Abstract
Background
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends at least annual spirometry for patients with chronic obstructive pulmonary disease (COPD). Since spirometry acquisition is variable in clinical practice, identifying characteristics associated with annual spirometry may inform strategies to improve care for patients with COPD.
Methods
We included veterans hospitalized for COPD at Veterans Health Administration (VHA) facilities from 10/2012 to 09/2015. Our primary outcome was spirometry within 1 year of COPD hospitalization. Patient demographics, health factors, and comorbidities as well as practice and geographic variables were identified using Corporate Data Warehouse; provider characteristics were obtained from the Survey of Healthcare Experiences of Patients. We used logistic regression with a random intercept to account for potential clustering within facilities.
Results
Spirometry was completed 1 year before or after hospitalization for 20,683/38,148 (54.2%) veterans across 114 facilities. Patients with spirometry were younger, (mean=67.2 years (standard deviation (SD)=9.3) vs. 69.4 (10.3)), more likely non-white (21.3% vs. 19.7%), and more likely to have comorbidities (p<0.0001 for asthma, depression, and post-traumatic stress disorder). Pulmonary clinic visit was most strongly associated with spirometry (odds ratio (OR)=3.14 [95% confidence interval 2.99–3.30]). There was no association for facility complexity. In a secondary analysis including provider-level data (3862 patients), results were largely unchanged. There was no association between primary care provider age, gender, or type (physician vs. advanced practice registered nurse vs. physician assistant) and spirometry.
Conclusion
In a cohort of high-risk COPD patients, just over half completed spirometry within 1 year of hospitalization. Pulmonary clinic visit was most strongly associated with 1-year spirometry, though provider variables were not. Spirometry completion for high-risk COPD patients remains suboptimal and strategies to improve post-hospitalization care for patients not seen in pulmonary clinic should be developed to ensure guideline concordant care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07826-5.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is common in the USA with a prevalence of 5.1% and a cost burden of approximately $49.9 billion.1,2 Among the veteran population, the prevalence is even higher at 7.8%.3 Practice patterns in COPD are widely variable and suboptimal management of patients with COPD is associated with adverse effects on quality of life and increased mortality.4 Therefore, optimizing COPD monitoring and treatment could yield significant improvements in quality of care, outcomes, and cost.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines endorse the use of spirometry for prognostication and consideration of therapeutic approaches.5 Spirometry is recommended at least annually for patients with stable COPD and within 3 months after an acute exacerbation.5 Prior work has shown that spirometry is inconsistently performed, even in patients with newly diagnosed COPD, but may be more common among older persons and among patients with more respiratory symptoms.6–11 Patients who do undergo spirometry are more likely to receive a prescription for guideline-concordant COPD medications, including short and long acting beta-agonists, long acting anticholinergic medications, and inhaled corticosteroids, though this was not adjusted for illness severity.7 Thus, guideline concordant spirometry utilization is a high yield target for quality improvement.12
Despite the importance of spirometry in COPD, adherence to GOLD guidelines remains suboptimal. Our group identified potential disparities in spirometry completion among veterans who are women, non-white, and younger age and have comorbid alcohol or drug use diagnoses before hospitalization for COPD.13 Prior work has also shown that older and minority veterans with COPD are less likely to be referred to pulmonary specialists in general, as are those with depression and history of substance use disorder.14 Since hospital resources and culture may impact inclination to provide certain services, we suspected that practice and geographic factors such as attendance in pulmonary clinic, rurality, facility complexity, and region may impact spirometry acquisition. On a provider level, although one survey-based study of 500 primary care providers found that self-reported adherence to spirometry guidelines did not differ by provider demographics of sex, specialty, or years in practice, the association of spirometry and primary care provider characteristics has not been well described on a national level.15
Since US veterans have higher rates of COPD than non-veterans, our goal was to identify system- and provider-level characteristics associated with spirometry acquisition. Therefore, we determined how Veterans Health Administration (VHA) practice, geographic variables, and primary care provider characteristics are associated with spirometry completion in veterans with recent hospitalization for COPD. Understanding reasons for variability in COPD care is an important step toward both ensuring that patients with COPD receive appropriate care and in identifying system-based factors which can be targeted to improve quality of care.
