Abstract
Purpose
Diabetes and dyslipidemia are leading causes of mortality and morbidity. According to international guidelines, statins are the cornerstone of treatment in patients with diabetes and/or dyslipidemia. However, statins and antidiabetic agents have opposite pharmacological effects, because statins, particularly atorvastatin and rosuvastatin, impair glucose homeostasis, increasing the risk of new-onset diabetes, whereas antidiabetic drugs improve glycemic homeostasis. The aim of this study was to investigate the effect of atorvastatin, rosuvastatin, and pitavastatin on glucose homeostasis in patients with type 2 diabetes mellitus (T2DM) and dyslipidemia during stable treatment with hypoglycemic drugs.
Materials and Methods
The study was conducted as a pilot, prospective, randomized, open label, parallel group with blinded-endpoints (PROBE) study. Of 180 recruited patients with T2DM and dyslipidemia, 131 were randomized to atorvastatin (n=44), rosuvastatin (n=45), and pitavastatin (n=42) and treated for 6 months.
Results
Fasting plasma glucose (FPG) marginally decreased in patients assigned to atorvastatin (-3.5 mg/dL, p=0.42) and rosuvastatin (-6.5 mg/dL, p=0.17), while it decreased much more in patients treated with pitavastatin (-19.0 mg/dL, p<0.001). Mean glycated hemoglobin A1c (HbA1c ) values remained unchanged during treatment with atorvastatin (-0.10%, p=0.53) and rosuvastatin (0.20%, p=0.40), but were significantly reduced with pitavastatin (-0.75%, p=0.01). Atorvastatin, rosuvastatin, and pitavastatin significantly lowered (p<0.001) plasma levels of total cholesterol, low-density lipoprotein-cholesterol, and triglycerides, while high-density lipoprotein-cholesterol (HDL-C) levels increased significantly (p=0.04) only in the pitavastatin group.
Conclusion
The results of the present study suggest that pitavastatin affects FPG and HbA1c less than atorvastatin and rosuvastatin in patients with T2DM and concomitant dyslipidemia. Lipid-lowering efficacies were not significantly different among the three statins, with the exception of HDL-C, which increased significantly with pitavastatin. Although the pharmacological mechanism of pitavastatin on glucose homeostasis in patients with T2DM during stable antidiabetic therapy is not known, it can be assumed that pitavastatin has less drug interaction with hypoglycemic agents or that it increases plasma levels of adiponectin.
Keywords: Type 2 diabetes, fasting plasma glucose, glycated hemoglobin A1c, atorvastatin, rosuvastatin, pitavastatin
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and dyslipidemia are often concomitantly detected in the same patient and remain major risk factors for fatal and non-fatal cardio-renal events.1,2 Statins and glucose lowering drugs are first-line treatments for patients with T2DM associated with dyslipidemia, and current guidelines3,4,5 recommend specific targets for glycemic and lipid control to delay or prevent disease progression. Indeed, lowering low-density lipoproteins-cholesterol (LDL-C) by 1–3 mmol/L and glycated hemoglobin A1c (HbA1c) by 1% is associated with 21%–50% and 26% risk reductions in major cardiovascular events, respectively.6,7,8,9 However, several studies and meta-analyses10,11,12,13 have shown that statins may have a pro-diabetogenic effect that increases the incidence of new onset T2DM. While the relationship between high/moderate doses of statins and the risk of T2DM is well documented,14,15,16 the effect on glucose homeostasis in patients treated with hypoglycemic agents remains controversial.
Several studies17,18,19,20,21 have reported varying results that could be dependent on the use of different statins, doses, duration of follow-up, and particularly, changes in antidiabetic agents during treatment. These conflicting findings have been properly highlighted in recent systematic reviews.20,22,23,24
Among statins, moderate/high daily doses of atorvastatin and rosuvastatin have been shown to be associated with dysregulation of glucose homeostasis in patients with or without T2DM.19,23,24,25,26,27,28 Meanwhile, researchers suggest that the risk of new onset diabetes and deterioration of glucose homeostasis is less evident with pitavastatin14,16,24,29,30,31 and pravastatin.32,33,34 Based on this background, the present study was designed to investigate whether and to what extent atorvastatin, rosuvastatin, and pitavastatin affect fasting plasma glucose (FPG) and HbA1c in patients with T2DM and dyslipidemia during stable treatment with hypoglycemic drugs.
