Abstract
Background:
Few studies have examined survival outcomes in relapsed childhood acute myeloid leukemia (AML) in resource-limited countries. This study aimed to evaluate the prognostic factors and survival outcomes of relapsed childhood AML in Thailand.
Methods:
The medical records of AML patients aged 0-15 years treated in a major tertiary center in Southern Thailand between December 1979 and December 2019 were reviewed retrospectively. The overall survival (OS) was calculated using the Kaplan-Meier method.
Results:
A total of 316 AML patients were included and relapse occurred in 98 (31%) patients. Of these, 57 (58.2%) and 41 (41.8%) patients had early [≤1 year from first complete remission (CR1)] and late (>1 year from CR1) relapses, respectively. Only 54 (55.1%) patients received chemotherapy after relapse. The 3-year OS of all relapsed patients was 3.5%. The 3-year OS of patients with early and late relapse were 0% and 8.5%, respectively (p=0.002). The 3-year OS of patients who received chemotherapy and those who did not were 6.5% and 0%, respectively (p <0.0001). The median survival time of patients who did not receive chemotherapy was 1.7 months. The 3-year OS of patients who achieved second complete remission (CR2) and those who did not were 12.6% and 0%, respectively (p <0.001).
Conclusion:
The relapsed AML rate was 31% and the survival outcome was poor with a 3-year OS of 3.5%. The adverse prognostic factors were early relapse, failure to achieve CR2 and those who did not receive chemotherapy after relapse.
Key Words: Relapsed childhood acute myeloid leukemia, survival outcome, resource-limited countries
Introduction
Treatment outcomes of childhood acute myeloid leukemia (AML) have improved significantly over recent decades due to intensive chemotherapy, improved supportive care and the use of hematopoietic stem cell transplantation (HSCT) for high-risk patients (Moritake et al., 2021; Taga et al., 2016). However, relapse, which occurs in approximately 20-40% of cases, remains the most common cause of treatment failure and mortality (Abrahamsson et al., 2007; Alexander et al., 2017; Liang et al., 2006; Moritake et al., 2021; Nakayama et al., 2014; Taga et al., 2016; Van Weelderen et al., 2021; Xu et al., 2010). The survival outcome of relapsed childhood AML remains poor with long-term overall survival (OS) ranging from 23% to 42% in high-income countries (Creutzig et al., 2014; Hoffman et al., 2021; Jastaniah et al., 2018; Kaspers, 2014; Kaspers et al., 2013; Nakayama et al., 2014; Rasche et al., 2021). There is only one study reporting the survival outcomes of childhood relapsed AML in a resource-limited country, which showed a long-term OS of only 7% (Marjerrison et al., 2014). Additionally, there are no studies comparing the survival outcome among those who received and did not receive chemotherapy after relapse. To better understand the poor outcomes and evaluate survival among children with relapsed AML who did not receive chemotherapy after relapse, studies involving patients in resource-limited countries with restricted access to HSCT are needed. The aim of this study was to examine the prognostic factors and survival outcomes of relapsed childhood AML in Thailand.
Materials and Methods
Patients
We retrospectively reviewed the medical records of all patients diagnosed with AML (excluding those with acute promyelocytic leukemia) aged under 15 years between December 1979 and December 2019 at Songklanagarind Hospital, the largest tertiary health care institution and a university hospital in Southern Thailand.
The clinical characteristics and survival outcomes of patients with relapsed AML were collected including age, gender, timing of initial diagnosis, time from a first complete remission (CR1) to relapse, site of relapse, treatment for relapse, second complete remission (CR2) and second relapse (if any), and survival status at the last follow up.
Initial AML diagnosis
The initial diagnosis of AML was made based on morphological and immunochemical examinations of bone marrow material obtained through aspiration and/or biopsy, with the criterion of >20% myeloblasts used for definitive diagnosis. Even if the blast threshold of 20% was not met, a diagnosis of AML was made when evidence of recurrent genetic abnormalities such as t(8;21), inv(16), or t(16;16) was found (Creutzig et al., 2012).
Chemotherapy protocols for newly diagnosed AML
The chemotherapy regimens used to treat newly diagnosed AML patients were divided into four periods. Between 1979 and 1993, the AML-Berlin-Frankfurt-Münster (BFM)-78 regimen was used (Scheer et al., 1979). During 1994-2007, the AML-BFM-83 regimen was used (Creutzig et al., 1990). From 2008-2013, the AML-BFM 98 regimen was used (Creutzig et al., 2006), and between 2014 and 2019, the chemotherapy according to the Children’s Oncology Group was used (Cooper et al., 2012).