METHODS
Study Design, Population, and Data Source
We performed a retrospective observational study of patients receiving care in VHA facilities. All patients with a VA-based hospitalization for COPD exacerbation, as determined by International Classification of Disease, Ninth Revision (ICD-9) codes, were included, as previously described.16 For patient demographics and practice and geographic variables, data were obtained from the VHA Corporate Data Warehouse (CDW), as previously described.13 Provider-level data were obtained from the Survey of Healthcare Experiences of Patients (SHEP). SHEP is a survey of a random sample of patients about aspects of their experiences with the healthcare system and is linked to primary care provider demographics of age, sex, and degree.17 Patients from VHA fiscal years 2012 to 2015 (October 2012 through September 2015) were included in our analysis.
Our primary outcome was completion of spirometry within 1 year before or after hospitalization for COPD exacerbation in any VHA facility. This time period was selected based on GOLD guideline recommendations for obtaining spirometry at least annually for patients with stable COPD and within 3 months after an acute exacerbation.5 Because hospitalization for acute exacerbation of COPD is associated with poor prognosis and increased risk of death,18 this group was felt to represent a high-risk cohort in whom guideline adherence such as at least annual spirometry, either in the year preceding or following hospitalization, was particularly important. Additionally, hospitalization for COPD exacerbation has been correlated with pulmonary clinic referral in the following year.14 Our study included this time period to allow for evaluation of those patients referred to pulmonary specialists after hospitalization in order to reflect subsequent completion of spirometry after a pulmonary clinic referral.
Completion of spirometry was determined by using the following Current Procedural Terminology (CPT) codes: 94010, 94150, 94060, 94726, 94727, 94728, 94729, 93720, 93721, 93722, 94240, 94260, 94350, 94360, 94370, 94720, and 94725. Patient demographics (age, sex, race/ethnicity), and health factors and comorbidities associated with COPD admission (smoking status, diabetes, asthma, depression, post-traumatic stress disorder [PTSD], and history of substance use disorder) were included as covariates, as were readmissions and inhaler prescriptions, as proxies of disease severity.13,19
Our primary analysis focused on practice and geographic characteristics. These were determined by the hospital of the patient’s COPD admission. Prior studies have shown wide variation in ordering of tests by VHA characteristics including geographic region, rurality, and hospital complexity.20 Therefore, we evaluated characteristics including attendance in pulmonary clinic (clinic stop code=312), rurality (urban vs. rural), facility complexity, and geographic region (Northeast, Southeast, Midwest, Continental, Pacific), as described previously.21 Complexity was determined using established VHA complexity levels.22 VHA complexity levels range from level 1 facilities, which have the highest levels of teaching, research, subspecialists, and patient case-mix complexity, to level 3 facilities, which have the lowest volume and complexity of cases. Level 1 facilities are further subdivided into levels 1a to 1c, with 1a being the most complex, having the largest volume, most complex patients, and highest level of intensive care unit and surgical facilities.23 Presence of medical trainees in the facility was excluded as a separate covariate given its high correlation with facility complexity level.
Provider characteristics including degree, time since graduation, and sex have all been associated with variations in care including utilization of low value care and rapid uptake of new medications.24,25 Here we included provider-level variables of age, gender, and degree (physician vs. advanced practice registered nurse [APRN] vs. physician assistant [PA]), as determined by SHEP data. Provider information was added by linking de-identified provider numbers with the provider or clinician identified in the first question of the SHEP survey, as previously described.17
Statistical Analysis
Among patients who were hospitalized for COPD, we identified patients who completed spirometry 1 year before or after the index admission and compared their characteristics to those who did not complete spirometry using descriptive statistics. We examined practice and geographic characteristics associated with spirometry using a multi-level logistic regression model, with a random intercept to account for potential clustering within each facility; the final model adjusted for patient age, sex, race, smoking status, diabetes, asthma, depression, PTSD, and history of substance use disorder, readmission within 1 year, inhaler prescription from discharge through 1 year, and practice and geographic variables (pulmonary clinic visit, rurality, complexity, and geographic region). A secondary analysis was performed on a subset of patients who had provider-level data, including age, gender, and degree to determine whether associations between patient and practice and geographic factors changed after including provider variables. A similar random-intercept logistic regression as the patient-level analyses was used to examine the provider characteristics associated with spirometry. Statistical significance was defined as two-sided p value < 0.05. All analyses were performed using SAS version 9.4.
