Abstract
Background:
Oral cancer screening strategies help reduce associated mortality and could be performed by a trained frontline health worker (FHW). The present review aims to assess the diagnostic accuracy of commonly used screening modalities for oral cancer performed by FHW in apparently healthy individuals.
Methods:
Electronic databases PubMed, Scopus, Embase, Cochrane Library, and Google Scholar, were searched. The review included studies conducted where apparently healthy adult individuals were screened by the FHW for cancer or PMD of the lip and oral cavity by any of the four commonly used techniques – Conventional Oral Examination (COE), toluidine blue staining (TBS), Oral Cytology (OC), and Chemiluminescent Illumination (CLI).
Findings:
A total of 2,413 potentially relevant articles were retrieved from the search, among which five studies for COE were included in the review. Four out of those five studies were done before the year 2000. None of the studies fitted the inclusion criteria for TBS, OC, and CLI. Pooled sensitivity of oral screening by COE performed by an FHW (n=5) was 88.8% (95% CI: 71.6-96.1), whereas pooled specificity was 91.9% (95% CI: 78.3-97.3). On subgroup analysis, the pooled sensitivity and specificity of studies where the prevalence of disease was <50% (n=4) was 84.5% (95% CI: 62.6 – 94.7) and 94.1% (95% CI: 82.2 – 98.2), respectively.
Interpretation:
COE by trained FHW had high pooled sensitivity and specificity for screening of oral cancer and PMDs. The screening techniques TBS, OC, and CLI, were not studied for mass screening by trained FHW. COE by trained FHW could be utilized for oral screening in limited-resource settings. However, the FHW should be sufficiently trained to get the desired benefits of early detection.
Funding:
Department of Health Research, Ministry of Health & Family Welfare, Government of India.
Key Words: Mouth neoplasm, premalignant condition, oral cancer screening, diagnostic accuracy
Introduction
Lip and oral cavity cancers are responsible for more than 170,000 deaths globally in 2020 (GLOBOCAN, 2020). The age-standardised incidence of lip and oral cavity cancer is highest in the WHO South-East Asia region, followed by WHO Europe and WHO East-Mediterranean region (GLOBOCAN, 2020). Most patients with lip & oral cavity cancer present in advanced stages, requiring expensive and aggressive combined modality treatment, leading to low cure rates, severe morbidity, and poor quality of life.
Screening facilitates the identification of precancerous lesions, early changes of malignant transformation, and oral neoplasia in pre-invasive or early preclinical invasive stages (Sankaranarayanan et al., 2015). WHO recommends various strategies for the screening of oral cancer (IARC, 2008). In Conventional Oral Examination (COE), the oral cavity is visualized under adequate light with the help of a disposable instrument (Rajaraman et al., 2015). Toluidine blue staining (TBS), oral cytology (OC), and light-based detection are supplementary to COE and require staining of the mucosa, scrapping of the mucosa, and illumination equipment, respectively (Allegra et al., 2009; Babshet et al., 2011; Sridharan and Shankar, 2012; Chaudhry and M, 2014; Shashidara et al., 2014; Vashisht et al., 2014; Macey et al., 2015; Sukegawa et al., 2020).
The majority of countries with a high burden of oral cancer are from the developing world. Developing countries have a scarcity of trained healthcare professionals (WHO, 2016; NHM, 2018-19). This scarcity is even more pronounced in rural areas (WHO, 2016; NHM, 2018-19). Hence, the frontline health workforce (FHW) is being utilized for the many field-level activities at the grass-root level. The oral cancer screening strategies (and sample collection in case of oral cytology) can be performed by an FHW (Joseph, 2002; NPCDCS, 2013). Multiple national-level agencies recommend that oral cavity screening should be performed by FHW (Moyer, 2014). Hence, it is crucial to assess the performance of these tests in terms of diagnostic accuracy.
Studies have been conducted in multiple settings to understand the diagnostic accuracy of oral screening techniques. Macey (2015) estimated the diagnostic accuracy of oral screening tests in patients presenting with clinically evident lesions (Macey et al., 2015). The tests in the included studies were conducted in secondary health care settings and by experienced specialists. Another review by Walsh (2013) evaluated the diagnostic accuracy of COE in apparently healthy adults (Walsh et al., 2013). However, the authors included studies irrespective of the person (medical healthcare professional/ frontline healthcare worker) performing screening.
