Abstract
Objective:
This study evaluated differences in Claudin-1 expression between follicular adenoma (FA), follicular thyroid carcinoma (FTC), follicular variant papillary thyroid carcinoma (FV-PTC), and papillary thyroid carcinoma (PTC).
Material and methods:
This study used a cross-sectional approach. Immunostaining using the polyclonal antibody Claudin-1 was performed on 75 samples divided into 20 samples for follicular adenoma, follicular thyroid carcinoma, papillary carcinoma, and 15 samples of follicular variant thyroid carcinoma, respectively.
Results:
Claudin-1 expression is detected on the cytoplasmic membrane of tumor cells and appears to be varied among thyroid neoplasms. The claudin-1 expression score revealed a statistically significant difference between FA against FV-PTC, FA versus (vs) PTC, and FTC vs PTC, with median values of 4 vs 6 (p = 0.016), 4 vs 8 (p = 0.001), and 5 vs 8 (p = 0.002), respectively. However, there was no statistically significant difference in scores between the FA and the FTC (4 vs 5), or between the FTC and the FV-PTC groups (5 vs 6 (p=1,000).
Conclusion:
These results suggest that Claudin-1 may be capable of discriminating follicular adenoma from classic and follicular variant of papillary thyroid carcinoma. It can also differentiate follicular thyroid carcinoma and papillary thyroid carcinoma, especially for cases challenging to assess by hematoxylin and eosin staining. It still holds promise in providing targeted cancer therapy.
Key Words: Claudin-1, follicular adenoma, follicular thyroid carcinoma, papillary thyroid carcinoma
Introduction
Histopathological analysis has been used to diagnose thyroid neoplasms for more than 50 years. Hematoxylin and eosin (H&E) staining is the gold standard for establishing thyroid tumor differential diagnostic criteria (Prasad et al., 2005). Thyroid neoplasms are produced mostly from thyroid follicular epithelial derivatives that are classified as benign, borderline, or malignant. On the basis of clinical and histological evidence, the malignant group is further divided as well-differentiated, poorly differentiated, and undifferentiated (Dralle et al., 2015).
Morphological characterization of tumor cells on typical papillary thyroid carcinoma (PTC) is straightforward by examining the nucleus’s optical clarity, elongation, overlapping or crowded nucleoli, and irregular contours with grooves and pseudoinclusions (Cheung et al., 2001; Radu et al., 2015). Differentiating thyroid neoplasms can be accomplished by examining the nucleus morphology, capsule morphology, or blood vascular invasiveness. However, this technique remains subjective and occasionally perplexing (Manimaran et al., 2014). Furthermore, Follicular differentiated thyroid neoplasms are a group of tumors with overlapping morphologies that are frequently difficult to discriminate histomorphologically. For example, differentiating FTC lesions from Follicular Variant of Papillary Thyroid Carcinoma (FV-PTC) is a significant challenge for pathologists in the majority of cases, particularly when the histology is ambiguous.
Contrary to follicular thyroid carcinoma, which can spread via hematogenous, particularly to the lung and bone, follicular adenoma (FA) is a benign neoplasm with a favorable prognosis (McHenry & Phitayakorn, 2011). FA can be identified not only based on their cytomorphological characteristics; but also by excluding capsule and vascular invasion (Nikiforov et al., 2017; Ma et al., 2014; Manimaran et al., 2014). Due to the detrimental impact of over- or under-diagnosis on patient treatment and prognosis, immunohistochemical examination as a tumor marker is required to assist in the differentiation of these lesions and may reveal the pathogenesis of thyroid cancer in the development of targeted cancer therapy (Ma et al., 2014; Morin, 2005; Na et al., 2020).