RESULTS
Patient Characteristics
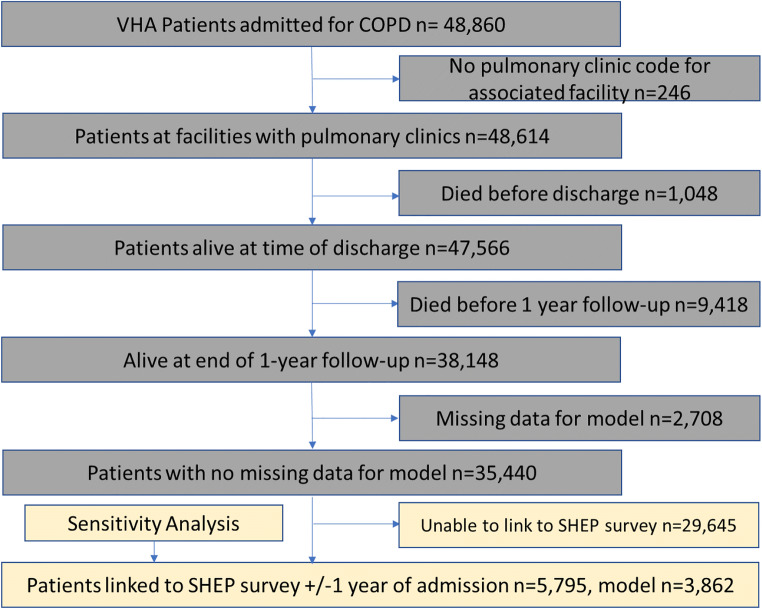
After applying exclusions (facilities with missing data on pulmonary clinic, patients who died either during hospitalization or in the year following hospitalization for COPD exacerbation, patients with missing data relating to patient race, smoking status, and region and rurality), 35,440 patients from 114 facilities were included in the analytic sample of patients who received primary care through the VHA and were hospitalized for a COPD exacerbation (Fig. 1). The mean age was 68.2 years (standard deviation (SD) 9.8) and the majority of patients were male (95.8%), white race (79.4%), and current or formers smokers (89.6%) (Table 1). There was a high prevalence of comorbid diabetes (42.3%), asthma (20.4%), depression (15.0%), PTSD (18.9%), and history of substance use disorder (27.6%).
Figure 1.
Flow diagram of patient exclusions. VHA, Veterans Health Administration; COPD, chronic obstructive pulmonary disease; SHEP, Survey of Healthcare Experiences of Patients.
Table 1.
Baseline Characteristics of Patients Included in Primary Analyses
| Total | No Spirometry | Spirometry within 1 year | p-value | Missing | |
|---|---|---|---|---|---|
| n=38,148 | n=17,465 | n=20,683 | |||
| Patient demographics, health factors, and comorbidities | |||||
| Patient age in years, mean (SD) | 68.2 (9.8) | 69.4 (10.3) | 67.2 (9.3) | <.0001 | |
| Patient sex (%) | 0.6658 | ||||
| Female | 4.2 | 4.2 | 4.2 | ||
| Male | 95.8 | 95.8 | 95.8 | ||
| Patient race (%) | 0.0002 | 2169 | |||
| White | 79.4 | 80.3 | 78.7 | ||
| Non-white | 20.6 | 19.7 | 21.3 | ||
| Smoking status (%) | 0.5720 | 376 | |||
| Current | 62.9 | 63.2 | 62.7 | ||
| Former | 26.7 | 26.6 | 26.9 | ||
| Never | 10.3 | 10.2 | 10.4 | ||
| Comorbidities (%) | |||||
| Diabetes | 42.3 | 41.9 | 42.7 | 0.0965 | |
| Asthma | 20.4 | 18.1 | 22.4 | <.0001 | |
| Major depression | 15.0 | 14.1 | 15.7 | <.0001 | |
| PTSD | 18.9 | 17.4 | 19.9 | <.0001 | |
| Substance use | 27.6 | 27.2 | 28 | 0.0779 | |
| Time to readmission, in days (median [IQR]) | 66 [10–174] | 64 [8–176] | 67 [12–173] | 0.0201 | |
| Readmission within 30 days | 19.7 | 19.3 | 20.0 | 0.0873 | |
| Readmission within 1 year | 53.1 | 50.4 | 55.4 | <.0001 | |
| Inhaler prescription | |||||
| LAMA | 79.8 | 75.6 | 83.3 | <.0001 | |
| LABA | 63.0 | 56.0 | 68.9 | <.0001 | |
| ICS | 15.3 | 14.9 | 15.6 | 0.0686 | |
| ICS/LABA | 77.3 | 72.5 | 81.4 | <.0001 | |
| Practice and geographic variables | |||||
| Pulmonary clinic attendance (%) | 58.2 | 43.2 | 70.8 | <.0001 | |
| Rurality (%) | <.0001 | 225 | |||
| Urban | 94.0 | 9.3 | 94.7 | ||