At the completion of the induction phase, bone marrow aspiration and/or biopsy was performed. The patient was considered to have achieved CR1 if they had <5% blast cells in the bone marrow and there were no blast cells in the cerebrospinal fluid (CSF).
Diagnosis of relapsed AML
The diagnosis of relapse was established at any time after achieving CR1. Isolated bone marrow relapse was defined as the presence of more than 20% blast cells in the bone marrow. Isolated central nervous system (CNS) relapse was defined as the presence of ≥5/µL leukocytes with identifiable blast cells in a cytocentrifuged preparation of CSF, or the development of cranial nerve palsies. Isolated testicular relapse was defined as the presence of unilateral or bilateral testicular enlargement, with biopsy-proven leukemic involvement in the absence of bone marrow involvement (<5% blast cells). The presence of ≥5% blast cells in the bone marrow and extramedullary disease was categorized as a combined relapse. Early and late relapse were defined as relapse within and after one year of achieving CR1, respectively (Gorman et al., 2010; Nakayama et al., 2014).
Chemotherapy protocols for relapsed AML
Patients who relapsed were retreated with the same chemotherapy regimen used following the initial diagnosis.
At the completion of the re-induction phase, bone marrow aspiration and/or biopsy was performed. The patient was considered to have achieved CR2 if they had <5% blast cells in the bone marrow and no blast cells in the CSF.
Statistical analysis
For continuous variables, descriptive statistics are presented using mean and standard deviation or median and interquartile range (IQR), as appropriate. For categorical variables, descriptive statistics are presented using frequency with percentage. The overall survival (OS) from the time of diagnosis of relapsed AML to various time points was depicted using the Kaplan–Meier method. The log rank test was used for comparing survival rates between groups. A p-value of less than 0.05 was considered significant.
Results
A total of 334 patients were diagnosed with AML in our center during the 40-year study period. We excluded 18 patients who had incomplete data, leaving a total of 316 patients for analysis. Of these, 98 (31.0%) had relapsed AML at a median age of 8.4 years (IQR 3.9 - 12.4). The percentages of males and females were 59.2% and 40.8%, respectively. Of the 98 relapsed patients, 57 (58.2%) had early relapse with a median (IQR) time from initial diagnosis of 7.2 (6.0-9.6) months and 41 (41.8%) had late relapse at a median (IQR) time from initial diagnosis of 25.2 (18.0-36.0) months. The most common site of relapse was isolated bone marrow in 88 patients (89.8%) followed by isolated CNS in 5 patients (5.1%) and combined sites in 5 patients (5.1%).
