Abstract
Background:
Angiogenesis is a complex biological process wherein novel capillary blood vessels mature from pre-existing vasculature for delivering tissues with oxygen and nutrients. Natural molecules that have anti-angiogenic activity and toxicity can raise the focus on using plant sources as essential therapeutic agent.
Objective:
The current research was intended to estimate the probable anti-angiogenic activity of abscisic acid alone and in combination with prednisolone, a well-known angiostatic glucocorticoid. Methods: two months old albino rats were used in this study. ABA and prednisolone stock solutions were prepared and added after embedding aortic rings in growth media. The ex vivo rat aorta ring assay (RAR) was applied to explore the anti-angiogenic effect of ABA. The in vivo chorioallantoic membrane assay (CAM) was applied to quantify the blood vessels inhibition zone by ABA effect. That zone was calculated as the mean inhibition region on eggs in mm ± SD.
Results:
Abscisic acid screened byex vivo and in vivo assays, revealed a significant dose-dependent blood vessels inhibition in comparison to the negative control with IC50= 7.5µg/ml and a synergism effect when combined with prednisolone.
Conclusion:
The synergism activity of ABA with prednisolone can significantly inhibit blood vessels growth in RAR and CAM assays. These results shed the light on the potential clinic values of ABA, and prednisolone combination in angiogenesis-dependent tumors.
Key Words: Angiogenesis, abscisic acid, prednisolone, CAM, rat aortic assay
Introduction
Cancer is a hypernym describing a various class of illnesses where cells go through uncontrolled growth with the potential to turn into malignant through the acquirement of several unusual characteristics during their development as a result of hereditary or ecological factors (Um, 2015). According to the statistics of World Health Organization 2020, cancer is the main leading cause of death around the globe for 10 million out of 19 million cases, including 33,000 incidence case with 60% mortality rate in Iraq alone (Sung et al., 2021).
Angiogenesis is a complex, multi-step, tightly-regulated process which is balanced by regulators that act either to stimulate (e.g., VEGF) or to inhibit (e.g., endostatin) blood vessel growth. Inadequate angiogenesis results in insufficient vascular growth that can cause ischemic diseases, while exaggerated vascular growth associated with cancer and inflammatory ailments (Fiedler and Pellet-many, 2022).
The general concept pronounces that tumor cells develop an angiogenic phenotype through which the pro-angiogenic mechanisms overwhelm the down-regulatory processes. Tumor angiogenesis functions in multi-aspects of tumor biology as endothelial cell apoptosis, tumor metastasis, and cancer stem cell proliferation. Along these lines, anti-angiogenesis-based treatments have attracted great attention as potential anti-cancer therapies. (Teleanu et al., 2020; Li et al., 2021).
Unfortunately, serious adverse effects have been related to anti-angiogenic drugs, including renal, visual, hepatic, cardiac and bone marrow toxicities which direct the efforts to uncover safe and efficacious therapeutic agents (Chaqour et al., 2018), rather than developing new synthetic drugs that target non-selectively angiogenic or non-angiogenic signaling pathways, a complementary and alternative remedies are gaining more attention in oncology management which mostly act through multiple cell-signaling mechanisms, but negligibly disturb the overall health of tissues (Khalid et al., 2016).
Plants are composed of numerous bioactive compounds such as steroids, alkaloids, flavonoids, and terpenes. Between these, terpenes be owned by the biggest category of secondary metabolites with diverse chemical structure, so most of them have biological activity as anti-tumor drugs such as Taxol and its derivatives (Muhseen and Li, 2020).
Abscisic acid, a terpenoid phytohormone is an example of terpenes. In plant it takes important role in monitoring of stomatal closure and regulation of seed germination. ABA recently recognized as an endogenous hormone in human. It has displayed arole on the inflammatory response, immuno-regulatory and antioxidantproperties as well asit have an important role in the maintenance of glycemic control in pre-clinical models of inflammation and diabetes (Chaqour et al., 2018; Baliño et al., 2019).
The goal of the current work is to identify the anti - angiogenic activity of abscisic acidand prednisolone in ex vivo rat aorta ring (RAR) and in vivo chorioallantoic membrane (CAM) animals study, and to investigate the synergistic/additive effect of abscisic acid and prednisolone as anti – angiogenic compounds in ex vivo rat aorta ring (RAR) assay and in vivo chorioallantoic membrane (CAM) assay.
Materials and Methods
All materials and equipment used in this study and their origins are listed in Table (1) and (2) below.
Table 1.
Chemicals
| Abscisic acid powder | Hyperchem, China |
|---|---|
| Aminocaproic acid solution | Hyperchem, China |
| Aprotinin powder | Hyperchem, China |
| Chloroform solution | Alpha chemika , India |
| Dimethyl sulfoxide (DMSO) solution | Alpha chemika , India |
| Ethanol (70%, 96%) solution | Turkey |
| Fibrinogen powder | Hyperchem, China |
| Foetal bovine serum | hyperchem, China |
| Gentamicin 80 mg/2ml amp | Troge , Germany |
| L-Glutamine solution | hyperchem, China |
| Povidone iodine 10% spray | Unirex, Turkey |
| Prednisolone powder | Hyperchem, China |
| Sodium chloride 9% bottle | Relcade, India |
| Thrombin 100 IU vial | Hyperchem, China |
Table 2.
Apparatus and Equipment
| 48 - well tissue culture plates | Orange Scientifics, Belgium |
|---|---|
| Autoclave | Lab Tech, Korea |
| Beakers | Simax, Chez |
| CO2 incubator | Lab Tech, Korea |
| Dissecting set | AVEAids, Malaysia |
| Eppendorf tubes 1ml | Eppendorf, Germany |
| Filter paper | Whatmann, England |
| Filter syringe 0.45 µm | China, Spain |
| Flask | Simax, Chez |
| Inverted light microscope | Optika, Italy |
| Laminar clean bench | Lab Tech, Korea |
| Micropipette tips | Oxford epigene, British |
| Micropipettes | Eppendorf, Germany |
| Petri dish | China |
| Racks | AFCO, Jordan |
| Sensitive Digital balance | Sartorius, Canada |
| Spatula | Simax, Chez |
| Sterile disposable test tubes (10,50) ml | AFCO, China |
| Syringes ( 5,10,20,50 ) ml G 23, G 21 | China, UAE, Italy |
| Vortex mixer | Taiwan |
| Water bath with shaker | Lab Tech, Korea |
Source of experimental animals
Animal: Albino male rats
Animals were supplied from Iraqi Center for Cancer and Medical Genetics Research. They were allowed for free access for water and food and kept in cages (after divided randomly) in animal house at 25-30°C. The Ethical committee approval had been certified from College Of Pharmacy/Al-Nahrain University.