| Rural | 6.0 | 6.7 | 5.3 | ||
| Complexity (%) | <.0001 | ||||
| 1a | 47.9 | 47.2 | 48.4 | ||
| Non-1a | 52.1 | 52.8 | 51.6 | ||
| Region n (%) | <.0001 | 225 | |||
| Northeast | 9104 (24.0) | 4215 (24.3) | 4889 (23.8) | ||
| Continental | 5831 (15.4) | 2839 (16.4) | 2992 (14.6) | ||
| Midwest | 9137 (24.1) | 3693 (21.3) | 5444 (26.5) | ||
| Pacific | 5676 (14.9) | 2665 (15.3) | 3011 (14.5) | ||
| Southeast | 8175 (21.6) | 3948 (22.7) | 4227 (20.6) |
Abbreviations: SD, standard deviation; PTSD, post-traumatic stress disorder; IQR, interquartile range; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta agonist; ICS, inhaled corticosteroid
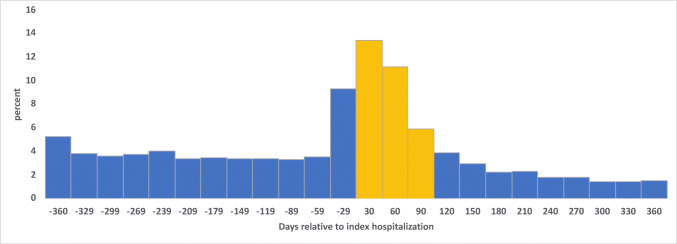
Among the cohort included in our analysis, 20,683 (54.2%) underwent spirometry within 1 year before or after hospitalization. Of this group, 10,295 (49.8%) were done in the year after hospitalization and 6305 (30.5% of all who underwent spirometry) were within 3 months after hospitalization (Fig. 2). Compared to those without spirometry, patients with spirometry were younger (mean age=67.2 years (SD=9.3) vs. 69.4 (10.3)), more likely non-white (21.3% vs. 19.7%), and more likely to have comorbidities of asthma, major depression, and PTSD (p<0.0001 for all). Smoking status did not differ with spirometry completion (overall current smoking=62.9%, former=26.7%, never=10.3%; p=0.5720).
Figure 2.
Distribution of spirometry completion over time in days relative to date of hospitalization (n=12,568 patients with spirometry completed). Yellow bars indicate spirometry acquisition within 90 days after hospital admission.
Practice and Geographic Characteristics and Associations with 1-Year Spirometry
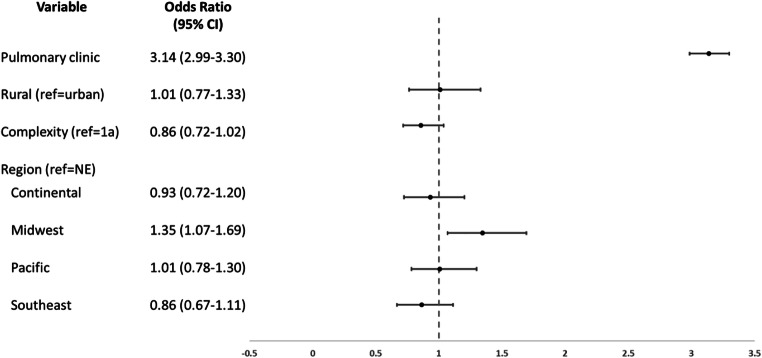
Our primary analysis of practice and geographic characteristic included 35,440 patients. Patients who were seen in pulmonary clinic were significantly more likely to complete spirometry within 1 year of COPD hospitalization (odds ratio (OR) 3.14 [95% confidence interval 2.99–3.30]) (Fig. 3, Supplemental Table 2). There were no significant associations between spirometry and rurality (reference=urban facilities OR 1.01 [0.77–1.33]) or hospital complexity (levels 1b–3 (reference=1a)=OR 0.86 [0.72–1.02]). Patients at facilities in the Midwest region were more likely to complete spirometry (reference=Northeast; OR 1.35 [1.07–1.69]); there was no significant difference in any other region of the country. To provide insight about how our included predictors may explain observed spirometry variability, we obtained a maximal-rescaled R2 of 12.1% using a downgraded logistic regression without random intercept.
