Abstract
Background:
Persistent infection with high-risk (HR) Human Papilloma Virus (HPV) genotypes has been shown to play a significant role in the development of cervical intraepithelial neoplasia (CIN) and CC (cervical cancer). The present study aimed to determine the distribution and quantification of viral load of HPV genotypes in numerous genital samples obtained from women undergoing routine gynaecological care in different regions of Turkey.
Methods:
HPV typing was done by HPV QUANT-21 Quantitative RT-PCR Kit®, which is intended for the specific identification and quantification of low-risk (HPV 6, 11, 44) and high-risk (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) from female subjects in Turkey.
Results:
From the total of 5,975 samples, 2,777 were positive for at least one HPV genotype, with an overall frequency of 46.4%. 1695 patients were positive for only one or more LR-HPV genotypes (61%) and 812 patients were positive for one or more HR-HPV genotypes (29%). The frequency of LR-HPV genotypes was 31.4%, while the frequency of HR-HPV genotypes was 118.8%. Our tecnology had a positive advantage to calculate the concentration of each genotypes. Although genotype 52 ranked fifth in frequency, it showed the highest mean concentration, with a value of 5.38 log (copies/sample).
Conclusion:
The presence and genotype of viruses before HPV vaccination have also gained importance. The data obtained would provide guidance for prevention strategies, mainly of vaccination. We decided to add a new estimate to the effectiveness of currently available HPV vaccines and the development of screening programs to prevent and decrease the incidence of CC in Turkey. Further studies would be planned to measure and define the high infection level that can lead to the development of cervical neoplasia. Using this tecnology could give us a clinical desicion to degree the cytological changes.
Key Words: Cervical cancer, cervical intraepithelial neoplasia, HPV, HPV DNA, HPV genotype
Introduction
Cervical cancer (CC) is the 12th most common cancer among women worldwide, with an estimated 604,127 new cases and 341,831 deaths in 2020 (Sung et al., 2021). About 2,532 new CC cases are diagnosed annually in Turkey (estimations for 2020). Also, CC ranks as the 12th leading cause of female cancer and the 5th most common female cancer in women aged 15 to 44 years, in Turkey (Ferlay et al., 2020). Approximately, 75-80% of CC cases are seen in developing countries where efficient cervical screening is insufficient (Parkin et al., 2005). Early diagnosis of precancerous lesions is important because the progression to invasive neoplasia takes as long as 15-20 years giving enough time for treatment (Munger et al., 2004).
Persistent infection with various Human Papilloma Virus (HPV) genotypes has been shown to play a significant role in the development of high-risk (HR) and low-risk (LR) cervical intraepithelial neoplasia (CIN) and CC (Zur Hausen, 2002; Bosch et al., 2002, Koutsky et al., 1992). To date, more than 220 HPV genotypes have been described (Bzhalava et al., 2015), of which at least 40 genotypes infect the female genital tract (Bosh et al., 1995; Clifford et al., 2003). Although this virus is present in more than 90% of CC cases, only a small proportion (1%) of infected women develop cancer (Haverkos, 2005). According to the International Agency for Research in Cancer (IARC) and the World Health Organization (WHO), 12 HPV genotypes, namely 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59, are considered high-risk HPV genotypes and belong to the group I of human biologic carcinogens that are found in 99.7% of precancerous squamous intraepithelial lesions (Bosch et al., 1995). HPV 68 is classified as probably carcinogenic (Group 2A) and genotypes 26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, and 97 are classified as possibly carcinogenic (Group 2B) (Annunziata et al., 2018). Human Papilloma Virus (HPV) is also divided into two groups according to its neoplastic potential. HPV 6 and 11, in particular, are referred to as LR-HPV genotypes, whereas HPV 16 and HPV 18 are referred to as “high risk” HR-HPV genotypes those that detected in 55% and 18% of all CC cases, respectively (Munoz et al., 2003).
Moreover, the prevalence and genotypic distribution of HPV infections vary greatly between populations; for example, HPV 31 and 33 are more prevalent in Europe and the United States, while genotypes 35 and 45 are more frequent in Africa and 52 and 58 in Asia (De Sanjose et al., 2010). In previous studies on HPV epidemiology in Turkey, many authors have identified a number of genotypes that were not only HPV 16 and 18 (Cilingir et al., 2013; Colakoglu et al., 2017; Duran et al., 2017).
Regional data on the prevalence and genotypic distribution of HPV are essential for estimating the impact of vaccines on CC and screening programmes. Because there is a causal relationship between HPV and CC and HPV immunity is genotype specific, determining LR- and HR-HPV prevalence in different geographic areas is critical. As the frequency of genotypes other than HPV 16 and 18 was still not clear in Turkey, the present study aimed to determine the relative frequency and distribution of HPV genotypes in numerous genital samples obtained from women undergoing routine gynaecological care in different regions of Turkey. Also, quantification of viral genome was a critical value to make a critical desicion.
Materials and Methods
Cervical sample collection
Our study consisted of an unknown open population, and the collection of the samples was conducted from January 2021 to May 2022. Open-population samples were obtained from women seeking routine gynaecologic care at several special healthcare centers or government hospitals and sent to a special laboratory as ordered to analyse HPV genotyping from all over the country. We have no detailed information about the patient examinations or findings that all samples were included in the data except cases with insufficient data (without age) or poor DNA quality or quantity were excluded. A total of 5975 genital brushings were obtained according to medical indication, from women aged in the range of 14-85 years old (years) (median 34 years) and consecutively enrolled in the study.
