Abstract
The cochlear implant (CI) is widely considered to be one of the most innovative and successful neuroprosthetic treatments developed to date. Although outcomes vary, CIs are able to effectively improve hearing in nearly all recipients and can substantially improve speech understanding and quality of life for patients with significant hearing loss. A wealth of research has focused on underlying factors that contribute to success with a CI, and recent evidence suggests that the overall health of the cochlea could potentially play a larger role than previously recognized. This article defines and reviews attributes of cochlear health and describes procedures to evaluate cochlear health in humans and animal models in order to examine the effects of cochlear health on performance with a CI. Lastly, we describe how future biologic approaches can be used to preserve and/or enhance cochlear health in order to maximize performance for individual CI recipients.
Keywords: Auditory prosthesis, Cochlear electrical stimulation, Spiral ganglion neuron, Measures of cochlear implant function, Genetic and environmental deafness, Gene therapy for hearing loss
Introduction
Cochlear implantation is widely considered to be the most successful means to partially restore hearing in patients with significant hearing loss. As of 2019, over 700,000 patients had received cochlear implants (CIs) worldwide [1], and it is reasonable to expect that this number will continue to grow exponentially due to expanding candidacy criteria and efforts to educate referring providers regarding referral guidelines and candidacy [2–5]. The increased number of CI recipients has allowed researchers and clinicians to better understand the factors that contribute to the success of CIs as well as the limitations of the technology. While several factors are known to contribute to CI performance outcomes in children and adults including duration of hearing loss and age at implantation [6–10], developing methods to estimate and/or improve neural health of implanted ears is likely to enhance outcomes for many patients, which would reduce the incidence of sub-optimal performance.
“Cochlear health” as it is used in this review, is a comprehensive term that includes neural, sensory, and other biological factors that are known or suspected to be important for CI function. Examples of “measures of neural health” include spiral ganglion neuron (SGN) density, neuronal cell size, myelination, presence/absence of peripheral process, and synaptic function. “Sensory health” includes number and condition of inner hair cells (IHCs) or outer hair cells (OHCs). Other attributes, such as stria vascularis function, help to maintain cochlear hemostasis and therefore are also important to consider. Also, we discuss that the effects of anatomical and structural characteristics of the implanted cochlea influence outcomes; for example, the presence of fibrous tissue or new bone, congenital cochlear anomalies such as cochlear hypoplasia, common cavity, incomplete partition (IP), and enlarged vestibular aqueduct (EVA), might all affect outcomes or measures of cochlear health to some extent.
When CIs were initially approved by the Food and Drug Administration (FDA) in 1984, they were reserved for a group of patients with no residual hearing on a pure-tone audiogram and zero percent word recognition with the use of hearing aids [11]. The impact of cochlear health on performance in early CI recipients was likely not very important because the population was relatively homogeneous with regards to pre-operative audiometric and speech recognition data. It should be acknowledged, however, that post-operative performance still varied to some extent across subjects [12]. Present day FDA criteria have expanded to include adults with significant residual hearing pre-operatively via audiometric pure-tone thresholds and residual open-set speech recognition scores. Several studies have shown significant benefit of cochlear implantation in patients with residual hearing function [13–16]. Thus, contemporary CI recipients are likely more heterogeneous with respect to pre-operative functional hearing [16], and it is logical that cochlear health factors, such as neural and sensory health, also vary across this group of patients. A better understanding of how cochlear health influences outcomes in CI recipients will help to advance technology used to reduce damage during implantation, develop biologically based therapies, and improve programming protocols, which will ultimately improve outcomes.
In this review article, we will first define and review features of cochlear health that are known or hypothesized to be particularly important for CI function and discuss what is known about cochlear health associated with common etiologies of hearing loss. We then describe how to evaluate cochlear health using animal models and human subjects and examine its effect on performance with a CI. Lastly, we explore how biologic approaches can be used to preserve or even improve the cochlear infrastructure needed for optimal performance by individual CI users. While this paper examines effects of peripheral neural health on CI outcomes, it is of course certain that central encoding of information (and central neural health) also plays a role in CI outcomes and performance [17–27]. Further research shows that cognitive function influences perception with a CI [28–32]. While these factors are important to acknowledge, the current paper focuses on peripheral cochlear function in CI patients.
Attributes of Cochlear Health Important for Electrical Hearing
Neural Health
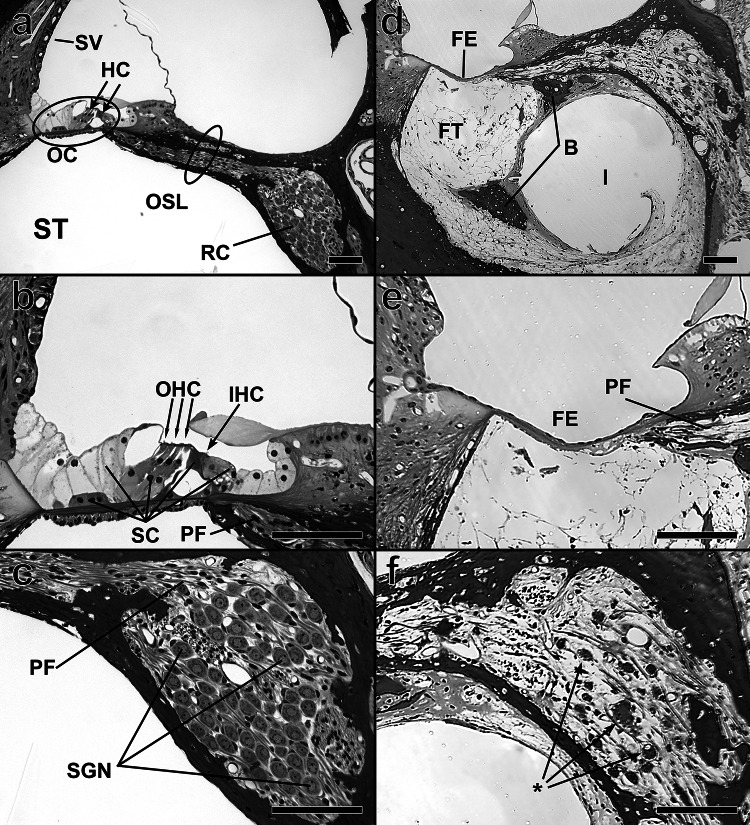
Because a CI directly stimulates the auditory nerve, it is logical that the condition of the SGNs would play a key role in how sound is perceived using CI technology. The most common anatomical measure of neural health in human temporal bones and in animal studies is SGN cell body survival. Typically, this is quantified as SGN density, (i.e., the number of SGN cell bodies in a cross section of Rosenthal’s canal, divided by the area of the region containing those cell bodies). SGNs are classified as type I with several subtypes [33] and type II; they are differentiated by morphology, function, and innervation within the cochlea. Given that type I SGNs comprise at least 90% of the overall population, the remainder of the discussion below will focus on type I cells illustrated schematically in Fig. 1. Animal and human temporal bone studies show that sensory (hearing) loss may be accompanied by degeneration of SGNs. In humans, there is evidence that this process can take several years, and likely occurs slower than the time course observed in most animal models [34–38]. Variation in SGN density across animal subjects has been shown to account for about 50% of the variance in simple psychophysical and electrophysiological measures of CI function [39–43], and those same functional measures have been shown to be predictive of speech recognition when applied across human subjects who use CIs [44, 45]. These specific functional measures of health will be reviewed in depth later in this paper. SGN density is a very useful anatomical measure of cochlear health, but it is likely that other anatomical features of the hearing-impaired, implanted cochlea are also of importance. Figure 2 shows a normal, healthy cochlea (a–c) and a deafened, implanted ear (d–f) for comparison. Figure 2d, f illustrate the location of the SGN components in relation to the location of a CI electrode array. The electrode array has been withdrawn but its previous location is evident from the empty space surrounded by fibrous tissue and new bone.
Fig. 1.
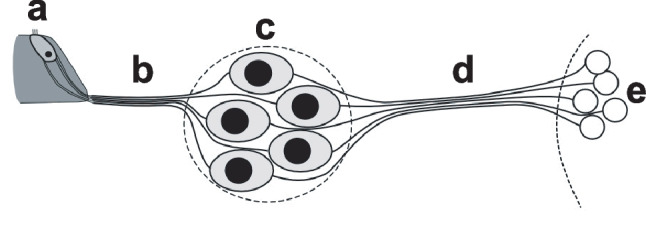
Schematic representation of type I spiral ganglion neurons in the context of their peripheral and central projections. The neurons are bipolar, with peripheral processes (b) that transport information from inner hair cells in the organ of Corti (a) via the cell bodies in Rosenthal’s canal (c) to the central processes (d), which then carry the information to neurons in the cochlear nucleus (e). Further details are provided in the manuscript text and in Fig. 2
Fig. 2.
Some anatomical and structural features of the implanted cochlea that are potentially important for CI function. Images in the left column are from a control guinea pig cochlea (no implant or treatment) while those in the right column are comparable images from a guinea pig cochlea that has been deafened with neomycin and received a CI. All scale bars indicate 100 µm. a Cross section of control cochlea, showing stria vascularis (SV), locations of hair cells (HC) in the organ of Corti (OC), and positions of Rosenthal’s canal (RC), and the osseus spiral lamina (OSL) adjacent to the scala tympani (ST). b Higher magnification image showing the ordered arrangement of inner and outer hair cells (IHC and OHC) and supporting cells (SC) in the OC. Distal ends of the myelinated portions of peripheral fibers (PF) of spiral ganglion neurons (SGN) can be seen in the OSL. c High magnification image showing dense packing of SGN cell bodies in RC and peripheral fibers exiting RC and entering the OSL. d Cochlea of a deafened and implanted guinea pig. The complexly structured organ of Corti has been replaced by a flat epithelium (FE), a simple sheet of thin cells, and the densities of SGNs and PFs are greatly reduced. The scala tympani is filled with fibrous tissue (FT) except for the space previously occupied by the implant (I). The dark regions embedded in the FT are regions with new bone. e High magnification image showing the simple structure of a flat epithelium and a few surviving peripheral fibers in the OSL. f High magnification image showing sparse surviving SGN cell bodies, some with uneven margins (*), suggesting abnormal cellular function
One prominent feature of SGN health, which varies across etiologies of deafness or experimental treatments, is the condition of the peripheral processes. The normal type I SGN is a bipolar neuron with a peripheral process that extends peripherally from the cell body in Rosenthal’s canal to the base of the IHC and centrally to the cochlear nucleus (Fig. 1). The peripheral process is myelinated within Rosenthal’s canal and the osseous spiral lamina (OSL in Fig. 2a), ending with a short final segment extending to the IHCs that is not myelinated. In human cases of cochlear pathology, the peripheral process can die back to the cell body [46], although this does not always happen in some animal models [47, 48]. If the peripheral process has died back, the cell body can remain intact as a unipolar neuron for an extended period of time; this has been observed in animal models [49, 50] and in human temporal bones [46, 51]. Experimental and modeling studies suggest that the effects of peripheral process survival on CI function are potentially significant but complex [52, 53]. These effects are discussed later in this paper. Other morphological features of the neural population including myelination and cell size can also vary in humans or animals with hearing loss, and these variables can potentially affect the responses to electrical stimulation [54, 55].
Wise and colleagues [56] administered an ototoxic drug cocktail to guinea pig ears to understand the effects of hair cell loss on SGN morphology and function, at 2, 6, and 12 weeks following deafening. This preparation showed degeneration of the SGN peripheral fibers, which occurred prior to the degeneration of the cell body. These results provide evidence that, at least in some cases, the cell bodies can remain relatively intact in the presence of degeneration of the peripheral process. Two key findings were present with respect to myelination: (1) that myelination degeneration had sometimes occurred in 6- and 12-week deafened animals and (2) that the axoplasm often degenerated leaving behind a myelin sheath. In contrast, Ramekers and colleagues [50] deafened guinea pigs with a procedure similar to that used by Wise and colleagues, but they found simultaneous degeneration of SGN peripheral processes and cell bodies instead of the sequential degeneration observed by Wise and colleagues described above [50]. However, in a subsequent experiment [57], they found that if animals were treated with a neurotrophin (BDNF via an osmotic pump) 2 weeks after deafening, there was significant survival of SGN cell bodies and central axons, but less robust survival of peripheral processes. Further studies are needed to better explore the sequence and timing of degeneration of neuronal structures and the underlying factors contributing to the demise or survival of these structures.
Simultaneous versus sequential degeneration of SGN fibers is important to CI stimulation because there is some evidence that the site of excitation along the SGN will differ depending on electrical current stimulus parameters and pulse shape [52, 58, 59]. For example, modeling studies propose that when the auditory nerve is healthy, and the peripheral process is present, cathodic stimuli are preferential; cathodic-leading biphasic pulses, similar to those used for CI stimulation, are those with a negative leading phase (Fig. 3c). In the case of poorer neural health (i.e., degenerated peripheral process), the site of excitation is the cell body, which is preferentially excited by anodic stimuli; anodic-leading biphasic pulses are those with a positive leading phase (Fig. 3). Single-unit recordings of healthy SGNs show that the site of excitation at threshold occurs at the peripheral process (if present) and moves to the axonal region at higher stimulation levels. Latency characteristics also change with an increase in stimulus level [52]. Therefore, the anatomy of the SGN fibers and stimulation level (charge) should influence the site of excitation, and degeneration would affect stimulation properties.
Fig. 3.