Claudin-1 is a crucial component of adjacent cells because it works as tight junction and apical adhesion molecules and contributes to cell polarity maintenance (Fujibe et al., 2004; Tsukita et al., 2001). Numerous data indicate that many cancers affect claudin-1 expression (Abd El Atti and Shash, 2012; Alikanoglu et al., 2015; Németh et al., 2010). It is a sign of cancer development and malignant transformation of epithelial cells, showing different expressions in normal, hyperplastic, benign, and malignancy that shows epithelial differentiation. Claudin-1 level was elevated in PTC entities. However, the rationale for the overexpression of claudin-1 in all papillary carcinomas is unclear (Németh et al., 2010). Based on a previous report, Claudin-1 expression in cancer varies according to the stage, type, and grading of cancer (Turksen and Troy, 2011).
The aim of the work was to compare claudin-1 expression in several follicular differentiation thyroid neoplasm types, specifically FA, FTC, FVPTC, and PTC. It is expected that with the usage of claudin-1 can serve as a suggestive biomarker, enabling for an accurate diagnosis of various thyroid tumor entities.
Materials and Methods
A total of 75 paraffin-block samples were separated into four groups: 20 samples of FA, twenty samples of FTC, fifteen samples of FV-PTC, and 20 samples of classic PTC.
Immunohistochemical procedure
Claudin-1 expression was evaluated immunohistochemically using a polyclonal antibody directed against claudin-1 that was detectable on tumor cell membranes. Claudin-1 immunoexpression was expressed in semi-quantitative by scoring system, using color intensity of Claudin-1 expression and percentage of area. The both were into 9 grade scores: 0 = no tumor epithelial cells were stained until the area was stained <5%; 1 = weak intensity, area <5% to 25%; 2 = weak intensity, area> 25% to 50%; 3 = weak intensity, area> 50%; 4 = moderate intensity, area <5% to 25%; 5 = moderate intensity, area> 25% to 50%; 6 = moderate intensity, area> 50%; 7 = strong intensity, area <5% to 25%; 8 = strong intensity, area> 25 to 50%; 9 = strong intensity, area> 50%. Total score (expression): Negative (0) = 0; Weak (+1) = 1-2; Medium (+2) = 3-5; Strong (+3) = 6-7; Very strong (+4) = 8-9. Two anatomic pathologists were determined to score Claudin-1 expression. All slides were examined under a light microscope at a magnification of 400x (Olympus CX-43).
Statistical Analysis
The Differences in Claudin-1 expression scores between FA, FTC, FV-PTC, and PTC were analyzed by Chi-Square, Kruskal Wallis following Multiple Comparison of Bonferroni. If the p-value is less than 0.05, it is deemed significant.
Results
Expression of Claudin-1 in Thyroid Neoplasms
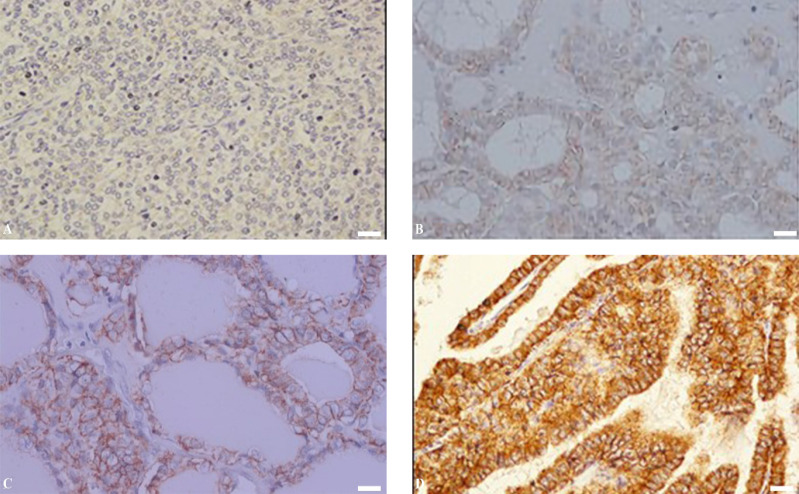
Our findings regarding Claudin-1 expression in thyroid tumors appear to vary in terms of the brown luminescence detected on the cell cytoplasmic membrane. Claudin-1 expression was negative in FA and low in the FTC entity, respectively (Figure 1A and 1B). Claudin-1 expression was moderate in FV-PTC samples (Figure 1C), whereas in PTC lesions, Claudin-1 expression was robust (Figure 1D). These findings suggest a tendency for claudin-1 expression discrepancies amongst FA, FTC, FV-PTC, and conventional PTC lesions.