Cervical samples were collected during a gynaecological examination. The sampling using a device for self-sampling is carried out in accordance with the instructions for use of special sterile disposable urogenital swabs. Before the sampling procedure, it is necessary to remove the mucus with a cotton tampon and, then, treat the cervix with a sterile physiological solution. The sampling swab is inserted into the cervical canal to a depth of 0.5-1.5 cm. Contact of the swab with the walls of the vagina should be excluded.
Procedural limitations: local application of medicine, vaginal ultrasound less than 24 hours before the procedure. Women must not perform hygiene procedures or syringing prior to the sampling procedure.
Transportation and Storage of the Samples
In the case of usage of transport media biological material samples were transported and stored according to the instructions for the transport medium used intended for subsequent sample analysis by PCR. Daily, samples were transferred to the microbiology laboratory in STOR-F transport medium (DNA-Tecnology, Russia). Samples should be stored at temperatures ranging from 2 to 4°C for no more than 24 hours. When it was impossible to deliver the material in the laboratory during the day, a one-time freezing of the material stored at temperatures of minus 20°C for one month was allowed.
DNA Extraction
DNA extraction was performed following the methodology recommended by the manufacturer using a commercial kit (Prep-NA Plus, DNA-Tecnology, Russia).
HPV Genotype Detection and Quantification
Open-population samples were analysed by a device named DTlite real-time PCR (DNA-Technology, Russia). HPV genotyping was done by HPV QUANT-21 Quantitative REAL-TIME PCR Kit® (DNA-Tecnology, Russia), which is intended for the specific identification and quantification of low-risk (HPV 6, 11, 44) and high-risk (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) with regard to their oncogenic properties of HPV. In the samples containing HPV DNA (specific product), the absolute quantity of this virus type was was given as log (copies/sample) (the degree of concentration common logarithm, number of copies of the HPV DNA per sample).
Ethic Committe
There was no need to obtain informed consent from participants as the data was retrieved from daily HPV genotyping routines. All samples were analysed in a special reference laboratory, named Special Gelisim Laboratories, located in İstanbul, Turkey. Prior to data collection, the laboratory requested a written confirmation report confirming that all participant data was handled confidentially. This study was approved by the Ethics and Research Committees of Nisantasi University, School of Medicine.
Statistical Analysis
Data was analysed using IBM SPSS Statistics version 22.0 software (IBM Corp., Armonk, NY). Where applicable, data were expressed as median (interquartile range, IQR) and mean (standard deviation). Comparisons between groups were performed using the chi-square test or Fisher’s exact test. A statistically significant p-value of 0.05 was considered (two-tailed test).
Table 5.
Frequency of Low Risk-HPV and High Risk-HPV Genotypes
| HPV Genotypes |
n | *Mean Value (SD) | *Minimum-Maximum Values |
|---|---|---|---|
| LR-HPV | |||
| Type 6 | 448 | 4.69 (1.97) | 0.4 - 3.9 |
| Type 11 | 252 | 4.92 (1.92) | 0.7 - 12.1 |
| Type 44 | 172 | 4.79 (1.47) | 0.7 - 11.2 |
| Total | 872 | 4.80 (0.09) | 0.4 - 11.2 |
| HR-HPV | |||
| Type 16 | 533 | 4.75 (1.93) | 1.93 - 13.3 |
| Type 18 | 300 | 5.12 (2.24) | 1.7 - 11.4 |
| Type 26 | 80 | 4.71 (1.99) | 1.6 - 12.6 |
| Type 31 | 173 | 5.10 (2.37) | 0.3 - 12.2 |
| Type 33 | 74 | 4,50 (1,73) | 1 - 11.8 |
| Type 35 | 127 | 4.40 (1.74) | 1.74 - 10.9 |
| Type 39 | 186 | 4.20 (1.62) | 0.5 - 11.6 |
| Type 45 | 149 | 5.12 (2.01) | 0.7 - 11.6 |
| Type 51 | 267 | 4.97 (1.70) | 1.7 - 11.7 |
| Type 52 | 210 | 5.38 (1.82) | 1 - 11.9 |
| Type 53 | 194 | 4.99 (1.71) | 0.1 - 11.6 |
| Type 56 | 215 | 4.78 (1.92) | 1.9 - 11.3 |
| Type 58 | 135 | 4.70 (2.13) | 2.1 - 11.4 |
| Type 59 | 166 | 4.79 (2.11) | 1.4 - 11.3 |
| Type 66 | 189 | 4.76 (1.91) | 1.7 - 11.5 |
| Type 68 | 166 | 4.14 (1.72) | 1.72 - 11.5 |
| Type 73 | 77 | 5.12 (2.23) | 1.8 - 11.6 |
| Type 82 | 55 | 4.99 (2.43) | 1.8 - 11.5 |
| Total | 3296 | 4.80 (0.32) | 0.3 - 13.3 |
*log (copies/sample)
Results
A total of 5,975 female subjects seeking routine gynaecologic care at the family planning and gynaecological clinics of different hospitals in Turkey were recruited. From the total samples, 2,777 were positive for at least one HPV genotype, with an overall frequency of 46.4%. The other part of the samples from 3198 women was HPV negative (54.4%). 1,695 patients were positive for only one or more LR-HPV genotypes (Genotypes 6, 11, and 44) (relative frequency of 61%) and 812 patients were positive for one or more HR-HPV genotypes (a relative frequency of 29%).