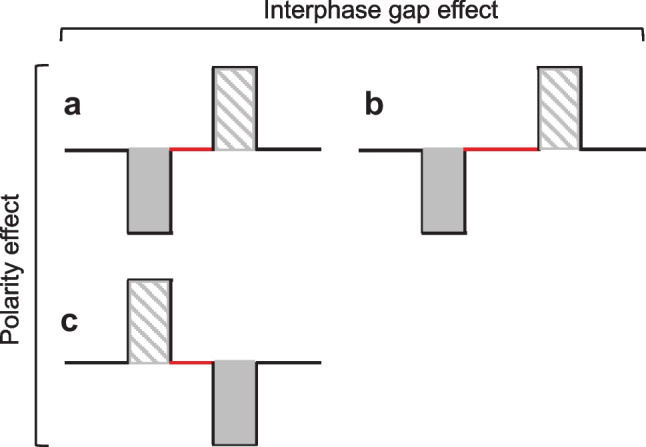
Schematic of a biphasic pulse, similar to those used in several cited studies. Within each pulse, the shaded gray area is the cathodic/negative phase, and the grey striped area is the anodic/positive phase. The solid red horizontal line represents the location of the interphase gap (IPG). a A cathodic-leading biphasic pulse. b The same a but with a longer IPG duration. Specific ECAP measurements such as the threshold, amplitude, slope, or latency of the amplitude-growth function change as the IPG duration increases (IPG Effect = difference between 3a and 3b). c An anodic-leading biphasic pulse. Specific ECAP measures previously mentioned might also change with a changing leading polarity phase (polarity effect = difference between Fig. 3a, c)
Sensory-neural Health
Early CI candidates had profound bilateral sensorineural hearing loss and often no residual hearing at the limits of audiometric testing. Based on temporal bone studies in humans with profound hearing loss [60], it can be assumed that for these early CI candidates, survival of OHCs and IHCs was quite poor. Over the past 30 years, candidacy criteria have expanded to include adult and pediatric patients with good pre-operative acoustic hearing levels, particularly in the lower frequencies while hearing levels in the high frequencies fall within the severe to profound hearing loss range [16]. Pre-operatively, contemporary candidates often demonstrate audiometric thresholds ranging from within normal limits, to moderate or moderate-to-severe in the low frequencies. These candidates likely exhibit a higher proportion of residual hair cells and supporting cells compared to those with significant and long-term hearing loss prior to implantation [61]. Several studies demonstrate that there can be variation in residual hearing or implant function that is independent of the level of hair cell survival [46, 62]. There is much research outlining the advantages of CI technology in patients with residual hair cell function and post-operative acoustic hearing, particularly for those with sufficient post-operative residual hearing who are able to take advantage of electro-acoustic stimulation (EAS) (e.g., acoustic stimulation of low frequencies and electrode stimulation of high frequencies, within the same ear) [63–65]. A focus on EAS benefit and processing is beyond the scope of this paper; however, here we provide a brief explanation of how sensory health might affect CI stimulation in the electric-only and EAS conditions.
Post-operatively, some adult and pediatric patients, as well as various animal models, continue to demonstrate residual acoustic hearing to various degrees [40, 66–69]. Therefore, it can be assumed that hair cell function as well as basilar membrane mechanics is at least somewhat preserved in this cohort. Insertion of the electrode has been shown to create intracochlear trauma, particular to the IHCs, OHCs (even those apical to the electrode), and peripheral processes, while the supporting cells are less affected although in some cases a flat epithelium is present [70]. Even in the absence of measurable acoustic hearing via traditional air-conduction audiometry, it is possible that sensory cells are still present and relatively intact in some patients. In such cases, the insertion of the electrode array could potentially affect basilar membrane mechanics to the extent that mechanosensory transmission is disrupted, yet the sensory cells are still present and healthy to some extent [71–74]. If insertion trauma sufficiently disrupts cochlear mechanics, perception evoked by an acoustical stimulus would not be possible, but the sensory cells would still be receptive to electrical stimulation. Although recent evidence suggests that there might be little effect of doing so [62], healthy IHCs could be directly stimulated by the electrical stimulus or indirectly stimulated via residual basilar membrane mechanics (if intact) causing them to release transmitter and stimulate the auditory nerve (“electrophonic” response) [52, 75–77]. These studies show complex interactions between electroneural and electrophonic stimulation, and potential masking of the electroneural signal. These interactions are not yet well understood, but they could certainly lead to more complicated sound perception in contemporary CI recipients [52, 78–80].
Furthermore, the presence of hair cells in the implanted cochlea can have important effects on the function of the surviving auditory neurons by generating “spontaneous activity” in the nerve. In a deaf ear, the auditory nerve tends to be silent in the resting state, so an electrical stimulus from the CI will cause all of the neurons within its receptive field to fire synchronously. In contrast, if IHCs are present and there is spontaneous activity in the nerve fibers, some fibers will be in a refractory state at the time that the electrical stimulus is delivered, so they will not respond to the electrical stimulus. Thus, the ensemble response of the nerve will be reduced but be more stochastic and thus more natural [81–83].
There has been significant debate regarding the interdependence of sensory and neural structures following insult, disease, or injury to the cochlea [84]. Collectively, results have shown that the interdependence might be influenced by the pathology and/or species; but it is clear that neural and sensory health can certainly vary independently. Historically, it was presumed that IHCs were important for SGN survival given that they provide neurotrophic support, and that following injury to the cochlea, hair cells would become quickly and easily damaged, soon followed by SGN loss. However, post-mortem temporal bone studies in humans show that SGNs can persist for decades in regions of the cochlea where hair cells are no longer present [61, 85]. Limited evidence from animal studies generally supports these findings. Specifically, the diphtheria toxin receptor (DTR) mouse [86] has the human DTR gene under control of Pou4f3, a hair-cell specific transcription factor. Studies in adult DTR mice show near complete preservation of SGNs is possible even in the complete absence of hair cells [47, 48, 86]. In two different mouse models, hair cell loss is neither correlated nor collocated with a SGN loss in the cochlea at least for 2–3 months after deafening [48, 84]. Preservation of the neurons after IHC loss is likely a result of neurotrophins that are secreted by the supporting cells. Hearing loss induced by some ototoxic drugs is associated with a rapid loss of hair cells, which is quickly followed by SGN degeneration even when supporting cells survive [87, 88]. McFadden et al. [88] noted intact supporting cells (Deiters’ and pillar) for several weeks post administration of ethacrynic acid and gentamicin in the chinchilla, at which point much of the SGN and hair cell populations were degenerated.
The importance of the work discussed in this section as it relates to cochlear implantation is the notion that the condition and degeneration of SGNs and sensory cells can differ substantially within and across ears. Depending on the species and etiology of hearing loss, attributes of cochlear health might degenerate in quick succession, but in other instances changes might present more independently of one another. Taken together, it is important to understand the effects of both sensory and neural elements to CI function given that their interdependence might vary widely based on multiple factors.
Non-neural Features of Cochlear Health
There are several non-neural factors related to neural and sensory health that are important to consider with regard to CI function. The health of neural and sensory cells within the cochlea is maintained, at least in part, by regulatory mechanisms that help to support homeostasis [89–92]. Proper function of the stria vascularis is necessary in order to maintain the endocochlear potential and hair cell transduction. Although it is unclear if stria function directly affects perception with a CI, studies have shown that damage to the stria vascularis during electrode insertion does disrupt cochlear homeostasis and contributes to post-operative changes in acoustic residual hearing in the animal model [93, 94]. Specifically, these studies showed that threshold changes of up to 40–50 dB post implantation were not directly related to changes in any anatomical feature other than to the stria vascularis. These studies found that post-operative changes in acoustic residual hearing were associated with reduced stria vascularis blood vessel density, but not to hair cells counts or SGN density. Although not yet tested directly, it is logical that damage to the stria would be more prevalent in lateral compared to perimodiolar arrays.
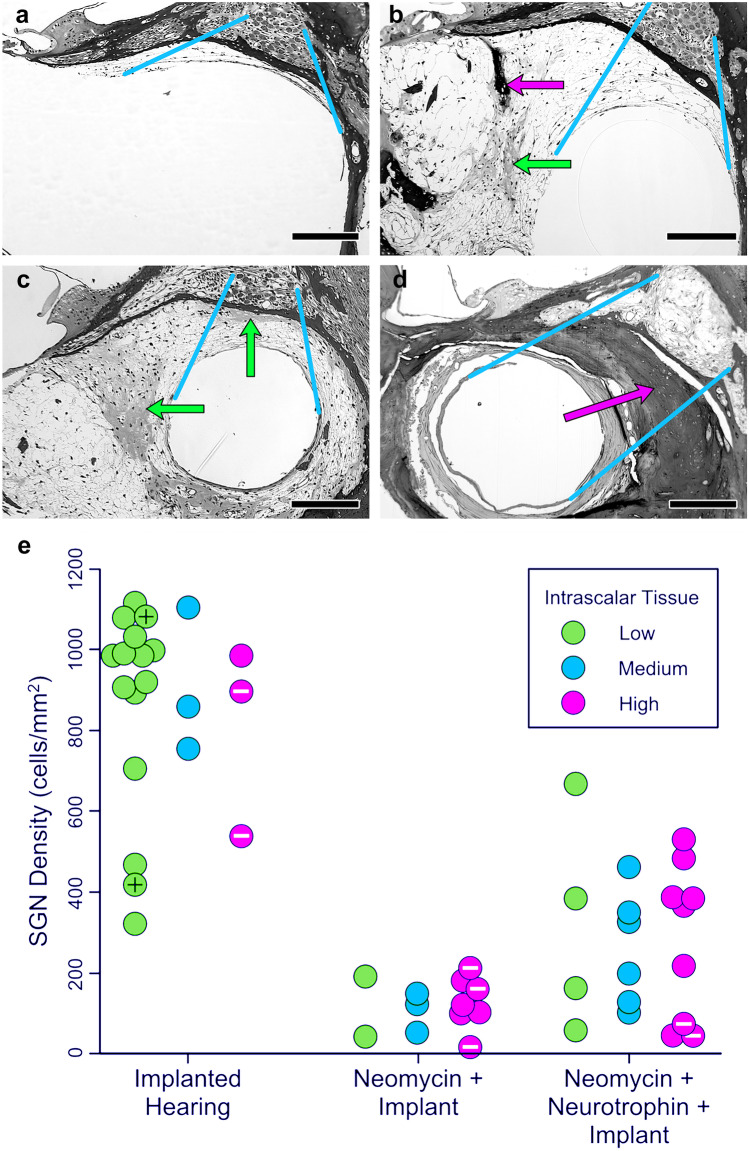
Several other studies note intracochlear changes following insertion of the electrode array, which could potentially disrupt homeostasis and perception of electroneural, electrophonic, or acoustical stimulation. Temporal bone studies in humans often reveal either fibrous or bony tissue surrounding the electrode array [37, 38, 95–98], and the prevalence and degree of post-operative ossification increases in patients with an abnormal insertion (e.g., “tip roll over”) [99]. Likewise, similar post-operative changes related to tissue growth have been noted in animal models of cochlear implantation [100, 101]. Related studies in animals and humans have postulated that post-operative growth of tissue and bone in the scala tympani occurs subsequent to an influx of an immune response due to trauma-induced inflammation. For example, a few studies have noted the active role of macrophages following cochlear implantation [96, 102]. Figure 4a–c, and d illustrate the range of variation in the presentation of post-operative tissue growth observed during histological examination of 52 cochlear implanted guinea pigs [101]. The tissue arising from implantation varied from a thin band to thick layers of fibrous protein with zero to large amounts of bone. A review of early studies revealed how biocompatibility of materials can also influence intracochlear damage and inflammatory responses, which could also lead to both residual loss of hearing and growth of tissue and bone [103].
Fig. 4.
Examples of intrascalar tissue formation and SGN densities found in three guinea pig models differing in treatment and implantation procedures: animals implanted in a normal-hearing ear; animals deafened by local infusion of neomycin and then implanted; and animals deafened with neomycin, treated with neurotrophin to help preserve SGN cell bodies, and implanted. The photomicrographs (a–d) demonstrate the variability in density and type of intrascalar tissue observed in these animal models. a Minimal tissue. b More extensive tissue with patches of bone (dark stain and laminated) and dense fibrosis (lighter and unlaminated). c Similar to “b,” but with dense fibrosis between the implant and the spiral ganglion. d Implant completely surrounded by bone that fills most of the scala. Arrows indicate patches of dense fibrosis (green) and bone (magenta) in the intrascalar tissue. Blue lines indicate the region of interest (between Rosenthal’s canal and the area previously occupied by the implant) in which intrascalar tissue development was scored (low, medium, high). Bar = 200 µm. e Results for each individual in each treatment group, illustrating the across-subject variability in SGN density near the implant and intrascalar tissue classifications in the region of interest. Special cases of intrascalar tissue are indicated by marks inside the symbols (black + = no visible intrascalar tissue, white-= bone surrounding the implant filled the remaining space in the scala tympani). This figure is a combination of Figs. 2 and 4 from Swiderski et al. [101], reproduced with permission from the publisher
It has been hypothesized that abnormal and substantial tissue growth within the cochlea, either before or after electrode insertion could lead to a loss of residual hearing, hair cells, SGNs, and support structures [101, 104, 105]. In humans, post-mortem temporal bone studies provide conflicting evidence. Some findings point to overall decreased SGN and IHC counts in ears with ossification along with collocation of the greatest ossification with lowest remaining SGN densities while other studies contradict these findings and show good SGN survival in some ears with significant ossification [106–109]. One explanation for these mixed findings could be that the trauma-induced immune response, or the trauma itself, which likely varies in each case, could also affect survival of SGNs. To investigate the relationship between intrascalar tissue and SGN density, the 52 animals examined by Swiderski et al. [101] were divided into three implantation and treatment groups (1, implant only no deafening treatment; 2, neomycin deafened and implanted; and 3, neomycin deafened + neurotrophin treatment + implant), and the density of tissue between the implant and Rosenthal’s canal was scored in three levels (low, medium, and high). Results shown in Fig. 4e illustrated the expected differences in SGN density between procedures (F(2,43) = 64.99, p < 0.001), and more important, showed that variation in SGN density within treatment groups was independent of the variation in density of the intrascalar tissue (F(4,43) = 0.127, p = 0.972) (for additional statistical analysis, see Swiderski et al. [101]).
Lastly, several studies reveal that, although the intended placement of the electrode array is within the scala tympani, the array often translocates into the scala media or vestibuli [110–113]. However, these studies also reveal that adequate speech recognition remains possible even when the electrode is placed in the scala vestibuli. Translocation of the electrode array could potentially cause damage to the neural and sensory elements [114–119], and it is possible that trauma induced by an electrode translocation or tip-foldover, would induce an immune response and possibly an increased accumulation of tissue surrounding the electrode array. Translocation of the electrode array is more common for perimodiolar (42%) compared to lateral (11%) arrays [120], and the incidence of tip fold-over is about 1–2% [121]. Taken together, it is possible that etiology of hearing loss and/or the trauma subsequent to electrode insertion could potentially disrupt cochlear homeostasis, which could in turn influence the health of neural and sensory elements.
Cochlear Health and Hearing-loss Etiology
SGN density, the condition of the peripheral processes, myelination, cell size, and other potentially important cochlear health attributes in the deaf ear, depend on the specific etiology of hearing loss in humans or on the specific treatments used in animal models of hearing loss. Studies show some correlation between etiology and outcome, along with a large variability within each cohort [122, 123]. It is estimated that 30% of congenital hearing losses are syndromic, while the remainder are non-syndromic genetic causes of hearing loss. Some etiologies entail mutations to genes that affect the hair cells directly (ACTG1, CDH23, LOXHD1, MYO15A, MYO6, MYO7A, OTOF), while others affect supporting cells (GJB2, CCDC50), SGNs (PMP22), or some combination of these (TMPRSS3, CHD7). Among patients with congenital hearing loss, approximately half are attributed to genetic etiology [124]. Mutations in the GJB2 gene, which encode the connexin 26 (Cx26) protein, are among the most common causes of congenital hearing loss [125]. Humans with GJB2 deafness usually perform well with a CI indicating that their SGNs are relatively intact and functional. In contrast, most mouse models of Cx26-related deafness reveal that the SGNs degenerate very early [126–129] although a more recent model with inducible conditional deletion of GJB2 presents a less severe phenotype [130]. Nadol [37] used post-mortem temporal bone studies to show average SGN densities in humans with various etiologies of hearing loss; these findings show that humans with sudden idiopathic hearing loss or aminoglycoside ototoxicity exhibit near normal counts of SGNs. Conversely, those with postnatal viral labyrinthitis or bacterial labyrinthitis demonstrate significantly lower SGN counts [37].