Figure 3.
Practice and geographic factors associated with spirometry completion within 1 year of COPD hospitalization. NE, Northeast; CI, confidence interval. Analyses also adjusted for patient characteristics of demographics, smoking, substance use, diabetes, asthma, depression, and PTSD.
Provider Characteristics and Associations with 1-Year Spirometry
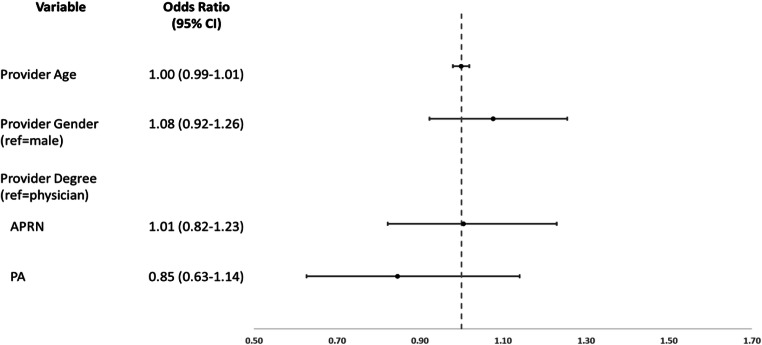
This group initially included 5795 patients; 1933 were excluded due to missing data leaving 3862 patients with provider-level data included in this analysis. Among these patients, baseline patient characteristics were similar to the primary cohort, with the majority male, white, and current or former smokers with similar rates of comorbidities (data not shown). In adjusted models, there were similar associations in spirometry completion with those who attended a pulmonary clinic (OR 3.31 [2.83–3.86]). There was no significant variation in spirometry completion at the provider level by provider age (OR for each 1-year increase in age=1.00 [0.99–1.01]) or gender (women provider OR 1.08 [CI 0.92–1.26]) (Fig. 4). Additionally, there was no significant difference in spirometry completion by provider degree including for APRN (reference=physician) (OR 1.01 [0.82–1.23]) or PA (OR 0.85 [0.63–1.14]).
Figure 4.
Provider-level characteristics associated with spirometry completion within 1 year of COPD hospitalization. APRN, advanced practice registered nurse; PA, physician assistant; CI, confidence interval. Analyses also adjusted for patient characteristics of demographics, smoking, substance use, diabetes, asthma, depression, and PTSD and practice and geographic characteristics of pulmonary clinic visit, rurality, complexity, and region.
DISCUSSION
In a large cohort of veterans hospitalized for COPD, we found that spirometry was obtained much less frequently than recommended by GOLD guidelines, with nearly half of all patients not having completed spirometry within 1 year before or after their COPD exacerbation hospitalization. We also found significant variation among practice and geographic variables, with pulmonary clinic visits most strongly associated with spirometry. We found no significant difference in patient completion of spirometry based on differences in facility complexity or rurality. A sensitivity analysis including provider characteristics did not significantly change these findings and notably also did not show any associations between provider characteristics and spirometry completion, though interpretation of these latter findings is limited due to the high number of missing values for provider characteristics.
Our study confirms prior work suggesting low overall rates of spirometry, despite chart-diagnosis codes for COPD in these hospitalized patients. Although our rates of spirometry were higher than in prior VA-based studies, this may be related to greater COPD severity in our cohort in the setting of hospitalization.7–9 We also found that attendance in pulmonary clinic was the strongest factor associated with spirometry, something which has been noted previously in patients with newly diagnosed COPD, and to a similar magnitude.7 This suggests subspecialty pulmonary services may serve as spirometry facilitators, leading to more guideline-concordant management of COPD, including spirometry at recommended intervals, both for patients with recently diagnosed COPD and those with a recent hospitalization for an acute exacerbation of COPD. A recent evaluation of over 180,000 veterans over age 65 with COPD found that referral to pulmonary care was more likely among those with hospitalizations within the prior year.14 This may represent an area for performance improvement for high-risk patients, including those with a recent hospitalization, through expanded virtual subspecialty services, as referral to pulmonary specialists may allow for improvement in guideline adherence in this population. In fact, a recent clinical trial utilizing a multidisciplinary team including pulmonologists to provide treatment recommendations to patients recently hospitalized at a VHA hospital for an acute exacerbation of COPD was able to improve quality of life in those patients, though the study was underpowered to determine a definitive effect on readmissions or death.26,27
Our evaluation of geographic variation was different from prior evaluations of newly diagnosed COPD in that we found less variation relative to prior VA-based studies.7,9 However, this may be related to differences in geographic categories where prior studies used more granular, individual VHA service networks while we limited our analyses to only five geographic regions. We also found that patients in the Midwest region had higher likelihood for spirometry compared to the Northeast. The reasons for this are unclear and may be due to chance, potentially due to the use of p-value thresholds that were not adjusted for multiplicity. Further research is needed to confirm and explain these findings.