The participants ranged from 14 to 85 years and were analysed in 4 different groups in order to evaluate the age distribution of the HPV positivity and negativity. The mean age of HPV negative population was 36,40 years, whereas the HPV positive population was 33.51 years, ending in age 74th. According to the four age groups, HPV positivity rates were: < 31 years (45%), 31-40 years (33.6%), 41-50 years (15.5%), and over 51 years (5.9%) (Table 1). Women aged under 31 years showed the highest overall HPV positivity with a rate of 45%. Comparing the presence of HPV genotypes in different age groups as statistically significant with p values of 0.000.
Table 1.
Frequency of HPV-Positive or HPV- Negative Samples According to Age Groups and Presence of One or Multiple Genotypes
| Age Groups (years) | HPV (+) numbers (%) |
HPV (-) numbers (%) |
p value |
|---|---|---|---|
| < 31 | 1250 (45) | 994 (31.1) | 0 |
| 31 - 40 | 932 (33.6) | 1216 (38) | 0 |
| 41 - 50 | 431 (15.5) | 756 (23.6) | 0 |
| > 51 | 164 (5.9) | 232 (7.3) | 0 |
Although 1,182 samples (64%) were identified as a single HPV genotype, more than one HPV genotype was detected in 1595 samples (36%). The age distribution in single and multiple genotypes was significant, as shown in Table 2.
Table 2.
HPV Positivity Rate in One and Multiple Genotypes
| Age Groups (years) | Single numbers (%) |
Multiple numbers (%) |
p value |
|---|---|---|---|
| < 31 | 328 (26.3) | 922 (73.7) | 0 |
| 31 - 40 | 621 (66.6) | 311 (33.4) | 0 |
| 41 - 50 | 135 (31.3) | 296 (68.7) | 0 |
| > 51 | 98 (59.7) | 66 (40.3) | 0 |
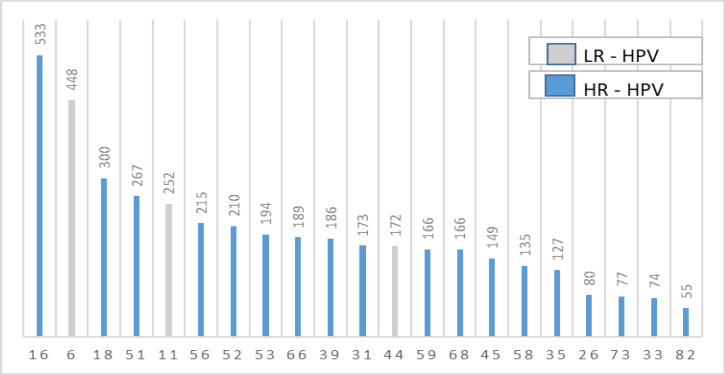
Twenty-one HPV genotyes ordered from the highest to lowest were given as frequency in Figure 1. While, the frequency of LR-HPV genotypes was 31.4%, the frequency of HR-HPV genotypes was as high as 118.8%. Genotype 16 was at the top and genotype 82 was at the bottom. The most common five HR-HPV genotypes were HPV 16, with 533 counts (relative frequency of 19.2%), followed by HPV 18, with 300 counts (relative frequency of 10.8%), HPV 51 with 267 counts (relative frequency of 9.7%), HPV 56 with 215 counts (relative frequency of 7.7% and HPV 52 with 210 counts (relative frequency of 7.5%) (Table 3). Those five genotypes represented over half of the most frequent HPV HR-positive genotypes (54.9%). HPV 33 and HPV 82 were the least seen genotypes, with 74 and 55 cases, respectively. The most common LR-HPV genotype was HPV 6, with 488 cases (relative frequency of 16.1%).
Figure 1.
Legend?
Table 3.
Frequency of Low Risk-HPV and High Risk-HPV Genotypes from 2,777 Positive Women
| HPV genotypes | n | % |
|---|---|---|
| LR-HPV | ||
| Type 6 | 448 | 16.1 |
| Type 11 | 252 | 9.1 |
| Type 44 | 172 | 6.2 |
| HR-HPV | ||
| Type 16 | 533 | 19.2 |
| Type 18 | 300 | 10.8 |
| Type 26 | 80 | 2.9 |
| Type 31 | 173 | 6.3 |
| Type 33 | 74 | 2.7 |
| Type 35 | 127 | 4.5 |
| Type 39 | 186 | 6.6 |
| Type 45 | 149 | 5.3 |
| Type 51 | 267 | 9.7 |
| Type 52 | 210 | 7.5 |
| Type 53 | 194 | 6.9 |
| Type 56 | 215 | 7.7 |
| Type 58 | 135 | 4.9 |
| Type 59 | 166 | 6.0 |
| Type 66 | 189 | 6.9 |
| Type 68 | 166 | 6.0 |
| Type 73 | 77 | 2.9 |
| Type 82 | 55 | 2.0 |
Age distribution percentages according to four groups for each HPV genotype were given in Table 4. Significant differences were observed for genotypes 16 and 56 (p=0.000).