Figure 1.
Shows Representative claudin-1 Expression in Thyroid Neoplasms. A, shows negative expression in FA while B exhibit weak expressions in FTC, respectively; C, demonstrates a moderate level of expression in FV-PTC entities, whereas D demonstrates a high level of expression in conventional PTC (Obj. 40x; Scale bar 100 µm; Olympus CX43)
Claudin-1 Expression Score Analysis
Following that, we determined whether there were changes in Claudin-1 expression scores based on histological findings. According to the data in (table 1), the mean + SD Claudin-1 score for PTC entities (7.6 + 1.5) is greater than that for FA (4 + 1.86), FTC (4.7 + 3.33), and FV-PTC (6.5 + 2.45). Kruskal-Wallis analysis found a statistically significant difference in the median Claudin-1 score between the two group entities, with p = 0.001.
Table 1.
Analysis of the Differences between Claudin-1 IHC Score and Histopathological Feature Groups
| IHC Score of Claudin-1 | Histopathology Features | |||
|---|---|---|---|---|
| FA | FTC | FV-PTC | PTC | |
| Mean | 4 | 4.7 | 6.5 | 7.6 |
| SD | 1.86 | 3.33 | 2.45 | 1.5 |
| Median | 4 | 5 | 6 | 8 |
| Min | 1 | 0 | 2 | 5 |
| Max | 6 | 9 | 9 | 9 |
Note. Kruskal-Wallis Test p=0,001 (p<0,05); FA, Follicular Adenoma; FTC, Follicular Thyroid Carcinoma; FV-PTC, Follicular Variant of Papillary Thyroid Carcinoma; PTC, Papillary Thyroid Carcinoma
Next on, we examined the differences between each thyroid neoplasm entity group as observed in (Table 2). The median value of Claudin-1 expression scores was significantly different between FA and FV-PTC (p=0.016); FA and PTC (p=0.001); and FTC and PTC (p=0.002). Meanwhile, there was no significant discrepancy in scores between the FA and the FTC and the FTC and the FV-PTC group entities (p=1,000). These findings suggest that Claudin-1 expression differs widely between benign and malignant tumor types.
Table 2.
Comparative Study of Claudin-1 Expression Scores between Histopathological Feature Groups
| No. | Comparative Groups (Score) | p-value |
|---|---|---|
| 1 | FA (4) vs FTC (5) | P=1,000 |
| 2 | FA (4) vs FV-PTC (6) | P=0,016 |
| 3 | FA (4) vs PTC (8) | p=0,001 |
| 4 | FTC (5) vs FV-PTC (6) | P=1,000 |
| 5 | FTC (5) vs PTC (8) | P=0,002 |
| 6 | FV-PTC (6) vs. PTC (8) | P=1,000 |
Note. Kruskal-Wallis Test p=0,001 (p<0,05); FA, Follicular Adenoma; FTC, Follicular Thyroid Carcinoma; FV-PTC, Follicular Variant of Papillary Thyroid Carcinoma; PTC, Papillary Thyroid Carcinoma
Discussion
Thyroid cancer is one of the leading 10 causes of new cases worldwide (Globocan, 2020). About 76.7% of all thyroid cancers diagnosed in pathology are PTC, followed by FTC, anaplastic, and medullary carcinomas, 19.3%, 3.3%, and 0.7%, respectively (Hayati Othman et al., 2020). The H&E staining modality for differentiating the diagnosis of thyroid malignancies occasionally involves linkage of clinical data and other investigations if the tumor growth pattern demonstrates a similar pattern, such as a follicular pattern. The significance of conclusive diagnosis of thyroid cancer entities has consequences for metastatic risk, prognosis, and therapeutic selection (El-Naby et al., 2022).