Another common congenital hearing loss etiology which affects approximately 5–15% of children with hearing loss is enlarged vestibular aqueduct (EVA). In these patients, EVA is considered to be related to the underlying deficiency of pendrin, which is an anion exchange protein expressed in the inner ear and is important for maintaining homeostasis and regulation of endolymph [131, 132]. These patients typically present with progressive hearing loss and are shown to have good outcomes when undergoing cochlear implantation in a timely manner [133]. In fact, studies generally show positive outcomes for patients with etiologies that do not include abnormalities in the auditory nerve whereas poorer outcomes are associated with etiologies known to specifically affect the neural elements [122, 123].
Auditory neuropathy spectrum disorder (ANSD) often involves specific demyelination of the auditory nerve, and outcomes among CI recipients with ANSD are mixed likely due to variations in the specific site of lesion [134]. Diseases associated with demyelination of the peripheral nervous system such as Charcot Marie Tooth (CMT) [135] are also associated with abnormal auditory nerve function as evidenced by auditory brainstem response (ABR) testing [136]. PMP22 mutations which cause CMT are known to primarily influence Schwann cells, leading to abnormal interactions between Schwann cells and axons [137]. Further studies using an existing mouse model may help elucidate the pathological mechanism in CMT and design treatments [138]. Patients with CMT who undergo cochlear implantation show mixed outcomes [139, 140]. Likewise, children born with cochlear nerve deficiency (CND), otherwise known as cochlear nerve hypoplasia, often have poorer outcomes and sometimes are unable to develop spoken language even when implanted at an appropriate age [141].
Specific etiologies of hearing loss are also associated with the accumulation of tissue in the scala tympani. Specifically, meningitis and sudden hearing losses caused by viral labyrinthitis often exhibit abnormal ossification of the otherwise typically fluid filled spaces in the cochlea, even prior to insertion of the cochlear-implant electrode array [106, 142]. Severe cases of ossification require additional drilling during electrode insertion, or in some cases insertion into a less-ossified portion of the cochlea such as the scala vestibuli [143]. It is logical that the medium surrounding the electrode would systematically influence impedance during electrical stimulation; however, the relationship does not seem to be straightforward [97, 144]. Patients who received a CI subsequent to meningitis require an increased stimulus amplitude to induce effective stimulation, and demonstrate higher impedances [145], and in the cases of severe ossification, outcomes with cochlear implantation can be quite poor [146]. Poorer outcomes when significant ossification is present are also affected by placement and/or limited insertion of the electrode array as well as duration of hearing loss and concomitant neurological involvement [147].
Evaluating the Effects of Cochlear Health on Cochlear Implant Function
Different types of studies help us to better understand how cochlear health influences CI function. Early studies in humans involved analysis of post-mortem temporal bone histology in order to better describe the condition of the human implanted ear [36–38, 148–150]. These early studies examined how structural conditions of the cochlea grossly relate to CI outcomes using word recognition scores but resulted in mixed findings. A recent meta-analysis collectively revealed a lack of correlation between SGN density and word recognition scores, using post-mortem temporal bone studies [151].
Animal studies, often using guinea pigs, rats, mice, or gerbils, can help to more precisely describe the relationship between cochlear health and its influence on electrical hearing using non-speech stimuli. These studies used various electrophysiological or psychophysical measures in implanted animals, and then compared those measures directly to histological findings. For comparison of histological results to functional measures, animal models have the advantage of better preservation of the temporal bones, and the ability to monitor the stability of the functional measures up to the time of euthanasia. Animal models utilize a variety of methods to achieve hearing loss similar to that found in human CI users. These include implantation in a hearing ear, deafening the ear with aminoglycoside antibiotics, and deafening followed by neurotrophin treatment to reduce the degeneration of SGNs [40, 43, 101]. Using multiple procedures, one can achieve a large range of SGN densities across a population of animals, and there is also considerable across-subject variation in SGN density for any given procedure (e.g., Fig. 4e). There is also considerable variation in other features of cochlear health including survival of IHCs and peripheral processes.
However, animal models fail to help us understand how the condition of the cochlea affects speech recognition outcomes. Fortunately, many of the electrophysiological or psychophysical measures that are made in cochlear implanted animals can also be assessed in human CI users. Therefore, we can estimate the health of an implanted human ear using the measures that have known relationships to cochlear health in animals and then directly compare those results to speech recognition outcomes or perception of complex speech-like sounds. We can also use those measures to help us understand how to improve biological conditions in the cochlea or alter programming to improve CI performance. Here, we focus on recent studies that have used electrophysiological and/or psychophysical measures in cochlear-implanted animals, and how they relate to cochlear health; we call those measures “functional measures.” Then, we review how we can apply those same functional measures to help us better understand how cochlear health influences perception of complex speech signals in cochlear-implanted humans.
Changes in Functional Measures of Cochlear Health Over Time
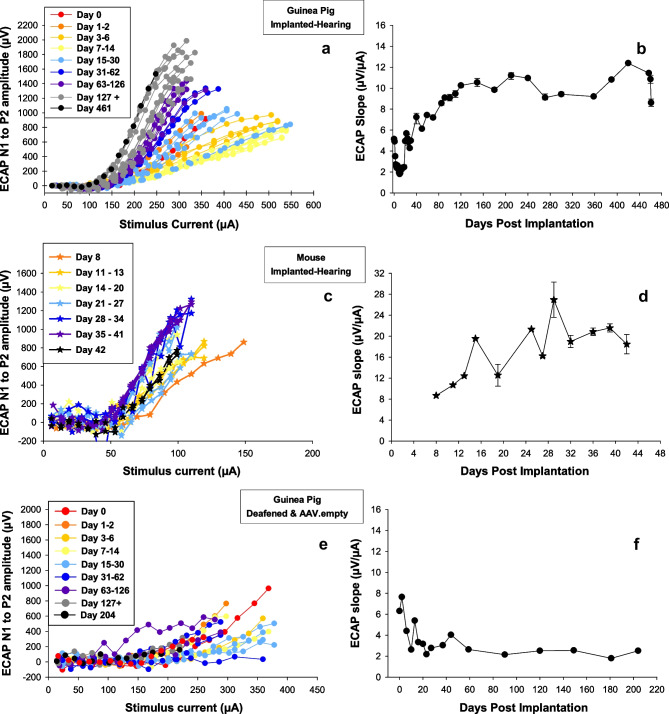
We have observed large fluctuations in functional measures (psychophysical detection thresholds and electrophysiological measures) over time, particularly in the months after implantation. Often, the sensitivity to electrical stimulation declines over days after implantation and then slowly improves over the course of several weeks. Psychophysical detection thresholds for electrical stimulation typically increase during the first week or two after implantation and gradually return to low levels and then remain stable. Slopes of electrically evoked compound action potential (ECAP) amplitude growth functions (AGFs) decline over the first weeks after implantation and then “recover” becoming steeper and then stabilizing (Fig. 5a, b). These changes in response to electrical stimulation over time after implantation have been seen in non-human primates [152, 153], guinea pigs [41, 154], and mice [155]. Examples of changes in objective electrophysiological responses are shown in Fig. 5. Once the functional measures have recovered from the initial effects of implantation and other treatments, they typically remain stable for long periods of time. It is important to demonstrate that they are stable up to the time of euthanasia and histological analysis if the goal is to relate structure to function.
Fig. 5.
Changes over time after treatment and implantation in electrically evoked compound action potential (ECAP) amplitude-growth functions (AGFs). Left column shows ECAP AGFs (input–output functions) for individual days post implantation (see inset), and the right column shows the slopes of the steeper part of the AGFs (µV/µA) as a function of post-implantation time. In long-term implanted animals, the magnitudes of the AGF slopes are typically positively correlated with SGN survival. a, b From a guinea pig implanted in a hearing ear. ECAP AGF slopes decrease over time after implantation and then recover to steeper levels and then stabilized. At the time of euthanasia, this animal had good nerve survival. Figure c, d show similar results obtained from an implanted mouse except that the electrophysiological recordings were not begun until 8 days after surgery and proceeded to only 42 days after implantation. This animal also had good nerve survival at the time of euthanasia. e, f From a guinea pig that was deafened and treated with an empty adeno-associated virus at the time of implantation. AGF slopes decreased over time after implantation but showed no recovery. This animal showed poor nerve survival at the time of euthanasia. Parts of this figure have been published previously and are reproduced here with permission: c from Colesa et al. [155] and e, f from Pfingst et al. [154]
Functional Measures of Cochlear Health in Animals and Humans
Electrically Evoked Compound Action Potentials (ECAPs)
Early studies showed that SGN density was positively and significantly correlated with the slope of the electrically evoked auditory brainstem response (EABR) AGF as well as its peak-amplitude [156, 157]. The EABR wave I is analogous to the ECAP, a measure frequently used in the clinic with CI patients both intra- and post-operatively. Subsequent studies have shown that attributes of the ECAP response are correlated with SGN densities [40–43, 158, 159]. The results in Fig. 6 [42] show the relationship between SGN density and ECAP measures in a group of 34 cochlear-implanted guinea pigs that underwent one of three treatments. Nine of the animals received an implant in a normal-hearing ear (highest SGNs). Two of the animals were deafened using neomycin delivered into the perilymph. Neomycin typically causes hair cell loss and an almost complete loss of SGNs and produces other morphological and physiological changes in the cochlea [160]. Twenty-three of the animals were deafened with neomycin, and then received neurotrophin treatments (AAV.BDNF or AAV.Ntf3) that helped to protect the SGNs from ototoxic effects of the neomycin [43, 161, 162]. The results shown in Fig. 6a–c (left column) are similar to results reported by others [40, 43, 156, 157] and demonstrate that the ECAP AGF slope, peak amplitude, and latency of the N1 response are positively and significantly correlated with residual SGN density across the animals. Related work in acutely implanted guinea pigs has shown that a loss of SGN fibers can affect temporal recovery time constants assessed using ECAP masker-probe paradigm and by examining the alternating pattern of ECAP amplitude in response to each pulse [159].
Fig. 6.
Linear regression analyses showing the relationships between SGN density and ECAP measures. The left column (panels a, b, and c) shows data for ECAP responses to biphasic pulsatile stimuli with a 2.1 µs IPG and the right column (panels d, e, and f) shows data for the IPG Effect (data for a 30 µs IPG minus data for a 2.1 µs IPG). The three rows represent three different measures derived from the ECAP amplitude growth function (AGF): top row: linear slope; middle row: ECAP AGF peak amplitude; bottom row: ECAP AGF N1 latency. Colors (red and blue) indicate if hair cells were present or absent, respectively. Solid regression lines are for analyses using data from all animals, regardless of IHC status. Regression statistics are shown in each figure. Taken, with permission, from Schvartz-Leyzac et al. [42]
We and others have also examined how ECAP AGF amplitude, slope, and latency change as the interphase gap (IPG) is increased (ECAP “IPG Effect”). The underlying contributions to the IPG Effect are not yet well understood, but theoretically would relate to the ability of the stimulated cell to recover from depolarization which would likely be affected by overall neural health as reviewed in previous literature [40, 163]. Previous studies have shown that increasing the IPG results in a reduction in the thresholds of auditory nerve fibers [164], and typically causes an increase in loudness perception [165]. While not completely understood, the IPG Effect is thought to be dependent on membrane characteristics, and thus reflect temporal response properties of the auditory nerve [166].
Similar to ECAP measures using a fixed IPG, SGN density and cell size in cochlear-implanted guinea pigs account for a significant proportion of variance (about 40–60%) when measuring the IPG Effect for ECAP amplitude and slope (Fig. 6d, e), but not latency (Fig. 6f) [40, 42]. Similarly, it has been observed that the charge required to evoke an equal-amplitude ECAP when using a short or long IPG also correlates with SGN density in acutely implanted guinea pigs [158]. It is proposed that, while the IPG Effect might be affected by temporal response properties of the auditory nerve, it also reflects SGN density based on these animal studies. Given that the IPG Effect is a measure performed within the same channel/electrode, it could be advantageous to use, when compared to ECAPs measured with a fixed IPG, as it should be less influenced by non-neural conditions that vary across the electrode array (e.g., electrode impedance, fibrous growth, electrode position) [113]; more on this topic is discussed later in this paper.
Most of the work looking at how cochlear health influences ECAPs has focused on the single attribute of SGN density. However, some work has shown that health of the IHCs also influences characteristics of the ECAP response. Hu and colleagues [83] measured ECAP responses in normal-hearing guinea pigs, and then used furosemide to temporarily disable hair cell function and examined the change in ECAP responses. When hair cell function was disabled, ECAPs exhibited steeper peak amplitudes and slopes. Furthermore, temporal characteristics also changed as adaptation, magnitude of amplitude alteration (using successive pulses), and refractoriness increased. The authors concluded that hair cells helped to provide desynchronization of the auditory nerve response, which could be advantageous to electrical hearing. Further work is needed to better quantify how other cochlear health attributes, such as demyelination, affect CI function and to characterize demyelination using ECAP responses.