As the concentration was calculated and given as a log (copies/sample), the mean values for each genotypes were shown in Table 6. Although genotype 52 ranked fifth in frequency, it showed the highest mean concentration, with a value of 5.38 log (copies/sample). The total mean value for LR and HR genotypes was almost the same, 4,80 log (copies/sample) (SD 0,09 and 0,32, respectively) (Table 6). The concentrations of LR-HPV and HR-HPV were mostly accumulated between 3,1 and 6,0 log (copies/sample) (p=0.000).
Table 6.
Distribution of LR-HPV and HR-HPV Concentrations
| *HPV concentration ranges | n | *Mean Values (SD) | p value |
|---|---|---|---|
| LR-HPV | |||
| <3.0 | 134 | 2.49 (0.51) | 0 |
| 3.1-6.0 | 559 | 4.20 (0.8) | |
| >6.1 | 179 | 7.70 (1.43) | |
| HR-HPV | |||
| <3.0 | 538 | 2.54 (0.39) | |
| 3.1-6.0 | 2062 | 4.46 (0.84) | |
| >6.1 | 696 | 7.61 (1.51) |
*log (copies/sample)
Discussion
Although, the most frequently used test for screening of CIN and CC is a cytological one, it is believed that nearly one third of CC cases are diagnosed in women who were screened regularly during cytological examination and thus false negative results for these patients were obtained (Koliopoulos et al., 2017; Macios et al., 2021). Therefore, DNA diagnostics of HPV infection is currently considered the basis for CC screening and prevention. Besides, knowing the HPV genotypes that predominate in each country’s regions is crucial.
Human papillomaviruses are small double-stranded DNA viruses, grouped into cutaneous and mucosal genotypes according to the infection site, with a further subdivision into high-risk (HR) and low-risk (LR) genotypes, depending on their association with malignancy (Sung et al., 2021). Molecular methods including hybridization, polymerase chain reaction (PCR), and hybrid capture are the most accurate methods to detect the virus’ DNA. The HPV QUANT-21 Quantitative Real Time-PCR Kit is an in vitro DNA test, which is intended for the specific identification and quantification of low-risk (HPV 6, 11, 44) and high-risk (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) with regard to their oncogenic properties of HPV. Therefore, in this study, we divided the HPV positive samples into LR and HR HPV genotypes according to manufacturer recommendations.
Since 2001, the molecular infection department has been established at Special Gelisim Laboratories. In this department, over 20,000 HPV screenings were carried out between 2001 and 2019. We collected the recent data from January 2021 to May 2022. Most of the samples were sent to the laboratory with little or no information on the participants’ clinical conditions, previous diagnosis, or treatment, besides gender and age. This lack of those informations were the main limitation of the study. The HPV frequency found in women’s samples (46.4%) and reported here with a sample size of 5975 was higher than in previous observational studies, using similar methodology. In Turkey, HPV prevalence has been reported between 2% and 40 % in some regional studies (Ozcelik et al., 2003;Inal et al., 2007; Dursun et al., 2009; Dursun et al., 2013). Altun et al., (2011) have found an HPV DNA positive rate of 5.2% in a study with 460 women using consensus PCR with MY09/11 and GP5+/6+primers. In another study, the frequency of HPV was found to be 10.8% among 527 HPV-positive cases (Vural et al., 2021). Yuce et al. reported HPV prevalence as 25.7% (Yüce et al., 2012) and Eroglu detected a positive rate of 32.5% in Kayseri (Eroğlu et al., 2011). Determination of human papilloma viruses DNA and genotypes in genital samples with PCR was reported as 46.3% with a linear array method, which is almost the same as our result (Duran et al., 2017). We believed that high prevelance percentage detected in our study among the others was due to having a large number of participants, and besides, this was possible due to the use of a multiplex PCRbased method that showed high sensitivity and specificity.
Different rates of infection have been recorded in diverse parts of the world. Studies from those countries which have all used PCR based methods report rates as 7.8% in Iran (Khodakarami et al., 2012), 10.3% in India (Sauvaget C et al., 2011), 19.4% in Portugal (Pista A et al., 2011), 35.9% in Italy (Piana et al., 2011), 64.1% in France (Casalegno et al., 2011), 12.2% in Canada (Ogilvie et al., 2013), 34.5% in Peru (Iwasaki et al., 2014), 12.1% in Mexico (Molina-Pineda et al., 2020) and 9.3% in Australia (Brotherton et al., 2019) and 4.6% in Japan (Aoyama-Kikawa et al., 2018). There is a wide range of HPV prevalence rates. This may be due to differences in geographic areas, social and cultural varities, and risk factors for cervical cancer or to the different methods used and their molecular sensitivity as showed in a study (Becker et al.,1994).
In this study, the frequency of HPV genotypes in different age groups was statistically significant with p values of 0.000. Similar studies with 400 women showed no difference that could be explained by having a smaller population than our study (García Muentes et al., 2019). Therefore, we suggested to focus on ages under 30 to prevent the disease, because nearly half of the accumulation was seen in this group (45%).