MATERIALS AND METHODS
This study was designed as a pilot, prospective, randomized, open label, parallel groups, with blinded-endpoints (PROBE) study to assess the effect of atorvastatin, rosuvastatin, and pitavastatin on FBG and HbA1c in patients with T2DM associated with dyslipidemia. One hundred and eighty patients, of both genders, were recruited from individuals referred by general practitioners to an outpatient specialist clinic of Internal Medicine at Kartal Hospital in Istanbul, Turkey. Patients were considered eligible for enrollment if they met the following criteria: consensus to participate in the study, age >20 years, and confirmed diagnoses of T2DM and dyslipidemia. Major exclusion criteria were type 1 diabetes, triglycerides (TG) ≥500 mg/dL, clinical manifestations of atherosclerotic cardiovascular disease (previous myocardial infarction or coronary revascularization, stable angina, cerebrovascular accident, heart failure, peripheral artery disease), abnormal thyroid function, renal disease, hepatic dysfunction, pregnancy, and concomitant medications that would interfere with glucose homeostasis.
After collection of demographic data, medical history, and total cardiovascular risk assessment, patients underwent clinical examination and received advice on healthy dieting. For laboratory tests, blood samples were collected in fasting conditions, two times on different days, before the intake of any drugs. Plasma glucose and lipid concentrations were assessed with available automated enzymatic colorimetric methods, and HbA1c was evaluated with high performance liquid chromatography. T2DM was diagnosed in patients with FPG level ≥126 mg/dL, HbA1c ≥6.5% or on- or pre-treatment with antidiabetic drugs,3,4 and dyslipidemia was diagnosed as LDL-C ≥70 mg/dL.35 Patients that fulfilled the inclusion and exclusion criteria were randomly assigned to receive moderate doses of atorvastatin (20 mg), rosuvastatin (10 mg), or pitavastatin (2 mg), once daily, and followed for 6 months. The doses were chosen as suggested by international guidelines4,35 for patients without atherosclerotic cardiovascular disease. Subjects on lipid-lowering therapy were shifted, at random, to study statins, and in patients with inadequate glycemic control, glucose-lowering drugs were started or modified according to international guidelines.3,4 Hypoglycemic drugs, statins, and doses were maintained stable during the 6 months of follow-up. After 3 months, individuals who required statins, antidiabetics, or dosage modifications were excluded from the study. All adverse events occurring before or at the trial closure visit were recorded and analyzed.
The primary endpoints of our trial were 1) change in FPG and 2) change in HbA1c levels from baseline. Secondary endpoints included changes, from baseline, in total cholesterol (TC), LDL-C, high-density lipoprotein-cholesterol (HDL-C), and TG. The study was approved by a local independent Ethics Committee (Kartal Dr. Lütfi Kırdar City Hospital Ethical Committee NUMBER: 2019/514/150/25) and conducted in accordance with the Declaration of Helsinki.