With this background, we planned this study to assess the diagnostic accuracy of commonly used screening modalities for oral cancer, i.e., COE, TBS, OC, and Chemiluminescent Illumination (CLI) screened by FHW in apparently healthy individuals. This study will provide evidence for policymakers to draw upon national or regional guidelines to suggest an appropriate strategy for oral screening.
Material and Methods
The protocol of this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), having registration number CRD42021267620, and the article was written according to PRISMA guidelines. (Table S5.1)
Search strategy
The search strategy was in three steps in the review. The initial search was through PubMed, where terms such as “oral cancer”, “premalignant disorders”, “screening”, “diagnostic accuracy” were used through Boolean operators like AND, OR, NOT for the retrieval of the initial few articles. This search was followed by exploring the controlled vocabulary and text words in the titles and abstracts. A subsequent search using all identified keywords and index terms was conducted in PubMed, Scopus, Embase, Cochrane Library, and Google Scholar (Table S2.1-6). Additionally, the reference list of all identified papers, reports, and articles was explored for bibliographic search. Studies published till December 2020 fitting the inclusion criteria were included in the review. Search results from electronic databases and other sources were exported into Rayyan software (Ouzzani et al., 2016).
Selection Criteria
The review included studies fulfilling the following criteria: (1) the study population was apparently healthy adult individuals being screened for cancer or PMD of the lip and oral cavity, (2) the FHW did the screening by any of the four commonly used techniques – COE, TBS, OC, and CLI, as a part of mass screening, (3) the diagnostic accuracy of the test was compared with an evaluation by a specialist or histopathological examination as the reference standard, (4) sufficient data were available to calculate sensitivity, specificity, positive predictive value, and negative predictive value (NPV), and (5) studies published in English language or summary in English. The operational definition of FHW used in the study was: those healthcare workers who directly provide nonspecialized basic health services at the community level. Various terms used for such health workers included Basic Health Worker, Accredited Social Health Activist (ASHA), Auxiliary nurse midwife (ANM), Multipurpose Health Workers (Male/Female), and Primary health care workers (PHCW) among others. Exclusion criteria were: (1) Studies conducted among the patient population with oral cancer or PMD, (2) studies where screening tests were conducted by dentists, doctors, or specialists, (3) Conference proceedings, reviews, case studies were excluded from the review.
Study selection and data extraction
Duplicates were removed after verifying the most recent and complete version. Two reviewers (DS and PD) independently screened the articles based on selection criteria. Any disagreements about selection were resolved by the third author (AL). Reviewers contacted the authors of the primary studies to collect missing data if it was unavailable in the reports. The final inclusion in the review was based on the full-text reading.
The data extraction was done on the pretested spreadsheet to collect information on essential details of the publication, socio-demographic characteristics of the population, details of index tests, comparator, the disease being studied, and outcome estimates such as true positive, true negative, false positive, and false negative. Two authors (DS and PD) independently extracted the data, and any disagreements were resolved by the third reviewer (AL).
Study quality assessment
The methodological quality of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool for the quality assessment of diagnostic accuracy studies (Whiting et al., 2011). Studies were rated high, unclear, and low on the risk of bias scale according to the four key domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing of participants through the study. Each domain was assessed in terms of its risk of bias and applicability.
Statistical analysis
Indicators of diagnostic accuracy, i.e., the number of true positives, true negatives, false positives, and false negatives for each test in each study, were entered into RevMan software (Review Manager, version 5.4.1; Nordic Cochrane Center, Copenhagen, Denmark). Summary estimates of sensitivity, specificity, PPV, and NPV were estimated with 95% confidence intervals. Forest plots were used to graphically display the point estimate and sensitivity and specificity confidence intervals. The summary receiver operating characteristic (SROC) curve was also plotted for the joint distribution. To address the heterogeneity, subgroup analysis was performed based on the prevalence of the disease and study location. The pooled results were estimated using MetaDTA version 1.27 (Freeman et al., 2019).