From the 2,777 positive samples, single and multiple HPV genotypes, which we have started to see more recently, were found to be 46%, and 54%, respectively (Table 2), and these values were (0.3–0.8%) less common (Chelimo et al., 2013; Rob et al., 2017; Haeggblom et al., 2019; Broomall et al., 2010; Karlsen et al., 1994;Anderson LA et al., 2016; Coutlée et al., 2011; Dunne et al., 2007; Tjalma et al. 2013). There were some studies supporting and also compatible with the results of our study from Turkey (Cilingir et al., 2013; Colakoglu et al., 2017; Duran et al., 2017; Hancer et al., 2018). Other studies from Turkey showed a high rate of multiple HPV infections such as 23.6% and 17.8% (Yuce et al., 2012; Eroglu et al, 2011) for multiple-genotype infections. However, Altun et al. report a low rate of 1.1% (Altun et al., 2011) which may be due to their method of DNA sequencing. DNA sequencing is considered the gold standard for detecting HPV genotypes but was insufficient in showing multiple infections (Giuliani et al., 2006). Only the dominant genotype was shown, but multiple-genotypes cannot be differentiated. Multiple probes are used in reverse hybridisation techniques to overcome this difficulty, and multiple HPV genotyping can be done (Chinchai et al., 2011). This HPV detection assay we used was also very sensitive to detecting multiple infections as it is based on the use of genotypespecific primers rather than consensus or degenerated primers.
On the other hand, the most commonly detected HPV genotypes that we found by RT-PCR in the open population group were 16, 18, 51, 56, and 58 (Figure 1). These genotypes have been classified by the IARC as carcinogenic to humans (IARC Working Group, 2002). Expanding on the findings of each common genotype identified in this study, HPV 16 was the most prevalent genotype found in all diagnostic groups, as previously reported in Turkey (Altun et al., 2011; Akcali et al., 2013) and worldwide (Bosch et al., 1995; De Sanjose et al., 2010; Forman et al., 2012; de Sanjose et al., 2007) by different authors. We have found HPV 16 as the most frequent genotype with a rate of 19.2%, followed by genotypes 18 and 51 with rates of 10.8% and 9.7%, respectively. Furthermore, the distribution of HPV 16 and 56 was significant below the age of 31, indicating a renewed focus on this age group (Table 4). Our results are comparable to other studies done at Turkey 28.5% (Akcali et al., 2013) and 33.3% (Altun et al., 2011) rates. We have detected HPV 18 in the second rank, as in previous studies, with a frequency of 10% (Akcali et al., 2013). HPV genotypes vary between countries. Spain reports HPV 16 and 31 as the most frequent geenotypes (Annunziata et al., 2018), England HPV 16 and 18 (Li et al., 2011), and the USA HPV 62, 84 and 53 (Dunne et al., 2007).
Table 4.
Distribution of HPV Genotypes According to Age Groups Given as %
| Age Groups (years) | HPV genotypes in % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 11 | 16 | 18 | 26 | 31 | 33 | 35 | 39 | 44 | 45 | 51 | 52 | 53 | 56 | 58 | 59 | 66 | 68 | 73 | 82 | |
| < 31 | 46.8 | 40.5 | 50.1* | 49.6 | 43.7 | 42.8 | 40.5 | 51.2 | 51.1 | 47.1 | 51 | 46.8 | 49.5 | 46.4 | 54.9* | 51.1 | 50 | 49.7 | 42.2 | 45.5 | 54.5 |
| 31 - 40 | 30.4 | 36.8 | 33.8* | 33 | 36.3 | 39.8 | 37.8 | 33.9 | 31.7 | 33.1 | 30.2 | 31.8 | 33.8 | 34.5 | 26* | 24.4 | 30.7 | 32.3 | 33.1 | 32.4 | 29.1 |
| 41 - 50 | 16.1 | 18.3 | 12* | 11.7 | 12.5 | 13.9 | 18.9 | 10.2 | 12.4 | 15.1 | 13.4 | 13.5 | 13.8 | 14.4 | 16.3* | 18.6 | 12.7 | 14.3 | 15.1 | 13 | 10.9 |
| > 51 | 6.7 | 4.4 | 4.1* | 5.7 | 7.5 | 3.5 | 2.8 | 4.7 | 4.8 | 4.7 | 5.4 | 7.9 | 2.9 | 4.7 | 2.8* | 5.9 | 6.6 | 3.7 | 9.6 | 9.1 | 5.5 |
*p value statistically significant
It is important to mention that HPVs 16 and 18 are the most commonly detected high-risk genotypes worldwide, accounting for approximately 75% of all CC cases (Gutiérrez-Campos et al., 2019). In our study, the prevalance of these genotypes was 30%, totally. After HPV16/18, the six most common HPV genotypes are the same in all world regions, namely 31, 33, 35, 45, 52, and 58; these account for an additional 20% of CC worldwide (Clifford et al., 2006).
We noticed, however, that possibly carcinogenic (Group 2B) genotypes according to the International Agency for Research in Cancer (IARC) and the World Health Organization (WHO), including genotypes 26, 53, 66, and 82, appeared to be as frequent as much as 18.7%, nearly one fifth of total positive types. Some HPV genotypes associated with cancer, including HPV 39 and HPV 58, were found as 11.5% in this study, whereas it appeared to be more frequent according to the locality (Mclemore, 2006).