Of the 98 relapsed patients, 54 (55.1%) received chemotherapy after relapse, while 44 (44.9%) did not receive chemotherapy after relapse. As shown in Table 1, of the 54 patients who received chemotherapy after relapse, 25 (46.3%) achieved CR2, 19 (35.2%) did not, and 10 (18.5%) died during re-induction chemotherapy.
Table 1.
Characteristics of Study Patients with Relapsed Acute Myeloid Leukemia (n = 98)
| Characteristic | n (%) |
|---|---|
| Age (years), median (IQR) | 8.4 (3.9-12.4) |
| Sex | |
| Male | 58 (59.2) |
| Female | 40 (40.8) |
| Timing of relapse | |
| Early | 57 (58.2) |
| Late | 41 (41.8) |
| Site of relapse | |
| Bone marrow | 88 (89.8) |
| CNS | 5 (5.1) |
| Combined (bone marrow with other site(s)) | 5 (5.1) |
| Treatment at relapse | |
| Chemotherapy | 54 (55.1) |
| Achieved CR2 | 25 (46.3) |
| Failed CR2 | 19 (35.2) |
| Re-induction death | 10 (18.5) |
| Alternative medicine | 44 (44.9) |
| Second relapsea | 18 (33.3) |
| Final status | |
| Alive | 3 (3.1) |
| Dead | 95 (96.9) |
a, Among patients who received chemotherapy only (n = 54); CNS, central nervous system; CR2, second complete remission; IQR, interquartile range
Survival outcomes
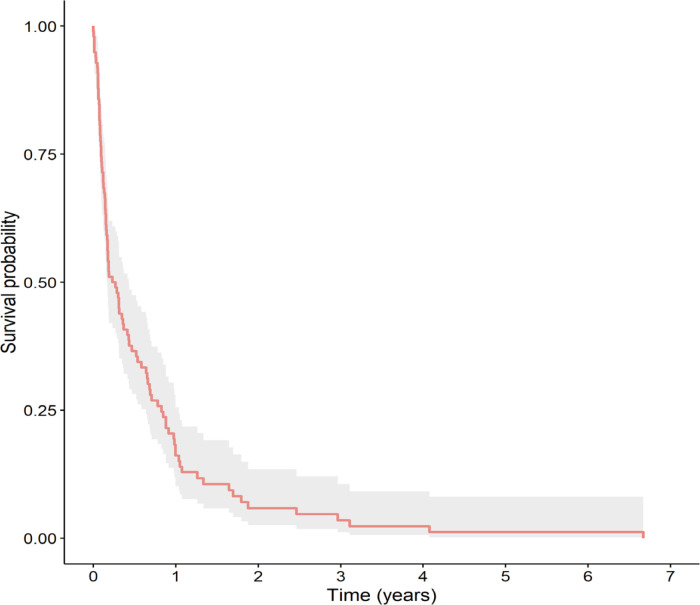
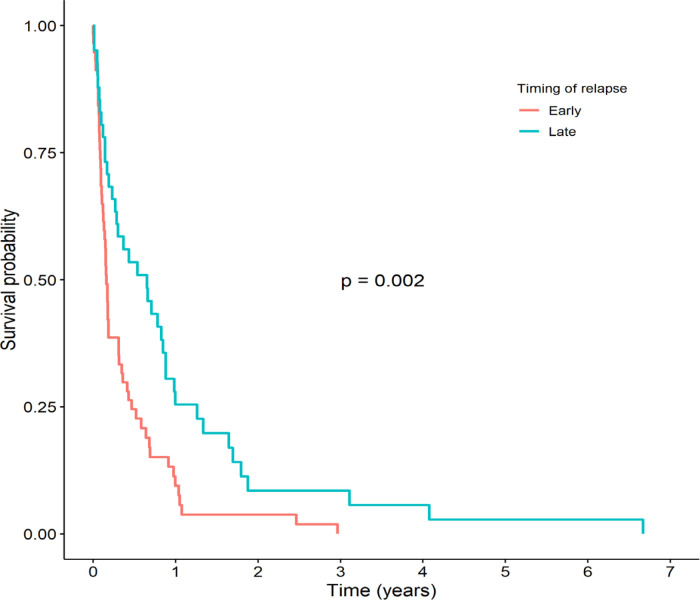
The 3-year and 5-year OS rates were 3.5% and 1.2%, respectively (Figure 1), with a median (IQR) survival time of 3.0 (2.0-5.2) months. The main prognostic factor associated with survival was the timing of the relapse. As shown in Figure 2, the 3-year OS among patients with early relapse was significantly lower than in patients with late relapse (0% vs. 8.5%, respectively, p = 0.002). The median (IQR) survival time of patients with early relapse was 1.9 (1.5-3.7) months while that for those with late relapse was 7.8 (3.4-10.6) months.
Figure 1.
Kaplan-Meier Survival Curve of Total Study Patients with Relapsed Acute Myeloid Leukemia (n=98)
Figure 2.
Kaplan-Meier Survival Curves Stratified by Timing of Relapse between Patients with Early and Late Relapsed Acute Myeloid Leukemia
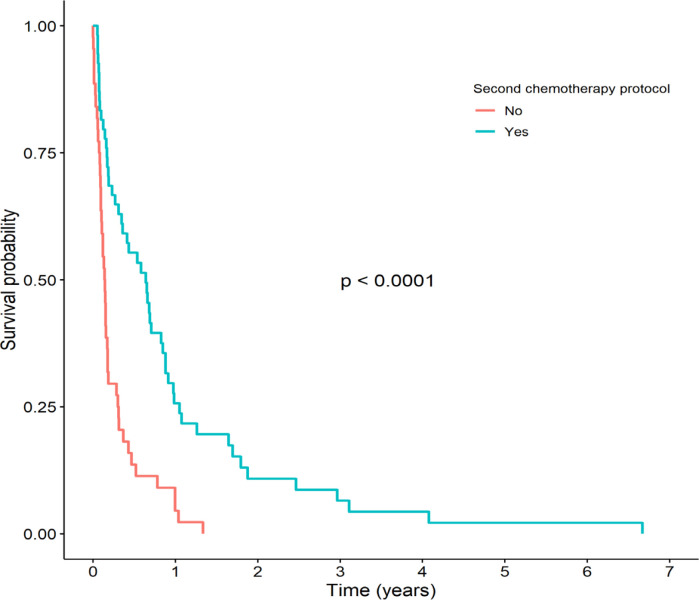
The patients who did not receive relapse chemotherapy had very poor survival outcomes. As shown in Figure 3, the 1- and 3-year OS among patients who received chemotherapy after relapse was significantly higher than in the patients who did not (4.6% vs. 25.7% and 0% vs. 6.5%, respectively, p <0.0001). The median (IQR) survival time of patients who received chemotherapy after relapse was 7.7 (4.2-10.6) months. Among the patients who did not receive chemotherapy after relapse, all died within 16 months with a median (IQR) survival time of 1.7 (1.2-2.1) months.