Data analysis
Data analysis was performed in all randomized patients that completed the 6 months of treatment. Distribution of data was tested using the Kolmogorov–Smirnov test. Continuous and categorical variables are expressed as means (SD) and percentages, respectively. Plasma glucose and HbA1c data not normally distributed are expressed as medians and 25th–75th percentiles. ANOVA, Kruskal-Wallis, and chi-square tests were used to compare continuous and categorical variables between treatments. Bonferroni correction was used for post-hoc test. Changes from baseline were compared using the paired t-test or Wilcoxon signed rank test. Two-sided p values<0.05 were considered statistically significant. All statistical tests were performed with the statistical package SPSS 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
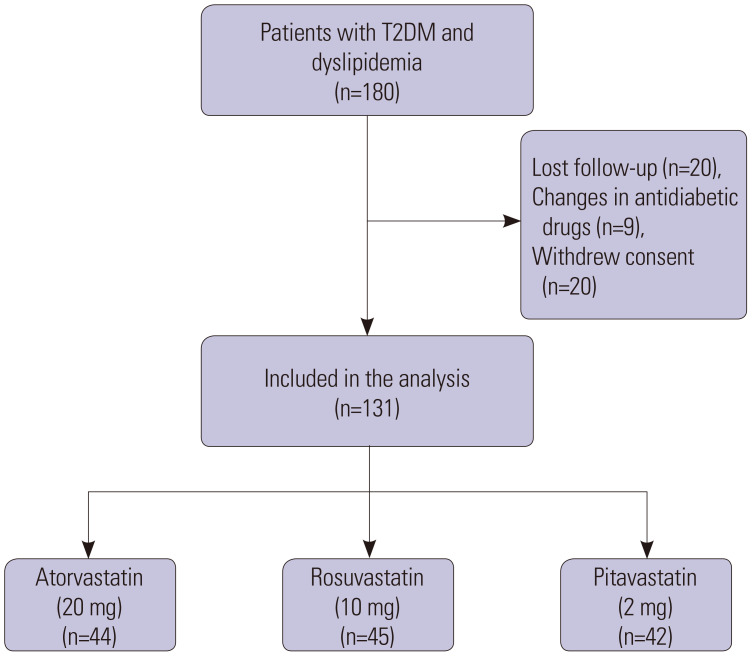
Of the180 patients recruited, 49 were excluded: 20 lost to follow-up, nine for changes in hypoglycemic drugs, and 20 for withdrawing consent during the study (Fig. 1). Thus, 131 patients completed the 6 months of treatment: 44 were assigned to atorvastatin, 45 to rosuvastatin, and 42 to pitavastatin as an add-on to hypoglycemic agents. The baseline demographic, clinical, and laboratory characteristics of patients are reported in Table 1. The three drug groups were homogeneous, particularly in terms of age, sex, treatment allocation, rate of hypertension, FPG, HbA1c, lipids, and anti-diabetic agents.
Fig. 1. Flowchart. T2DM, type 2 diabetes mellitus.
Table 1. Demographic, Clinical, and Biochemical Characteristics of the Study Participants at Baseline.
| Parameters | Atorvastatin (20 mg) (n=44) | Rosuvastatin (10 mg) (n=45) | Pitavastatin (2 mg) (n=42) | p value | |
|---|---|---|---|---|---|
| Male | 20 (45.4) | 13 (28.8) | 14 (33.3) | 0.24 | |
| Age, yr | 58.8±9.7 | 58.7±12.0 | 57.9±9.4 | 0.91 | |
| Hypertension | 15 (34.1) | 17 (37.8) | 11 (26.2) | 0.50 | |
| Antidiabetic drugs | 35 (79.5) | 32 (71.1) | 33 (75.6) | ||
| Metformin | 8 (22.9) | 10 (31.3) | 9 (27.3) | 0.88 | |
| Metformin combinations* | 23 (65.7) | 20 (62.5) | 21 (63.6) | 0.75 | |
| Others† | 4 (11.4) | 2 (6.2) | 3 (9.1) | 0.68 | |
| FPG, mg/dL | 134.0 (122.5–149.7) | 134.0 (121.0–147.0) | 139.0 (112.0–185.3) | 0.59 | |
| HbA1c, % | 6.0 (6.5–7.8) | 6.1 (6.8–8.2) | 7.5 (6.4–9.4) | 0.12 | |
| Total cholesterol, mg/dL | 255.9±42.2 | 256.6±47.8 | 259.1±39.8 | 0.99 | |
| LDL-C, mg/dL | 173.8±35 | 174.5±39.4 | 176.1±33 | 0.98 | |
| HDL-C, mg/dL | 47.2±10.2 | 48.9±9.1 | 49±8.7 | 0.60 | |
| Triglycerides, mg/dL | 177.5±64.4 | 172.6±68.2 | 179.5±66.9 | 0.88 | |
FPG, fasting plasma glucose; HbA1c, Hemoglobin A1c; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol.
Data are presented as means±SD, medians (Q1–Q3), or n (%). P-values refers to comparisons among drugs.
*Metformin+ GLP-1 RA or DPP4 or SGLT-2 inhibitors; †Insulin+ metformin or DPP4 inhibitors.
