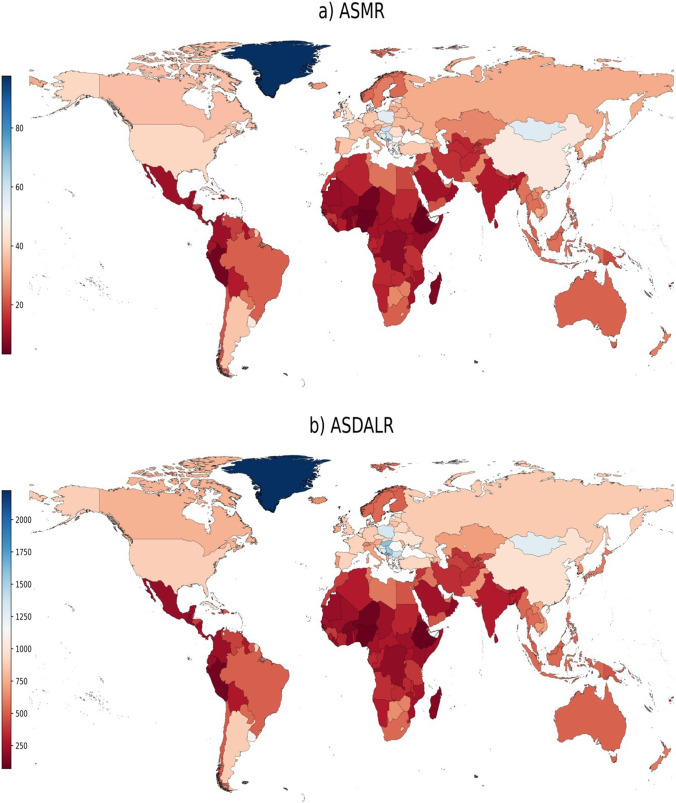
China, United States, and India were the three leading countries in terms of mortality, with 900,362, 230,494, and 138,980 deaths, respectively, in 2019 (Supplementary Table 2). These three countries were also the top three countries in terms of DALYs, with 20.4 million, 4.8 million, and 3.5 million DALYs, respectively, in 2019. In 8/204 countries (China, United States, India, Japan, Russia, Germany, United Kingdom, and Brazil), more than 50,000 cancer deaths were attributable to tobacco smoking in 2019. The ASMR was the highest in Greenland, Montenegro, and Monaco at 97.6/100,000, 68.4/100,000, and 68.3/100,000, respectively, and the lowest ASMR occurred in Ethiopia (3.3/100,000), Nigeria (3.8/100,000), and Niger (4.8/100,000) in 2019 (Fig. 3). In heavily burdened countries such as China and United States, the ASMR was at 45.5/100,000 and 40.5/100,000, respectively, in 2019. The ASDALR varied 31-folds across countries, from 72.2/100,000 in Ethiopia to 2224.0/100,000 in Greenland. In terms of ASDALR, Montenegro (1708.0/100,000) and Hungary (1588.6/100,000) were also the top-ranked countries after Greenland in 2019. The ASDALR was generally lower in SSA countries, with Nigeria (80.6/100,000) and Niger (102.2/100,000) being the other two countries with low ASDALR besides Ethiopia in 2019 (Fig. 3).
Fig. 3.
Age-standardized rates of neoplasms due to tobacco smoking in 2019. ASMR: age-standardized mortality rate; ASDALR: age-standardized DALY rate. Data source: Global Burden of Disease 2019 Study
Between 1990 and 2019, the burden of cancers due to tobacco smoking increased the most in United Arab Emirates, which reported a growth in deaths by 776.2% and DALYs by 860.8% between 1990 and 2019 (Supplementary Table 2). All-age deaths more than doubled (growth > 100%) in 68/204 countries/territories, and all-age DALYs doubled or more in 58/204 countries/territories between 1990 and 2019. In 28/204 countries/territories, all-age DALYs decreased between 1990 and 2019, led by Kazakhstan (− 32.5%) and Latvia (− 32.0%). Similarly, all-age deaths decreased the most in Kazakhstan (− 26.3%) and Ukraine (− 27.3%) between 1990 and 2019. Supplementary Fig. 3 presents geographically the percent changes in age-standardized rates between 1990 and 2019. The ASMR increased the most by 54.6% in Sao Tome and Principe and 44.0% in Lesotho, with ASMR increasing by 10% or more in 25/204 countries. The ASMR decreased the most in decreased the most in Singapore (−60.1%), and Colombia (−57.6%), with 126/204 countries posting negative growth of 10% or more (< − 10%) between 1990 and 2019. Among eight countries with more than 50,000 deaths in 2019, Brazil posted the highest reduction in ASMR (− 42.4%), followed by Japan (− 37.6%). The ASDALR decreased the most in Singapore (− 63.7%), followed by Colombia (− 59.6%) and Bahrain (− 57.7%), and increased the most in Sao Tome and Principe (53.4%), Lesotho (47.6%), and Egypt (32.2%) between 1990 and 2019.