Fertilized chicken eggs
They were collected from the Poultry Field of College Of Veterinary Medicine, University of Baghdad.
Methodology
The current study had been started on October/2021 and lasts for five months. The work conducted in cell culture lab in Al-Nahrain University / College of Medicine.
Rataortaring (RAR) assay
This assay was performed according to Kathryn J. Brown protocol (Brown et al., 1996) with a minor modifications accomplished by Hayder B Sahib (Abdul et al., 2017). It was started by taking five Albino male rats (2 months old),which authenticated via cervical dislocation after anesthetized with chloroform inside the anesthesia chamber. The thoracic aorta is then extirpated, rinsed with serum-free media, cleaned from any residual periadventitial fibroadipose tissue and cross-sectioned into 1 mm thickness rings. The assay was performed in a48-well tissue culture plate. 300µl of (3 mg/ml fibrinogen with 5 mg/ml of Aprotinin in serum free medium [M199]) is added to each well. Each ring tissue is placed in the center of the well. To each well 10μl of thrombin; prepared at 50 NIH U/ml in 0.15 M NaCl was added and then was incubated for 10 min at 37°C in a humidified 5%CO2 incubator for solidification and formation of fibrin gel and ideally the vessel fragment remained suspended in the center of that gel.
Then 0.3 ml/well of Medium M199 supplemented with 20% of heat inactivated fetal bovine serum (HIFBS), 0.1% E-aminocaproic acid, 1% L-glutamine and 0.6% gentamycin was added.The test substance preparation (ABA or Prednisolone) was achieved by dissolving it in dimethyl sulfoxide (DMSO), and diluting in M199 growth medium to make the final DMSO concentration 1%.ABA or prednisolone was added to the complete growth medium at concentration of 100μg/ml and each treatment was performed in five replicates. The concentration used for combination represents the summation of IC50 of ABA and IC50 of prednisolone together. The culture plates then returned to 5% CO2 humidified incubator at 37°C for five days. On day 4, the top layer medium was replaced with fresh medium because if it is not replaced, the angiogenesis response and the stability of neovessels are enhanced due to accumulation of endogenous growth factors in the system. The DMSO (1%v/v) and prednisolone (100 μg/ml) were used as negative and positive controls respectively. Vessel growth was quantified manually under (40x) magnification on day 5, with aid of mobile camera and software. The magnitude of blood vessel growth inhibition was determined according to the technique developed by Nicosia (Nicosia et al., 1997).
Quantification of rat aortic assay
The quantification of angiogenesis was accomplished by the detection of the extent and number of newly blood vessels outgrowth from the primary tissue explants, manual measurement and processing was done at day 5 of the experiment when the blood vessels growth achieved its extreme growth to quantify the growth of micro-vessels (Blacher et al., 2001). The results are presented as mean percent inhibition to the negative control ± SD. The experiment was repeated three times using five replicates / sample. The percentage of blood vessels inhibition was determined according to the formula:
Blood vessels inhibition = 1- (A0/A) ×100
Where: A0 = distance of blood vessels growth for the test substance in mm, A = distance of blood vessels growth in the control in mm.
Dose Response study on abscisic acid and prednisolone with rat aortic ring anti-angiogenesis assay
Serial dilutions of the test substances were prepared in the concentrations: 100, 50, 25, 12.25 and 6.25 μg/ml, from the original stock samples that were dissolved in DMSO, and diluted in the M199 growth medium to make the final DMSO concentration 1%. Wells without test samples were received medium with 1% DMSO used as the negative control. The data was represented as mean ± SD. The concentration that inhibits 50% of the growing blood vessels “IC50” was calculated by using the linear regression equation or the logarithmic equation for the substances. Where Y= the percentage of inhibition, and X= concentration. (Stiffey-Wilusz et al., 2001)
Chick Chorioallantoic Membrane (CAM) Assay
The CAM assay was performed by using fertilized chicken eggs that was kept in a humidified incubator at a constant humidity of 60 % and at a temperature of 37°C for 72 hours where eggs were placed in horizontal position. On D4 a small pinpoint hole was created at the apex of the eggs and 2-3 ml of albumin was removed and sealed to lower the embryo and CAM away for better visualization of the formed CAM, where the CAM would split from the sack that is linked to the egg shell. The eggs were incubated for further 24 hours.On D5 a small square window (3-4cm) of the shell was made and the test samples that were soaked previously in round discs of filter papers was placed on the CAM and the window covered with a sterile surgical adhesive tape to ensure sterility and maintain humidity, then eggs were incubated again for another 48 hours.
This method of cultivation was slightly invasive on the growing chick embryo, providing a quite unchanged environment for its growth, and generally improves the survival rate for the experiment. Finally the inhibition zone photographed and calculated. The test samples which were abscisic acid, prednisolone and their combination were prepared as 10mg/ml and 50μl (final dose was 0.5mg/disc) of abscisic acid and prednisolone and 25μl of each substance was taken to make the combination (final dose was 0.25mg for each one/disc) which placed on the filter paper discs and left to dry before being transferred to the CAM. The whole procedure was performed under aseptic condition. (Lokman et al., 2012)
Quantification and Imaging of CAM
The responses were graded + (3-6 mm); ++ (6 – 9mm); +++ (> 10mm) and the results were presented as Mean ± Standard errors of means. (Murray, 2001)
Statistical Analysis
The research design used for our study was Rationalized Complete Block Design (RCBD). Results were displayed as mean ± SD. The differences among groups were compared through the one way ANOVA followed by Tukey Post-hoc test (t-test) and consider significant at P < 0.05. The statistical analysis was carried out by using SPSS (edition 21.0). A computerized application (compusyn) also used to determine the synergism/additive or antagonism effect of ABA- prednisolone combination.