A unique aspect of this study is our evaluation of facility complexity. This designation by VHA stratifies hospitals and associated clinics by their patient volume and resources. We did not find a significant difference in the most complex (i.e., 1a) facilities as compared to others. This was a surprising finding, given that 1a facilities would be expected to have ready access to higher numbers of pulmonary specialists and pulmonary testing such as spirometry, but did not appear to have significantly different outcomes. Though it is encouraging that non-1a facilities, which account for more than half of all VHA facilities,22 are effectively delivering a similar standard of care for patients with COPD as 1a facilities, the overall low rate of spirometry completion suggests that there is significant room for improvement across the spectrum of hospital complexity.
Our analysis of provider characteristics found no significant variation in spirometry by provider degree, though interpretation of these findings is limited by the relatively small sample size and high number of missing values. This has also been shown to be the case for patients with COPD in referral to pulmonary clinic, with no difference between physician and non-physician providers.14 Prior work evaluating the quality of diabetes care among physicians, APRNs, and PAs within the VHA found no significant differences in outcomes of blood pressure control, cholesterol levels, or hemoglobin A1c.28 We add to this literature in finding that there were also no significant differences by provider age or sex in adherence to GOLD-based spirometry recommendations.
Our study has several limitations. Primarily, we looked only at completion of spirometry; it is possible that there were unmeasured facility and provider characteristics associated with similar rates of referral but lower rates of completion, which we were unable to evaluate. Similarly, while we evaluated only primary care provider characteristics, spirometry may be ordered by any provider on a patient’s care team including inpatient providers or other outpatient subspecialty providers. Our evaluation was also limited to spirometry completed within the VHA system as part of pulmonary function testing and thus we were unable to account for patients who had handheld spirometry performed, as is available at many VHA clinic sites, or had spirometry ordered by non-VA providers or completed outside of VHA facilities through community care programs, including the Choice Act which was passed in 2014. In a sample of 489,508 veterans from fiscal year 2015, community care for any service was utilized by 72,650 (14.8%) patients.29 Prior work by Rinne et al. estimated non-VA care utilization of 17.8% for any service among dual-enrolled (Medicare and VA) patients admitted for COPD.30 These findings suggest that, while utilization of any non-VA care is not rare, it is unlikely to affect our primary findings.
We were also limited by the data available in CDW and SHEP; our analysis used a static year for provider characteristics and facility urban vs. rural status, which could have changed over time. Additionally, our inclusion of patients within 1 year before or after hospitalization allowed for a slightly broader population than the strict 3-month post-hospitalization recommendation from GOLD in order to take into account some leniency in delays caused by scheduling availability at pulmonary function testing facilities. It has been shown previously that hospitalizations are more common in patients with COPD who have not completed spirometry7 and it is not clear if this is a direct cause and effect relationship with adherence to GOLD spirometry guidelines preventing hospitalizations, or if patients who are more likely to be hospitalized are less likely to complete spirometry. If the latter is the case, it is possible that our study which was limited to patients with a hospitalization, whom we considered to be a high-risk group, may not be generalizable to the broader population of patients with COPD. Our study uses data only from a population of VA patients with COPD; though prior work has shown similarities to the non-VA population in age and readmission frequency,31 other differences between the veteran and non-veteran population may limit generalizability of our findings. In addition, testing such as spirometry may be easier in the VA system compared to systems outside the VA due to healthcare access and insurance coverage concerns. Additionally, the modest R2 of 12% suggests that other important variables that relate to spirometry completion in this population have yet to be discovered. Finally, it should be noted that spirometry in patients with COPD should be individualized based on patient circumstances; it is most helpful for assessment of progression of disease and to determine inhaler optimization and reversibility of symptoms with appropriate treatment. It is possible that patient-centered discussions of the necessity of spirometry took place even though spirometry was not completed.