Results
Search results and excluded studies
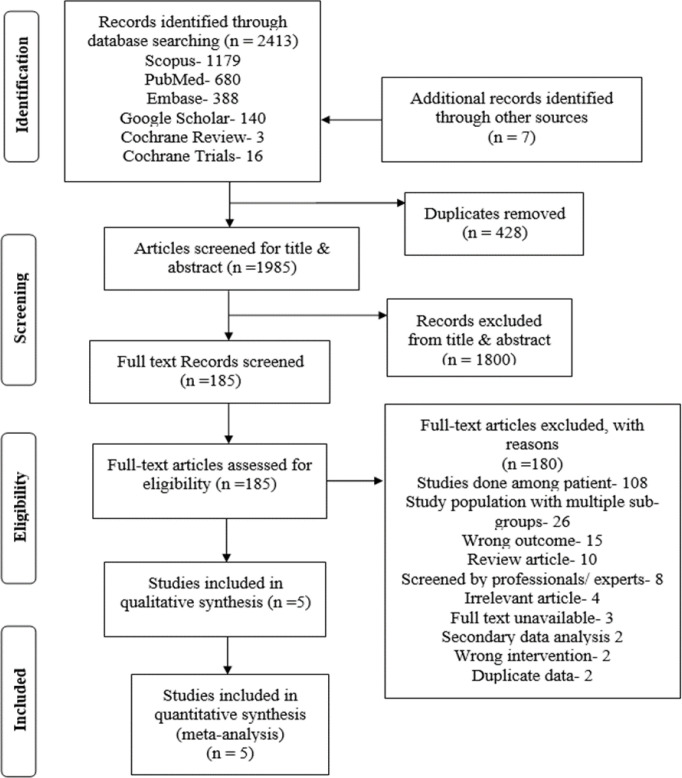
A total of 2,413 potentially relevant articles were considered for the review. Full texts of 185 articles were screened, of which 180 were excluded, and finally, five studies were included in the systematic review and meta-analysis (Figure 1). The most common reason (60%) for excluding full-text articles was the study population being patients seeking healthcare (Table S6. 1-2).
Figure 1.
PRISMA Flow Diagram for Selection of Studies
Characteristics of included studies
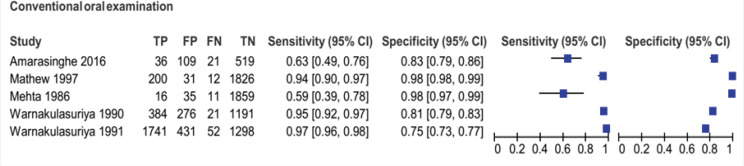
Five articles included in the review were about COE, where FHW conducted screening among apparently healthy individuals in a community setting. No studies fit the inclusion criteria for TBS, OC, and CLI. All the included studies were done in South-East Asia, two in Kerala, India, and three in Sri Lanka. Indian studies included populations above 35 years of age, whereas Sri Lankan studies included adults more than 20 years of age. The prevalence of oral cancer and PMD in the included studies ranged between 1.4 and 50.9%. The sample size of the included studies ranged from 685 to 3,543. (Table 1) The sensitivity and specificity in the included studies ranged from 59% to 97% and 73% to 98%, respectively (Figure 2).
Table 1.
Characteristics of Included Studies in the Review (COE)
| Author and Year | Location | Sample size | Age group | Prevalence (%) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| Mehta et al., 1986 | Ernakulam, Kerala, India | 1921 | 35+ years | 1.4 | 59% (39-78) | 98% (97-99) |
| Warnakulasuriya et al., 1990 | Kadugannawa, Sri Lanka | 1872 | 20-59 years | 21.6 | 95% (92-97) | 81% (79-83) |
| Warnakulasuriya et al., 1991 | Galle, Sri Lanka | 3543 | 20+ years | 50.9 | 97% (96-98) | 75% (73-77) |
| Mathew et al., 1997 | Trivandrum, Kerala, India | 2069 | 35-64 years | 10.3 | 94% (90-97) | 98% (98-99) |
| Amarasinghe et al., 2016 | Sabaragamuwa, Sri Lanka | 685 | 30+ years | 8.3 | 63% (50-75) | 83% (80-85) |
COE, Conventional Oral Examination
Figure 2.