Since the discovery of Claudin, the link between variation of claudin expression and cancer has been widely mentioned (Ding et al., 2013; Singh and Dhaswan, 2015). Claudins are a cluster of membrane proteins that contribute to the formation of taut connections in epithelial or endothelial cells. This protein is required for the modulation of transcellular transport throughout the epithelial or endothelial, as well as the proliferation and differentiation of cells (Bhat et al., 2020). Claudin deficiency inhibits TJ activity and leads to carcinogenesis by enhancing the multiplication, migration, and invasiveness of cancer cells (Oliveira and Morgado-Díaz, 2007). Also, Loss of adhesion between cells leads to an irregular pattern or undifferentiated phenotype (Tzelepi et al., 2008). On either hand, while the relationship between increased Claudin expression and tumorigenesis is not fully understood, it is claimed that activation of matrix metalloproteinases as a result of Claudin overexpression raises cancer’s invasiveness (Dhawan et al., 2010). These findings imply that dynamic expression of Claudin-1, either down- or up-regulated, plays a critical role in thyroid tumor development.
Numerous studies have indicated that Claudin-1 may be valuable as a biomarker for PTC (Abd El Atti and Shash, 2012; Ma et al., 2014; Németh et al., 2010). Our findings corroborate prior research indicating that Claudin-1 expression is more abundant and greater in PTC than in other thyroid neoplasms (Table 1). Claudin-1 expression was increased in lesions of PTC but not in dedifferentiated thyroid carcinoma (Fluge et al., 2006). On either side, we observed that FA lesions express less Claudin-1 than PTC lesions or their follicular variant (Table 2). In agreement with this result, several other studies found that Claudin-1 expression was either absent or poorly expressed in the membranes of FA, FTC, and other thyroid nodules neoplasms (Abd El Atti and Shash, 2012; Cheung et al., 2001; Németh et al., 2010; Tzelepi et al., 2008).
Our study shows a significant difference in Claudin-1 in FA and papillary neoplasms (Table 2). The notion that FA has a lower Claudin-1 expression score than FV-PTC and PTC implies a propensity for dynamic expression to rise between benign and follicularly differentiated malignant thyroid tumors. Additionally, (Zwanziger et al., 2015) examined Claudin-1 expression in the membrane, cytoplasm, and nucleus of tumor cells, demonstrating that expression and location of Claudin-1 were altered in follicular thyroid neoplasms and metastatic FTC. Nuclear localization of Claudin-1 may have been associated with cellular and metastatic behavior (Dhawan et al., 2010).
Strong expression of Claudin-1 in PTC and weak expression in follicular neoplasms suspected that differences in mutation pathways in the two tumor groups influenced Claudin-1 expression (Dralle et al., 2015). (Hucz et al., 2006) and (Németh et al., 2010) published comparable findings, demonstrating that PTCs express a high level of claudin-1. These findings may indicate that Claudin-1 plays a critical role in carcinogenesis and interacts with a variety of molecules implicated in the pathogenesis of PTC.
Most follicular neoplasms have RAS mutations in the PI3K pathway, while papillary neoplasms with their variants generally have BRAF mutations in the MAPK pathway (Dralle et al., 2015; Romitti et al., 2013). Furthermore, The Cancer Genome Atlas has offered a fresh knowledge in the molecular perspective of PTC which classifies its phenotypic into 2 types namely BRAF-like and RAS-like (Agrawal et al., 2014). BRAF-like neoplasms include papillary thyroid carcinomas and various non-follicular variations that all express high levels of Claudin-1. Meanwhile, RAS-like includes FV-PTC, FTC, and FA, accounting for around 70% of the Claudin-1-expressing cells (Gucer et al., 2016). Our results demonstrate that there is no difference in Claudin-1 scores between the RAS-like tumor groups FA vs FTC and FTC vs FV-PTC (Table 2). This finding may necessitate tracing the RAS mutation status in order to determine whether the results are consistent with those from earlier investigations.