Overall, patients with a hospitalization for COPD exacerbation had low rates of spirometry completion, significant differences based on several factors including attendance in pulmonary clinic, and no differences based on provider-level factors. Our study offers important insights and raises the question of whether pulmonary referral after hospitalization for COPD exacerbation, either through face-to-face or telemedicine encounters, should become the standard of care. With the increasingly available telemedicine options, this may ensure patients receive appropriate, guideline-concordant, high-quality COPD care. Further evaluation of variation in adherence to guidelines in other aspects of COPD care will be necessary to understand the variation in outcomes of patients with COPD.
Supplementary Information
(DOCX 25 kb)
Funding
This research was supported by the Department of Veterans Affairs through a Pain Research, Informatics, Multimorbidities, and Education (PRIME) Center (CIN 13-407) Locally Initiated Project grant (Project ID: LIP 96-059 (BR)). Dr. Han is supported in part by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG021342 NIH/NIA (LH)). The views expressed in this article represent only those of the authors and do not necessarily represent those of the US government.
Declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akinbami L, Liu X. Chronic Obstructive Pulmonary Disease Among Adults Aged 18 and Over in the United States, 1998–2009. NCHS Data Brief. 2011. [PubMed]
- 2.VA/DoD Clinical Practice Guideline for the Mangement of Chronic Obstructive Pulmonary Disease. Department of Veterans Affiars, Department of Defense. 2014. https://www.healthquality.va.gov/guidelines/CD/copd/VADoDCOPDCPG2014.pdf. Accessed December 23, 2019.
- 3.Finklestein J, Cha E. Association of Veteran status with COPD prevalence stratified by gender. Am J Respir Crit Care Med. 2013;187.
- 4.Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest. 2000;117:33S-7S. [DOI] [PubMed]
- 5.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report). 2020. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf. Accessed December 23, 2019.
- 6.Arne M, Lisspers K, Stallberg B, Boman G, Hedenstrom H, Janson C, Emtner M. How often is diagnosis of COPD confirmed with spirometry? Respir Med. 2010;104:550-6. [DOI] [PubMed]
- 7.Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129:1509-15. [DOI] [PubMed]
- 8.Han MK, Kim MG, Mardon R, Renner P, Sullivan S, Diette GB, Martinez FJ. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-9. [DOI] [PubMed]
- 9.Joo MJ, Au DH, Fitzgibbon ML, McKell J, Lee TA. Determinants of spirometry use and accuracy of COPD diagnosis in primary care. J Gen Intern Med. 2011;26:1272-7. [DOI] [PMC free article] [PubMed]
- 10.Pretto JJ, McDonald VM, Wark PA, Hensley MJ. Multicentre audit of inpatient management of acute exacerbations of chronic obstructive pulmonary disease: comparison with clinical guidelines. Intern Med J. 2012;42:380-7. [DOI] [PubMed]
- 11.Schneiderman AI, Dougherty DD, Fonseca VP, Wolters CL, Bossarte RM, Arjomandi M. Diagnosing chronic obstructive pulmonary disease among Afghanistan and Iraq Veterans: Veterans Affair’s Concordance with clinical guidelines for Spirometry Administration. Mil Med. 2017;182:e1993-e2000. [DOI] [PMC free article] [PubMed]
- 12.Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM, Estudi del Factors de Risc d'Aguditzacio de la Mi. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100-5. [DOI] [PMC free article] [PubMed]
- 13.Bade BC, DeRycke EC, Ramsey C, Skanderson M, Crothers K, Haskell S, Bean-Mayberry B, Brandt C, Bastian LA, Akgun KM. Sex differences in veterans admitted to the hospital for chronic obstructive pulmonary disease exacerbation. Ann Am Thorac Soc. 2019;16:707-714. [DOI] [PMC free article] [PubMed]
- 14.Nunez ER, Johnson SW, Qian SX, Powell WR, Walkey AJ, Wiener RS, Rinne ST. Patterns of pulmonary consultation for veterans with incident COPD. Ann Am Thorac Soc. 2021;18(7):1249-52. [DOI] [PMC free article] [PubMed]