Results
Rat aortic ring assay
Comparison between abscisic acid, positive and negative control
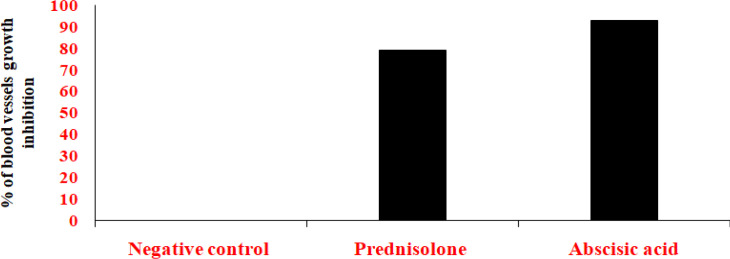
Figure 1, showed that there was a significant difference between ABA and the negative control, moreover there also was a significant difference between and prednisolone and the negative control. Also, regarding the blood vessel inhibition the ABA was significantly higher in comparing to prednisolone. The following table revealed the percentage of blood vessels inhibition of ABA, prednisolone (as positive control) and DMSO (as negative control) as seen in Table 3, and Image 1.
Figure 1.
Anti – Angiogenic Effect of ABS, Prednisolone and DMSO in ex vivo Rat Aortic Ring assay as a Percentage of Blood Vessels Growth Inhibition
Table 3.
The Inhibition Percentage of Blood Vessels Growth Produced by the Abscisic Acid, Positive and Negative Controls
| Compound | Percentage of inhibition ± SD |
|---|---|
| ABA | 93 + 0.57 |
| Prednisolone | 79+ 1.83 |
| Negative control | 0 |
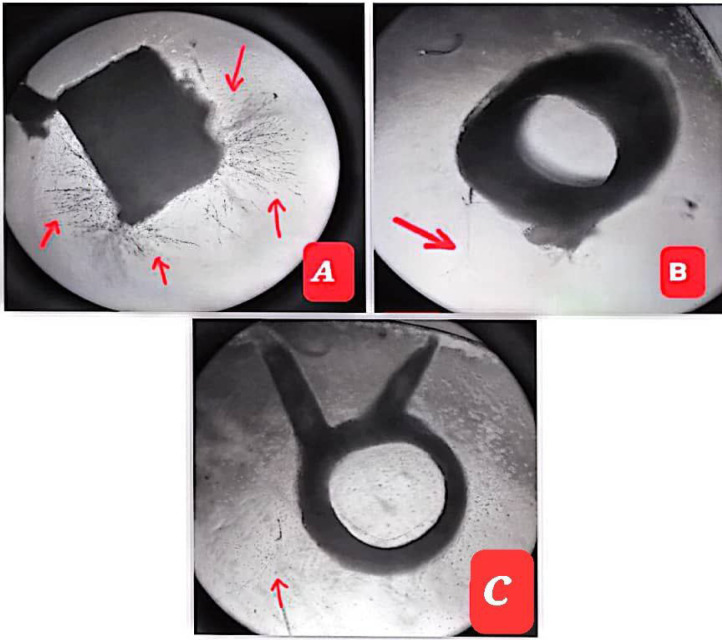
Image 1.
Anti – Angiogenic Effect of Abscisic Acid in ex vivo Rat Aortic Ring. Where A: negative control, B: prednisolone; (positive control) and C: Abscisic acid. Note: black arrows represent the growth of micro-blood vessels
Dose response effect of rat aortic ring assay
Dose response effect of Abscisic acid
ABA showed significant dose response relationship regarding blood vessels inhibition when compared to the negative control at day five of the experiment. The results are shown in Table 4, and Image 2.
Table 4.
Serial Concentrations and Their Respective Inhibition Percentage for Abscisic Acid
| Concentration (µg/ml) | Percentage of inhibition + SD |
|---|---|
| 100 | 93.2 + 0.57 |
| 50 | 75.9 + 1.75 |
| 25 | 68.3 + 2.24 |
| 12.5 | 60.1 + 2.17 |
| 6.25 | 46.8 + 2.98 |
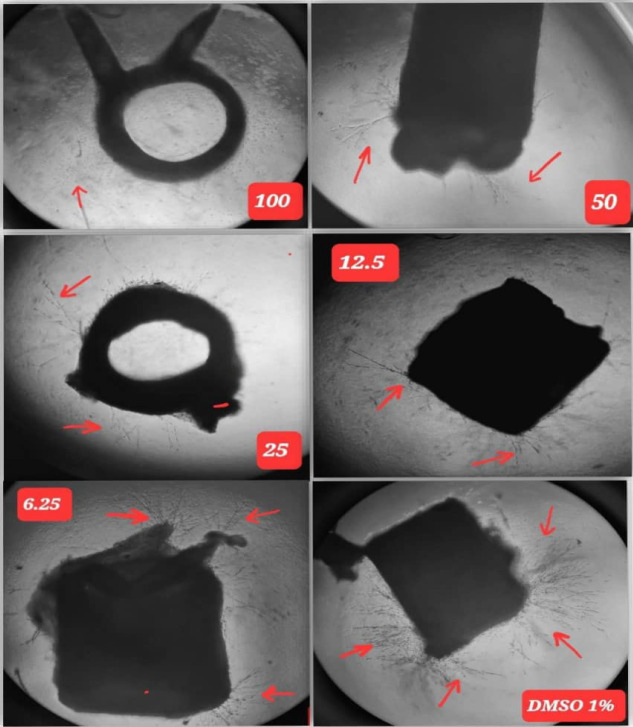
Image 2.