At end of the study (Table 2), the median change in FPG, compared with baseline values, decreased only with pitavastatin (-19 mg/dL, p<0.001) (atorvastatin -3.5 mg/dL, p=0.42; rosuvastatin -6.5 mg/dL, p=0.17). Median changes in HbA1c (Table 2) were not significant with atorvastatin (-0.10%, p=0.53) and rosuvastatin (0.20%, p=0.40), while levels were significantly lowered by pitavastatin (-0.75%, p=0.01). At the end of follow-up, the differences among atorvastatin, rosuvastatin, and pitavastatin were statistically significant for both FPG (p=0.03) and HbA1c (p=0.01). Therefore, pitavastatin deteriorated glycemic control less than atorvastatin and rosuvastatin in patients with T2DM throughout concomitant stable treatment with hypoglycemic agents. Among the secondary endpoints of our trial (Table 3), atorvastatin, rosuvastatin, and pitavastatin significantly decreased (p<0.05) plasma levels of total TC, LDL-C, and TG, while HDL-C slightly increased with atorvastatin (0.65 mg/dL, p=0.28) and rosuvastatin (0.38 mg/dL, p=0.75) and significantly with pitavastatin (1.1 mg/dL, p=0.04), compared to baseline.
Table 2. FPG and HbA1c Changes from Baseline.
| Parameters | Drugs | Change (baseline vs. end of treatment) | p value among drugs† | |
|---|---|---|---|---|
| Median (Q1–Q3) | p value* | |||
| FPG, mg/dL | Atorvastatin | -3.5 (-21.7–6.7)* | 0.42 | 0.03 |
| Rosuvastatin | - 6.5 (-13.0–3.0) | 0.17 | ||
| Pitavastatin | -19.0 (-40.0– -1.5) | <0.001 | ||
| HbA1c, % | Atorvastatin | -0.10 (-0.5–0.2)* | 0.53 | 0.01 |
| Rosuvastatin | 0.20 (-0.8–0.5)* | 0.40 | ||
| Pitavastatin | -0.75 (-1.3–0.1) | 0.01 | ||
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin.
*Wilcoxon sign test; †Kruskal-Wallis test. For other explanations see previous table.
Table 3. Changes in Lipids from Baseline.
| Parameters | Drugs | Change (baseline vs. end of treatment) | p value among drugs† | |
|---|---|---|---|---|
| Mean (SD) | p value* | |||
| Total cholesterol, mg/dL | Atorvastatin | -74.2 (31.8) | <0.001 | 0.38 |
| Rosuvastatin | -70.9 (31.9) | <0.001 | ||
| Pitavastatin | -71 (49.5) | <0.001 | ||
| LDL-C, mg/dL | Atorvastatin | -65.7 (21.5) | <0.001 | 0.58 |
| Rosuvastatin | -64.6 (28.2) | <0.001 | ||
| Pitavastatin | -62.6 (30.5) | <0.001 | ||
| HDL-C, mg/dL | Atorvastatin | 0.65 (4.1) | 0.28 | 0.86 |
| Rosuvastatin | 0.38 (8.0) | 0.75 | ||
| Pitavastatin | 1.1 (3.1) | 0.04 | ||
| Triglycerides, mg/dL | Atorvastatin | -32.8 (71.4) | 0.001 | 0.92 |
| Rosuvastatin | -27.7 (64.4) | 0.001 | ||
| Pitavastatin | -33.1 (73.8) | 0.001 | ||
LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol.
*Paired Student’s t test; †ANOVA test. For explanations see previous tables.
No clinical adverse events, particularly muscle symptoms or abnormal liver function, were observed with the treatment (data not shown).