Figure 3.
Kaplan-Meier Survival Curves Stratified by Relapse Chemotherapy between Patients who Received Relapse Chemotherapy and Those who Did not
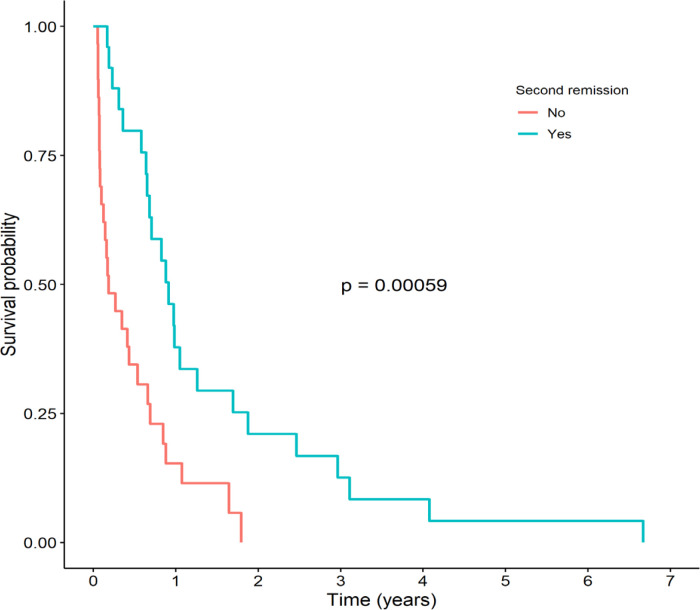
The patients who achieved CR2 had a significantly higher 3-year OS than those who did not (12.6% vs. 0%, respectively, p <0.001), as shown in Figure 4. The median (IQR) survival time of patients who achieved CR2 was 11.0 (8.2-22.6) months, while the median (IQR) survival time of patients who did not achieve CR2 was 2.2 (1.5-7.9) months.
Figure 4.
Kaplan-Meier Survival Curves Stratified by Second Complete Remission (CR2) between Patients who Achieved CR2 and Those who Did not
Discussion
The prevalence of relapsed AML in our study was 31%, which was not different from other studies which reported rates ranging from 20% to 40% (Abrahamsson et al., 2007; Alexander et al., 2017; Liang et al., 2006; Moritake et al., 2021; Nakayama et al., 2014; Taga et al., 2016; Van Weelderen et al., 2021; Xu et al., 2010). The OS in our study was less than 5%, which is significantly lower than previous studies from high-income countries, which reported rates of 23-42% (Creutzig et al., 2014; Hoffman et al., 2021; Jastaniah et al., 2018; Kaspers, 2014; Kaspers et al., 2013; Nakayama et al., 2014; Rasche et al., 2021). In comparison with other resource-limited countries, our study demonstrated a similar survival outcome compared to a study by Marjerrison et al. (2014) from Central America, which found an OS of 7% in the setting with limited access to minimal residual disease (MRD), HSCT and immunotherapy. A lower survival rate is possible due to the effect of a lack of access to these modern therapies and MRD-directed risk stratification treatments.