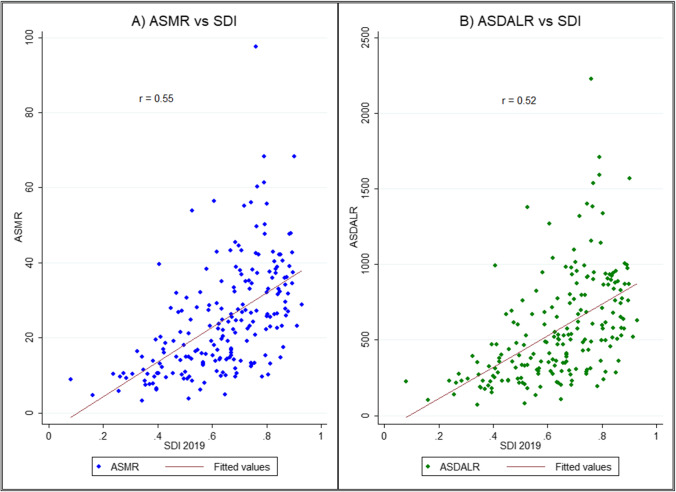
Supplementary Fig. 4 shows the burden of neoplasms by SDI quintile in 2019 and percent changes between 1990 and 2019. In general, DALYs and deaths as well as age-standardized rates increased with SDI, with the highest absolute count and age-standardized rate in upper-middle SDI quintile. In contrast, the percent change was the highest in middle SDI quintile (deaths: 123.56%; DALYs: 98.53%), followed by lower-middle SDI and low SDI quintile. In contrast to absolute counts, the age-standardized rates decreased between 1990 and 2019 in all SDI quintiles, with the highest negative growth posted by high SDI quintile (ASMR: − 32.94%; ASDALR: − 37.96%), followed by upper-middle SDI quintile (ASMR: − 20.42%; ASDALR: − 27.81%). Fig. 4 shows the bivariate plot of age-standardized rates and SDI in 2019. In general, SDI had a positive correlation with ASMR () and ASDALR (), showing that countries with higher SDI levels, on average, had higher age-standardized rates of neoplasms due to tobacco smoking. By contrast, percent change in ASMR and ASDALR was negatively correlated with SDI with a pairwise correlation of − 0.28 and − 0.31, respectively (Supplementary Fig. 5).
Fig. 4.
Bivariate association between age-standardized rates of neoplasms due to tobacco smoking in 2019. ASMR: age-standardized mortality rate; ASDALR: age-standardized DALY rate. Fitting line represents the estimated values as per linear fit. SDI: Socio-demographic index. Data source: Global Burden of Disease 2019 Study
Sex-wise burden of neoplasms due to tobacco smoking in regions in 2019
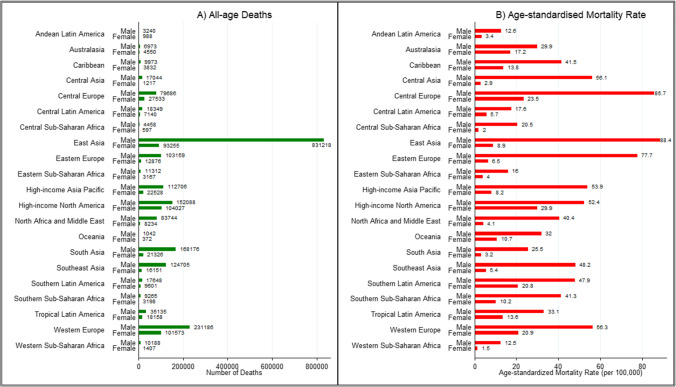
Males had higher number of deaths as well as age-standardized rates than females in all 21 regions in 2019, with death count and age-standardized rate among males being 1.5 to 10-folds higher than females across regions (Fig. 5A, B). Among males, East Asia had the highest absolute burden with 831,218 deaths, followed by 231,186 deaths in Western Europe. Among females, high-income North America (104,027), Western Europe (101,573), and East Asia (93,255) were the three leading regions (Fig. 5A). The ASMR among males was the highest in East Asia (88.4/100,000), Central Europe (85.7/100,000), and Eastern Europe (77.7/100,000), whereas high-income North America (29.9/100,000), Central Europe (23.5/100,000), and Western Europe (20.9/100,000) were the three leading regions among females (Fig. 5B). The male–female differences in death count and age-standardized rate were much wider in Asia and SSA regions than in Europe and America (Fig. 5A, B).
Fig. 5.
Sex-wise all-age deaths and age-standardized rate of mortality of neoplasms by region, 2019. Data source: Global Burden of Disease 2019 Study
Burden of neoplasms by age
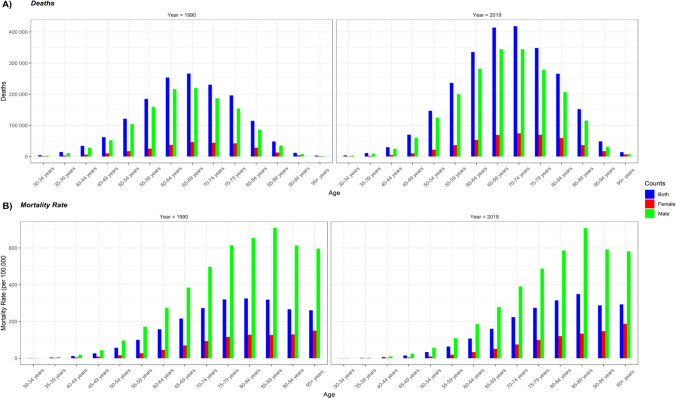
Figure 6 demonstrates number of deaths and age-specific mortality rate in different age groups in 1990 and 2019. Four key observations emerge from this figure. First, number of deaths as well as age-specific mortality rate of neoplasms due to tobacco smoking was much lower in females than males both in 1990 and 2019. Second, between 1990 and 2019, the number of deaths increased in all age groups, whereas age-specific mortality rate declined in all age groups except among those above the age of 85 years. Third, number of deaths in both sexes combined peaked at 65–69 years and 70–74 years in 1990 and 2019, respectively, and declined thereafter; the age-specfic mortality rate, however, peaked by 80–84 years and 85–89 years in 1990 and 2019, respectively, and declined marginally thereafter. In slight deviation from males, the age-specific mortality rate among females continued to increase with age both in 1990 and 2019, resulting in the highest age-specific mortality rate among those aged 95 years and above (Fig. 6B).
Fig. 6.