DISCUSSION
Our study shows that moderate doses of atorvastatin, rosuvastatin, and pitavastatin exert different effects on glucose homeostasis in patients with T2DM and dyslipidemia under stable treatment with hypoglycemic drugs. Herein, significantly (p<0.01) greater decreases in FPG and of HbA1c were obtained with pitavastatin, compared to atorvastatin and rosuvastatin. It is noteworthy that, unlike other studies and meta-analyses,17,23,24,27,28,31,33,34,35,36,37,38 that have reported a dysregulation of glycemic control with low-moderate doses of atorvastatin and rosuvastatin in patients with T2DM, we did not observe this effect. On the contrary, our results show that glycemic homeostasis was not significantly impaired by atorvastatin and rosuvastatin, but, surprisingly, also not improved as expected during concomitant glucose lowering therapy. This finding is in agreement with the results of other studies that have shown modest changes in HbA1c with low to moderate doses of atorvastatin and/or rosuvastatin in patients with diabetes in stable therapy with antidiabetic agents.19,20,26,39,40,41 Notably, different from other trials,19,29,30,31,42 we found no evidence that pitavastatin has a neutral effect on glucose homeostasis. Our results indicate a positive effect on glycemic control, as shown by significant reductions in FPG and HbA1c. This suggests that pitavastatin, compared to atorvastatin and rosuvastatin, could have counteracted the effect of glucose-lowering medications less. The pharmacological mechanism of pitavastatin on glucose homeostasis in patients with T2DM during stable antidiabetic therapy is not known. It could be assumed that pitavastatin has fewer drug interactions with hypoglycemic agents or that it increases plasma levels of adiponectin.43,44 Atorvastatin, rosuvastatin, and pitavastatin exhibited similar effects on lipids, except for a significant increase in HDL-C with pitavastatin. This finding is consistent with the results of other studies.45,46,47,48
Our study has some potential limitations and strengths. Limitations include 1) that we did not calculate sample size, being a pilot trial; 2) that the study was not performed in a double-blind manner; 3) the absence of oral glucose tolerance test; and 4) small study groups. The strengths include 1) that the study was conducted as a simultaneous head-to-head comparison; 2) that antidiabetic therapy was maintained stable throughout the study; and 3) that FPG and HbA1c plasma levels were assessed in blinded conditions.
In conclusion, the results of our study suggest that not all statins are the same. Although the lipid lowering efficacies of atorvastatin, rosuvastatin, and pitavastatin are not statistically different, pitavastatin appears to less negatively affect the action of hypoglycemic drugs in patients with T2DM. This observation has important clinical relevance and may help in choosing the most appropriate statin in patients with dyslipidemia and diabetes. Moreover, our data emphasize the importance of closely monitoring glycemic control during statin therapy, being ready to change therapeutic strategy.
It is worth mentioning that the results of the present pilot study have to be considered as preliminary and that additional double-blind trials are needed to confirm our data.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Francesco Fici.
- Data curation: Bahar Arican Tarim.
- Formal analysis: Nicolás Roberto Robles.
- Funding acquisition: Elif Ari.
- Investigation: Yurdaer Ozcan, Istemihan Tengiz, Elif Ari, and Bahar Arican Tarim.
- Methodology: Guido Grassi.
- Project administration: Bahar Arican Tarim.
- Resources: Elif Ari.
- Software: Saadet Avunduk.
- Supervision: Elif Ari.
- Validation: Gokhan Faikoglu.
- Visualization: Gokhan Faikoglu.
- Writing—original draft: Francesco Fici.
- Writing—review & editing: Francesco Fici and Nicolás Roberto Robles.
- Approval of final manuscript: all authors.
References
- 1.Shahwan MJ, Jairoun AA, Farajallah A, Shanabli S. Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab Syndr. 2019;13:2387–2392. doi: 10.1016/j.dsx.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Athyros VG, Doumas M, Imprialos KP, Stavropoulos K, Georgianou E, Katsimardou A, et al. Diabetes and lipid metabolism. Hormones (Athens) 2018;17:61–67. doi: 10.1007/s42000-018-0014-8. [DOI] [PubMed] [Google Scholar]