The Dose Response Effect to Serial Concentrations of Abscisic Acid in Rat Aortic Rings. Note: black arrows represent the growth of micro-blood vessels
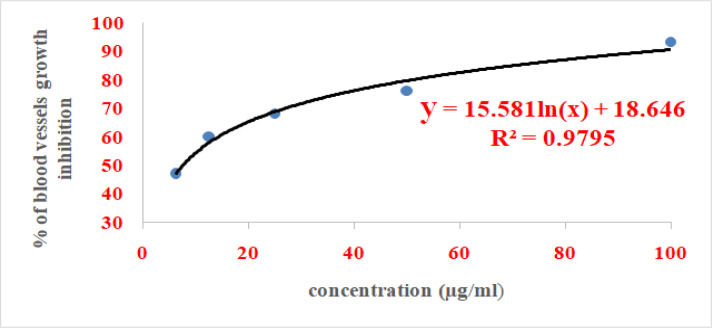
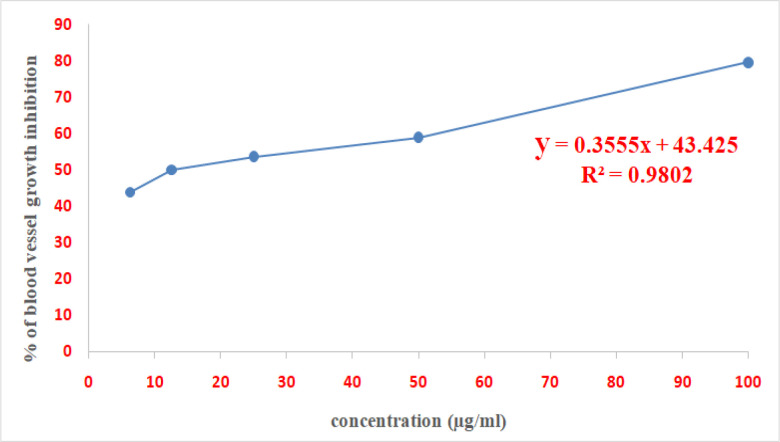
IC50 value of ABA was calculated from the logarithmic equation shown in Figure 2 which was found to be 7.5μg/ml
Figure 2.
The Dose Response Curve of Serial Dilutions of Abscisic Acid in Rat Aortic Rings Assay
Dose response effect of prednisolone
Prednisolone exhibited a significant dose response relationship as regards to aortic blood vessels inhibition comparing to the negative control at 5th day. The results presented in Table 5, and Image 3.
Table 5.
Serial Concentrations and Their Respective Inhibition Percentage for Prednisolone
| Concentration (µg/ml) | % of inhibition + SD |
|---|---|
| 100 | 79.6 + 1.83 |
| 50 | 59 + 2.25 |
| 25 | 53.6 + 2.69 |
| 12.5 | 50 + 3.21 |
| 6.25 | 43.8 + 2.65 |
Image 3.
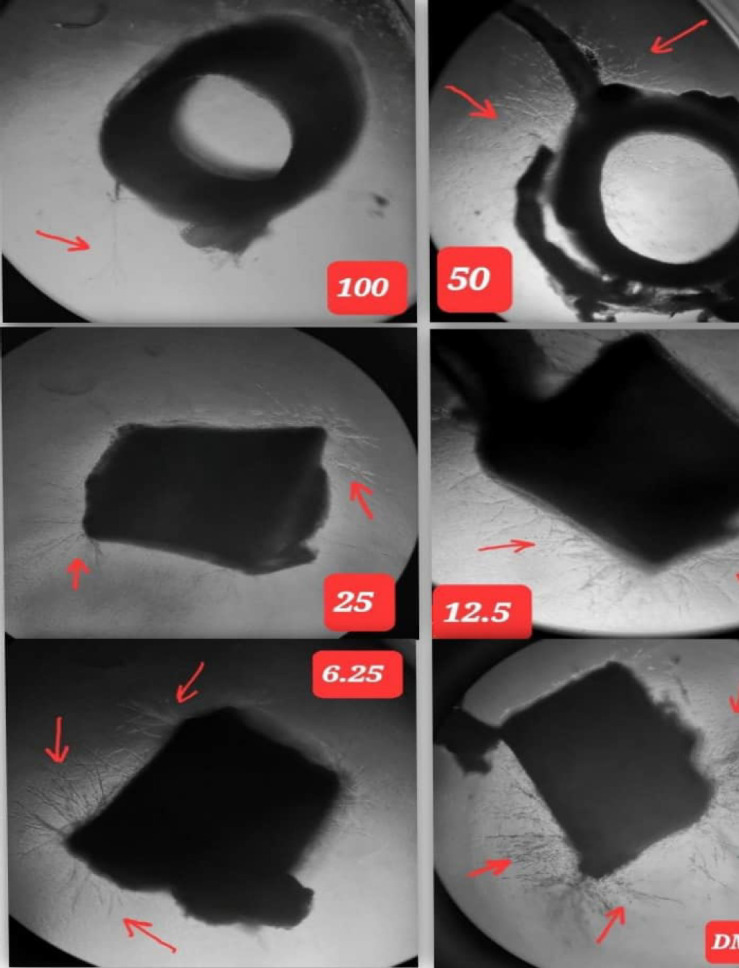
The Dose Response Effect to Serial Concentrations of Prednisolone in Rat Aortic Rings. (Black arrows represent the growth of micro-blood vessel)
IC50 value of prednisolone was calculated from the linear regression equation shown in Figure 3 which was found to 18.5µg/ml.
Figure 3.
The Dose Response Curve of Serial Dilutions of Prednisolone in Rat Aortic Rings Assay
The combination effect of abscisic acid and prednisolonein rat aortic ring assay
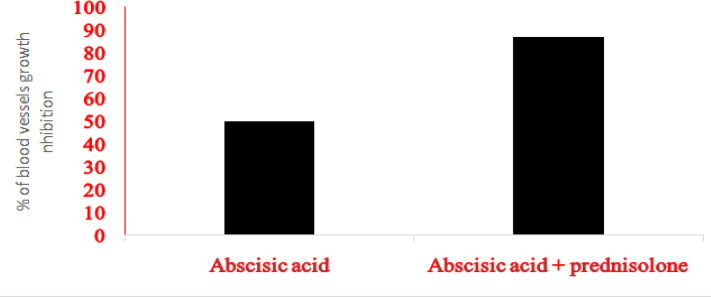
Abscisic acid and prednisolone (50 µg/ml from each)combinations were exhibited significantinhibition of blood vessels growth in comparison to 50µg/mlABA alone at day five of the experiment as presented below in Figure 4, and Image 4. The CI index for this combination using Compusyn was below 1 indicating synergism effect. (The application put CI>1 for antagonism, CI=1 for additive and CI<1 for synergism effect of combined drugs)
Figure 4.