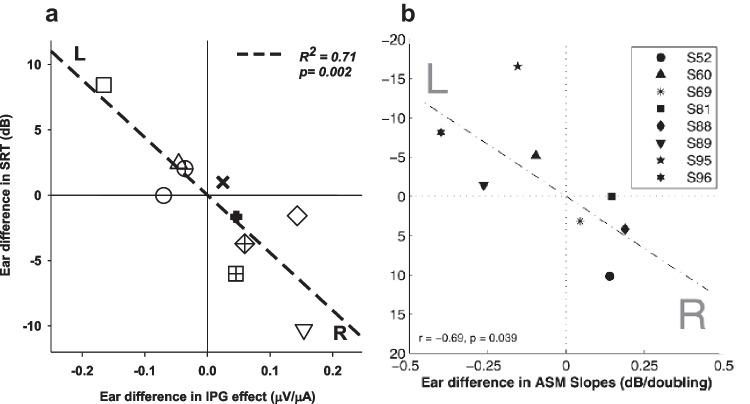
ECAPs can be easily measured in the clinical setting but have been typically limited to measuring ECAP thresholds. However, research shows that ECAP thresholds provide rather poor correlation with behavioral programming levels [167–169] do not necessarily reflect cochlear health in animal models [40], and are not related to speech understanding with a CI [170]. Supra-threshold measures, such as the AGF slope and peak amplitude or N1 latency, which have been shown to reflect cochlear health, are not typically used in the clinical setting. Collectively, there is mixed evidence when examining the relationship between supra-threshold ECAP measures and speech recognition performance in CI users [170]. Some studies show a relationship between ECAP peak-amplitude or slope using a fixed IPG and phoneme or sentence recognition performance [171–173], and other studies show no relationship between ECAP measures and the speech recognition outcomes [174]. We propose that these discrepancies are due, at least in part, to variables such as central auditory processing and cognition that contribute to speech recognition performance but vary among CI listeners [25, 26, 30, 175, 176]. These findings and factors emphasize the advantages of using within-subject designs [44, 177]. Figure 7a shows results from a study that examined the relationship between ECAP suprathreshold measures and speech recognition [45]. Within a bilaterally implanted listener, the ear with a higher average ECAP IPG Effect for AGF linear slope across the electrode array also had better speech recognition. These findings show that for each CI user, the ear estimated to have a higher density of SGNs was also the ear that could more accurately process complex speech signals, such as sentences in noise; this finding was statistically significant.
Fig. 7.
Results of experiments examining the relationship between measures of speech reception thresholds (SRTs) in human subjects and two measures of neural health (as inferred from animal studies). Both studies were conducted in human subjects with bilateral CIs. Scatterplot of ear differences (R-L for a and L-R for b) in the dB signal-to-noise ratio (SNR) at 50% correct for CUNY Sentences in Noise on the y axis (scales are reverse between the two graphs) are compared to measures estimating SGN density on the x axis. In a, the measure estimating SGN density is the IPG Effect for ECAP AGF linear slope. In b, the measure estimating SGN density is across-site mean (ASM) MPI slope. Each data point corresponds to one subject. The dashed lines show the fitted linear function of the regression analysis, and statistics (regression coefficient and p value) are also provided. Figure a shows data from Schvartz-Leyzac and Pfingst [45] and Figure b shows data from Zhou and Pfingst [44]. Figures were copied, with permission from the original publications
It has been observed that ECAPs can be useful to capture differences between populations that may be related to differences in cochlear health. For example, a recent study showed that pediatric patients with CND had smaller ECAP peak amplitudes and shallower AGF slopes when compared to children without CND [178]. Another study found that CI patients with Cx26-related deafness had larger ECAP amplitudes and steeper ECAP AGF slopes when compared to non-Cx26 (EVA) patients, suggesting better neural survival in patients with connexin-related deafness [179]. These findings could help to explain why patients with connexin-related deafness tend to perform quite well with CIs, particularly when implanted at an early age [180–183]. Jahn and Arenberg [184] reported steeper AGF slopes and amplitudes in adult patients who were implanted in childhood, compared to adult patients implanted in adulthood. The same study found a similar pattern for the IPG Effect for ECAP amplitude, but not for slope or threshold [184]; none of these ECAP measures were correlated with vowel recognition. These studies help to support animal data that shows that specific ECAP measures can be useful to estimate cochlear health in humans, and particularly when large disparities in cochlear health are expected between two groups. However, within subject designs are ideal when comparing ECAP measures to speech recognition performance with a CI.
Recent work from our laboratory examined the effects of medial–lateral electrode distance on ECAP measures in human CI users [113]. Specifically, we found that ECAP AGF slopes (a suprathreshold measure) increased with increasing distance between the electrode and mid-modiolar axis when ECAPs were measured using a fixed IPG. A more recent study also reported somewhat similar findings [185]; specifically, they reported that the ECAP thresholds, but not necessarily suprathreshold (e.g., AGF slope) ECAP measures were correlated with medial–lateral distance. The IPG Effect for ECAP thresholds and AGF slope were independent of these factors. It is possible that IPG Effect measures are better suited for human application, given that electrode location differs in humans both within and across ears [110, 111, 113, 186–189].
A novel and promising ECAP measure, called the “panoramic” ECAP (PECAP) was developed to take into account neural and non-neural factors [190]. The panoramic PECAP is examined by taking the ECAP amplitude for every probe and masker combination across the entire length of the electrode array, which theoretically provides information regarding not only the neural survival but electrical current field overlap/interaction; the latter of which would also be affected by distance between the electrode and the neural population. While this measure does show some promise when compared to other physiologic data measured in humans, it has yet to be validated using an animal model. Using computer modeling, Garcia et al. [191] showed that PECAP measures in humans could correctly identify simulated neural cochlear dead regions. This study also compared PECAP measures to a neural health estimate using low-rate detection thresholds as well as medial–lateral electrode distance as measured via post-operative imaging. Results across subjects are highly variable and do not show a clear relationship between the PECAP measure and neural health estimates nor medial–lateral distance of the electrode for most CI users evaluated.
Ensemble Spontaneous Activity (ESA)
Another useful electrophysiological measure of cochlear health is ensemble spontaneous activity (ESA). This activity can be recorded from an electrode on the round window, or from one of the CI electrodes, in the absence of any external stimulus. Studies using guinea pigs [192, 193] have indicated that the presence of a spectral peak near 900 Hz in these recordings represents spontaneous activity at the level of the auditory nerve. In cochlear-implanted guinea pigs, ESA can be recorded in cochleae with good IHC survival and high SGN density [39, 43, 66]. It is reasonable to assume that IHCs generate spontaneous activity in the auditory nerve and that functioning auditory nerve fibers must be present in sufficient quantities to generate measurable “ensemble” activity. Low levels of ESA have been observed in deaf guinea pigs suggesting very low levels of spontaneous activity in the auditory nerve in the absence of IHCs [43].
Electrocochleography
Electrocochleography (an evoked potential elicited using acoustic stimulation), and in particular, the cochlear microphonic (CM), can also be used to assess the status of sensory health. The CM reflects contributions of the OHCs and IHCs, and reflects basilar membrane displacement at least in a normal hearing, non-pathological ear [194, 195]. More recent work in cochlear-implanted, noise-exposed animals showed the presence of CM when tested intraoperatively [114, 196]. Noise-exposed animals demonstrated decreased hearing, abnormal OHC morphology, and the absence of latency delays in CM recordings, suggesting that residual OHCs were contributing to CM responses in noise-exposed animals [196]. Results from these studies and others [197] suggest that CM measures can be used in the clinical setting in order to monitor residual hearing and/or residual hair cell function either intra- or post-operatively. A recent paper by Tejani and colleagues examined CM and auditory nerve neurophonic (ANN) responses in seven patients with residual hearing immediately post-implantation, but who demonstrated a progressive hearing loss over several months post-implantation. The ANN is a sustained phase-locked neural response, also measured via electrocochleography. There is evidence among these patients that the CM remains present to some extent, even when residual hearing cannot be measured behaviorally via standard audiometry [74]. In the same study, patients with present CMs had no ANN responses. Hence, electrocochleography responses could be useful to help better understand individual contributions of sensory and neural components to CI function, particularly among patients with significant residual, acoustic hearing either before or after CI surgery.
In humans, intraoperative electrocochleography is predictive of post-operative speech recognition with a CI, likely reflecting preserved hair cell function and associated preserved neural health across the electrode array. Among pediatric patients, intraoperative electrocochleography measures accounted for 32% of the variance in post-operative word recognition performance [198]. Similar studies in adults show that intraoperative electrocochleography accounts for 40–47% of the variance in post-operative word recognition performance [199, 200]. Several studies have proposed that intraoperative cochleography can be used to monitor trauma during electrode insertion [118, 119, 201], and related studies have shown that the same measures predict post-operative audiometric thresholds (residual hearing) in pediatric and adult recipients [202, 203]. Lastly, intraoperative electrocochleography measures were predictive of electrode scalar location and translocation in 32 adults with CIs [117]; the study examined the amplitude and phase changes, and a model predicted the final scalar location. Results showed that the model successfully predicted final location of the electrode array in 82% of the ears tested as confirmed with post-operative imaging.
Psychophysical Measures
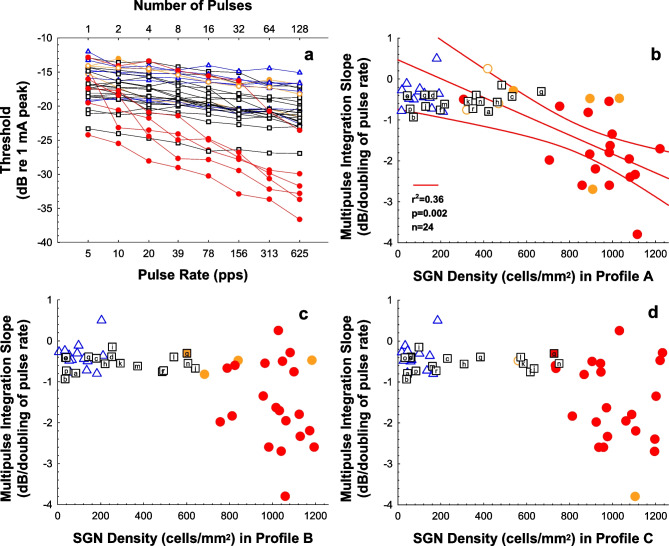
Psychophysical measures have also been shown to reflect cochlear health status in implanted animals. Multipulse integration (MPI) refers to a decrease (improvement) in the psychophysical detection threshold with increasing pulse rate for fixed-duration pulse trains in CI stimulation (Figs. 8 and 9a). MPI slopes (amount of threshold decrease per unit of pulse-rate increase) are typically calculated for pulse rates over a range from a few pulses per second (pps) up to about 1000 pps. The proposed mechanism underlying threshold change likely differs for pulse rates above 1000 pps [39, 204]. Studies in animal models have shown that the slope of the MPI function (rate of change in psychophysical detection threshold as a function of increase in pulse rate) differs between animals with lower and higher SGN densities [39, 43, 66, 101, 205, 206]. Similar to MPI findings, temporal integration (detection thresholds vs stimulus duration with a fixed pulse rate, (Fig. 8)) is also related to cochlear health [39].
Fig. 8.
Schematic of a biphasic, cathodic leading pulse train similar to that shown in Fig. 3. The first pulse in a–c shows the cathodic and anodic phase (along with the interphase gap) consistent with Fig. 3. The x axis is current level, and the y axis is a duration of time (unspecified). Temporal integration is described by comparing the difference in psychophysical threshold between a and b, in which case the pulse rate remains constant, but the stimulus duration is longer. In this example, the stimulation duration is twice as long in b compared to a. Multipulse integration (MPI) is described by comparing the difference in psychophysical thresholds between a and c, in which case the pulse rate increases for the same duration of time. In this case, the pulse rate is doubled for a fixed duration of time
Fig. 9.
Examples of multi-pulse integration (MPI) functions (decreases in psychophysical detection threshold as a function of pulse rate for fixed-duration pulse trains) and their relation to SGN density. a MPI functions for 200-ms pulse trains of biphasic pulses at 25 µs/phase and pulse rates ranging from 5 to 625 pps. Data were obtained from cochlear implanted guinea pigs with varying degrees of cochlear health that resulted from the following treatments: implanted in a hearing ear (red and orange circles); deafened with neomycin, inoculated with AAV, Ntf3; and implanted (black squares; letters indicate identifiers as detailed in Pfingst et al. [43]), and deafened with neomycin, no inoculation or inoculated with an AAV.empty, and implanted (blue triangles). b, c, d The slopes of MPI functions plotted as a function of SGN density in Rosenthal’s canal for cochlear locations in the area of the electrode used for the MPI measurements (profile A); a half turn apical to that electrode (profile B), and a full turn above that electrode (profile C). In all panels, filled symbols indicate the presence of IHC (red fill ≥ 80% survival, orange fill 1 to 79% survival), and open symbols indicate the absence of IHCs in that profile. b The red regression line and corresponding statistics show the significant correlation between MPI slope and SGN-A density for the animals implanted in a hearing ear; red curving lines are 95% confidence ranges. For profiles B and C, this correlation was not significant. Taken, with permission, from Pfingst et al. [43]
Studies in humans have shown that MPI slopes are predictive of speech recognition. Similar to results shown for the ECAP IPG Effect, Fig. 7b shows results obtained in bilateral CI users; within each subject, the ear with steeper across-site-mean (ASM) MPI slopes also had better speech reception thresholds (SRT) performance on speech recognition in noise. Note that the y-axis scales are opposite in panels a and b because the ear difference in panel a was calculated as right–left, whereas the ear difference in panel b was calculated as left–right. These findings show that, when looking for each CI user, the ear estimated to have a higher density of SGNs was also the ear that could more accurately process complex speech signals, such as sentences in noise. Related studies have shown that MPI in humans is not related to medial–lateral distance of the electrode array via post-operative imaging [113].
However, animal studies also show a potential influence of sensory health; specifically, the relationship between MPI slopes and SGN density is only significant when animals with healthy IHCs are included in the analysis (see Fig. 9). In the data shown in Fig. 9, only animals with SGN densities greater than 706 cells/mm2 demonstrated good MPI slopes (steeper than − 1 dB/doubling). For those animals, all had acoustic hearing before and after cochlear implantation, and it is unknown if the high counts of IHCs, SGNs, or both of these variables contributed to the results.
Data are needed in animals to determine how simple psychophysical detection thresholds for low and high pulse rates measured using other electrode configurations (e.g., bipolar or tripolar) relate to SGN density. Studies performed in CI humans have proposed that psychophysical detection thresholds measured using focused current (e.g., bipolar, tripolar) reflect the underlying condition of the electrical-neural interface, which includes cochlear health [207–211]. Lower psychophysical detection thresholds using focused current but higher pulse rates (~ 900 pps) have been shown to correlate with polarity sensitivity (i.e., polarity effect) in CI users [212] and can characterize differences in the electrical-neural interface between groups of listeners [213]. Jahn et al. [213] reported that cochlear-implanted children with EVA syndrome had higher detection thresholds using focused current when compared to a group of pediatric CI recipients with connexin-related deafness, suggesting better neural health in patients with EVA [213]. Studies using focused thresholds (e.g., bipolar and tripolar configurations) in humans are compelling and do support the hypothesis that focused thresholds reflect the condition of the electrical neural interface. Despite these studies, Bierer and Litvak [209] did not show that performance among CI listeners improved when programming was specifically altered based on psychophysical thresholds measured using focused stimuli [209]. To date, there lacks support from an animal model to clarify the relationship between focused thresholds and cochlear health.