In terms of tumorigenesis, (Alikanoglu et al., 2015) found that Claudin-1 is involved in the early stages of thyroid cancer, beginning with the epithelial mesenchymal transition process. Additionally, it was discovered that Claudin-1 expression persists in tumor cells in secondary lymph node locations, indicating that it plays a role at the metastatic process (Németh et al., 2010). These data suggest that more research into the significance of Claudin-1 as a theranostic marker in thyroid neoplasms is warranted.
Our data should be taken with caution due to the limited sample size and use of a single protein detection approach, which are shortcomings of this investigation. Additional study using a variety of molecular detection techniques is required to ascertain the relevance of Claudin-1 expression at the transcriptional and translational levels.
These findings imply that Claudin-1 may be useful in distinguishing follicular adenoma from follicular and classic variant of papillary thyroid carcinoma. Additionally, it can separate follicular thyroid carcinoma from papillary thyroid carcinoma, which is helpful in situations that are difficult to assess using H&E staining. It retains promise as a means of offering targeted cancer therapy.
Author Contribution Statement
The methodology was planned and designed by UM, AS, and HD; UM, AS, DI, and HC were involved in data gathering, processing, and reporting. TD and CK conducted a comprehensive conceptual and editorial evaluation; the finalization of the article was amended and approved by all of the contributors.
Acknowledgements
The Anatomical Pathology Laboratory at Hasanuddin University Hospital contributed to the feasibility of this study in part.
Study Approval
The research committee of the Faculty of Medicine at Hasanuddin University approved this project.
Ethical approval
The Ethics Committee of the Faculty of Medicine granted informed consent for this study (Protocol #UH18010017, Archive No. 21/H4.8.4.5.31/PP36- KOMETIK).
Availability of Data
On reasonable request, the associated author will release the datasets used in this work.
Conflict of Interest
All contributors report having no competing interests.
References
- Abd El Atti RM, Shash LS. Potential diagnostic utility of CD56 and claudin-1 in papillary thyroid carcinoma and solitary follicular thyroid nodules. J Egypt Natl Canc Inst. 2012;24:175–84. doi: 10.1016/j.jnci.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Agrawal N, Akbani R, Aksoy BA, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikanoglu AS, Gunduz S, Demirpence O, et al. Expression pattern and prognostic significance of claudin 1, 4 and 7 in pancreatic cancer. Asian Pac J Cancer Prev. 2015;16:4387–92. doi: 10.7314/apjcp.2015.16.10.4387. [DOI] [PubMed] [Google Scholar]
- Bhat AA, Syed N, Therachiyil L, et al. Claudin-1, a double-edged sword in cancer. Int J Mol Sci. 2020;21:1–24. doi: 10.3390/ijms21020569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Ezzat S, Freeman JL, et al. Immunohistochemical diagnosis of papillary thyroid carcinoma. Modern Pathol. 2001;14:338–42. doi: 10.1038/modpathol.3880312. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Sharma A. Claudin family of proteins and cancer: An overview. J Oncol. 2010;2010:1–11. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Lu Z, Lu Q, et al. The claudin family of proteins in human malignancy: A clinical perspective. Cancer Manag Res. 2013;5:367–75. doi: 10.2147/CMAR.S38294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dralle H, MacHens A, Basa J, et al. Follicular cell-derived thyroid cancer. Nature Rev Dis Primers. 2015;1:1–19. doi: 10.1038/nrdp.2015.77. [DOI] [PubMed] [Google Scholar]
- El-Naby N, Mohammed M. A metastatic papillary thyroid carcinoma could be confused clinically with a primary renal neoplasm; A Case Report and Literature Review. Asian Pac J Cancer Biol. 2022;7:185–89. [Google Scholar]
- Fluge Ø, Bruland O, Akslen LA, et al. Gene expression in poorly differentiated papillary thyroid carcinomas. Thyroid. 2006;16:161–75. doi: 10.1089/thy.2006.16.161. [DOI] [PubMed] [Google Scholar]