Anti – Angiogenic Effect of Abscisic Acid and Prednisolone Combination Compared to Abscisic Acid. in ex vivo rat aortic ring assay as a percentage of blood vessels growth inhibition
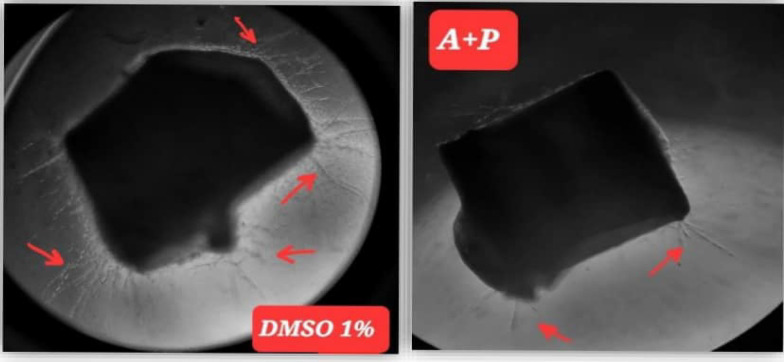
Image 4.
Anti – Angiogenic Effect of Abscisic Acid + Prednisolone Combination Compared to Negative Control in Rat Aortic Ring Assay. (A+P) represent the combination. Note: black arrows represent the growth of micro-blood vessels
Chick Chorioallantoic Membrane (CAM) Assayof abscisic acid and prednisolone
The zone of inhibition for ABA and prednisolone were measured at day 7 of the experiment. CAM Blood vessels be likely to regress by test molecule effect. The inhibition was noticed by the presence of a vascular zone surrounding the disc that enriched with 0.5 µg/ml of abscisic acid or/and prednisolone, then the extent of inhibition zone was measured according to the scoring system mentioned previously. It was found that ABA, prednisolone produced a significant inhibition zone effect on blood vessels in the CAM and their combination exhibit an synergism effect comparing to abscisic acid effect as shown below in Tables 6, 7 and 8) and image 5.
Table 6.
The Zone of Inhibition of Blood Vessels Growth of Abscisic Acid and the Corresponding Scoring Using the Chick Chorioallantoic Membrane (CAM) Assay
| Egg no. | Zone of inhibition area (mm) | Scoring |
|---|---|---|
| 1 | 11 | +++ |
| 2 | 9 | ++ |
| 3 | 12 | +++ |
| 4 | 13 | +++ |
| 5 | 11 | +++ |
| 6 | 9 | ++ |
| Mean ± SD | 10.6 ± 1.86 | +++ |
Table 7.
The Zone of Inhibition of Blood Vessels Growth of Prednisolone and the Corresponding Scoring Using the Chick Chorioallantoic Membrane (CAM) assay
| Egg no. | Zone of inhibition area (mm) | Scoring |
|---|---|---|
| 1 | 9 | ++ |
| 2 | 11 | +++ |
| 3 | 8 | ++ |
| 4 | 13 | +++ |
| 5 | 8 | ++ |
| 6 | 10 | ++ |
| Mean ± SD | 9.5 ± 1.37 | ++ |
Table 8.
The Zone of Inhibition of Blood Vessels Growth of Abscisic Acid + Prednisolone Combination and the Corresponding Scoring Using the Chick Chorioallantoic Membrane (CAM) Assay
| Egg no. | Zone of inhibition area (mm) | Scoring |
| 1 | 13 | +++ |
| 2 | 11 | +++ |
| 3 | 10 | ++ |
| 4 | 12 | +++ |
| 5 | 14 | +++ |
| 6 | 9 | ++ |
| Mean ± SD | 11.5 ± 1.87 | +++ |
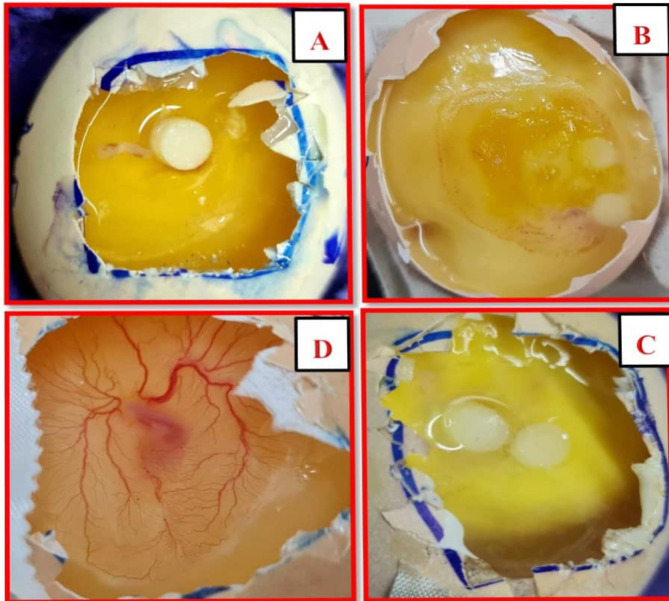
Image 5.
In vivo (CAM) Assay; (A) represent the treated group with abscisic acid, (B) represent group treated with prednisolone and (C) represent the treated group with abscisic acid + prednisolone combination and they showed a marked vessel regression. (D) Represent the negative control "received DMSO 1%”
Discussion
Rat aorta ring anti – angiogenesis assay
The main practical challenge in any study of angiogenesis is the selection of the most suitable assay so despite the increasing numbers of both in vitro and in vivo assays, a ’’gold-standard’’ angiogenesis assay has not been developed yet, for that reason, a combination of assays are essential to identify the full range of effects of a test substance or to detect the molecular and cellular mechanisms in angiogenesis.
The explants assays are considered closest to mimicking the in vivo conditions since they include the surrounding non-endothelial cells (e.g., myocytes and pericytes) in addition to the supportive matrix. Furthermore, endothelial cells do not proliferate during explanation make them more representative for in vivo conditions (Staton et al., 2004).