Global age-wise deaths and mortality rate in 1990 and 2019. Data source: Global Burden of Disease 2019 Study
Discussion
The PPPM approach to cancer management is bringing a paradigm shift to cancer management and control [7, 8]. Under this approach, we need not wait for cancer to proliferate and start therapy; in fact, the PPPM approach focuses on targeting the risk factors such that the probability of cancer initiation and progression can be minimized. In this direction, our study brings forth the temporal patterns of the burden of cancers attributable to tobacco smoking at the global level in 21 regions and 204 countries. At the global as well as regional level, the age-standardized rates of deaths and DALYs due to cancers caused by tobacco smoking decreased, whereas due to population growth, the absolute count of deaths and DALYs increased between 1990 and 2019. This finding follows a similar observation of increasing number of tobacco smokers but decreasing prevalence rates worldwide [1]. In 8/21 regions, there were more than 100,000 deaths due to cancers attributable to tobacco smoking, led by East Asia and Western Europe in 2019. The regions of SSA (except southern SSA) had one of the lowest absolute counts of deaths, DALYs, and age-standardized rates. In 2019, TBL, esophageal, stomach, colorectal, and pancreatic cancer were the top 5 neoplasms attributable to tobacco smoking, with different burden in regions as per their development status.
We observed that the cancer deaths attributable to tobacco smoking increased the most (absolute counts) in middle SDI quintile, followed by lower-middle and low SDI quintiles between 1990 and 2019, implying that tobacco control efforts need to be accelerated in these countries. A study has found that tobacco companies employ a range of activities (e.g., marketing/promotion, economic and political, and manipulative/deceptive) to block the effects of tobacco control policies in low and middle-income countries [25]. Furthermore, the financial resources spent on tobacco control efforts might not be sufficient. As per a WHO report, globally, taxes on tobacco generated government revenues of US$ 269 billion in 2013–14, yet only US$ 1 billion was spent on tobacco control efforts [26]. It shows that more resources, innovative strategies, and political-will are required to curtail the tobacco smoking prevalence in these countries. Among low and middle-income countries, a few exemplar countries have adopted stringent policies to curtail tobacco smoking. Madagascar, for instance, is the only low-income country maintaining tax on tobacco sales at more than 75% of the price [27]. Similarly, increased tax rate on tobacco products in countries such as Georgia and Morocco has been effective in reducing tobacco consumption [27]. Among low and middle-income countries, Brazil is also an exemplar country, which posted a reduction in adult smoking prevalence by 72.5% in males and 74.7% in females between 1990 and 2019 [1]. Consequently, Brazil posted one of the biggest reductions in ASMR and ASDALR of neoplasms attributable to tobacco smoking. Brazil’s remarkable performance could be attributed to “national program to fight smoking” initiated in 1986 (later renamed tobacco control plan in 2006), focused on tobacco taxes and other tobacco legislations [1, 28, 29].
Males account for 4/5th of the total cancer deaths and DALYs due to tobacco smoking in 2019, reflecting greater smoking prevalence among males than females [1, 27]. The male–female differences in cancer burden varied from 1.5 to 10-folds in different regions, reflecting the differential prevalence of tobacco smoking in males and females. Consequently, the male–female differences in death count and age-standardized rate were much wider in regions of Asia due to much higher smoking prevalence in males than females compared to lesser male–female differences in Europe and America. For instance, adult tobacco smoking prevalence is nearly similar between males and females in countries such as France (males: 35.2%; females: 31.9%) and Germany (males: 24.6%; females: 20.4%), whereas it is widely different in China (males: 45.6%; females: 1.7%), India (males: 15.6%; females: 1.6%), and Indonesia (males: 62.0%; females: 2.6%) (Supplementary Table 3).
PPPM approach to disease management and control suggests adopting targeted prevention approaches. Towards targeted prevention, the underlying reasons for differences in smoking prevalence among males and females must be more researched upon. The results from a neuroimaging study indicated that smoking activates pathways of male’s rewards more than female’s [30]. Fewer male–female differences in smoking prevalence in high-income countries and regions may also be linked with greater female labor force participation and hence greater economic independence among females. In low and middle-income countries of Asia and North Africa, females have lesser participation in the labor force [31], which results in low earning capacity and autonomy. In contrast to Asian countries, female labor force participation is high in the SSA region; however, low earning capacity and income levels might have led to low tobacco smoking rates in both males and females. The overall effects of gender equality are positive for the health of both males and females [32]; however, a study found that as gender inequality decreased in Spain, the smoking prevalence among males and females began to converge [33]. However, we abstain from providing a causal interpretation of the linkage between country’s economic levels, gender equality, and smoking prevalence among females; these interlinkages (or lack of) require further research.
Globally, the number of deaths due to neoplasms increased with age and peaked among those aged 65–74 years, and it is evident because tobacco smoking has a latency period to manifest its harmful effects. Research has identified that most people start smoking at younger ages [34–36] and there are life-long consequences of early smoking initiation in the form of respiratory problems, cardiovascular diseases, and cancers. As per a global study, there were 155 million smokers of age 15 to 24 years worldwide in 2019, with prevalence rate varying from 4.95% in females to 20.1% among males [37]. As there is a low probability of a person becoming a smoker after the age of 25 years, the tobacco control policies must target these age groups (15 to 24 years), which can have tremendous effects on smoking-induced disease burden in the future [37]. One such public health policy measure is increasing the legal age of tobacco sale. In 2019, United States passed legislation named Tobacco 21, under which the minimum age for selling tobacco products was raised from 18 to 21 years [38]. By contrast, in most European Union countries, the legal age for tobacco sale is 18 years [39, 40].
Apart from these public health efforts, the PPPM approach to medicine suggests that more targeted and individualized prevention techniques must be applied as several individual-specific factors predict smoking behavior among adolescents. Among individual-specific factors, research has identified the role of four personality-related factors for substance abuse among young and adolescents—namely, impulsivity, sensation-seeking, hopelessness, and anxiety [41]. A selective personality-targeted preventive approach termed “preventure” successfully reduced tobacco uptake and intention to use tobacco among adolescents even after 36 months of baseline [42]. Those among the “preventure” treatment group also reported high self-efficacy in resisting peer pressure to smoke even after three years of the treatment [42]. The personality-targeting preventive approaches represent the trait-based approach of health interventions that seek to target the underlying psychological processes to help regulate and shape behaviors [43]. A recent study has also found that personalized genetically informed risk tool can potentially reduce tobacco smoking [44].