Therefore, the currently available vaccines may not be as effective in Turkey, and special attention should be paid to different genotypes that are not covered by the vaccine. Considering these results, regional data on the prevalence and genotypic distribution of HPVs are essential for estimating the impact of vaccines on CC and screening programs. Vaccination programmes with a quadrivalent vaccine (HPV 6, 11, 16, and 18) and a bivalent vaccine (HPV 16 and 18), which have been approved by the FDA, have been implemented in over 40 countries (Markowitz et al., 2013). However, in 2012, Serrano et al. described the potential impact of a nine-valent (9-HPV) vaccine against HPVs 6, 11, 16, 18, 31, 33, 45, 52, and 58, reporting that this vaccine could prevent almost 90% of CC cases worldwide (Serrano et al., 2012). Nevertheless, this vaccine confers no protection against other HPV genotypes frequently found in women in the present study. Therefore, it is necessary to continue analysing the geographical distribution of HPV genotypes in Turkey and worldwide to design effective HPV screening systems and develop new HPV vaccines. This is becoming increasingly important as new technologies are rapidly detecting new HPV genotypes that cannot be detected by commercial detection tests (Ambulos et al., 2016;Payungporn et al., 2018; Flores-Miramontes et al., 2020).
Besides, analysing the frequency of LR- and HR-HPV HPV genotypes, our tecnology had a positive advantage to calculate the concentration of each genotypes, given as log (copies/sample). In order to get positive or negative results, it was vulnarable to see the value in a quantative form to trace the changes. Although, we have not the oppurtinity to compare the quantative results to cytological grade, virus copy number showed a minimum concentration of 0,1 log (copies/sample) with a high sensitivity to a maximum concentration of 13,3 log (copies/sample). Further studies would be planned to measure and define the high infection level that can lead to the development of cervical neoplasia. Using this tecnology could give us a clinical desicion to degree the cytological changes.
In conclusion, precancerous lesions caused by persistent HR-HPV infections are early indicators of CC. The overall HPV positivity rate was 46.4% in our country in a high-risk population. Among these positive results, 118.8% were HR-HPV genotypes. Early detection of HPV and early diagnosis of precancerous lesions is important. Country-wide screening programs are essential in the prevention of cervical cancer.
In this context, one of the most important prevention strategies is the implementation of HPV vaccination programs. Due to the circulation of genotypes different from the ones covered by the available vaccines, a broader immunization strategy should be considered. The differences in HPV prevalence and genotypic distribution described in this study could have a potential impact on the design of new vaccines and screening programs to facilitate prevention of CC in Turkey. On the other hand, it is necessary to promote knowledge among the female population and raise awareness about the risk factors for CC development in addition to HPV infection.
The quantification of genotype results using this RT-PCR technology would provide an early indication of the traceability of therapeutic efficiency and recovery. Besides, we need to classify the degree of cytological status according to given results in further studies.
Author Contribution Statement
Nilgun TEKKESİN, Safak GÖKTAS, Tuğba GÜRBÜZ and Pasa GÖKTAS were conceived and designed the analysis. Senem KOÇ contributed data and analysis tools. Veysi ALKIŞ performed the analysis. Nilgun TEKKESIN wrote the paper.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This study was approved by the Ethics and Research Committees of Nisantasi University, School of Medicine. The authors whose names are listed certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants;participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
We acknowledge our technical personnel at the Molecular Biology Department of Gelisim Laboratory. Also, the authors thank for sharing the data for this study to the laboratory director.
References
- Aguilar-Lemarroy A, Vallejo-Ruiz V, Cortés-Gutiérrez EI, et al. Human papillomavirus infections in Mexican women with Normal cytology, precancerous lesions, and cervical cancer : type-specific prevalence and HPV Coinfections. J Med Virol. 2015;87:871–84. doi: 10.1002/jmv.24099. [DOI] [PubMed] [Google Scholar]
- Akcali S, Goker A, Ecemis T, Kandiloglu AR, Sanlidag T. Human papilloma virus frequency and genotype distribution in a Turkish population. Asian Pac J Cancer Prev. 2013;14:503–6. doi: 10.7314/apjcp.2013.14.1.503. [DOI] [PubMed] [Google Scholar]
- Altun Z, Yarkın F, Vardar MA, Uğuz AH. The prevalence of human papilloma virus infection among women who admitted to çukurova university faculty of medicine hospital. J Med Sci. 2011;31:307–14. [Google Scholar]
- Ambulos NP, Schumaker LM, Mathias TJ, et al. Next-generation sequencing-based HPV genotyping assay validated in formalin-fixed, paraffin-embedded oropharyngeal and cervical cancer specimens. J Biomol Tech. 2016;27:46–52. doi: 10.7171/jbt.16-2702-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, O’Rorke MA, Wilson R, Jamison J, Gavin AT. Northern Ireland HPV Working Group HPV prevalence and type-distribution in cervical cancer and premalignant lesions of the cervix: A population-based study from Northern Ireland. J Med Virol. 2016;88:1262–70. doi: 10.1002/jmv.24447. [DOI] [PubMed] [Google Scholar]