The important finding in our study is the survival outcomes in patients who did not receive chemotherapy after relapse. In our study, 44 of 98 (44.9%) patients did not receive chemotherapy after relapse, which is significantly higher than previous studies that found rates ranging from 4% to 29% (Abrahamsson et al., 2007; Aladjidi et al., 2003; Goemans et al., 2008; Gorman et al., 2010; Marjerrison et al., 2014; Rasche et al., 2021; Rubnitz et al., 2007; Sander et al., 2010; Webb et al., 1999). Abrahamsson et al. (2007) reported that 21 of 146 (14.4%) relapsed AML patients did not receive chemotherapy after relapse, and all of them died with a median survival time of 1.8 months. Another study by Aladjidi et al., (2003) reported that 10 of 106 (9.4%) relapsed AML patients did not receive chemotherapy after relapse, and all died with a median survival time of 2.5 months. Similarly, relapsed AML patients who did not receive chemotherapy after relapse in our study had a median survival time of 1.7 months. However, no previous studies have reported on OS among patients who did not receive chemotherapy after relapse. Our study found that patients who did not receive chemotherapy after relapse had a very poor survival outcome with a 1-year OS of only 4.6%, which is significantly lower in than those who received chemotherapy after relapse. Thus, a high incidence of patients who did not receive chemotherapy after relapse, in addition to the modern therapies and MRD-directed risk stratification treatments, is another factor associated with a lower survival outcome in our study.
We found that the adverse prognostic factors for relapsed AML were early relapse and failure to achieve CR2. Our findings on the time of relapse were consistent with those of other studies that demonstrated early relapse to be a poor predictive factor for survival outcome (Gorman et al., 2010; Nakayama et al., 2014). The patients in our study with late relapse had a longer survival time than those who relapsed earlier. In our study, achieving CR2 following re-induction chemotherapy was associated with a better survival, which is consistent with prior studies (Creutzig et al., 2014; Jastaniah et al., 2018; Moritake et al., 2021; Nakayama et al., 2014; Rasche et al., 2021).
In the absence of risk-adapted, MRD-directed risk stratification treatment, HSCT, and immunotherapy, our study found that patients with relapsed AML had a poor prognosis. As a result, we believe that MRD-directed risk stratification treatment, HSCT, and immunotherapy might be beneficial to individuals with relapsed AML (Bekadja et al., 2022). Moreover, patients with late relapse, who had a better survival outcome than those who relapsed earlier, should receive intensive re-induction chemotherapy to achieve CR2 and strategies should be developed to assist healthcare providers to persuade these patients and their families to accept this life-prolonging treatment.
There were certain limitations to our study. First, there is the potential bias inherent in all retrospective studies. Second, our study covered a 40-year period during which advances in diagnosis, chemotherapy regimens and supportive care were made. As a result, our data may be considered to be heterogeneous in terms of diagnosis, chemotherapy regimen, and supportive care during the study period. Third, our study did not include molecular studies and MRD, which are important for identifying AML risk and treatment. Fourth, due to the small number of patients who had prolonged survival after relapse, multivariate analysis to predict mortality was not possible. Finally, there is a possibility of referral bias because our setting was a major tertiary health care facility and university hospital that treats predominantly high-risk and complicated cases with AML.
Over the 40-year study period, the overall relapsed AML rate was 31%. The outcome of relapsed AML was poor with a 3-year OS of 3.5%. The adverse prognostic factors were early relapse, failure to achieve CR2 and those who did not receive chemotherapy.
Author Contribution Statement
N.S., P.S., S.C., E.M. and T.C. all contributed significantly to the conception and design, acquisition of data, and/or analysis and interpretation of data. N.S. and T.C. were primarily responsible for drafting and revising the manuscript for critical intellectual content. The manuscript was given final approval by all authors before it was submitted for publication.
Acknowledgments
We would like to thank Mr. Dave Patterson of the Office of International Affairs, Faculty of Medicine, Prince of Songkla University for proofreading the manuscript.
Ethical declaration
Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
Data availability statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflict of interest
The authors report there are no competing interests to declare. There was no funding support for this study.