Ex vivo assays were established to link the gap between in vivo and in vitro models, providing a more physiologically relevant model. One of the most widely used is the aortic ring assay. First recognized in 90s was by Nicosia and Ottinetti (Nicosia and Ottinetti 1997) using a rat tissue, then adapted to further species such as: chicken and mouse tissue. It permits easy quantification of vascular sprouting, investigation of inter-cellular interactions with the supportive cells, ignores inflammatory components, and it is fast and low-price. It is unique in that it run through all of the main steps in the process (matrix degradation, migration, proliferation and reorganization also it considers greatly adaptable thus allowing the study of the effects of different circumstances on vascular sprouting (Fiedler and Pellet-many, 2022).
From the current work, the anti-angiogenic effect of abscisic acid was first investigated using the rat aortic ring model, as well as the possible additive or synergism effect of ABA when combined with the glucocorticoid member, prednisolone.
The results quantified after five days of ring implants culturing, by determining the length of the blood vessel extensions. As the blood vessel growth reached their extreme on day 5, this interval is considered as the most appropriate for quantification (Nicosia et al., 1997). Prednisolone was used as a positive control in this study since it has been shown to act as anti-angiogenic agent that is mediated by the glucocorticoid receptor in rodents at supra-physiological and physiological concentrations and in human endothelial cells (Morgan et al., 2018).
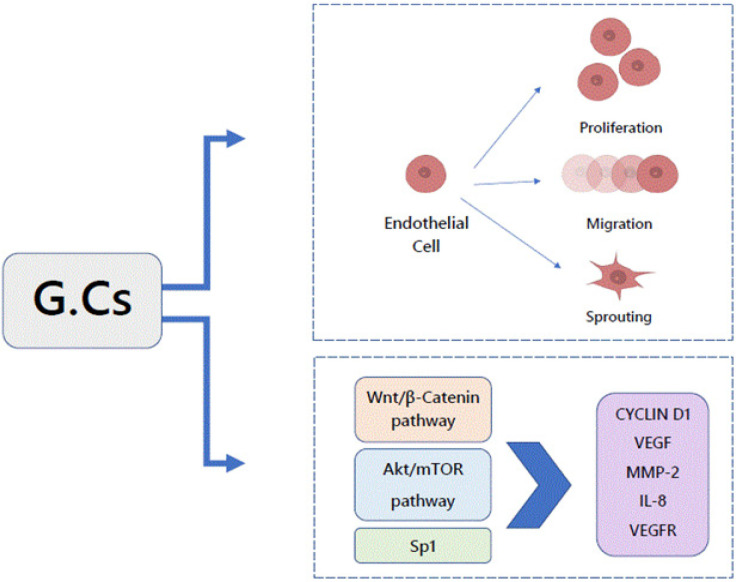
Shikatani and his coworkers stated that glucocorticoid reduced the capillaries number in rodent skeletal muscle and inhibited proliferation, migration, and sprouting of micro-vascular endothelial cells in vitro through diminished VEGF levels, as well as reduced production and stimulation of MMP-2. The glucocorticoid down-regulated expression of transcriptional regulator of both VEGF and MMP-2.In vitro glucocorticoid decreased the protein expression of cellular and soluble VEGF and VEGFR-1 through the Akt/mTOR pathway in a dose and time dependent fashion (Shikatani et al.,2012; Liu and Goodwin 2020).
In 2020, Meszaros and Patocs (2020) mentioned that glucocorticoid induces upregulation of the Wnt signaling inhibitor. Another previous literature proposes that the Wnt/β-catenin signaling pathway is critical in vascular endothelial cells so once the signaling is triggered, the expression of key regulators of angiogenesis, such as VEGF, IL-8, Cyclin D1 and MMP-2 is changed. With loss of endothelial glucocorticoid, the Wnt signaling upregulation is occur in both in vitro and in vivo, which confirm the strong correlation between glucocorticoid, Wnt proteins and angiogenesis, Figure 5.
Figure 5.
The Regulatory Effects of GCs on Angiogenesis
The results of this study demonstrated that ABA at 100 µg/ml exhibit a massive inhibition of angiogenesis in ring implants and therefore evaluated at five dilutional concentrations ranging from 6.25µg/ml to 100µg/ml. At concentrations of 12.5, 25 and 50 µg/ml, significant inhibition was accomplished, but at concentrations of 6.25µg/ml, the inhibition decreased indicating a dose -dependent manner of inhibition.
In 2015, a study mentioned that in human granulocytes and other immune system cells, ABA motivates cell specific functions, like chemotaxis, phagocytosis, and reactive oxygen species (Vigliarolo et al., 2015). Magnone and hiscollegues displayedlately that ABA have been demonstrated to activate human granulocytes through a signaling pathway including activation of adenylyl cyclase to produce cAMP, then ADPribosyl cyclase (ADPRC) phosphorylation which converts NAD+ to cADPR, leading to an increase of both extra-cellular Ca+ entry and intracellular Ca+ mobilization eventually. ABA-induced Ca+2 upsurge triggers ROS and NO release, phagocytosis, and chemotaxis. The increment of intracellular ABA in stressed granulocytes and ABA release during phagocytosis point to that ABA behaves as a new human pro-inflammatory hormone (Magnone et al., 2009).
Dr. Josep Bassaganya-Riera and his coworkers shed more light on ABA as a potential cancer treatment due to its ability to modulate calcium signaling and reinforce the idea of cADPR mechanism. What is more, Calcium, which is an ABA second messenger, is a key regulator of apoptosis which its distribution in the cell initiates cellular cascades that leads to cell death (Bassaganya-Riera et al., 2010). Growing evidence proposed that ABA is critical signaling controller targeting mesenchymal stem cells (MSC) in the bone marrow. When Stress signals (e.g., TNF-α) secreted by lymphocytes or monocytes have shown to stimulate MSC to release ABA. The new secreted molecule in turn enhances hematopoietic progenitors’ number by the way of cADPR/ Ca2+- mediated signaling pathway. In addition, ABA- treated early hematopoietic progenitor cells are known to release VEGF, which regulates the growth of hematopoietic cells, stromal and endothelial cells (Jung et al., 2021).