Related to this work, others have hypothesized how psychophysical thresholds using low rate (< 100 pps) stimuli reflect the underlying condition of the auditory nerve in CI patients. Zhou and colleagues have shown promising work in humans that psychophysical detection thresholds using low pulse rates (e.g., 80 pps) also reflects the underlying condition of the auditory nerve [214, 215]. Zhou [215] reported improved speech recognition in nine CI users after using an experimental map in which electrodes were deactivated based on psychophysical thresholds using low-rate, monopolar thresholds [215]. However, similar to thresholds measured with focused current, there is a paucity of direct evidence from an animal model that specifically examines how cochlear health contributes to psychophysical threshold measures using low-rate stimuli. As mentioned previously, low-rate thresholds are also influenced by medial distance and potentially electrode configuration and might not reflect only neural conditions [112, 113]. Similar work shows that focused thresholds reflect medial–lateral distance [172]. Further work is needed in this area to maximize clinical application of these measures.
Lastly, strength duration functions (detection thresholds vs pulse phase duration) have the potential for diagnosing several aspects of the health of the implanted cochlea. A recent study [211] showed that psychophysical strength duration function slopes were significantly shallower in guinea pigs implanted in a hearing ear compared to those implanted in a deafened ear that was treated with neurotrophin. In animals deafened with neomycin and treated with neurotrophin, which typically had no surviving inner hair cells (IHCs), the slopes of the psychophysical strength-duration functions were correlated with spiral ganglion neuron (SGN) density, being steeper in cases with higher SGN densities. However, in animals implanted in a hearing ear, which typically had surviving IHCs, slopes of the strength-duration functions were not correlated with SGN density. These data suggest opposing effects of SGN density and IHC presence on the slopes of psychophysical strength-duration functions, but further experiments are needed to better understand these relationships.
Polarity Effect
Based on modeling and animal studies [58, 59, 216, 217], some researchers have proposed that differential sensitivity to cathodic or anodic pulses (polarity sensitivity or polarity effect; Fig. 3) is also an indicator of cochlear health and can be used to improve CI performance. This concept would apply to both psychophysical and electrophysical measures. These modeling studies hypothesize that differential sensitivity to either anodic or cathodic stimuli reflects the underlying condition of the auditory nerve. In studies using acutely deafened animals, where the neural anatomy might still be relatively intact, there is evidence that the cathodic rather than the anodic phase of the biphasic pulse elicits greater excitability [216, 218]. In a healthier neuron where the peripheral process is present, then the cathodic phase preferentially excites the peripheral process. Conversely, the anodic phase is thought to preferentially excite the cell body; therefore, in cases of neural degeneration, the anodic phase might be the primary excitatory phase. Additional work in electrically stimulated cats and guinea pigs supports the idea that cathodic- or anodic-leading phase stimuli excite different points along the auditory nerve [217].
In humans, results are often opposite of those reported in animals, and show preferential excitation to anodic-leading biphasic pulses using electrophysiological and psychophysical studies [219–223], but polarity sensitivity for threshold measures can vary within an ear [224, 225]. As described in previous studies [163, 219], there are several potential reasons for this difference including position of the electrode relative to the stimulated neural population, electrode geometry, overall health of the neurons (which could be influenced by deafening method/etiology), and stimulus level. Jahn and Arenberg found that polarity sensitivity to psychophysical thresholds was not related to electrode location [212]. Goehring et al. [226] examined the efficacy of selecting electrode sites for activation based on polarity sensitivity to psychophysical thresholds, but results were mixed and did not show a clear advantage of this method when subjects were measured on speech recognition tasks or a spectro-temporal ripple task [226].
Additional Commentary on Functional Health Measures
Various electrophysiological and psychophysical measures of cochlear health described above do not necessarily reflect the same underlying variables. The fact that two measures correlate with SGN density does not necessarily mean that SGN density is the underlying causal variable [227, 228]. SGN density typically accounts for only 50% of the across-subject variability in any given measure. Other features of cochlear health including neuronal cell size, fibrosis, and osteoneogenesis are not as strongly correlated with these measures but may not account for additional variance once the effects of SGN density have been taken into account [42, 101]. Also, it should be noted that the relationship between SGN density and various functional measures differs across measures. For example, the correlation of MPI with SGN density depends on inclusion of cases with very high SGN densities and surviving IHCs while the correlation of ECAP AGF slopes with SGN density holds across a broad range of SGN densities [43]. Presence of IHCs seems to facilitate MPI slopes but may reduce slopes of ECAP AGFs, as noted previously [83].
Each of the measures above could be influenced by tissue and fluids surrounding the electrode array, including fibrous tissue or bone. These factors are difficult to parse because they are often related to one another. For example, insertion trauma has been shown to elicit an immune response [115, 229, 230], which could both increase intrascalar tissue and decrease SGN density. Kamakura and Nadol [231] examined post-mortem temporal bones in cochlear-implanted ears and found that post-operative CNC word scores were negatively correlated with percent volume of new bone within the cochlea, but not with percent of fibrous tissue. The percent of new bone was correlated with intrascalar trauma, and particularly trauma to the basilar membrane [231]. Collectively, these studies show that application of cochlear health measures in humans is quite complex, and therefore efforts to use these measures to improve outcomes often yield mixed results [209, 214, 215, 226, 232].
Biological Approaches for Preserving or Improving Cochlear Health
There are several cochlear domains that should be considered as targets for preserving or enhancing cochlear health in a way that could potentially benefit CI outcomes. The most obvious targets are the sensory epithelium (hair cells and supporting cells) and the SGNs [37]. The sensory epithelium is a target because of clear evidence for a positive influence of surviving HCs on outcomes in both humans [70] and animals [66] after cochlear implantation. The synapses in the auditory periphery also need to be considered as targets for therapy since changes in synapses associated with CI stimulations have been observed [233]. The stria vascularis and other lateral wall structures could also be additional therapeutic targets to address decline in performance and/or loss of residual hearing after cochlear implantation [93, 94]. The relationship between the number of surviving SGNs and CI outcomes is complex but by examining both independent studies and literature reviews, there is evidence using well controlled paradigms that the health of SGNs positively influences speech understanding with a CI [44, 45, 169, 170, 234]. At least some neurons need to survive for the CI to function, and the physiological health of these neurons should be preserved, making them an important target for therapy. The therapies mentioned below have the potential to enhance CI outcomes addressing the conditions of one or more of the targets mentioned above.
One approach is the use of dexamethasone, which, among the options listed below, is the only treatment currently used in the clinical setting. Dexamethasone is administered by many CI surgeons routinely intra- and post-operatively, and has been shown to have positive effects by enhancing hearing preservation, reducing inflammation, and preserving neurons [235, 236]. Another approach for enhancing survival and health of CI relevant cochlear structures is via gene therapy, which has the potential for inducing long-term over-expression of therapeutic genes. In vivo gene therapy is based on local (cochlear) delivery of gene vectors (usually viruses), which results in forced expression of the transgenes that either act entirely within the target cells or cause those cells to produce a diffusible product that can affect the entire cochlea. Gene vectors vary in their extent of toxicity and the duration of gene expression. Currently, the most commonly used gene therapy vectors are adeno-associated viruses (AAVs), due to their low toxicity and long-term gene expression. Several reviews summarize the use of gene therapy in the cochlea in general [237–240] and in relation to CIs [162, 241].
An alternative for inserting genes via a gene vector is infusing the therapeutic reagents directly into the cochlea. This can be done during the CI insertion surgery, either by direct infusion, or by use of special implants that, in addition to delivering electrical stimulation, can also serve as eluting vehicles. Such implants have been tested for delivering dexamethasone [242–244] as well as other molecules such as IGF-1, which has been shown to increase neuronal survival and CI outcomes [245]. A recent review summarizes developments of drug delivery via the CI [246]. Another goal of future treatments is to prevent or reduce the fibrosis that is often found to surround the electrode array. This can likely be accomplished by reducing the immune response to the CI insertion and reducing the surgical trauma [115]. Once the signaling molecules that cause the fibrosis are identified, it should be possible to use gene therapy or eluting electrodes to reduce this negative outcome of the CI surgery.
Stem cell therapies are being developed for several inner ear applications, including the replacement of lost SGNs. Proof of the principle has been shown in the gerbil model, in which elimination of the neurons while preserving IHCs was feasible [247, 248]. Oubain was used to eliminate most SGNs, and then stem cells were injected into the modiolus. The authors demonstrated differentiation of the stem cells into neuronal phenotypes, establishment of connection both centrally (cochlear nucleus) and peripherally (IHC), and improvement in ABR thresholds. Similarly, restoration of the population of hair cells, even in part, may assist in preservation and functionality of the auditory nerve and/or provide some acoustic hearing. The field of hair cell regeneration in the cochlea is not ready for clinical use but progress is being made, with clear evidence for the possibility to generate new hair cells [249–252].
Summary and Conclusions
CI technology has seen tremendous advancements over the past 40 years, and hundreds of thousands of individuals worldwide have benefited from cochlear implantation. Combined behavioral, electrophysiological, and histological studies in animal models coupled with broadened candidacy criteria in humans have shed light on the importance of the health of the cochlea for the success of cochlear implantation; a relationship which was once thought relatively unimportant. Advanced animal models in combination with the development of biological approaches will yield more sophisticated methods to understand how cochlear health can guide stimulation parameters, and ultimately how we can optimize outcomes in CI users. These methods can also be used to understand and improve CI outcomes for specific populations; for example, by using mouse models of genetic hearing loss etiologies that are also common in humans. Biologic approaches can be used in combination with cochlear implantation in an effort to preserve cochlear health and to limit intracochlear trauma associated with surgical insertion of the implant. Further work is needed to continue to fully realize the promise as well as the limitations of these approaches, and to further understand how we can exploit features of cochlear health to maximize performance for each CI recipient.
Acknowledgements
We thank our valued colleagues and collaborators Lisa Beyer, Christopher Buswinka, Jenna Devare, and Teresa Zwolan for their invaluable contributions to this work. We are grateful to our research participants who have generously provided their time and interest in our studies.
Funding
Much of the work reviewed in this paper was funded by the National Institute on Deafness and Other Communication Disorders, with additional support from Cochlear LTD and MED-EL.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NIDCD (2019) NIDCD fact sheet, hearing and balance: cochlear implants. Available from: https://www.nidcd.nih.gov/health/cochlear-implants. Accessed 24 Jun 2020
- 2.Gubbels SP, Gartrell BC, Ploch JL, Hanson KD. Can routine office-based audiometry predict cochlear implant evaluation results? Laryngoscope. 2017;127:216–222. doi: 10.1002/lary.26066. [DOI] [PubMed] [Google Scholar]
- 3.Zwolan TA, Kallogjeri D, Firszt JB, Buchman CA. Assessment of cochlear implants for adult medicare beneficiaries aged 65 years or older who meet expanded indications of open-set sentence recognition: a multicenter nonrandomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2020;146:1–9. doi: 10.1001/jamaoto.2020.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwolan TA, Schvartz-Leyzac KC, Pleasant T. Development of a 60/60 guideline for referring adults for a traditional cochlear implant candidacy evaluation. Otol Neurotol. 2020;41:895–900. doi: 10.1097/mao.0000000000002664. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajan VV, Sydlowski SA, Li MM, Anne S, Adunka OF. Evolving criteria for adult and pediatric cochlear implantation. Ear Nose Throat J. 2021;100:31–37. doi: 10.1177/0145561320947258. [DOI] [PubMed] [Google Scholar]
- 6.Niparko JK, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden LK, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34:342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnard JM, et al. A prospective longitudinal study of U.S. children unable to achieve open-set speech recognition 5 years after cochlear implantation. Otol Neurotol. 2015;36:985–992. doi: 10.1097/mao.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geers AE, Mitchell CM, Warner-Czyz A, Wang NY, Eisenberg LS. Early sign language exposure and cochlear implantation benefits. Pediatrics. 2017 doi: 10.1542/peds.2016-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas ES, Zwolan TA. Communication mode and speech and language outcomes of young cochlear implant recipients: a comparison of auditory-verbal, oral communication, and total communication. Otol Neurotol. 2019;40:e975–e983. doi: 10.1097/mao.0000000000002405. [DOI] [PubMed] [Google Scholar]
- 11.Eshraghi AA, et al. The cochlear implant: historical aspects and future prospects. Anat Rec (Hoboken) 2012;295:1967–1980. doi: 10.1002/ar.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkany T, Hodges AV, Luntz M. Update on cochlear implantation. Otolaryngol Clin North Am. 1996;29:277–289. doi: 10.1016/S0030-6665(20)30391-1. [DOI] [PubMed] [Google Scholar]