In most regions, TBL is the most dominant malignancy caused by tobacco smoking, followed by cancers of esophagus, stomach, and colorectal. In the carcinogenic process, besides the direct carcinogenic effects of tobacco smoking, heightened inflammation due to tobacco smoking might also be responsible for the increased risk of several cancers, including lung cancer [12, 45, 46]. PPPM approach to medicine suggests that cancer predisposing syndromes particularly the monogenic subtypes must be identified to categorize at-risk population for preventive measures and screening [13]. Studies have indicated that smokers with arylamine N-acetyltransferase 2 (NAT2) slow acetylation genotype have a modestly increased risk of bladder cancer [47, 48], which emanates from the role of NAT2 in the metabolism of aryl amines present in the tobacco smoke. Similarly, the association between susceptibility single nucleotide polymorphisms (SNPs) and risk of colorectal cancer might be modified by smoking behavior [49]. A recent study concluded that abstinence from smoking could attenuate to a great extent the genetic risk of colorectal cancer [50].
In case of lung cancer, several SNPs were identified in the European Prospective Investigation into Cancer and Nutrition trials, which influence lung cancer survival in smokers and non-smokers [51]. Genome-wide association studies (GWAS) have found one or two copies of 15q25.1 locus as the susceptibility region for lung cancer, smoking behavior, and nicotine addiction [52–57]. However, some studies suggested no association between 15q25.1 susceptibility locus and risk of lung cancer among never-smokers [58, 59], which suggests that the risk of lung cancer due to 15q25.1 variant might be mediated through smoking or through higher intensity of nicotine that an individual with this variant can extract from cigarette smoke [57]. The potential pathogenic pathways through which 15q25.1 susceptibility locus influences the lung cancer risk must be identified to bridge the gap between cancer biology and patient care [60]. A recent GWAS study analyzing data of 38,602 smokers has found that individuals with DNA variant located at DNMT3B have higher predisposition to nicotine dependence, heavy smoking, and consequent lung cancer [61].
Recent research has highlighted the role of m6A in cancer pathogenesis and progression [62–64]. The m6A modification is one of the most important RNA modifications closely linked with lung carcinoma [65, 66], and studies have found that m6A regulator signatures have independent prognostic value to risk stratify patients and prognostic assessment and to offer personalized treatments [65–67]. Towards targeted prevention and personalized treatments, a recent study has also highlighted the role of m6A-related non-coding RNA as a potential biomarker for predicting bladder cancer prognosis as well as therapeutic response to immunotherapy [68]. Towards precision medicine, the role of m6A RNA methylation in human cancers can provide targeted treatment strategies in the future [69].
The PPPM approach to cancer management suggests not only targeted prevention but also focuses on personalized and precision medicine. Towards this end, nomograms—based on individual patient profiles and disease characteristics—have been found to be useful in oncology in providing single probability estimate for events such as lymph node metastasis, cancer recurrence or relapse, probability of death, effect of treatment on survival, and quality of life [70]. Studies have found nomograms to be useful in predicting the recurrence of bladder cancer [71], assessing risk of indolent prostate tumor [72] and risk of recurrence of ductal carcinoma in situ or invasive cancer after surgery [73]. Recently, a study [74] has developed nomogram to predict the probability of lung cancer in non-smoking females in the Chinese context. Nomograms have also been used to predict smoking cessation and smoking relapse [75, 76]. A recent study in Chinese context has found that cohabitation and depression were associated with greater risk of smoking relapse [76].
WHO Framework Convention on Tobacco Control
At the global level, tobacco control efforts were accelerated by the WHO Framework Convention on Tobacco Control (FCTC) [27], which focuses on a slew of a measures for demand reduction of tobacco use across countries: banning tobacco advertisements, labeling health warnings, rehabilitation of former tobacco users, taxes on tobacco products, and banning illicit trade of tobacco products. Ever since the WHO FCTC came into force in 2005, the adult tobacco prevalence has decreased substantially across countries, yet adult tobacco prevalence increased in 13 countries for males and 13 countries for females between 2005 and 2019 (Supplementary Table 3). One of the Sustainable Development Goals targets strengthening the implementation of WHO FCTC for all countries [77]. WHO tracks the progress of countries on the MPOWER framework—M: monitor tobacco use and prevention policies, P: protect people from tobacco smoke, O: offer help to quit tobacco, W: warn about the dangers of tobacco, E: enforce bans on tobacco advertising, promotion, and sponsorship, R: raise taxes on tobacco [27].
As per the WHO report, by 2020, 146 countries have adopted at least one MPOWER measure at the highest level, yet 49 nations have not adopted any MPOWER measure at the highest level. Taxation has been recognized as one of the most potent means of reducing tobacco use [27] as it reduces affordability, leading to either quitting or lesser consumption of tobacco. As per a WHO report, the number of countries with taxes on tobacco > 75% of the selling price increased from 23 to 40 between 2008 and 2020 and only 1 belonged to the low-income, 15 to middle-income, and 24 to high-income category [27]. Between 2010 and 2020, cigarette affordability decreased in 34/62 high-income, 42/104 middle-income, and 8/29 low-income countries [27]. The varying progress on the MPOWER framework across countries shows tremendous potential and requirement of more concerted efforts to reduce smoking prevalence and as a result, reduce the burden of smoking-induced cancers.
Strengths and limitations of the study
The main strength of this study is that the GBD estimates are based on all available data from cancer registries, vital statistics, and verbal autopsy, and spatiotemporal modeling is used along with covariates to arrive at the estimates of incidence, deaths, and DALYs. Additionally, GBD uses available epidemiological studies on the link between risk and disease. A major strength of this study is that estimates are examined on a temporal basis, allowing a more nuanced analysis of how the cancer burden attributable to tobacco smoking has been changing in the last three decades. However, it should also be noted that carcinogenesis involves a complex interplay of several factors, including genetic, environmental, and behavioral factors apart from smoking. Therefore, our results do not indicate that a particular cancer death or DALY occurred solely because of tobacco smoking and the role of interaction among risk factors in carcinogenesis can be an agenda for future research. The main limitation of the study relates to the availability of quality data from cancer registries in low and middle-income countries. For instance, deaths are attributed to the risks by multiplying absolute count of deaths with PAF; however, if death estimates are downward biased for a location, then it would reflect that tobacco smoking has caused fewer cancer deaths in a particular country or region. This might be the case with several low and middle-income countries in Asia, Africa, Oceania, and America. The role of cancer registration, therefore, becomes crucial in properly coding the cause of death in low and middle-income countries and thereby understanding the underlying risk factors.