It is practically known that ABA signaling is mediated by as a minimum two receptors, lanthionine synthetase C-like 2 (LANCL2) and peroxisome proliferator-activated receptors (PPARγ). LANCL2 is a peripheral protein attached to the intracellular membrane of RBC; and its hormone ligand ABA, is an anion at physiological pH thus, for ABA entry into RBC and bind to its receptor, ABA transport through the plasma membrane is necessary. Actually, influx of ABA has been established through the transmembrane bicarbonate/chloride exchanger 1 (AE1, band 3 protein) in RBCs (Fresia et al., 2016).
Zou et al., (2020) exposed that mTOR signaling pathway is related to tumor’s cell growth, metabolism, apoptosis and autophagy and they noticed pregulation of PI3K/Akt/mTOR pathway in most malignant tumor. A number of studies were shown that mTOR inhibitors displayed high anti-tumor activity in clinical trials. Also ABA-PPAR receptor signaling powerfully suppresses mTOR activity, thus inducing prostate cancer cellular dormancy. In retina, ABA significantly increased OIR-induced M1 and M2 macrophage markers expression and since ischemia is usually associated with increased VEGF levels, ABA treatment reduced the ischemic response by significantly reducing VEGF expression (Nakamura et al., 2015).
From another sight of view, our results accept the claims of Chaqour et al., (2018) that ABA treatment blocked significant steps in the initiation of sprouting angiogenesis including EC differentiation, migration and proliferation. ABA inhibited EC sprouting in dose-dependent style without affecting cell viability even at micro-molar concentrations of ABA, it retained antiangiogenic properties both in vitro and in vivo. In notable recent study, it was stated that the ABA levels were two-fold higher in low-grade gliomas than in high-grade gliomas. Remarkably, both differentiation and cellular apoptosis were amplified in the glioblastoma cells after ABA treatment. These data suggest that ABA may perhaps show an anti-cancer role in glioma (Zhou et al., 2021).
The use of herb-drug combination to improve therapeutic outcome is of great interest, mainly in cancer chemo-therapy and common reasons for this use are to improve clinical results, enhance quality of life, reduce adverse toxic effectsof anti-cancer therapies, and potentially enhance their effects by means of decreasing the dose needed to achieve the same effect (Chen et al., 2014).
In the current study results regarding the impact of ABA-Prednisolone combination, it appears that the 50:50 µg/ml effects on blood vessels inhibition was significant synergism effect, which is predictable due to anti-angiogenic activity of each solo molecule.
Chick Chorioallantoic Membrane (CAM) Assay
The usage of CAM assay for such a range of pro- and anti-angiogenic factors: drugs, metabolites surely enforces the applicability in addition to efficiency of the CAM as a screening tool to shape the micro-circulation and angiogenic properties of different substances (Kennedy et al., 2021). The CAM assay can be realized as a bridge which binds cell based in vitro studies with in vivo animal investigation. The main factors dictating the choice of this technique are its cost, ease of use, reproducibility, and dependability (Ribatti, 2010). Due to incompletely-developed immunity until development day 18, the CAM is capable of holding immune-incompetent medium or tissue graft. For that reason, this is the ideal time to accomplished CAM assay to accurately assess an angiogenic response and avoid immune reactions (Kennedy et al., 2021).
So up to now, no study has been observed about anti-angiogenic effect of ABA or prednisolone – as a positive control – byCAM analysis. In our study results, The CAM regions were reduced significantly in treated eggs by using ABA, prednisolone and their combination.
The results measured according to scoring method mentioned previously, which displayed the inhibition effect of ABA as (+++) and less for prednisolone (++) which gave ABA an synergism effect on CAM vessels when used together as combination this is because of using a half dose which provided a proximate result when compared to ABA-only results. Li et al., (2011) examine the glucocorticoid effect when used in combination with other known anti-angiogenic molecule, where it shows a significantly positive results in tube formation of HUVECs, rat aortic sprouting and CAM assay much more potent than known monotherapy.
Another study which pronounced that glucocorticoid inhibited angiogenesis at the ulcer margin, delayed gastric ulcer healing and associated with a significant decrease of VEGF which isa prominent factor in neovascularization (Luo et al., 2004). Different results suggested that glucocorticoid act by inhibiting the final common angiogenesis pathway which initiates tube formation. Since VEGF stimulate angiogenesis through separate second messenger pathways (phospholipase C, cAMP, and IP3 respectively), it seems to be predictable that glucocorticoid affect a final common pathway (e.g. extracellular signal-regulated kinase-mitogen activated protein kinase (ERK-MAPK)) that including calcium exchange and its signaling effects. (Logie et al., 2010).
Regarding to ABA, One of the most interesting findings in a past study was that connective tissue growth factor (CTGF) remarkably decreased VEGF-induced angiogenesis in a complex form with VEGF .This inhibition by CTGF was efficient since only small concentration was enough to display greatest inhibition of VEGF-induced angiogenesis (Kinashi et al., 2017; Chaqour et al., 2018).
In conclusion, the current study displayed that ABA was capableof inhibiting angiogenesis in both ex vivo and in vivo assay with significant activity (at p value ≤0.05). Our results indicate the potential clinic values of ABA and prednisolone combination in angiogenesis-dependent tumors and these findings offer useful documents for additional studies to investigate the molecular mechanism behind its anti-angiogenic effect and discuss the potential possibilities for using of ABA in cancer diseases therapies.
Author Contribution Statement
ZJ: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft. HS: Supervision, Validation, Writing – review & editing.
Acknowledgements
The authors would like to acknowledge the Department of Pharmacology, College of Medicine, Al-Nahrain University, for offering the logic support to finish this work.
Approval
This paper is part of the dissertation of the corresponding author at the College of Medicine at Al-Nahrain University in Partial Fulfilment of the Requirements for the Degree of MSc in Science in Pharmacology.
Ethic statement
Handling of the animals and the experimental design were carried out based on the guidelines reported in “Guide for the care and use of laboratory animal” and according to the protocol approved by the Al-Nahrain University/college of pharmacy, Baghdad, Iraq with the ethical clearance number S. Y/2/1/658 dated 20 July 2022 approved by Ass/ Prof. Dr. Rafal Shakeeb AbdulWahab (Vic Dean for Scientific Affairs).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Study Registration
There is no registration number or support for this project.