- 13.Firszt JB, Holden LK, Reeder RM, Cowdrey L, King S. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 2012;33:521–533. doi: 10.1097/AUD.0b013e31824b9dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firszt JB, Reeder RM, Holden LK, Dwyer NY. Results in adult cochlear implant recipients with varied asymmetric hearing: a prospective longitudinal study of speech recognition, localization, and participant report. Ear Hear. 2018;39:845–862. doi: 10.1097/aud.0000000000000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson ML, et al. Evidence for the expansion of pediatric cochlear implant candidacy. Otol Neurotol. 2015;36:43–50. doi: 10.1097/mao.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 16.Holder JT, Reynolds SM, Sunderhaus LW, Gifford RH. Current profile of adults presenting for preoperative cochlear implant evaluation. Trends Hear. 2018;22:2331216518755288. doi: 10.1177/2331216518755288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown CJ, et al. Cortical auditory evoked potentials recorded from nucleus hybrid cochlear implant users. Ear Hear. 2015;36:723–732. doi: 10.1097/aud.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han JH, Dimitrijevic A. Acoustic change responses to amplitude modulation in cochlear implant users: relationships to speech perception. Front Neurosci. 2020;14:124. doi: 10.3389/fnins.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JH, Lee J, Lee HJ. Noise-induced change of cortical temporal processing in cochlear implant users. Clin Exp Otorhinolaryngol. 2020 doi: 10.21053/ceo.2019.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby AE, Middlebrooks JC. Unanesthetized auditory cortex exhibits multiple codes for gaps in cochlear implant pulse trains. J Assoc Res Otolaryngol. 2012;13:67–80. doi: 10.1007/s10162-011-0293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middlebrooks JC. Auditory cortex phase locking to amplitude-modulated cochlear implant pulse trains. J Neurophysiol. 2008;100:76–91. doi: 10.1152/jn.01109.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middlebrooks JC. Chronic deafness degrades temporal acuity in the electrically stimulated auditory pathway. J Assoc Res Otolaryngol. 2018;19:541–557. doi: 10.1007/s10162-018-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middlebrooks JC. Cochlear-implant high pulse rate and narrow electrode configuration impair transmission of temporal information to the auditory cortex. J Neurophysiol. 2008;100:92–107. doi: 10.1152/jn.01114.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olds C, et al. Cortical activation patterns correlate with speech understanding after cochlear implantation. Ear Hear. 2016;37:e160–e172. doi: 10.1097/aud.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheperle RA, Abbas PJ. Peripheral and central contributions to cortical responses in cochlear implant users. Ear Hear. 2015;36:430–440. doi: 10.1097/aud.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheperle RA, Abbas PJ. Relationships among peripheral and central electrophysiological measures of spatial and spectral selectivity and speech perception in cochlear implant users. Ear Hear. 2015;36:441–453. doi: 10.1097/aud.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Z, Stakhovskaya O, Goupell MJ, Anderson S. Aging effects on cortical responses to tones and speech in adult cochlear-implant users. J Assoc Res Otolaryngol. 2021 doi: 10.1007/s10162-021-00804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claes AJ, et al. Impaired cognitive functioning in cochlear implant recipients over the age of 55 years: a cross-sectional study using the repeatable battery for the assessment of neuropsychological status for hearing-impaired individuals (RBANS-H) Front Neurosci. 2018;12:580. doi: 10.3389/fnins.2018.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claes AJ, et al. The repeatable battery for the assessment of neuropsychological status for hearing impaired individuals (RBANS-H) before and after cochlear implantation: a protocol for a prospective, longitudinal cohort study. Front Neurosci. 2016;10:512. doi: 10.3389/fnins.2016.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosetti MK, et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging. 2016;11:603–613. doi: 10.2147/cia.s100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moberly AC, Reed J. Making sense of sentences: top-down processing of speech by adult cochlear implant users. J Speech Lang Hear Res. 2019;62:2895–2905. doi: 10.1044/2019_jslhr-h-18-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moberly AC, Houston DM, Castellanos I. Non-auditory neurocognitive skills contribute to speech recognition in adults with cochlear implants. Laryngoscope Investig Otolaryngol. 2016;1:154–162. doi: 10.1002/lio2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrestha BR, et al. Sensory neuron diversity in the inner ear is shaped by activity. Cell. 2018;174:1229–1246.e17. doi: 10.1016/j.cell.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 35.Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann N Y Acad Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- 36.Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 37.Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/S0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- 38.Nadol JB, Jr, Eddington DK. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol. 2006;64:31–49. doi: 10.1159/000094643. [DOI] [PubMed] [Google Scholar]
- 39.Pfingst BE, et al. Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am. 2011;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramekers D, et al. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 2014;15:187–202. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schvartz-Leyzac KC, et al. Changes over time in the electrically evoked compound action potential (ECAP) interphase gap (IPG) effect following cochlear implantation in Guinea pigs. Hear Res. 2019 doi: 10.1016/j.heares.2019.107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schvartz-Leyzac KC et al (2020) How electrically evoked compound action potentials in chronically implanted guinea pigs relate to auditory nerve health and electrode impedance. J Acoust Soc Am 148 [DOI] [PMC free article] [PubMed]
- 43.Pfingst BE, et al. Neurotrophin gene therapy in deafened ears with cochlear implants: long-term effects on nerve survival and functional measures. J Assoc Res Otolaryngol. 2017;18:731–750. doi: 10.1007/s10162-017-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou N, Pfingst BE. Relationship between multipulse integration and speech recognition with cochlear implants. J Acoust Soc Am. 2014;136:1257. doi: 10.1121/1.4890640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schvartz-Leyzac KC, Pfingst BE. Assessing the relationship between the electrically evoked compound action potential and speech recognition abilities in bilateral cochlear implant recipients. Ear Hear. 2018;39:344–358. doi: 10.1097/AUD.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- 47.Tong L, et al. Selective deletion of cochlear hair cells causes rapid age-dependent changes in spiral ganglion and cochlear nucleus neurons. J Neurosci. 2015;35:7878–7891. doi: 10.1523/jneurosci.2179-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurioka T, et al. Selective hair cell ablation and noise exposure lead to different patterns of changes in the cochlea and the cochlear nucleus. Neuroscience. 2016;332:242–257. doi: 10.1016/j.neuroscience.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goycoolea MV, Stypulkowski P, Muchow DC. Ultrastructural studies of the peripheral extensions (dendrites) of type I ganglion cells in the cat. Laryngoscope. 1990;100:19–24. doi: 10.1288/00005537-199002001-00002. [DOI] [PubMed] [Google Scholar]
- 50.Ramekers D, Klis SFL, Versnel H. Simultaneous rather than retrograde spiral ganglion cell degeneration following ototoxically induced hair cell loss in the guinea pig cochlea. Hear Res. 2020;390:107928. doi: 10.1016/j.heares.2020.107928. [DOI] [PubMed] [Google Scholar]
- 51.Felder E, Kanonier G, Scholtz A, Rask-Andersen H, Schrott-Fischer A. Quantitative evaluation of cochlear neurons and computer-aided three-dimensional reconstruction of spiral ganglion cells in humans with a peripheral loss of nerve fibres. Hear Res. 1997;105:183–190. doi: 10.1016/s0378-5955(96)00209-2. [DOI] [PubMed] [Google Scholar]
- 52.van den Honert C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerve. II Single fiber recordings Hear Res. 1984;14:225–243. doi: 10.1016/0378-5955(84)90052-2. [DOI] [PubMed] [Google Scholar]
- 53.Heshmat A, et al. Dendritic degeneration of human auditory nerve fibers and its impact on the spiking pattern under regular conditions and during cochlear implant stimulation. Front Neurosci. 2020;14:599868. doi: 10.3389/fnins.2020.599868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroon S, et al. Degeneration of auditory nerve fibers in guinea pigs with severe sensorineural hearing loss. Hear Res. 2017;345:79–87. doi: 10.1016/j.heares.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Resnick JM, Rubinstein JT. Simulated auditory fiber myelination heterogeneity desynchronizes population responses to electrical stimulation limiting inter-aural timing difference representation. J Acoust Soc Am. 2021;149:934. doi: 10.1121/10.0003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise AK, Pujol R, Landry TG, Fallon JB, Shepherd RK. Structural and ultrastructural changes to type i spiral ganglion neurons and schwann cells in the deafened guinea pig cochlea. J Assoc Res Otolaryngol. 2017;18:751–769. doi: 10.1007/s10162-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vink HA, Versnel H, Kroon S, Klis SFL, Ramekers D. BDNF-mediated preservation of spiral ganglion cell peripheral processes and axons in comparison to that of their cell bodies. Hear Res. 2021;400:108114. doi: 10.1016/j.heares.2020.108114. [DOI] [PubMed] [Google Scholar]
- 58.Rattay F, Lutter P, Felix H. A model of the electrically excited human cochlear neuron. I. Contribution of neural substructures to the generation and propagation of spikes. Hear Res. 2001;153:43–63. doi: 10.1016/S0378-5955(00)00256-2. [DOI] [PubMed] [Google Scholar]
- 59.Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 1999;89:335–346. doi: 10.1016/S0306-4522(98)00330-3. [DOI] [PubMed] [Google Scholar]
- 60.Santos F, Nadol JB. Temporal bone histopathology of furosemide ototoxicity. Laryngoscope Investig Otolaryngol. 2017;2:204–207. doi: 10.1002/lio2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quesnel AM, et al. Delayed loss of hearing after hearing preservation cochlear implantation: human temporal bone pathology and implications for etiology. Hear Res. 2016;333:225–234. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu PZ, et al. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience. 2019;407:8–20. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irving S, et al. Electroacoustic stimulation: now and into the future. Biomed Res Int. 2014;2014:350504. doi: 10.1155/2014/350504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auinger AB, et al. Masking release with changing fundamental frequency: electric acoustic stimulation resembles normal hearing subjects. Hear Res. 2017;350:226–234. doi: 10.1016/j.heares.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Tejani VD, Brown CJ. Speech masking release in hybrid cochlear implant users: roles of spectral and temporal cues in electric-acoustic hearing. J Acoust Soc Am. 2020;147:3667. doi: 10.1121/10.0001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang SY, et al. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanetti D, Nassif N, De Zinis LR. Factors affecting residual hearing preservation in cochlear implantation. Acta Otorhinolaryngol Ital. 2015;35:433–441. doi: 10.14639/0392-100x-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlson ML, et al. Hearing preservation in pediatric cochlear implantation. Otol Neurotol. 2017;38:e128–e133. doi: 10.1097/mao.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 69.Thompson NJ, et al. Electric-acoustic stimulation after reimplantation: hearing preservation and speech perception. Otol Neurotol. 2019;40:e94–e98. doi: 10.1097/mao.0000000000002094. [DOI] [PubMed] [Google Scholar]
- 70.Kamakura T, O'Malley JT, Nadol JB., Jr Preservation of cells of the organ of Corti and innervating dendritic processes following cochlear implantation in the human: an immunohistochemical study. Otol Neurotol. 2018;39:284–293. doi: 10.1097/mao.0000000000001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbas PJ, Tejani VD, Scheperle RA, Brown CJ. Using neural response telemetry to monitor physiological responses to acoustic stimulation in hybrid cochlear implant users. Ear Hear. 2017;38:409–425. doi: 10.1097/aud.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choudhury B, et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33:1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeMason C, et al. Electrophysiological properties of cochlear implantation in the gerbil using a flexible array. Ear Hear. 2012;33:534–542. doi: 10.1097/AUD.0b013e3182498c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tejani VD, et al. Residual hair cell responses in electric-acoustic stimulation cochlear implant users with complete loss of acoustic hearing after implantation. J Assoc Res Otolaryngol. 2021;22:161–176. doi: 10.1007/s10162-021-00785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato M, Baumhoff P, Kral A. Cochlear implant stimulation of a hearing ear generates separate electrophonic and electroneural responses. J Neurosci. 2016;36:54–64. doi: 10.1523/jneurosci.2968-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imsiecke M, Krüger B, Büchner A, Lenarz T, Nogueira W. Electric-acoustic forward masking in cochlear implant users with ipsilateral residual hearing. Hear Res. 2018;364:25–37. doi: 10.1016/j.heares.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Kipping D, Krüger B, Nogueira W. The role of electroneural versus electrophonic stimulation on psychoacoustic electric-acoustic masking in cochlear implant users with residual hearing. Hear Res. 2020;395:108036. doi: 10.1016/j.heares.2020.108036. [DOI] [PubMed] [Google Scholar]
- 78.McAnally KI, Clark GM, Syka J. Hair cell mediated responses of the auditory nerve to sinusoidal electrical stimulation of the cochlea in the cat. Hear Res. 1993;67:55–68. doi: 10.1016/0378-5955(93)90232-p. [DOI] [PubMed] [Google Scholar]
- 79.Nuttall AL, Ren T. Electromotile hearing: evidence from basilar membrane motion and otoacoustic emissions. Hear Res. 1995;92:170–177. doi: 10.1016/0378-5955(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 80.Le Prell CG, Kawamoto K, Raphael Y, Dolan DF. Electromotile hearing: acoustic tones mask psychophysical response to high-frequency electrical stimulation of intact guinea pig cochleae. J Acoust Soc Am. 2006;120:3889–3900. doi: 10.1121/1.2359238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. Am J Otol. 1997;18:S30–S34. [PubMed] [Google Scholar]
- 82.Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 83.Hu N, et al. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear Res. 2003;185:77–89. doi: 10.1016/S0378-5955(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 84.Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rask-Andersen H, Liu W, Linthicum F. Ganglion cell and ‘dendrite’ populations in electric acoustic stimulation ears. Adv Otorhinolaryngol. 2010;67:14–27. doi: 10.1159/000262593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golub JS, et al. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci. 2012;32:15093–15105. doi: 10.1523/jneurosci.1709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dupont J, Guilhaume A, Aran JM. Neuronal degeneration of primary cochlear and vestibular innervations after local injection of sisomicin in the guinea pig. Hear Res. 1993;68:217–228. doi: 10.1016/0378-5955(93)90125-k. [DOI] [PubMed] [Google Scholar]
- 88.McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 89.Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 1981;91:529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- 90.Quraishi IH, Raphael RM. Generation of the endocochlear potential: a biophysical model. Biophys J. 2008;94:L64–L66. doi: 10.1529/biophysj.107.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patuzzi R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res. 2011;277:4–19. doi: 10.1016/j.heares.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review) Hear Res. 2016;338:52–63. doi: 10.1016/j.heares.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanaka C, Nguyen-Huynh A, Loera K, Stark G, Reiss L. Factors associated with hearing loss in a normal-hearing guinea pig model of hybrid cochlear implants. Hear Res. 2014;316:82–93. doi: 10.1016/j.heares.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiss LA, et al. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired Guinea pig model. Hear Res. 2015;327:163–174. doi: 10.1016/j.heares.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li PM, Somdas MA, Eddington DK, Nadol JB., Jr Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116:731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- 96.Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35:1545–1551. doi: 10.1097/mao.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishai R, Herrmann BS, Nadol JB, Jr, Quesnel AM. The pattern and degree of capsular fibrous sheaths surrounding cochlear electrode arrays. Hear Res. 2017;348:44–53. doi: 10.1016/j.heares.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahman MT, et al. Cochlear implants: causes, effects and mitigation strategies for the foreign body response and inflammation. Hear Res. 2022;422:108536. doi: 10.1016/j.heares.2022.108536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trakimas DR, Kozin ED, Ghanad I, Nadol JB, Jr, Remenschneider AK. Human otopathologic findings in cases of folded cochlear implant electrodes. Otol Neurotol. 2018;39:970–978. doi: 10.1097/mao.0000000000001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shepherd RK, Clark GM, Black RC, Patrick JF. The histopathological effects of chronic electrical stimulation of the cat cochlea. J Laryngol Otol. 1983;97:333–341. doi: 10.1017/s0022215100094202. [DOI] [PubMed] [Google Scholar]