Conclusions and expert recommendations
Even after 50 years of the United States surgeon general’s report on health implications of tobacco, 2.5 million deaths occurred worldwide due to neoplasms caused by tobacco smoking in 2019. The prevalence rates of tobacco smoking are higher in high-income countries and regions, whereas populous countries at low and middle-income spectrum have the highest absolute number of tobacco smokers. As tobacco smoking is the single biggest risk factor of several neoplasms, which entail large healthcare costs, more concerted efforts at a war-footing level are required to curtail the tobacco prevalence especially given that the smoking epidemic is unfolding in parts of low and middle-income countries of Africa, Asia, and Oceania. Smoking cessation demands a multi-pronged approach from the policymakers in the form of banning advertisements, increasing taxes, increasing legal age of selling tobacco, smoking bans at public places, and labeling cigarette packs. However, these efforts alone might not be sufficient to reduce tobacco prevalence and the disease burden attributable to tobacco smoking. The PPPM approach to medicine suggests that the behavioral, psychological, socio-economic, and cultural factors behind different smoking prevalence across countries and regions must be understood such that country and context-specific personalized and targeted prevention approaches can be devised and adopted.
Most tobacco smokers begin smoking at younger ages, suggesting that interventions during early ages might produce large public health dividends in the future. To this end, targeted personality-based prevention approaches have tremendous potential by identifying vulnerable youth and providing targeted prevention solutions. Importantly, there are several approaches for helping people quit tobacco smoking, such as nicotine replacement therapy, behavior counseling, and group-based smoking cessation programs [78]. Recent research has pointed toward the success of some tobacco cessation strategies focusing on the personality and behavior of smokers. A meta-analysis found that behavioral interventions can assist smokers in quitting; however, interventions tailored for those with low socio-economic status were ineffective in helping disadvantaged smokers [79]. A recent review highlighted the importance of strategies such as text-message interventions, quit and win contests, and multiple behavioral interventions on tobacco cessation among youth [80].
Behavior or personality-targeting programs for smoking cessation currently face few challenges. First, misconceptions and risk denial among smokers present one of the biggest challenges encountered while encouraging smokers to quit [81–83]. Second, the long-term effects of behavioral therapies in tobacco cessation are inconclusive. A review of Cochrane database has found limited evidence of the effectiveness of behavioral interventions among young people to quit smoking on long-term sustained basis [84]. Third, there is limited or no research on the cost-effectiveness and scalability of these approaches at the country level. For instance, the total population of adult tobacco smokers is estimated to be 341 million in China [1]; research, as of now, is silent on the effectiveness and scalability of trait-based targeted prevention methods in countries such as China. Therefore, towards PPPM approach to cancer management and control, future research can focus on three dimensions: (1) behavioral interventions for those with low socio-economic status, (2) whether behavioral interventions lead to sustained tobacco cessation on a long-term basis, and (3) the scope, scalability, and cost-effectiveness of personality or behavior-based preventive medicine at the country level.
Lastly, cancer burden due to tobacco smoking is widely different across regions and countries due to different prevalence of tobacco smoking. Moreover, burden of cancers is higher among males than females reflecting differential smoking prevalence. Apart from this, the role of several individual-specific factors modulates the risk of smoking initiation and progression as well as risk of cancers due to tobacco smoking. The PPPM approach suggests health risk assessment of those at higher risk of smoking initiation as well as smoking progression, followed by targeted interventions tailored to individual patient profiles. Among genetic factors, GWAS studies have highlighted the role of SNPs located at 15q25, 19q13, and 8p11 in nicotine addiction and smoking behaviors [54, 55]. A study has found that early smokers with high genetic risk score (GRS)—identified through GWAS—are more likely to progress to heavy smoking with high nicotine dependence, and for those individuals with higher GRS, smoking cessation was more difficult than those with low GRS [85]. However, a previous research study has pointed out that personalized medicine based only on genomics has limited applicability for PPPM in oncology [86]. Rather, towards personalized and precision medicine, multiple data from genomics, transcriptomics, proteomics, metabolics, and radiomics can better help in patient stratification and providing personalized medicine [86, 87].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Institute of Health Metrics and Evaluation for providing GBD 2019 estimates in the public domain.
Abbreviations
- PPPM
Predictive, preventive, and personalized medicine
- DALYs
Disability-adjusted life years
- SDI
Socio-demographic index
- ASMR
Age-standardized mortality rate
- ASDLAR
Age-standardized DALY rate
- GBD
Global Burden of Disease
- PAF
Population attributable fraction
- RR
Relative risk
- TMREL
Theoretical minimum risk exposure level
- WHO
World Health Organization
- TBL
Tracheal, bronchus, and lung
- SSA
Sub-Saharan Africa
- GWAS
Genome-wide association studies
- GRS
Genetic risk score
- NAT2
Arylamine N-acetyltransferase 2
- SNPs
Single nucleotide polymorphisms
- FCTC
Framework convention on tobacco control
Author contribution
RS contributed to the design of the study, data collection, analysis and interpretation, writing, and critical revision of the manuscript. BR contributed to writing and critical revision of the manuscript. All authors approved the final version of the manuscript.
Data availability
All data is procured from GBD Results Tool (https://vizhub.healthdata.org/gbd-results/).
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
The research was conducted using data available in the public domain and did not include any human participants or animals. Therefore, no ethical approvals were required.