Forest Plot of Sensitivity and Specificity of Conventional Oral Examination of the Included Studies in the Review
Quality assessment
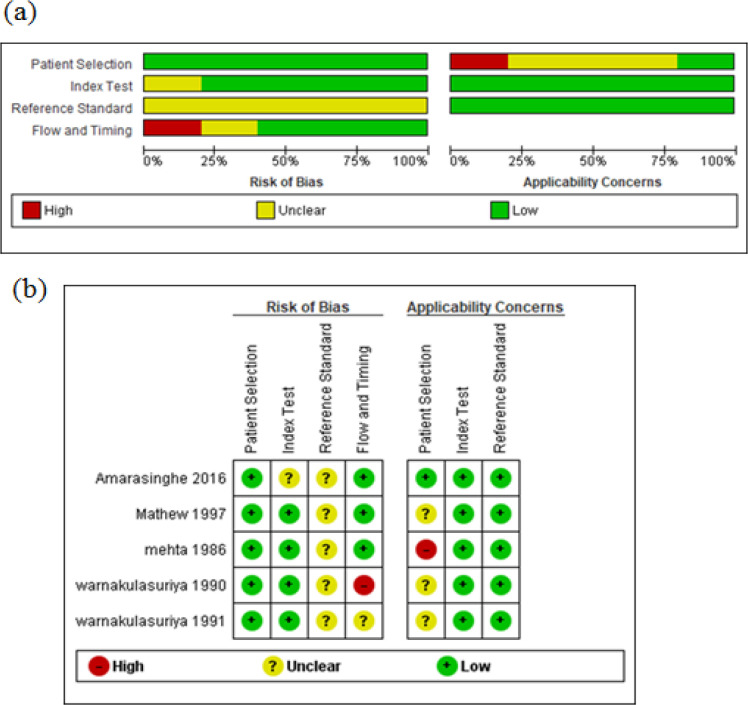
Among the included studies, there were low applicability concerns in the domains of index tests and reference standards. However, other studies did not have low applicability concerns in the patient selection domain except for one. The risk of bias was low in the domains of patient selection and index tests. In the index test and flow & timing domain, 80% and 60% of studies had a low risk of bias. However, none of the studies had a low risk of bias in the reference standard domain. (Figure 3)
Figure 3.
(a) and (b) Methodological quality graph of included studies
Diagnostic accuracy of common oral screening strategies
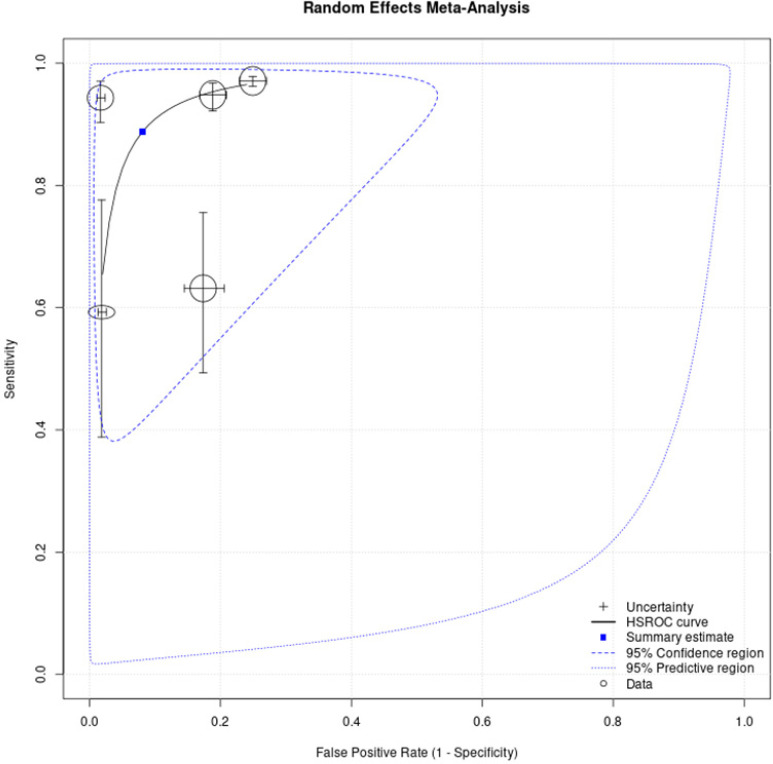
Pooled results were estimated from five studies with 10,069 participants above 20. Pooled sensitivity of oral screening by COE performed by an FHW on apparently healthy individuals was 88.8% (95% CI: 71.6-96.1), whereas pooled specificity was 91.9% (95% CI: 78.3-97.3) (Figure 4).
Figure 4.
Summary Receiver-Operating Characteristic (SROC) Curve
Indian studies (n=2) reported pooled sensitivity and specificity of COE of 83.9% (95% CI: 48.6 – 96.6) – and 98.2% (95% CI: 97.7 – 98.6) respectively. Likewise, Sri Lankan studies (n=3) reported pooled sensitivity and specificity of 91.2% (95% CI: 71.4 – 97.7) and 79.9% (95% CI: 75.7 – 83.5) respectively. To assess the effect of the prevalence of oral cancer and PMD on the sensitivity and specificity, we did a sub-group analysis for different prevalence. Studies having >10% prevalence (n=3) reported pooled sensitivity and specificity of 95.9% (95% CI: 93.7 – 97.3) and 90.1% (95% CI 67.1 – 97.6) respectively. On the other hand, the pooled sensitivity and specificity of studies having <50% prevalence (n=4) was 84.5% (95% CI: 62.6 – 94.7) and 94.1% (95% CI: 82.2 – 98.2) respectively. Studies with prevalence <50% and >10% (n=2) reported pooled sensitivity and specificity of 94.6% (95% CI 92.3 – 96.2) and 94.1% (95% CI 72 – 99) respectively.