- 101.Swiderski DL, Colesa DJ, Hughes AP, Raphael Y, Pfingst BE. Relationships between intrascalar tissue, neuron survival, and cochlear implant function. J Assoc Res Otolaryngol. 2020;21:337–352. doi: 10.1007/s10162-020-00761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryu KA, et al. Intracochlear bleeding enhances cochlear fibrosis and ossification: an animal study. PLoS ONE. 2015;10:e0136617. doi: 10.1371/journal.pone.0136617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark GM et al (1987) Biological safety, in The University of Melbourne-Nucleus multi-electrode cochlear implant. Adv Otorhinolaryngol. Karger: Basel 22–62 [PubMed]
- 104.O'Leary SJ, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 105.Yamahara K, et al. Hearing preservation at low frequencies by insulin-like growth factor 1 in a guinea pig model of cochlear implantation. Hear Res. 2018;368:92–108. doi: 10.1016/j.heares.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 106.Green JD, Jr, Marion MS, Hinojosa R. Labyrinthitis ossificans: histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg. 1991;104:320–326. doi: 10.1177/019459989110400306. [DOI] [PubMed] [Google Scholar]
- 107.Hinojosa R, Green JD Jr, Marion MS (1991) Ganglion cell populations in labyrinthitis ossificans. Am J Otol 12(Suppl):3–7; discussion 18–21 [PubMed]
- 108.Nadol JB, Jr, Hsu WC. Histopathologic correlation of spiral ganglion cell count and new bone formation in the cochlea following meningogenic labyrinthitis and deafness. Ann Otol Rhinol Laryngol. 1991;100:712–716. doi: 10.1177/000348949110000904. [DOI] [PubMed] [Google Scholar]
- 109.Hinojosa R, Redleaf MI, Green JD, Jr, Blough RR. Spiral ganglion cell survival in labyrinthitis ossificans: computerized image analysis. Ann Otol Rhinol Laryngol Suppl. 1995;166:51–54. [PubMed] [Google Scholar]
- 110.Finley CC, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teymouri J, Hullar TE, Holden TA, Chole RA. Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otol Neurotol. 2011;32:980–986. doi: 10.1097/MAO.0b013e3182255915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Long CJ, et al. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol. 2014;15:293–304. doi: 10.1007/s10162-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schvartz-Leyzac KC, et al. Effects of electrode location on estimates of neural health in humans with cochlear implants. J Assoc Res Otolaryngol. 2020;18:324–334. doi: 10.1007/s10162-020-00749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choudhury B, et al. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol Neurotol. 2011;32:1370–1378. doi: 10.1097/MAO.0b013e31822f09f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bas E, et al. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. 2015;9:303. doi: 10.3389/fncel.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O'Connell BP, Hunter JB, Wanna GB. The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol. 2016;1:169–174. doi: 10.1002/lio2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koka K, et al. Intra-cochlear electrocochleography during cochear implant electrode insertion is predictive of final scalar location. Otol Neurotol. 2018;39:e654–e659. doi: 10.1097/mao.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giardina CK, et al. Intracochlear electrocochleography: response patterns during cochlear implantation and hearing preservation. Ear Hear. 2019;40:833–848. doi: 10.1097/aud.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haumann S, et al. Monitoring of the inner ear function during and after cochlear implant insertion using electrocochleography. Trends Hear. 2019;23:2331216519833567. doi: 10.1177/2331216519833567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wanna GB, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(Suppl 6):S1–7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gabrielpillai J, Burck I, Baumann U, Stöver T, Helbig S. Incidence for tip foldover during cochlear implantation. Otol Neurotol. 2018;39:1115–1121. doi: 10.1097/mao.0000000000001915. [DOI] [PubMed] [Google Scholar]
- 122.Shearer AE, et al. Genetic variants in the peripheral auditory system significantly affect adult cochlear implant performance. Hear Res. 2017;348:138–142. doi: 10.1016/j.heares.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eppsteiner RW, et al. Prediction of cochlear implant performance by genetic mutation: the spiral ganglion hypothesis. Hear Res. 2012;292:51–58. doi: 10.1016/j.heares.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith RJ, Bale JF, Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/s0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 125.Hochman JB, et al. Prevalence of connexin 26 (GJB2) and Pendred (SLC26A4) mutations in a population of adult cochlear implant candidates. Otol Neurotol. 2010;31:919–922. doi: 10.1097/MAO.0b013e3181e3d324. [DOI] [PubMed] [Google Scholar]
- 126.Crispino G, et al. BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice. PLoS ONE. 2011;6:e23279. doi: 10.1371/journal.pone.0023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takada Y, et al. Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear Res. 2014;309:124–135. doi: 10.1016/j.heares.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Q, et al. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014;21:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iizuka T, et al. Perinatal Gjb2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum Mol Genet. 2015;24:3651–3661. doi: 10.1093/hmg/ddv109. [DOI] [PubMed] [Google Scholar]
- 130.Guo J, et al. GJB2 gene therapy and conditional deletion reveal developmental stage-dependent effects on inner ear structure and function. Mol Ther Methods Clin Dev. 2021;23:319–333. doi: 10.1016/j.omtm.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choi BY, et al. Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J Clin Invest. 2011;121:4516–4525. doi: 10.1172/jci59353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Griffith AJ, Wangemann P. Hearing loss associated with enlargement of the vestibular aqueduct: mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hear Res. 2011;281:11–17. doi: 10.1016/j.heares.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eshraghi AA, et al. Genotype-phenotype correlation for predicting cochlear implant outcome: current challenges and opportunities. Front Genet. 2020;11:678. doi: 10.3389/fgene.2020.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Norrix LW, Velenovsky DS. Auditory neuropathy spectrum disorder: a review. J Speech Lang Hear Res. 2014;57:1564–1576. doi: 10.1044/2014_jslhr-h-13-0213. [DOI] [PubMed] [Google Scholar]
- 135.Taioli F, Cabrini I, Cavallaro T, Acler M, Fabrizi GM. Inherited demyelinating neuropathies with micromutations of peripheral myelin protein 22 gene. Brain. 2011;134:608–617. doi: 10.1093/brain/awq374. [DOI] [PubMed] [Google Scholar]
- 136.Giuliani N, Holte L, Shy M, Grider T. The audiologic profile of patients with Charcot-Marie Tooth neuropathy can be characterised by both cochlear and neural deficits. Int J Audiol. 2019;58:902–912. doi: 10.1080/14992027.2019.1633022. [DOI] [PubMed] [Google Scholar]
- 137.Sahenk Z. Abnormal Schwann cell-axon interactions in CMT neuropathies. The effects of mutant Schwann cells on the axonal cytoskeleton and regeneration-associated myelination. Ann N Y Acad Sci. 1999;883:415–426. doi: 10.1111/j.1749-6632.1999.tb08602.x. [DOI] [PubMed] [Google Scholar]
- 138.Zhou Y, et al. A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice. Exp Neurol. 2019;321:113031. doi: 10.1016/j.expneurol.2019.113031. [DOI] [PubMed] [Google Scholar]
- 139.Anzalone CL, Nuhanovic S, Olund AP, Carlson ML. Cochlear implantation in Charcot-Marie-tooth disease: case report and review of the literature. Case Rep Med. 2018;2018:1760978. doi: 10.1155/2018/1760978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kobayashi M, et al. Cochlear implantation in patient with Charcot-Marie-tooth disease. Auris Nasus Larynx. 2021;48:327–330. doi: 10.1016/j.anl.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 141.Vesseur A, et al. Hearing restoration in cochlear nerve deficiency: the choice between cochlear implant or auditory brainstem implant, a meta-analysis. Otol Neurotol. 2018;39:428–437. doi: 10.1097/mao.0000000000001727. [DOI] [PubMed] [Google Scholar]
- 142.Trakimas DR, Knoll RM, Castillo-Bustamante M, Kozin ED, Remenschneider AK. Otopathologic analysis of patterns of postmeningitis labyrinthitis ossificans. Otolaryngol Head Neck Surg. 2020;194599820934748:-. doi: 10.1177/019459982093474. [DOI] [PubMed] [Google Scholar]
- 143.Wanna GB, et al. Implantation of the completely ossified cochlea: an image-guided approach. Otol Neurotol. 2013;34:522–525. doi: 10.1097/MAO.0b013e31827d8aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilk M, et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS ONE. 2016;11:e0147552. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Durisin M, et al. Cochlear implantation in children with bacterial meningitic deafness: the influence of the degree of ossification and obliteration on impedance and charge of the implant. Cochlear Implants Int. 2015;16:147–158. doi: 10.1179/1754762814y.0000000094. [DOI] [PubMed] [Google Scholar]
- 146.Nichani J, et al. Cochlear implantation after bacterial meningitis in children: outcomes in ossified and nonossified cochleas. Otol Neurotol. 2011;32:784–789. doi: 10.1097/MAO.0b013e31821677aa. [DOI] [PubMed] [Google Scholar]
- 147.Singhal K, Singhal J, Muzaffar J, Monksfield P, Bance M. Outcomes of cochlear implantation in patients with post-meningitis deafness: a systematic review and narrative synthesis. J Int Adv Otol. 2020;16:395–410. doi: 10.5152/iao.2020.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Linthicum FH, Jr, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991;12:245–311. [PubMed] [Google Scholar]
- 149.Khan AM, Whiten DM, Nadol JB, Jr, Eddington DK. Histopathology of human cochlear implants: correlation of psychophysical and anatomical measures. Hear Res. 2005;205:83–93. doi: 10.1016/j.heares.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 150.Linthicum FH, Jr, Doherty JK, Lopez IA, Ishiyama A. Cochlear implant histopathology. World J Otorhinolaryngol Head Neck Surg. 2017;3:211–213. doi: 10.1016/j.wjorl.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng YS, Svirsky MA. Meta-analysis-correlation between spiral ganglion cell counts and speech perception with a cochlear implant. Audiol Res. 2021;11:220–226. doi: 10.3390/audiolres11020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pfingst BE. Changes over time in thresholds for electrical stimulation of the cochlea. Hear Res. 1990;50:225–236. doi: 10.1016/0378-5955(90)90047-s. [DOI] [PubMed] [Google Scholar]
- 153.Pfingst BE, Donaldson JA, Miller JM, Spelman FA. Psychophysical evaluation of cochlear prostheses in a monkey model. Ann Otol Rhinol Laryngol. 1979;88:613–625. doi: 10.1177/000348947908800505. [DOI] [PubMed] [Google Scholar]
- 154.Pfingst BE, et al. Insertion trauma and recovery of function after cochlear implantation: evidence from objective functional measures. Hear Res. 2015;330:98–105. doi: 10.1016/j.heares.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Colesa DJ, et al. Development of a chronically-implanted mouse model for studies of cochlear health and implant function. Hear Res. 2021;404:108216. doi: 10.1016/j.heares.2021.108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. Ann Otol Rhinol Laryngol. 1983;92:19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- 157.Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-U. [DOI] [PubMed] [Google Scholar]
- 158.Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK. Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res. 2006;215:47–55. doi: 10.1016/j.heares.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ramekers D, Versnel H, Strahl SB, Klis SF, Grolman W. Recovery characteristics of the electrically stimulated auditory nerve in deafened guinea pigs: relation to neuronal status. Hear Res. 2015;321:12–24. doi: 10.1016/j.heares.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 160.Zappia JJ, Altschuler RA. Evaluation of the effect of ototopical neomycin on spiral ganglion cell density in the guinea pig. Hear Res. 1989;40:29–37. doi: 10.1016/0378-5955(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 161.Budenz CL, Pfingst BE, Raphael Y. The use of neurotrophin therapy in the inner ear to augment cochlear implantation outcomes. Anat Rec (Hoboken) 2012;295:1896–1908. doi: 10.1002/ar.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Budenz CL, et al. Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep. 2015;5:8619. doi: 10.1038/srep08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schvartz-Leyzac KC, Pfingst BE. Across-site patterns of electrically evoked compound action potential amplitude-growth functions in multichannel cochlear implant recipients and the effects of the interphase gap. Hear Res. 2016;341:50–65. doi: 10.1016/j.heares.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/S0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 165.McKay CM, Henshall KR. The perceptual effects of interphase gap duration in cochlear implant stimulation. Hear Res. 2003;181:94–99. doi: 10.1016/S0378-5955(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 166.van den Honert C, Mortimer JT. The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng. 1979;7:117–125. doi: 10.1007/BF02363130. [DOI] [PubMed] [Google Scholar]
- 167.Thai-Van H, et al. Relationship between NRT measurements and behavioral levels in children with the Nucleus 24 cochlear implant may change over time: preliminary report. Int J Pediatr Otorhinolaryngol. 2001;58:153–162. doi: 10.1016/s0165-5876(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 168.Jeon EK, et al. Comparison of electrically evoked compound action potential thresholds and loudness estimates for the stimuli used to program the Advanced Bionics cochlear implant. J Am Acad Audiol. 2010;21:16–27. doi: 10.3766/jaaa.21.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He S, Teagle HFB, Buchman CA. The electrically evoked compound action potential: from laboratory to clinic. Front Neurosci. 2017;11:339. doi: 10.3389/fnins.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Eijl RH, Buitenhuis PJ, Stegeman I, Klis SF, Grolman W. Systematic review of compound action potentials as predictors for cochlear implant performance. Laryngoscope. 2017;127:476–487. doi: 10.1002/lary.26154. [DOI] [PubMed] [Google Scholar]
- 171.Kim JR, et al. The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol. 2010;31:1041–1048. doi: 10.1097/MAO.0b013e3181ec1d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.DeVries L, Scheperle R, Bierer JA. Assessing the electrode-neuron interface with the electrically evoked compound action potential, electrode position, and behavioral thresholds. J Assoc Res Otolaryngol. 2016;17:237–252. doi: 10.1007/s10162-016-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scheperle R. Suprathreshold compound action potential amplitude as a measure of auditory function in cochlear implant users. J Otol. 2017;12:18–28. doi: 10.1016/j.joto.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Franck KH, Norton SJ. Estimation of psychophysical levels using the electrically evoked compound action potential measured with the neural response telemetry capabilities of Cochlear Corporation’s CI24M device. Ear Hear. 2001;22:289–299. doi: 10.1097/00003446-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 175.Heydebrand G, Hale S, Potts L, Gotter B, Skinner M. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiol Neurootol. 2007;12:254–264. doi: 10.1159/000101473. [DOI] [PubMed] [Google Scholar]