Consent for publication
The authors of the study have consent and responsibility for submission to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reitsma MB, Kendrick PJ, Ababneh E, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodchild M, Nargis N, d'Espaignet ET. Global economic cost of smoking-attributable diseases. Tob Control. 2018;27(1):58–64. doi: 10.1136/tobaccocontrol-2016-053305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma: a study of six hundred and eighty-four proved cases. JAMA. 1950;143(4):329–336. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits. Br Med J. 1954;1(4877):1451. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Public Health Service . Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: US Department of Health, Education, and Welfare; 1964. [Google Scholar]
- 6.International Agency for Research on Cancer Monographs 1–132. Last Updated July 1, 2022. Available at https://monographs.iarc.who.int/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf. (Accessed September 7, 2022).
- 7.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation-EPMA position paper 2016. EPMA J. 2016;7(1):1–3. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golubnitschaja O, Yeghiazaryan K, Costigliola V, Trog D, Braun M, Debald M, Kuhn W, Schild HH. Risk assessment, disease prevention and personalised treatments in breast cancer: is clinically qualified integrative approach in the horizon? EPMA J. 2013;4(1):1–24. doi: 10.1186/1878-5085-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US FDA. https://www.fda.gov/tobacco-products/products-ingredients-components/chemicals-cigarettes-plant-product-puff (Accessed September 17, 2022)
- 10.Rioux N, Castonguay A. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone modulation of cytokine release in U937 human macrophages. Cancer Immunol Immunother. 2001;49(12):663–670. doi: 10.1007/s002620000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Xiong R, Zhou J, Guan X, Jiang G, Chen Y, Yang Q. Involvement of m6A regulatory factor IGF2BP1 in malignant transformation of human bronchial epithelial Beas-2B cells induced by tobacco carcinogen NNK. Toxicol Appl Pharmacol. 2022;1(436):115849. doi: 10.1016/j.taap.2021.115849. [DOI] [PubMed] [Google Scholar]
- 12.Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10(4):365–381. doi: 10.1007/s13167-019-00194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, Vittadini G, Desiderio DM. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):1–31. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucera R, Pecen L, Topolcan O, Dahal AR, Costigliola V, Giordano FA, Golubnitschaja O. Prostate cancer management: long-term beliefs, epidemic developments in the early twenty-first century and 3PM dimensional solutions. EPMA J. 2020;11(3):399–418. doi: 10.1007/s13167-020-00214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bizzarri M, Fedeli V, Monti N, Cucina A, Jalouli M, Alwasel SH, Harrath AH. Personalization of medical treatments in oncology: time for rethinking the disease concept to improve individual outcomes. EPMA J. 2021;12(4):545–558. doi: 10.1007/s13167-021-00254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazurakova A, Koklesova L, Samec M, Kudela E, Kajo K, Skuciova V, Csizmár SH, Mestanova V, Pec M, Adamkov M, Al-Ishaq RK. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J 2022a:1–20. [DOI] [PMC free article] [PubMed]
- 17.Mazurakova A, Samec M, Koklesova L, Biringer K, Kudela E, Al-Ishaq RK, Pec M, Giordano FA, Büsselberg D, Kubatka P, Golubnitschaja O. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine—comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA J 2022b:1–26. [DOI] [PMC free article] [PubMed]
- 18.Zhang S, Wan X, Lv M, Li C, Chu Q, Wang G. TMEM92 acts as an immune-resistance and prognostic marker in pancreatic cancer from the perspective of predictive, preventive, and personalized medicine. EPMA J. 2022;13(3):519–534. doi: 10.1007/s13167-022-00287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Abbas KM, Abbasifard M, et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. 2020;396:1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray CJ, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) results. Seattle: Institute for Health Metrics and Evaluation (IHME). Source: Institute for Health Metrics Evaluation. Used with permission. All rights reserved. Available from https://vizhub.healthdata.org/gbd-results/ (Accessed: September 2022).
- 23.Kocarnik JM, Compton K, Dean FE, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organisation Global Health Observatory. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-tobacco-control-monitor-current-tobaccouse-tobaccosmoking-cigarrettesmoking-agestd-tobagestdcurr (Accessed September 10,2022).
- 25.Lee S, Ling PM, Glantz SA. The vector of the tobacco epidemic: tobacco industry practices in low and middle-income countries. Cancer Causes Control. 2012;23(1):117–129. doi: 10.1007/s10552-012-9914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. Geneva: World Health Organization; 2015. Available from: http://www.who.int/tobacco/global_report/2015/en
- 27.WHO Report on the Global Tobacco Epidemic, 2021: addressing new and emerging products. World Health Organization; 2021.
- 28.Portes LH, Machado CV, Turci SR, Figueiredo VC, Cavalcante TM, Silva VL. Tobacco Control Policies in Brazil: a 30-year assessment. Ciencia Saude Coletiva. 2018;23:1837–1848. doi: 10.1590/1413-81232018236.05202018. [DOI] [PubMed] [Google Scholar]
- 29.Divino JA, Ehrl P, Candido O, Valadao MA. Extended cost–benefit analysis of tobacco taxation in Brazil. Tobacco Control 2021 [DOI] [PubMed]
- 30.Cosgrove KP, Wang S, Kim S-J, et al. Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci Off J Soc Neurosci. 2014;34(50):16851–16855. doi: 10.1523/JNEUROSCI.3661-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verick S. Female labor force participation and development. IZA World Labor 2018;87 10.15185/izawol.87.v2
- 32.King TL, Kavanagh A, Scovelle AJ, Milner A. Associations between gender equality and health: a systematic review. Health Promot Int. 2020;35(1):27–41. doi: 10.1093/heapro/day093. [DOI] [PubMed] [Google Scholar]
- 33.Bilal U, Beltrán P, Fernández E, Navas-Acien A, Bolumar F, Franco M. Gender equality and smoking: a theory-driven approach to smoking gender differences in Spain. Tob Control. 2016;25(3):295–300. doi: 10.1136/tobaccocontrol-2014-051892. [DOI] [PubMed] [Google Scholar]
- 34.Marcon A, Pesce G, Calciano L, Bellisario V, Dharmage SC, Garcia-Aymerich J, Gislasson T, Heinrich J, Holm M, Janson C, Jarvis D. Trends in smoking initiation in Europe over 40 years: a retrospective cohort study. PLoS One. 2018;13(8):e0201881. doi: 10.1371/journal.pone.0201881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman KS, Nelson NM, Feldman LL. Smoking initiation among young adults in the United States and Canada, 1998–2010: a systematic review. Prev Chronic Dis. 2012;9:E05. [PMC free article] [PubMed] [Google Scholar]
- 36.United States Public Health Service. Office of the Surgeon General, National Center for Chronic Disease Prevention, Health Promotion (US). Office on Smoking. Preventing tobacco use among youth and young adults: a report of the surgeon general. US Government Printing Office; 2012.