Publication bias
The small number of studies prevented any reliable estimation of publication bias.
Discussion
Screening at the community level for oral cancer helps detect, early treatment and prevent further complications. Physicians or specialists may not be able to participate in oral cancer screening in community settings. There is prior evidence that oral cancer screening can be performed by FHW (Sankaranarayanan et al., 2005; Walsh et al., 2013). The current meta-analysis thus has a novel appeal to present the diagnostic accuracy of oral cancer screening conducted by an FHW. Our review found no study regarding the diagnostic accuracy of TBS, OC, and CLI done by FHW. However, five studies reported the diagnostic accuracy of COE by FHW for oral screening. Studies finalised for the review were from South Asian countries India and Sri Lanka. Pooled sensitivity of COE was 88.8% (95% CI: 71.6-96.1), and pooled specificity was 91.9% (95% CI: 78.3-97.3).
The sensitivity estimates reported in our study were higher than that of previous reviews by Downer (2004), Macey (2015), Moles (2002). On the contrary, the specificity estimates were lower than Downer (2004) and Moles (2002) and higher than Macey (2015). The difference is probably because of the different research questions and inclusion criteria of the reviews. Downer (2004) and Moles (2002) included studies irrespective of the person performing screening. On the other hand, Macey (2015) estimated the diagnostic accuracy of index tests (vital staining, Oral Cytology, Light-based detection, oral spectroscopy, Blood, and saliva analysis) for the detection of oral cancer and premalignant disorders of lip and oral cavity, in patients presenting with clinically evident lesions. Walsh (2013) did not provide pooled estimates of the sensitivity and specificity in the review for comparison.
Credibility of findings
Various factors add credibility to our study estimates. Firstly, carrying out a risk of bias assessment with the standard QUADAS-2 tool for all four included studies. Secondly, exploring multiple databases like PubMed, Scopus, Embase, Cochrane Library, and Google Scholar for retrieval of literature for the review. Lastly, a sub-group analysis which was done to assess the effect of prevalence and geographical location on results, strengthens the credibility of our study estimates. All these efforts increase our confidence in the validity of the estimates.
Clinical applicability
In our study, we have estimated the diagnostic accuracy of oral cancer screening strategies by the FHW. In countries with a high burden of lip and oral cavity cancer, the availability of doctors in remote and rural areas is low. Almost 68% of WHO Member States report having less than five dentists per 10,000 population. About 37% of Member States report having less than one dentist per 10,000 population (Health workforce, WHO). Dentist per 10,000 population in Bangladesh, India, Nepal, Pakistan, and Sri Lanka is 0.69, 1.62, 1.26, 1.23, and 1.07, respectively (Health workforce, WHO). Globally, 57 countries face a critical shortage of health service providers; 36 are in sub-Saharan Africa, with a relative need of almost 140% necessary to meet the threshold (WHO, 2006). The most severe in terms of absolute shortages are in South-East Asia, where the incidence of lip and oral cavity cancer is highest (WHO, 2006). According to Rural Health Statistics of 2018-19, almost 10% of the Primary Health Centres (PHC) and 16.7% of doctors at Urban Primary Health Centres (UPHC) in India are working without a doctor (NHM, 2018-19). The availability of doctors in India is highly skewed in terms of rural-urban distribution as well (Yadav and Rawal, 2016). The shortfall of doctors in other South-East Asia countries like Sri Lanka, Bangladesh, Nepal, and Pakistan is evident (Barria et al., 2018). FHWs are available at the grass-root level in many countries, and they have a wider reach than doctors (Barria et al., 2018). Hence, it is prudent to assess the performance of FHW so that oral screening services are readily available for the eligible population in developing high-burden countries.