- 176.Finke M, Buchner A, Ruigendijk E, Meyer M, Sandmann P. On the relationship between auditory cognition and speech intelligibility in cochlear implant users: an ERP study. Neuropsychologia. 2016;87:169–181. doi: 10.1016/j.neuropsychologia.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 177.Garadat SN, Zwolan TA, Pfingst BE. Across-site patterns of modulation detection: relation to speech recognitiona) J Acoust Soc Am. 2012;131:4030–4041. doi: 10.1121/1.3701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He S, et al. Responsiveness of the Electrically stimulated cochlear nerve in children with cochlear nerve deficiency. Ear Hear. 2018;39:238–250. doi: 10.1097/aud.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luo J, et al. The effects of GJB2 or SLC26A4 gene mutations on neural response of the electrically stimulated auditory nerve in children. Ear Hear. 2020;41:194–207. doi: 10.1097/aud.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 180.Fukushima K, et al. Better speech performance in cochlear implant patients with GJB2-related deafness. Int J Pediatr Otorhinolaryngol. 2002;62:151–157. doi: 10.1016/s0165-5876(01)00619-x. [DOI] [PubMed] [Google Scholar]
- 181.Yan YJ, Li Y, Yang T, Huang Q, Wu H. The effect of GJB2 and SLC26A4 gene mutations on rehabilitative outcomes in pediatric cochlear implant patients. Eur Arch Otorhinolaryngol. 2013;270:2865–2870. doi: 10.1007/s00405-012-2330-y. [DOI] [PubMed] [Google Scholar]
- 182.Popov TM, et al. Auditory outcome after cochlear implantation in patients with congenital nonsyndromic hearing loss: influence of the GJB2 status. Otol Neurotol. 2014;35:1361–1365. doi: 10.1097/mao.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 183.Abdurehim Y, Lehmann A, Zeitouni AG. Predictive value of GJB2 mutation status for hearing outcomes of pediatric cochlear implantation. Otolaryngol Head Neck Surg. 2017;157:16–24. doi: 10.1177/0194599817697054. [DOI] [PubMed] [Google Scholar]
- 184.Jahn KN, Arenberg JG. Electrophysiological estimates of the electrode-neuron interface differ between younger and older listeners with cochlear implants. Ear Hear. 2020;41:948–960. doi: 10.1097/aud.0000000000000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Imsiecke M, Büchner A, Lenarz T, Nogueira W. Amplitude growth functions of auditory nerve responses to electric pulse stimulation with varied interphase gaps in cochlear implant users with ipsilateral residual hearing. Trends Hear. 2021;25:23312165211014137. doi: 10.1177/23312165211014137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Skinner MW, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. doi: 10.1177/00034894071160S401. [DOI] [PubMed] [Google Scholar]
- 187.Noble JH, Labadie RF, Gifford RH, Dawant BM. Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Transactions on Neural Systems and Rehabilitation Engineering: a Publication of the IEEE Engineering in Medicine and Biology Society. 2013;21:820–829. doi: 10.1109/TNSRE.2013.2253333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Noble JH, Gifford RH, Hedley-Williams AJ, Dawant BM, Labadie RF. Clinical evaluation of an image-guided cochlear implant programming strategy. Audiol Neurootol. 2014;19:400–411. doi: 10.1159/000365273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Noble JH, et al. Initial results with image-guided cochlear implant programming in children. Otol Neurotol. 2016;37:e63–e69. doi: 10.1097/mao.0000000000000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cosentino S, Gaudrain E, Deeks JM, Carlyon RP. Multistage nonlinear optimization to recover neural activation patterns from evoked compound action potentials of cochlear implant users. IEEE Trans Biomed Eng. 2016;63:833–840. doi: 10.1109/tbme.2015.2476373. [DOI] [PubMed] [Google Scholar]
- 191.Garcia C, et al. The panoramic ECAP method: estimating patient-specific patterns of current spread and neural health in cochlear implant users. J Assoc Res Otolaryngol. 2021;22:567–589. doi: 10.1007/s10162-021-00795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dolan DF, Nuttall AL, Avinash G. Asynchronous neural activity recorded from the round window. J Acoust Soc Am. 1990;87:2621–2627. doi: 10.1121/1.399054. [DOI] [PubMed] [Google Scholar]
- 193.Searchfield GD, Muñoz DJ, Thorne PR. Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear Res. 2004;192:23–35. doi: 10.1016/j.heares.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 194.Patuzzi RB, Yates GK, Johnstone BM. Changes in cochlear microphonic and neural sensitivity produced by acoustic trauma. Hear Res. 1989;39:189–202. doi: 10.1016/0378-5955(89)90090-7. [DOI] [PubMed] [Google Scholar]
- 195.Dong W, Olson ES. Detection of cochlear amplification and its activation. Biophys J. 2013;105:1067–1078. doi: 10.1016/j.bpj.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bester C, et al. Cochlear microphonic latency predicts outer hair cell function in animal models and clinical populations. Hear Res. 2020;398:108094. doi: 10.1016/j.heares.2020.108094. [DOI] [PubMed] [Google Scholar]
- 197.Adel Y, Tillein J, Petzold H, Weissgerber T, Baumann U. Band-limited chirp-evoked compound action potential in guinea pig: comprehensive neural measure for cochlear implantation monitoring. Ear Hear. 2020;42:142–162. doi: 10.1097/aud.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 198.Formeister EJ, et al. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear. 2015;36:249–260. doi: 10.1097/aud.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 199.Fitzpatrick DC, et al. Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults. Otol Neurotol. 2014;35:64–71. doi: 10.1097/mao.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McClellan JH, et al. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol. 2014;35:e245–e252. doi: 10.1097/mao.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 201.Harris MS, et al. Patterns seen during electrode insertion using intracochlear electrocochleography obtained directly through a cochlear implant. Otol Neurotol. 2017;38:1415–1420. doi: 10.1097/mao.0000000000001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koka K, Saoji AA, Litvak LM. Electrocochleography in cochlear implant recipients with residual hearing: comparison with audiometric thresholds. Ear Hear. 2017;38:e161–e167. doi: 10.1097/aud.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 203.Coulthurst S, Nachman AJ, Murray MT, Koka K, Saoji AA. Comparison of pure-tone thresholds and cochlear microphonics thresholds in pediatric cochlear implant patients. Ear Hear. 2020;41:1320–1326. doi: 10.1097/aud.0000000000000870. [DOI] [PubMed] [Google Scholar]
- 204.Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- 205.Pfingst BE, et al. Cochlear infrastructure for electrical hearing. Hear Res. 2011;281:65–73. doi: 10.1016/j.heares.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou N, Kraft CT, Colesa DJ, Pfingst BE. Integration of pulse trains in humans and guinea pigs with cochlear implants. J Assoc Res Otolaryngol. 2015;16:523–534. doi: 10.1007/s10162-015-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bierer JA. Probing the electrode-neuron interface with focused cochlear implant stimulation. Trends Amplif. 2010;14:84–95. doi: 10.1177/1084713810375249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–258. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bierer JA, Litvak L. Reducing channel interaction through cochlear implant programming may improve speech perception: current focusing and channel deactivation. Trends Hear. 2016 doi: 10.1177/2331216516653389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.DiNino M, O’Brien G, Bierer SM, Jahn KN, Arenberg JG. The estimated electrode-neuron interface in cochlear implant listeners is different for early-implanted children and late-implanted adults. J Assoc Res Otolaryngol. 2019;20:291–303. doi: 10.1007/s10162-019-00716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Garadat SN, Colesa DJ, Swiderski DL, Raphael Y, Pfingst BE. Estimating health of the implanted cochlea using psychophysical strength-duration functions and electrode configuration. Hear Res. 2022;414:108404. doi: 10.1016/j.heares.2021.108404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jahn KN, Arenberg JG. Evaluating psychophysical polarity sensitivity as an indirect estimate of neural status in cochlear implant listeners. J Assoc Res Otolaryngol. 2019;20:415–430. doi: 10.1007/s10162-019-00718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jahn KN, Bergan MD, Arenberg JG. Auditory detection thresholds and cochlear resistivity differ between pediatric cochlear implant listeners with enlarged vestibular aqueduct and those with connexin-26 mutations. Am J Audiol. 2020;29:23–34. doi: 10.1044/2019_aja-19-00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou N. Deactivating stimulation sites based on low-rate thresholds improves spectral ripple and speech reception thresholds in cochlear implant users. J Acoust Soc Am. 2017;141:El243. doi: 10.1121/1.4977235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou N. Longitudinal effect of deactivating stimulation sites based on low-rate thresholds on speech recognition in cochlear implant users. Int J Audiol. 2019;58:587–597. doi: 10.1080/14992027.2019.1601779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Miller CA, Abbas PJ, Robinson BK, Rubinstein JT, Matsuoka AJ. Electrically evoked single-fiber action potentials from cat: responses to monopolar, monophasic stimulation. Hear Res. 1999;130:197–218. doi: 10.1016/S0378-5955(99)00012-X. [DOI] [PubMed] [Google Scholar]
- 217.Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: evoked compound action potential recordings. Hear Res. 2000;149:115–128. doi: 10.1016/S0378-5955(00)00172-6. [DOI] [PubMed] [Google Scholar]
- 218.Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- 219.Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Undurraga JA, van Wieringen A, Carlyon RP, Macherey O, Wouters J. Polarity effects on neural responses of the electrically stimulated auditory nerve at different cochlear sites. Hear Res. 2010;269:146–161. doi: 10.1016/j.heares.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 221.Undurraga JA, Carlyon RP, Wouters J, van Wieringen A. The polarity sensitivity of the electrically stimulated human auditory nerve measured at the level of the brainstem. J Assoc Res Otolaryngol. 2013;14:359–377. doi: 10.1007/s10162-013-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hughes ML, Choi S, Glickman E. What can stimulus polarity and interphase gap tell us about auditory nerve function in cochlear-implant recipients? Hear Res. 2018;359:50–63. doi: 10.1016/j.heares.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hughes ML, Goehring JL, Baudhuin JL. Effects of stimulus polarity and artifact reduction method on the electrically evoked compound action potential. Ear Hear. 2017;38:332–343. doi: 10.1097/aud.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Macherey O, Carlyon RP, Chatron J, Roman S. Effect of pulse polarity on thresholds and on non-monotonic loudness growth in cochlear implant users. J Assoc Res Otolaryngol. 2017;18:513–527. doi: 10.1007/s10162-016-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Carlyon RP, Cosentino S, Deeks JM, Parkinson W, Arenberg JA. Effect of stimulus polarity on detection thresholds in cochlear implant users: relationships with average threshold, gap detection, and rate discrimination. J Assoc Res Otolaryngol. 2018 doi: 10.1007/s10162-018-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goehring T, Archer-Boyd A, Deeks JM, Arenberg JG, Carlyon RP. A site-selection strategy based on polarity sensitivity for cochlear implants: effects on spectro-temporal resolution and speech perception. J Assoc Res Otolaryngol. 2019;20:431–448. doi: 10.1007/s10162-019-00724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brochier T, et al. Evaluating and comparing behavioural and electrophysiological estimates of neural health in cochlear implant users. J Assoc Res Otolaryngol. 2021;22:67–80. doi: 10.1007/s10162-020-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brochier T, McKay CM, Carlyon RP. Interpreting the effect of stimulus parameters on the electrically evoked compound action potential and on neural health estimates. J Assoc Res Otolaryngol. 2021;22:81–94. doi: 10.1007/s10162-020-00774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fayad JN, Makarem AO, Linthicum FH., Jr Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg. 2009;141:247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Souter M, et al. Systemic immunity influences hearing preservation in cochlear implantation. Otol Neurotol. 2012;33:532–538. doi: 10.1097/MAO.0b013e31824bac44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kamakura T, Nadol JB., Jr Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132–141. doi: 10.1016/j.heares.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schvartz-Leyzac KC, Zwolan TA, Pfingst BE. Using the electrically-evoked compound action potential (ECAP) interphase gap effect to select electrode stimulation sites in cochlear implant users. Hear Res. 2021;406:108257. doi: 10.1016/j.heares.2021.108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li Q, Lu T, Zhang C, Hansen MR, Li S. Electrical stimulation induces synaptic changes in the peripheral auditory system. J Comp Neurol. 2020;528:893–905. doi: 10.1002/cne.24802. [DOI] [PubMed] [Google Scholar]
- 234.Zhou N, Pfingst BE. Effects of site-specific level adjustments on speech recognition with cochlear implants. Ear Hear. 2014;35:30–40. doi: 10.1097/AUD.0b013e31829d15cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rah YC, et al. Extended use of systemic steroid is beneficial in preserving hearing in guinea pigs after cochlear implant. Acta Otolaryngol. 2016;136:1213–1219. doi: 10.1080/00016489.2016.1206965. [DOI] [PubMed] [Google Scholar]
- 236.Shaul C, et al. Glucocorticoid for hearing preservation after cochlear implantation: a systemic review and meta-analysis of animal studies. Otol Neurotol. 2019;40:1178–1185. doi: 10.1097/mao.0000000000002383. [DOI] [PubMed] [Google Scholar]
- 237.Ma Y, Wise AK, Shepherd RK, Richardson RT. New molecular therapies for the treatment of hearing loss. Pharmacol Ther. 2019;200:190–209. doi: 10.1016/j.pharmthera.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Askew C, Chien WW. Adeno-associated virus gene replacement for recessive inner ear dysfunction: progress and challenges. Hear Res. 2020;394:107947. doi: 10.1016/j.heares.2020.107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Delmaghani S, El-Amraoui A. Inner ear gene therapies take off: current promises and future challenges. J Clin Med. 2020 doi: 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang L, Wu X, Lin X. Gene therapy for genetic mutations affecting non-sensory cells in the cochlea. Hear Res. 2020;394:107858. doi: 10.1016/j.heares.2019.107858. [DOI] [PubMed] [Google Scholar]
- 241.Leake PA, Akil O, Lang H. Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness. Hear Res. 2020;394:107955. doi: 10.1016/j.heares.2020.107955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Manrique-Huarte R, et al. Cochlear implantation with a dexamethasone eluting electrode array: functional and anatomical changes in non-human primates. Otol Neurotol. 2020;41:e812–e822. doi: 10.1097/mao.0000000000002686. [DOI] [PubMed] [Google Scholar]
- 243.Simoni E, et al. Immune response after cochlear implantation. Front Neurol. 2020;11:341. doi: 10.3389/fneur.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.O'Leary SJ, et al. Systemic methylprednisolone for hearing preservation during cochlear implant surgery: a double blinded placebo-controlled trial. Hear Res. 2021;404:108224. doi: 10.1016/j.heares.2021.108224. [DOI] [PubMed] [Google Scholar]
- 245.Kikkawa YS, et al. Growth factor-eluting cochlear implant electrode: impact on residual auditory function, insertional trauma, and fibrosis. J Transl Med. 2014;12:280. doi: 10.1186/s12967-014-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dhanasingh A, Hochmair I. Drug delivery in cochlear implantation. Acta Otolaryngol. 2021;141:135–156. doi: 10.1080/00016489.2021.1888505. [DOI] [PubMed] [Google Scholar]
- 247.Chen W, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rivolta MN. New strategies for the restoration of hearing loss: challenges and opportunities. Br Med Bull. 2013;105:69–84. doi: 10.1093/bmb/lds035. [DOI] [PubMed] [Google Scholar]
- 249.Lee S, et al. Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea. Sci Rep. 2020;10:21397. doi: 10.1038/s41598-020-78167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shibata SB, et al. Gene therapy for hair cell regeneration: review and new data. Hear Res. 2020;394:107981. doi: 10.1016/j.heares.2020.107981. [DOI] [PubMed] [Google Scholar]
- 251.White PM. Perspectives on human hearing loss, cochlear regeneration, and the potential for hearing restoration therapies. Brain Sci. 2020 doi: 10.3390/brainsci10100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Iyer AA, Groves AK. Transcription factor reprogramming in the inner ear: turning on cell fate switches to regenerate sensory hair cells. Front Cell Neurosci. 2021;15:660748. doi: 10.3389/fncel.2021.660748. [DOI] [PMC free article] [PubMed] [Google Scholar]