- Fujibe M, Chiba H, Kojima T, et al. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res. 2004;295:36–47. doi: 10.1016/j.yexcr.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Gucer H, Bagci P, Bedir R, et al. The value of HBME-1 and Claudin-1 expression profile in the distinction of BRAF-like and RAS-like phenotypes in papillary thyroid carcinoma. Endocrine Pathol. 2016;27:224–32. doi: 10.1007/s12022-016-9433-8. [DOI] [PubMed] [Google Scholar]
- Hayati Othman N, Nadiah Abd Ghani N, Zaini Mohd N. Clinico-pathological characteristics and survival analysis of 300 thyroid cancer cases in one referral Hospital in Kelantan, Malaysia: A 10-year study. Asian Pac J Cancer Care. 2018;3:53. [Google Scholar]
- Hucz J, Kowalska M, Jarza̧b M, et al. Gene expression of metalloproteinase 11, claudin 1 and selected adhesion related genes in papillary thyroid cancer. Endokrynologia Polska. 2006;57:18–25. [PubMed] [Google Scholar]
- Ma H, Xu S, Yan J, et al. The value of tumor markers in the diagnosis of papillary thyroid carcinoma alone and in combination. Polish J Pathol. 2014;65:202–9. doi: 10.5114/pjp.2014.45782. [DOI] [PubMed] [Google Scholar]
- Manimaran D, Karthikeyan TM, Khan DM, Thulasi RR. Follicular variant of papillary thyroid carcinoma: Cytological indicators of diagnostic value. J Clin Diagnostic Res. 2014;8:46–8. doi: 10.7860/JCDR/2014/7477.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry CR, Phitayakorn R. Follicular adenoma and carcinoma of the thyroid gland. Oncologist. 2011;16:585–93. doi: 10.1634/theoncologist.2010-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–06. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- Na HY, Moon JH, Choi JY, et al. Preoperative diagnostic categories of fine needle aspiration cytology for histologically proven thyroid follicular adenoma and carcinoma, and Hurthle cell adenoma and carcinoma: Analysis of cause of under- or misdiagnoses. PLoS One. 2020;15:1–18. doi: 10.1371/journal.pone.0241597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh J, Németh Z, Tátrai P, et al. High expression of claudin-1 protein in papillary thyroid tumor and its regional lymph node metastasis. Pathol Oncol Res. 2010;16:19–27. doi: 10.1007/s12253-009-9182-9. [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Morgado-Díaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad ML, Pellegata NS, Huang Y, et al. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Modern Pathol. 2005;18:48–57. doi: 10.1038/modpathol.3800235. [DOI] [PubMed] [Google Scholar]
- Radu TG, Mogoantă L, Busuioc CJ, StĂnescu C, Grosu F. Histological and immunohistochemical aspects of papillary thyroid cancer. Romanian J Morphol Embryol. 2015;56:789–95. [PubMed] [Google Scholar]
- Romitti M, Ceolin L, Siqueira DR, et al. Signaling pathways in follicular cell-derived thyroid carcinomas. Int J Oncol. 2013;42:19–28. doi: 10.3892/ijo.2012.1681. [DOI] [PubMed] [Google Scholar]
- Singh AB, Dhawan P. Claudins and cancer: Fall of the soldiers entrusted to protect the gate and keep the barrier intact. Semin Cell Dev Biol. 2015;42:58–65. doi: 10.1016/j.semcdb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Junctions gone bad: Claudins and loss of the barrier in cancer. Biophys Acta Rev Cancer. 2011;1816:73–9. doi: 10.1016/j.bbcan.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Tzelepi VN, Tsamandas AC, Vlotinou HD, Vagianos CE, Scopa CD. Tight junctions in thyroid carcinogenesis: Diverse expression of claudin-1, claudin-4, claudin-7 and occludin in thyroid neoplasms. Modern Pathol. 2008;21:22–30. doi: 10.1038/modpathol.3800959. [DOI] [PubMed] [Google Scholar]
- Zwanziger D, Badziong J, Ting S, et al. The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness. Endocr Relat Cancer. 2015;22:819–30. doi: 10.1530/ERC-14-0502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On reasonable request, the associated author will release the datasets used in this work.