- 37.Reitsma MB, Flor LS, Mullany EC, Gupta V, Hay SI, Gakidou E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Pub Health. 2021;6(7):e472–e481. doi: 10.1016/S2468-2667(21)00102-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States FDA. Available at https://www.fda.gov/tobacco-products/retail-sales-tobacco-products/tobacco-21 (Accessed September 9, 2022).
- 39.Nuyts PA, Kuipers MA, Willemsen MC, Kunst AE. An increase in the tobacco age-of-sale to 21: for debate in Europe. Nicotine Tob Res. 2020;22(7):1247–1249. doi: 10.1093/ntr/ntz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Union Agency for Fundamental Rights. https://fra.europa.eu/en/publication/2017/mapping-minimum-age-requirements-concerning-rights-child-eu/purchasing-and-consuming-tobacco (Accessed September 10, 2022).
- 41.Conrod PJ, Nikolaou K. Annual research review: on the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry. 2016;57(3):371–94. doi: 10.1111/jcpp.12516. [DOI] [PubMed] [Google Scholar]
- 42.Debenham J, Grummitt L, Newton NC, Teesson M, Slade T, Conrod P, Kelly EV. Personality-targeted prevention for adolescent tobacco use: three-year outcomes for a randomised trial in Australia. Prev Med. 2021;153:106794. doi: 10.1016/j.ypmed.2021.106794. [DOI] [PubMed] [Google Scholar]
- 43.Rebele RW, Koval P, Smillie LD. Personality-informed intervention design: examining how trait regulation can inform efforts to change behavior. Eur J Pers. 2021;35(4):623–645. doi: 10.1177/08902070211016251. [DOI] [Google Scholar]
- 44.Ramsey AT, Bourdon JL, Bray M, Dorsey A, Zalik M, Pietka A, Salyer P, Chen LS, Baker TB, Munafò MR, Bierut LJ. Proof of concept of a personalized genetic risk tool to promote smoking cessation: high acceptability and reduced cigarette smoking. Cancer Prev Res. 2021;14(2):253–262. doi: 10.1158/1940-6207.CAPR-20-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ-and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Closas M, Rothman N, Figueroa JD, Prokunina-Olsson L, Han SS, Baris D, Jacobs EJ, Malats N, De Vivo I, Albanes D, Purdue MP. Common genetic polymorphisms modify the effect of smoking on absolute risk of bladder cancer. Cancer Res. 2013;73(7):2211–2220. doi: 10.1158/0008-5472.CAN-12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figueroa JD, Han SS, Garcia-Closas M, Baris D, Jacobs EJ, Kogevinas M, Schwenn M, Malats N, Johnson A, Purdue MP, Caporaso N. Genome-wide interaction study of smoking and bladder cancer risk. Carcinogenesis. 2014;35(8):1737–1744. doi: 10.1093/carcin/bgu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song N, Shin A, Jung HS, Oh JH, Kim J. Effects of interactions between common genetic variants and smoking on colorectal cancer. BMC Cancer. 2017;17(1):1–9. doi: 10.1186/s12885-017-3886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Jansen L, Guo F, Hoffmeister M, Chang-Claude J, Brenner H. Smoking, genetic predisposition, and colorectal cancer risk. Clin Transl Gastroenterol 2021;12(3). [DOI] [PMC free article] [PubMed]
- 51.Xun WW, Brennan P, Tjonneland A, Vogel U, Overvad K, Kaaks R, Canzian F, Boeing H, Trichopoulou A, Oustoglou E, Giotaki Z. Single-nucleotide polymorphisms (5p15. 33, 15q25. 1, 6p22. 1, 6q27 and 7p15. 3) and lung cancer survival in the European Prospective Investigation into Cancer and Nutrition (EPIC) Mutagenesis. 2011;26(5):657–66. doi: 10.1093/mutage/ger030. [DOI] [PubMed] [Google Scholar]
- 52.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat genet. 2008;40(5):616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 54.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobacco and Genetics Consortium Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat genet. 2010;42(5):436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.VanderWeele TJ, Asomaning K, TchetgenTchetgen EJ, Han Y, Spitz MR, Shete S, Wu X, Gaborieau V, Wang Y, McLaughlin J, Hung RJ. Genetic variants on 15q25. 1, smoking, and lung cancer: an assessment of mediation and interaction. Am J Epidemiol. 2012;175(10):1013–20. doi: 10.1093/aje/kwr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lan Q, Hsiung CA, Matsuo K, Hong YC, Seow A, Wang Z, Hosgood HD, Chen K, Wang JC, Chatterjee N, Hu W. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat genet. 2012;44(12):1330–1335. doi: 10.1038/ng.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung RJ, Spitz MR, Houlston RS, Schwartz AG, Field JK, Ying J, Li Y, Han Y, Ji X, Chen W, Wu X. Lung cancer risk in never-smokers of European descent is associated with genetic variation in the 5p15. 33 TERT-CLPTM1Ll region. J Thorac Oncol. 2019;14(8):1360–9. doi: 10.1016/j.jtho.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji X, Bossé Y, Landi MT, Gui J, Xiao X, Qian D, Joubert P, Lamontagne M, Li Y, Gorlov I, de Biasi M. Identification of susceptibility pathways for the role of chromosome 15q25. 1 in modifying lung cancer risk. Nat Commun. 2018;9(1):1–5. doi: 10.1038/s41467-018-05074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hancock DB, Guo Y, Reginsson GW, et al. Genome-wide association study across European and African American ancestries identifies a SNP in DNMT3B contributing to nicotine dependence. Mol Psychiatry. 2018;23:1911–1919. doi: 10.1038/mp.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Chai P, Jia R. Novel insights on m (6) A RNA methylation in tumorigenesis: a double-edged sword. Mol Cancer. 