- Annunziata C, Stellato G, Stefano Greggi S, et al. Prevalence of “unclassified” HPV genotypes among women with abnormal cytology. Infect Agents Cancer. 2018;13:2–8. doi: 10.1186/s13027-018-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama-Kikawa S, Fujita H, Hanley SJB. Comparison of human papillomavirus genotyping and cytology triage , COMPACT study: design, methods and baseline results in 14 642 women. Cancer Sci. 2018;109:2003–12. doi: 10.1111/cas.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TM, Wheeler CM, Mcgough NS, et al. Sexually transmitted diseases and other risk factors for cervical dysplasia among southwestern Hispanic and non-Hispanic White women. JAMA. 1994;271:1181–8. [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Manos M, Muñoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Broomall EM, Reynolds SM, Jacobson RM. Epidemiology, clinical manifestations, and recent advances in vaccination against human papillomavirus. Postgrad Med. 2010;122:121–9. doi: 10.3810/pgm.2010.03.2129. [DOI] [PubMed] [Google Scholar]
- Brotherton JM, Hawkes D, Sultana F, et al. Age-specific HPV prevalence among 116,052 women in Australia’s renewed cervical screening program: a new tool for monitoring vaccine impact. Vaccine. 2019;37:412–6. doi: 10.1016/j.vaccine.2018.11.075. [DOI] [PubMed] [Google Scholar]
- Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. J Virol. 2015;476:341–4. doi: 10.1016/j.virol.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Casalegno JS, Benchaib M, Le Bail Carval K, et al. Human papillomavirus genotype distribution among French women with and without cervical abnormalities. Int J Gynaecol Obstet. 2011;114:116–9. doi: 10.1016/j.ijgo.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Chelimo C, Wouldes TA, Cameron LD, Elwood JM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66:207–17. doi: 10.1016/j.jinf.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Chinchai T, Chansaenroj J, Junyangdikul P, et al. Comparison between direct sequencing and INNO-LiPA methods for HPV detection and genotyping in Thai Women. Asian Pac J Cancer Prev. 2011;12:989–94. [PubMed] [Google Scholar]
- Cilingir IU, Bengisu E, Agacfidan A, et al. Microarray detection of human papilloma virus genotypes among Turkish women with abnormal cytology at a colposcopy unit. J Turk Gynecol Assoc. 2013;14:23–7. doi: 10.5152/jtgga.2013.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;31:S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Clifford G, Smith J, Franceschi S, Plummer M. Human papillomavirus types in invasive cervical cancer worldwide : a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colakoglu S, Bolat FA, Coban G. Human papilloma virus (HPV) prevalence and genotype distribution. J Clin Anal Med. 2017;8:109–13. [Google Scholar]
- Coutlée F, Ratnam S, Ramanakumar AV, et al. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol. 2011;83:1034–41. doi: 10.1002/jmv.22081. [DOI] [PubMed] [Google Scholar]
- Cuschieri KS, Cubie HA, Whitley MW, et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol. 2004;57:68–72. doi: 10.1136/jcp.57.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- de Sanjose S, Quint WGV, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:7–11. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- Duran AC, Erdin BN, Sayıner AA. Determination of human papilloma viruses DNA and genotypes in genital samples with PCR. J Clin Anal Med. 2017;8:302–6. [Google Scholar]
- Dursun P, Ayhan A, Mutlu L, et al. HPV types in Turkey: multicenter hospital based evaluation of 6388 patients in Turkish gynecologic oncology group centers. Turk Patoloji Derg. 2013;29:210–6. doi: 10.5146/tjpath.2013.01188. [DOI] [PubMed] [Google Scholar]
- Dursun P, Senger SS, Arslan H, Kuscu E, Ayhan A. Human papillomavirus (HPV) prevalence and types among Turkish women at a gynecology outpatient unit. BMC Infect Dis. 2009;9:191–6. doi: 10.1186/1471-2334-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroğlu C, Keşli R, Eryılmaz MA, et al. Serviks kanseri için riski olan kadınlarda HPV tiplendirmesi HPV sıklığının risk faktörleri ve servikal smearla ilişkisi. Nobel Med. 2011;7:72–7. [Google Scholar]
- Flores-Miramontes MG, Olszewski D, Artaza-Irigaray C, et al. Detection of alpha, Beta, gamma, and unclassified human papillomaviruses in cervical cancer samples from Mexican women. Front Cell Infect Microbiol. 2020;10:1–15. doi: 10.3389/fcimb.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D, De Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Giuliani L, Coletti A, Syrjänen K, Favalli C, Ciotti M. Comparison of DNA sequencing and Roche Linear array in human papillomavirus (HPV) genotyping. Anticancer Res. 2006;26:3939–41. [PubMed] [Google Scholar]
- Gutiérrez-Campos R, Malacara-Rosas A, Gutierrez-Santillán E, et al. Unusual prevalence of high-risk genotypes of human papillomavirus in a group of women with neoplastic lesions and cervical cancer from Central Mexico. PLoS One. 2019;14:1–13. doi: 10.1371/journal.pone.0215222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggblom L, Attoff T, Yu J, et al. Changes in incidence and prevalence of human papillomavirus in tonsillar and base of tongue cancer during 2000-2016 in the Stockholm region and Sweden. Head Neck. 2019;41:1583–90. doi: 10.1002/hed.25585. [DOI] [PubMed] [Google Scholar]
- Hancer VS, Buyukdogan M, Bylykbashi I, Oksuz B, Acar M. Prevalence of human papilloma virus types in Turkish and Albanian women. J Cytol. 2018;35:252–4. doi: 10.4103/JOC.JOC_162_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkos HW. Multifactorial etiology of cervical cancer: a hypothesis. Medscape Gen Med. 2005;7 [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group. Biological agents Part B Areview of human carcinogens. IARC Monographs. 2012; 100B:1–441. [PMC free article] [PubMed] [Google Scholar]