- 3.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiorino MI, Longo M, Scappaticcio L, Bellastella G, Chiodini P, Esposito K, et al. Improvement of glycemic control and reduction of major cardiovascular events in 18 cardiovascular outcome trials: an updated meta-regression. Cardiovasc Diabetol. 2021;20:210. doi: 10.1186/s12933-021-01401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore MO, McGuire DK. A test in context: hemoglobin A1c and cardiovascular disease. J Am Coll Cardiol. 2016;68:2479–2486. doi: 10.1016/j.jacc.2016.08.070. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 11.Ko MJ, Jo AJ, Kim YJ, Kang SH, Cho S, Jo SH, et al. Time-and dose-dependent association of statin use with risk of clinically relevant new-onset diabetes mellitus in primary prevention: a nationwide observational cohort study. J Am Heart Assoc. 2019;8:e011320. doi: 10.1161/JAHA.118.011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy R, Ajithan A, Joseph A, Mateti UV, K S. Statin-induced new onset of diabetes in dyslipidemic patients: a retrospective study. Postgrad Med. 2019;131:383–387. doi: 10.1080/00325481.2019.1643636. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadizar F, Ochoa-Rosales C, Glisic M, Franco OH, Muka T, Stricker BH. Associations of statin use with glycaemic traits and incident type 2 diabetes. Br J Clin Pharmacol. 2019;85:993–1002. doi: 10.1111/bcp.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na E, Cho S, Kim DJ, Choi J, Han E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: a retrospective cohort study. Cardiovasc Diabetol. 2020;19:67. doi: 10.1186/s12933-020-01037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu WT, Lin C, Tsai MC, Cheng CC, Chen SJ, Liou JT, et al. Effects of pitavastatin, atorvastatin, and rosuvastatin on the risk of new-onset diabetes mellitus: a single-center cohort study. Biomedicines. 2020;8:499. doi: 10.3390/biomedicines8110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25:1131–1149. doi: 10.1002/pds.4020. [DOI] [PubMed] [Google Scholar]
- 17.Davis TM, Badshah I, Chubb SA, Davis WA. Dose-response relationship between statin therapy and glycaemia in community-based patients with type 2 diabetes: the Fremantle diabetes study. Diabetes Obes Metab. 2016;18:1143–1146. doi: 10.1111/dom.12710. [DOI] [PubMed] [Google Scholar]
- 18.Anyanwagu U, Mamza J, Donnelly R, Idris I. Effects of background statin therapy on glycemic response and cardiovascular events following initiation of insulin therapy in type 2 diabetes: a large UK cohort study. Cardiovasc Diabetol. 2017;16:107. doi: 10.1186/s12933-017-0587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Yuan Y, Cai RR, Huang Y, Xia WQ, Yang Y, et al. Statin therapy on glycaemic control in type 2 diabetes: a meta-analysis. Expert Opin Pharmacother. 2013;14:1575–1584. doi: 10.1517/14656566.2013.810210. [DOI] [PubMed] [Google Scholar]
- 20.Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2014;57:2444–2452. doi: 10.1007/s00125-014-3374-x. [DOI] [PubMed] [Google Scholar]
- 21.Cai R, Yuan Y, Sun J, Xia W, Huang R, Tian S, et al. Statins worsen glycemic control of T2DM in target LDL-c level and LDL-c reduction dependent manners: a meta-analysis. Expert Opin Pharmacother. 2016;17:1839–1849. doi: 10.1080/14656566.2016.1220539. [DOI] [PubMed] [Google Scholar]
- 22.Hammad MA, Abdo MS, Mashaly AM, Syed Sulaiman SA, Alghamdi S, Mangi AA, et al. The statins effects on HbA1c control among diabetic patients: an umbrella review of systematic reviews and meta-analyses of observational studies and clinical trials. Diabetes Metab Syndr. 2019;13:2557–2564. doi: 10.1016/j.dsx.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Angelidi AM, Stambolliu E, Adamopoulou KI, Kousoulis AA. Is atorvastatin associated with new onset diabetes or deterioration of glycemic control? Systematic review using data from 1.9 million patients. Int J Endocrinol. 2018;2018:8380192. doi: 10.1155/2018/8380192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui JY, Zhou RR, Han S, Wang TS, Wang LQ, Xie XH. Statin therapy on glycemic control in type 2 diabetic patients: a network meta-analysis. J Clin Pharm Ther. 2018;43:556–570. doi: 10.1111/jcpt.12690. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi F, Lamendola C, Harris CS, Harris V, Tsai MS, Tripathi P, et al. Statins are associated with increased insulin resistance and secretion. Arterioscler Thromb Vasc Biol. 2021;41:2786–2797. doi: 10.1161/ATVBAHA.121.316159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thongtang N, Tangkittikasem N, Samaithongcharoen K, Piyapromdee J, Srinonprasert V, Sriussadaporn S. Effect of switching from low-dose simvastatin to high-dose atorvastatin on glucose homeostasis and cognitive function in type 2 diabetes. Vasc Health Risk Manag. 