2018;17:101. doi: 10.1186/s12943-018-0847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L, Li J, Wang X, Ying Y, Xie H, Yan H, Zheng X, Xie L. The dual role of N6-methyladenosine modification of RNAs is involved in human cancers. J Cell Mol Med. 2018;22:4630–4639. doi: 10.1111/jcmm.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol cancer. 2019;18(1):1–5. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Zhan X. Identification of pathology-specific regulators of m6A RNA modification to optimize lung cancer management in the context of predictive, preventive, and personalized medicine. EPMA J. 2020;11(3):485–504. doi: 10.1007/s13167-020-00220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu R, Pang G, Zhao Q, Yang L, Chen S, Jiang L, Shen Y, Shao W. The momentous role of N6-methyladenosine in lung cancer. J Cell Physiol. 2021;236(5):3244–3256. doi: 10.1002/jcp.30136. [DOI] [PubMed] [Google Scholar]
- 67.Dong S, Wu Y, Liu Y, Weng H, Huang H. N6-Methyladenosine steers RNA metabolism and regulation in cancer. Cancer Commun. 2021;41(7):538–559. doi: 10.1002/cac2.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu M, Zhan H, Liu B, et al. N6-methyladenosine-related non-coding RNAs are potential prognostic and immunotherapeutic responsiveness biomarkers for bladder cancer. EPMA J. 2021;12:589–604. doi: 10.1007/s13167-021-00259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuncel G, Kalkan R. Importance of m N6-methyladenosine (m6A) RNA modification in cancer. Medical Oncol. 2019;36(4):1–6. doi: 10.1007/s12032-019-1260-6. [DOI] [PubMed] [Google Scholar]
- 70.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ha YS, Kim TH. The surveillance for muscle-invasive bladder cancer (MIBC). In Bladder Cancer 2018 (pp. 553–597). Academic Press.
- 72.Bokhorst LP, Steyerberg EW, Roobol MJ. Decision support for low-risk prostate cancer. In Prostate Cancer (second edition) 2016(pp. 207–213). Academic Press.
- 73.McCloskey SA, White J. Radiotherapy and ductal carcinoma in situ. In The Breast (Fifth Edition), Elsevier, 2018;671–676.e2
- 74.Guo L. Lung cancer risk prediction nomogram in Chinese female non-smokers. J Thoracic Oncol. 2022;17(9):S35–36. doi: 10.1016/j.jtho.2022.07.066. [DOI] [Google Scholar]
- 75.Zhu N, Lin S, Cao C, Xu N, Yu X, Chen X. Nomogram to predict successful smoking cessation in a Chinese outpatient population. Tobacco Induced Dis. 2020;18. [DOI] [PMC free article] [PubMed]
- 76.Hu N, Yu Z, Du Y, Li J. Risk factors of relapse after smoking cessation: results in China family panel studies from 2010 to 2018. Front Pub Health 2022;10. [DOI] [PMC free article] [PubMed]
- 77.World Health Organisation. https://www.who.int/data/gho/data/themes/topics/sdg-target-3_a-tobacco-control (Accessed September 9, 2022).
- 78.Boderie NW, van Kippersluis JL, Ceallaigh DT, Radó MK, Burdorf A, van Lenthe FJ, Been JV. PERSonalised Incentives for Supporting Tobacco cessation (PERSIST) among healthcare employees: a randomised controlled trial protocol. BMJ Open. 2020;10(9):e037799. doi: 10.1136/bmjopen-2020-037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kock L, Brown J, Hiscock R, Tattan-Birch H, Smith C, Shahab L. Individual-level behavioural smoking cessation interventions tailored for disadvantaged socioeconomic position: a systematic review and meta-regression. Lancet Pub Health. 2019;4(12):e628–e644. doi: 10.1016/S2468-2667(19)30220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villanti AC, West JC, Klemperer EM, Graham AL, Mays D, Mermelstein RJ, Higgins ST. Smoking-cessation interventions for US young adults: updated systematic review. Am J Prev Med. 2020;59(1):123–136. doi: 10.1016/j.amepre.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peretti-Watel P, Constance J, Guilbert P, Gautier A, Beck F, Moatti JP. Smoking too few cigarettes to be at risk? Smokers’ perceptions of risk and risk denial, a French survey. Tob Control. 2007;16(5):351–356. doi: 10.1136/tc.2007.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heikkinen H, Patja K, Jallinoja P. Smokers’ accounts on the health risks of smoking: why is smoking not dangerous for me? Soc Sci Med. 2010;71(5):877–883. doi: 10.1016/j.socscimed.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 83.Peretti-Watel P, Seror V, Verger P, Guignard R, Legleye S, Beck F. Smokers’ risk perception, socioeconomic status and source of information on cancer. Addict Behav. 2014;39(9):1304–1310. doi: 10.1016/j.addbeh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 84.Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, Hartmann-Boyce J. Tobacco cessation interventions for young people. Cochrane Database Sys Rev 2017(11). [DOI] [PMC free article] [PubMed]
- 85.Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, Houts R, Meier M, Sugden K, Williams B, Poulton R. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiat. 2013;70(5):534–542. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bizzarri M, Fedeli V, Monti N, et al. Personalization of medical treatments in oncology: time for rethinking the disease concept to improve individual outcomes. EPMA J. 2021;12:545–558. doi: 10.1007/s13167-021-00254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9:77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is procured from GBD Results Tool (https://vizhub.healthdata.org/gbd-results/).
Not applicable.