- Inal MM, Kose S, Yildirim Y, et al. The relationship between human papillomavirus infection and cervical intraepithelial neoplasia in Turkish women. Int J Gynecol Cancer. 2007;17:1266–70. doi: 10.1111/j.1525-1438.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki R, Galvez-Philpott F, Arias-Stella JJ, Arias-Stella J. Prevalence of high-risk human papillomavirus by Cobas 4800 HPV test in urban Peru. Braz J Infect Dis. 2014;18:469–72. doi: 10.1016/j.bjid.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen F, Rabbitts PH, Sundresan V, Hagmar B. PCR-RFLP studies on chromosome 3p in formaldehyde-fixed, paraffin-embedded cervical cancer tissues. Int J Cancer. 1994;58:787–92. doi: 10.1002/ijc.2910580606. [DOI] [PubMed] [Google Scholar]
- Khodakarami N, Clifford GM, Yavari P, et al. Human papillomavirus infection in women with and without cervical cancer in Tehran, Iran. Int J Cancer. 2012;131:156–61. doi: 10.1002/ijc.26488. [DOI] [PubMed] [Google Scholar]
- Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population (Review) Cochrane Database Syst Rev. 2017;8:CD008587. doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327:1272–8. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Li N, Franceschi S, Howell-jones R, Snijders PJF, Clifford GM. Human paillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- Macios A, Didkowska J, Wojciechowska U, et al. Risk factors of cervical cancer after a negative cytological diagnosis in Polish cervical cancer screening programme. Cancer Med. 2021;10:3449–60. doi: 10.1002/cam4.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz LE, Tsu V, Deeks SL et al. Human papillomavirus vaccine introduction – the first five years. Vaccine. 2012;305:F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Mclemore MR. Gardasil ®:introducing the new human papillomavirus vaccine. Clin J Oncol Nurs. 2006;10:559–60. doi: 10.1188/06.CJON.559-560. [DOI] [PubMed] [Google Scholar]
- Molina-Pineda A, López-Cardona MG, Limón-Toledo LP, et al. High frequency of HPV genotypes 59, 66, 52, 51, 39 and 56 in women from Western Mexico High frequency of HPV genotypes 59, 66, 52, 51, 39 and 56 in women from Western Mexico. BMC Infect Dis. 2020;20:2–10. doi: 10.1186/s12879-020-05627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muentes G, García M, Galárraga R, et al. Frequency and distribution of HPV genotypes in 800 genital samples of Ecuadorian men and women from the city of Guayaquil. Rev Inst Med Trop São Paulo. 2019;41:1–5. doi: 10.1590/S1678-9946201961041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjosé, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Eng J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Ogilvie GS, Cook DA, Taylor DL, et al. Population-based evaluation of type-specific HPV prevalence among women in British Columbia, Canada. Vaccine. 2013;31:1129–33. doi: 10.1016/j.vaccine.2012.09.085. [DOI] [PubMed] [Google Scholar]
- Ozcelik B, Serin IS, Gokahmetoglu S, Basbug M, Erez R. Human papillomavirus frequency of women at low risk of developing cervical cancer: A preliminary study from a Turkish university hospital. Eur J Gynaecol Oncol. 2003;24:157–9. [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Payungporn S, Poovorawan Y. Comparison of four human papillomavirus genotyping. Ann Lab Med. 2018;38:139–46. doi: 10.3343/alm.2018.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piana A, Sotgiu G, Castiglia P, et al. Prevalence and type distribution of human papillomavirus infection in women from North Sardinia, Italy. BMC Public Hlth. 2011;11:1–8. doi: 10.1186/1471-2458-11-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pista A, de Oliveira CF, Cunha MJ, et al. CLEOPATRE Portugal study group prevalence of human papillomavirus infection in women in Portugal: the CLEOPATRE Portugal study. Int J Gynecol Cancer. 2011;21:1150–8. doi: 10.1097/IGC.0b013e31821dd3b2. [DOI] [PubMed] [Google Scholar]
- Rob F, Tachezy R, Pichlík T, et al. High prevalence of genital HPV infection among long-term monogamous partners of women with cervical dysplasia or genital warts-Another reason for HPV vaccination of boys. Dermatol Ther. 2017;30:1–6. doi: 10.1111/dth.12435. [DOI] [PubMed] [Google Scholar]
- Sauvaget C, Nene BM, Jayant K, et al. Prevalence and determinants of high-risk human papillomavirus infection in middle-aged Indian women. Sex Transm Dis. 2011;38:902–6. doi: 10.1097/OLQ.0b013e318223be5f. [DOI] [PubMed] [Google Scholar]
- Serrano B, Alemany L, Tous S, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agents Cancer. 2012;38:1–13. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics-GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tjalma WA, Fiander A, Reich O, et al. HERACLES/SCALE Study Group Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;132:854–67. doi: 10.1002/ijc.27713. [DOI] [PubMed] [Google Scholar]
- Vural G, Polat N. Human Papilloma Virus Frequency and Genotypes; Evaluation of the 4879 Screenings Made with Polymerase Chain Reaction and Chip Array Between 2001 and 2019 in Istanbul. Med Bull Sisli Etfal Hosp. 2021;55:232–36. doi: 10.14744/SEMB.2021.67355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Roberson D, Boland J, et al. Development of the TypeSeq assay for detection of 51 human papillomavirus genotypes by next-generation sequencing. J Clin Microbiol. 2019;57:1–11. doi: 10.1128/JCM.01794-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuce K, Pinar A, Salman MC, et al. Detection and genotyping of cervical HPV with simultaneous cervical cytology in Turkish women: a hospital-based study. Arch Gynecol Obstet. 2012;286:203–8. doi: 10.1007/s00404-012-2280-z. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Natl Rev. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]