2020;16:367–377. doi: 10.2147/VHRM.S270751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa H, Matsui K, Saito Y, Sugiyama S, Jinnouchi H, Sugawara M, et al. Differences between rosuvastatin and atorvastatin in lipid-lowering action and effect on glucose metabolism in Japanese hypercholesterolemic patients with concurrent diabetes. Lipid-lowering with highly potent statins in hyperlipidemia with type 2 diabetes patients (LISTEN) study. Circ J. 2014;78:2512–2515. doi: 10.1253/circj.cj-14-0810. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, et al. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241:409–418. doi: 10.1016/j.atherosclerosis.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Chapman MJ, Orsoni A, Robillard P, Hounslow N, Sponseller CA, Giral P. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: insights from the CAPITAIN and PREVAIL-US studies. Curr Med Res Opin. 2014;30:775–784. doi: 10.1185/03007995.2013.874989. [DOI] [PubMed] [Google Scholar]
- 31.Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2008;15:269–275. doi: 10.5551/jat.e562. [DOI] [PubMed] [Google Scholar]
- 32.Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–1130. doi: 10.1016/j.amjcard.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Koh KK, Quon MJ, Sakuma I, Han SH, Choi H, Lee K, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2013;166:509–515. doi: 10.1016/j.ijcard.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Park JB, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis. 2009;204:483–490. doi: 10.1016/j.atherosclerosis.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Kostapanos MS, Milionis HJ, Agouridis AD, Rizos CV, Elisaf MS. Rosuvastatin treatment is associated with an increase in insulin resistance in hyperlipidaemic patients with impaired fasting glucose. Int J Clin Pract. 2009;63:1308–1313. doi: 10.1111/j.1742-1241.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 37.Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20-40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13:1047–1055. doi: 10.1111/j.1463-1326.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 38.Takano T, Yamakawa T, Takahashi M, Kimura M, Okamura A. Influences of statins on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13:95–100. doi: 10.5551/jat.13.95. [DOI] [PubMed] [Google Scholar]
- 39.Yokote K, Saito Y CHIBA. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study) J Atheroscler Thromb. 2009;16:297–298. doi: 10.5551/jat.e1008. [DOI] [PubMed] [Google Scholar]
- 40.Livingstone SJ, Looker HC, Akbar T, Betteridge DJ, Durrington PN, Hitman GA, et al. Effect of atorvastatin on glycaemia progression in patients with diabetes: an analysis from the collaborative atorvastatin in diabetes trial (CARDS) Diabetologia. 2016;59:299–306. doi: 10.1007/s00125-015-3802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu CH, Lee JK, Lam HC, Lu CC, Sun CC, Wang MC, et al. Atorvastatin does not affect insulin sensitivity and the adiponectin or leptin levels in hyperlipidemic type 2 diabetes. J Endocrinol Invest. 2008;31:42–47. doi: 10.1007/BF03345565. [DOI] [PubMed] [Google Scholar]
- 42.Jeong HS, Hong SJ, Son S, An H, Kook H, Joo HJ, et al. Incidence of new-onset diabetes with 1 mg versus 4 mg pitavastatin in patients at high risk of developing diabetes during a 3-year follow-up. Cardiovasc Diabetol. 2019;18:162. doi: 10.1186/s12933-019-0969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnaboldi L, Corsini A. Could changes in adiponectin drive the effect of statins on the risk of new-onset diabetes? The case of pitavastatin. Atheroscler Suppl. 2015;16:1–27. doi: 10.1016/S1567-5688(14)70002-9. [DOI] [PubMed] [Google Scholar]
- 44.Koh KK, Oh PC, Sakuma I, Lee Y, Han SH, Shin EK. Rosuvastatin dose-dependently improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Int J Cardiol. 2016;223:488–493. doi: 10.1016/j.ijcard.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Saku K, Zhang B, Noda K PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75:1493–1505. doi: 10.1253/circj.cj-10-1281. [DOI] [PubMed] [Google Scholar]
- 46.Adams SP, Alaeiilkhchi N, Wright JM. Pitavastatin for lowering lipids. Cochrane Database Syst Rev. 2020;6:CD012735. doi: 10.1002/14651858.CD012735.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan P, Shao L, Tomlinson B, Zhang Y, Liu ZM. An evaluation of pitavastatin for the treatment of hypercholesterolemia. Expert Opin Pharmacother. 2019;20:103–113. doi: 10.1080/14656566.2018.1544243. [DOI] [PubMed] [Google Scholar]
- 48.Hoy SM. Pitavastatin: a review in hypercholesterolemia. Am J Cardiovasc Drugs. 2017;17:157–168. doi: 10.1007/s40256-017-0213-8. [DOI] [PubMed] [Google Scholar]