References
- Abrahamsson J, Clausen N, Gustafsson G, et al. Improved outcome after relapse in children with acute myeloid leukaemia. Br J Haematol. 2007;136:229–36. doi: 10.1111/j.1365-2141.2006.06419.x. [DOI] [PubMed] [Google Scholar]
- Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21:4377–85. doi: 10.1200/JCO.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Alexander TB, Wang L, Inaba H, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123:3791–8. doi: 10.1002/cncr.30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekadja M, Mansour B, Ouldjeriouat H, et al. Prognostic impact on survival of early relapse after autologous stem cell transplantation with non-cryopreserved stem cells for multiple myeloma in real life: A single-center cohort study from Oran (Algeria) Asian Pac J Cancer Biol. 2022;7:15–20. [Google Scholar]
- Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children’s Oncology Group. Cancer. 2012;118:761–9. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Ritter J, Schellong G. Identification of two risk groups in childhood acute myelogenous leukemia after therapy intensification in study AML-BFM-83 as compared with study AML-BFM-78. AML-BFM Study Group. Blood. 1990;75:1932–40. [PubMed] [Google Scholar]
- Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol. 2006;24:4499–506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood. 2012;120:3187–205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Zimmermann M, Dworzak MN, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: Results of the international study Relapsed AML 2001/01. Haematologica. 2014;99:1472–8. doi: 10.3324/haematol.2014.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goemans BF, Tamminga RYJ, Corbijn CM, Hählen K, Kaspers GJL. Outcome for children with relapsed acute myeloid leukemia in the Netherlands following initial treatment between 1980 and 1998: Survival after chemotherapy only? Haematologica. 2008;93:1418–20. doi: 10.3324/haematol.12807. [DOI] [PubMed] [Google Scholar]
- Gorman MF, Ji L, Ko RH, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): A Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55:421–9. doi: 10.1002/pbc.22612. [DOI] [PubMed] [Google Scholar]
- Hoffman AE, Schoonmade LJ, Kaspers GJL. Pediatric relapsed acute myeloid leukemia: A systematic review. Expert Rev Anticancer Ther. 2021;21:45–52. doi: 10.1080/14737140.2021.1841640. [DOI] [PubMed] [Google Scholar]
- Jastaniah W, Bayoumy M, Alsultan A, et al. Identifying prognostic factors that influence outcome of childhood acute myeloid leukemia in first relapse in Saudi Arabia: Results of the multicenter SAPHOS study. Clin Lymphoma Myeloma Leuk. 2018;18:773–80. doi: 10.1016/j.clml.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Kaspers G. How I treat paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2014;166:636–45. doi: 10.1111/bjh.12947. [DOI] [PubMed] [Google Scholar]
- Kaspers GJL, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: Results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31:599–607. doi: 10.1200/JCO.2012.43.7384. [DOI] [PubMed] [Google Scholar]
- Liang DC, Chan TT, Lin KH, et al. Improved treatment results for childhood acute myeloid leukemia in Taiwan. Leukemia. 2006;20:136–41. doi: 10.1038/sj.leu.2403979. [DOI] [PubMed] [Google Scholar]
- Marjerrison S, Antillon F, Bonilla M, et al. Outcome of children treated for relapsed acute myeloid leukemia in Central America. Pediatr Blood Cancer. 2014;61:1222–6. doi: 10.1002/pbc.24942. [DOI] [PubMed] [Google Scholar]
- Moritake H, Tanaka S, Miyamura T, et al. The outcomes of relapsed acute myeloid leukemia in children: Results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 2021;68:e28736. doi: 10.1002/pbc.28736. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Tabuchi K, Tawa A, et al. Outcome of children with relapsed acute myeloid leukemia following initial therapy under the AML99 protocol. Int J Hematol. 2014;100:171–9. doi: 10.1007/s12185-014-1616-9. [DOI] [PubMed] [Google Scholar]
- Rasche M, Zimmermann M, Steidel E, et al. Survival following relapse in children with acute myeloid leukemia: A report from AML-BFM and COG. Cancers. 2021;13:2336. doi: 10.3390/cancers13102336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Razzouk BI, Lensing S, et al. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007;109:157–63. doi: 10.1002/cncr.22385. [DOI] [PubMed] [Google Scholar]
- Sander A, Zimmermann M, Dworzak M, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: Results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010;24:1422–8. doi: 10.1038/leu.2010.127. [DOI] [PubMed] [Google Scholar]
- Scheer U, Schellong G, Riehm H. Prognosis improvements in children with acute myelocytic leucemia after more intensive induction therapy. Klin Padiatr. 1979;191:210–6. [PubMed] [Google Scholar]
- Taga T, Tomizawa D, Takahashi H, Adachi S. Acute myeloid leukemia in children: Current status and future directions. Pediatr Int. 2016;58:71–80. doi: 10.1111/ped.12865. [DOI] [PubMed] [Google Scholar]
- Van Weelderen RE, Klein K, Natawidjaja MD, De Vries R, Kaspers GJL. Outcome of pediatric acute myeloid leukemia (AML) in low- and middle-income countries: A systematic review of the literature. Expert Rev Anticancer Ther. 2021;21:765–80. doi: 10.1080/14737140.2021.1895756. [DOI] [PubMed] [Google Scholar]
- Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999;13:25–31. doi: 10.1038/sj.leu.2401254. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Tang YM, Song H, et al. Long-term outcome of childhood acute myeloid leukemia in a developing country: Experience from a children’s hospital in China. Leuk Lymphoma. 2010;51:2262–9. doi: 10.3109/10428194.2010.518653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.