Conflict of interest
The authors declare that they have no competing interests.
References
- Abdul S, et al. Research Article, the Anti – Angiogenic Activity of Olea europaea Seeds Extracts: ex vivo and in vivo study. Int J Pharm Sci Rev Res. 2017;44:188–94. [Google Scholar]
- Baliño P, Gómez-Cadenas A, López-Malo D, Romero FJ, Muriach M. Is there a role for abscisic acid, a proven anti-inflammatory agent, in the treatment of ischemic retinopathies? Antioxidants (Basel) 2019;8:104. doi: 10.3390/antiox8040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassaganya-Riera J, Skoneczka J, Kingston DG, et al. 2010) Mechanisms of action and medicinal applications of abscisic acid. Curr Med Chem. 17:467–78. doi: 10.2174/092986710790226110. [DOI] [PubMed] [Google Scholar]
- Blacher S, Devy L, Burbridge MF, et al. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–42. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Maynes SF, Bezos A, et al. A novel in vitro assay for human angiogenesis. Lab Invest. 1996;75:539–55. [PubMed] [Google Scholar]
- Chaqour J, Lee S, Ravichandra A, Chaqour B. Abscisic acid - An anti-angiogenic phytohormone that modulates the phenotypical plasticity of endothelial cells and macrophages. J Cell Sci. 2018;131:jcs210492. doi: 10.1242/jcs.210492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang Z, Huang Y, et al. Ginseng and anticancer drug combination to improve cancer chemotherapy: A critical review. Evid based Complement Alternat Med. 2014;2014:168940. doi: 10.1155/2014/168940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresia C, Vigliarolo T, Guida L, et al. G-protein coupling and nuclear translocation of the human abscisic acid receptor LANCL2. Sci Rep. 2016;6:26658. doi: 10.1038/srep26658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Cackowski FC, Yumoto K, et al. Abscisic acid regulates dormancy of prostate cancer disseminated tumor cells in the bone marrow. Neoplasia. 2021;23:102–11. doi: 10.1016/j.neo.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DC, Coen B, Wheatley AM, McCullagh KJA. Microvascular experimentation in the chick chorioallantoic membrane as a model for screening angiogenic agents including from gene-modified cells. Int J Mol Sci. 2021;23:452. doi: 10.3390/ijms23010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid EB, Ayman EE, Rahman H, Abdelkarim G, Najda A. Natural products against cancer angiogenesis. Tumor Biol. 2016;37:14513–36. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- Kinashi H, Falke LL, Nguyen TQ, et al. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int. 2017;92:850–63. doi: 10.1016/j.kint.2017.03.029. [DOI] [PubMed] [Google Scholar]
- Li R, Song X, Guo Y, et al. Natural Products: A Promising Therapeutics for Targeting Tumor Angiogenesis. Front Oncol. 2021;11:772915. doi: 10.3389/fonc.2021.772915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Goodwin JE. The effect of glucocorticoids on angiogenesis in the treatment of solid tumors. J Cell Signal. 2020;1:42–9. [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Shang BY, Wang DC, et al. Endostar, a modified recombinant human endostatin, exhibits synergistic effects with dexamethasone on angiogenesis and hepatoma growth. Cancer Lett. 2011;301:212–20. doi: 10.1016/j.canlet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Logie JJ, Ali S, Marshall KM, et al. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS One. 2010;5:e14476. doi: 10.1371/journal.pone.0014476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokman NA, Elde AS, Ricciardelli C, Oehler MK. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int J Mol Sci. 2012;13:9959–70. doi: 10.3390/ijms13089959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JC, Shin VY, Liu ES, et al. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol. 2004;485:275–81. doi: 10.1016/j.ejphar.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Magnone M, Bruzzone S, Guida L, et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009;284:17808–18. doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros K, Patocs A. Glucocorticoids influencing Wnt/β-catenin pathway; multiple sites, heterogeneous effects. Molecules. 2020;25:1489. doi: 10.3390/molecules25071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, Keen J, Halligan D, et al. Species-specific regulation of angiogenesis by glucocorticoids reveals contrasting effects on inflammatory and angiogenic pathways. PLoS One. 2018;13:e0192746. doi: 10.1371/journal.pone.0192746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhseen ZT, Li G. Promising terpenes as natural antagonists of cancer: An in-silico approach. Molecules. 2020;25:155. doi: 10.3390/molecules25010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Sene A, Santeford A, et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Lin YJ, Hazelton D, Qian X. Endogenous regulation of angiogenesis in the rat aorta model: Role of vascular endothelial growth factor. Am J Pathol. 1997;151:1379–86. [PMC free article] [PubMed] [Google Scholar]
- Olsen JJ, Pohl SÖ, Deshmukh A, et al. The role of Wnt signalling in angiogenesis. Clin Biochem Rev. 2017;38:131–42. [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. The chick embryo chorioallantoic membrane as an in vivo assay to study antiangiogenesis. Pharmaceuticals. 2010;3:482–513. doi: 10.3390/ph3030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikatani EA, Trifonova A, Mandel ER, et al. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS One. 2012;7:e46625. doi: 10.1371/journal.pone.0046625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton CA, Stribbling SM, Tazzyman S, Hughes R. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85:233–48. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffey-Wilusz J, Boice JA, Ronan J, Fletcher AM, Anderson MS. An ex vivo angiogenesis assay utilizing commercial porcine carotid artery: Modification of the rat aortic ring assay. Angiogenesis. 2001;4:3–9. doi: 10.1023/a:1016604327305. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2020;9 doi: 10.3390/jcm9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliarolo T, Guida L, Millo E, et al. Abscisic acid transport in human erythrocytes. J Biol Chem. 2015;290:13042–52. doi: 10.1074/jbc.M114.629501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Wei Z, Qi Z, Chen L. Abscisic Acid-Induced Autophagy Selectively via MAPK/JNK Signalling Pathway in Glioblastoma. Cell Mol Neurobiol. 2021;41:813–26. doi: 10.1007/s10571-020-00888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Tao T, Li H, Zhu X. MTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020;10:31. doi: 10.1186/s13578-020-00396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.