- 15.Salinas GD, Williamson JC, Kalhan R, Thomashow B, Scheckermann JL, Walsh J, Abdolrasulnia M, Foster JA. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis. 2011;6:171-9. [DOI] [PMC free article] [PubMed]
- 16.Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, Au DH. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. [DOI] [PMC free article] [PubMed]
- 17.Bastian LA, Trentalange M, Murphy TE, Brandt C, Bean-Mayberry B, Maisel NC, Wright SM, Gaetano VS, Allore H, Skanderson M, Reyes-Harvey E, Yano EM, Rose D, Haskell S. Association between women veterans’ experiences with VA outpatient health care and designation as a women’s health provider in primary care clinics. Womens Health Issues. 2014;24:605-12. [DOI] [PMC free article] [PubMed]
- 18.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-31. [DOI] [PMC free article] [PubMed]
- 19.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233-9. [DOI] [PMC free article] [PubMed]
- 20.Chui PW, Esserman D, Bastian LA, Curtis JP, Gandhi PU, Rosman L, Desai N, Hauser RG. Facility variation in troponin ordering within the Veterans Health Administration. Med Care. 2020;58:1098-1104. [DOI] [PMC free article] [PubMed]
- 21.Alore EA, Massarweh NN, Ramsey DJ, Chen L, Chai CY, Singh H, Makris KI. Variation in surgical management of primary hyperparathyroidism in the US Department of Veterans Affairs healthcare system: A 15-year observational study. Surgery. 2020;168:838-844. [DOI] [PubMed]
- 22.Veterans Health Administration (VHA) Facility Complexity Model. Veterans Health Administration Office of Productivity Efficiency and Staffin. 2020. http://opes.vssc.med.va.gov/Pages/Facility-Complexity-Model.aspx. Accessed 09/01/2021.
- 23.National Academies of Sciences Engineering and Medicine; Committee on Facilities Staffing Requirements for Veterans Health and Administration. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. Washington (DC): National Academies Press (US); 2019. [PubMed]
- 24.Barreto TW, Chung Y, Wingrove P, Young RA, Petterson S, Bazemore A, Liaw W. Primary care physician characteristics associated with low value care spending. J Am Board Fam Med. 2019;32:218-225. [DOI] [PubMed]
- 25.Tamblyn R, McLeod P, Hanley JA, Girard N, Hurley J. Physician and practice characteristics associated with the early utilization of new prescription drugs. Med Care. 2003;41:895-908. [DOI] [PubMed]
- 26.Au DH, Collins MP, Berger DB, Carvalho PG, Nelson KM, Reinke LF, Goodman RB, Adamson R, Woo DM, Rise PJ, Coggeshall SS, Plumley RB, Epler EM, Moss BR, McDowell JA, Weppner WG. Health system approach to improve chronic obstructive pulmonary disease care after hospital discharge: Stepped-Wedge Clinical Trial. Am J Respir Crit Care Med. 2022;205:1281-1289. [DOI] [PubMed]
- 27.Lindenauer PK, Williams MV. Improving outcomes after a chronic obstructive pulmonary disease hospitalization: lessons in population health from the U.S. Department of Veterans Affairs. Am J Respir Crit Care Med. 2022;205:1257-1258. [DOI] [PMC free article] [PubMed]
- 28.Jackson GL, Smith VA, Edelman D, Woolson SL, Hendrix CC, Everett CM, Berkowitz TS, White BS, Morgan PA. Intermediate diabetes outcomes in patients managed by physicians, nurse practitioners, or physician assistants: a cohort study. Ann Intern Med. 2018;169:825–835. doi: 10.7326/M17-1987. [DOI] [PubMed] [Google Scholar]
- 29.Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act?: Differences between rural and urban veterans. Med Care. 2021;59:S270-S278. [DOI] [PMC free article] [PubMed]
- 30.Rinne ST, Elwy AR, Bastian LA, Wong ES, Wiener RS, Liu CF. Impact of multisystem health care on readmission and follow-up among veterans hospitalized for chronic obstructive pulmonary disease. Med Care. 2017;55 Suppl 7 Suppl 1:S20-S25. [DOI] [PubMed]
- 31.Jacobs DM, Noyes K, Zhao J, Gibson W, Murphy TF, Sethi S, Ochs-Balcom HM. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thorac Soc. 2018;15:837-845. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 25 kb)