There were no studies in which TBS, OC, and CLI were used for mass screening of lip and oral cavity cancers. These techniques supplement the screening by COE. In TBS, the mucosa is visualised after applying toluidine blue stain. After staining by FHW, this visualisation technique has been utilised for the mass screening of cervical cancer in developing countries (WHO, 2002; O’Donovan et al., 2019). VIA is the WHO-recommended method for cervical screening in LMICs (O’Donovan et al., 2019). However, we could not find studies documenting the performance of TBS for mass screening of oral cancers. OC requires the scrapping of cells from the oral mucosa with a brush, and cytology is prepared from the collected material and is seen by pathologists. Like OC, Papanicolaou staining also involves smear preparation of mucosal cells, which is evaluated by a pathologist (Al-Abbadi, 2011). These tests are resource-intensive and need evaluation by specialists (Al-Abbadi, 2011). Hence, they are not widely used for screening in the developing world. CLI is done using commercially available equipment for illumination to detect cancerous lesions. It is an electrically operated device that preferentially illuminates precancerous and cancerous lesions (Shashidara et al., 2014). The cost and expertise required to use the device make it a less desirable option for mass screening by FHW (Vashisht et al., 2014). Implementation of screening programs with these strategies requires exclusive training and more resources and equipment, leading to high costs and making it less feasible for mass screening in developing countries with a high burden of lip and oral cavity cancers (Vashisht et al., 2014).
On the other hand, COE requires fewer resources in terms of training, equipment, and time, making it a desirable option for large-scale implementation in the developing world (Sankaranarayanan et al., 2005). Pooled estimates of sensitivity and specificity demonstrate a high level of accuracy in detecting precancerous and cancerous oral lesions by FHW. The studies included in the review were from two South-East Asian countries – India & Sri Lanka and included populations where the prevalence of oral cancer and PMD varied widely. In the sub-group analysis by the site of the study and the prevalence of precancer and PMD, it was observed that the confidence intervals of the pooled estimates were overlapping. This demonstrates that the performance of this test was largely unaffected by these factors. Four out of these five studies were done more than 20 years ago (Mehta 1986; Warnakulasuriya 1990; Warnakulasuriya 1991; Mathew 1997). The recent study by Amarasinghe et al (2016) demonstrated 63% sensitivity and 82% specificity of COE. The authors concluded that there is a need for better training and facilities for oral screening. The importance of training FHW has been highlighted by previous studies as well and will play a major role in the successful implementation of the screening process (Warnakulasuriya 1984). All the included studies used different terms for the FHW who performed screening. However, all the studies included those workers who provide non-specialized care to the community.
The effectiveness of COE in reducing the morbidity and mortality due to oral cancer has been studied in the past in a randomised controlled study from Kerala, India (Sankaranarayanan et al., 2005). It was demonstrated that screening with COE in individuals with high-risk behaviour (tobacco and/or alcohol users) leads to a significant reduction in oral cancer incidence and mortality in the long run (Sankaranarayanan et al., 2005). In the community based oral cancer screening program conducted in Thailand, 88,201 individuals were screened by dental auxiliaries and 544 oral potentially malignant disorders (OPMDs) and 1,047 non-OPMDs were identified by the dentists (Klongnoi et al., 2021). A district-level oral cancer screening program conducted at 48 panchayats of Kannur district in Kerala, India, proved that trained FHW could be effectively used in oral cancer screening programs agreeing with our review (Philip et al., 2018). Almost half of the oral cancer patients detected were in the early stages of the disease in that population-based cancer screening program (Philip et al., 2018).
Hence, the high diagnostic accuracy of COE by trained FHW in detecting oral precancerous and cancerous lesions, along with the lesser requirement of resources in terms of training & equipment, and the ability of COE screening to reduce morbidity and mortality due to oral cancer makes it a possible option for mass screening programs, especially in low resource settings. The findings of our study further support the recommendations put forth by USPSTF and Indian and Sri Lankan guidelines for oral cancer screening, where oral cancer screening is recommended by the FHW (NPCDCS, 2013; Moyer, 2014; NCCP, 2019). However, screening is a long-term process that needs consistent effort and commitment from the health system. As per a national survey in India, the oral screening coverage in the rural and urban areas in 2019-20 was very low (0.8% and 1.2% for rural and urban areas, respectively) (NFHS-5, 2019-21). To obtain the desired outcome, it is essential to demonstrate a high level of screening coverage and to ensure that the FHW are adequately and regularly trained in detecting PMD and oral cancers.
Strengths and limitations
A replicable search strategy from multiple databases strengthened our review. Secondly, we used Rayyan software for screening and eligibility assessment of retrieved studies, making our process reliable and reproducible. We included most of the commonly used screening strategies for oral cancer and PMD in our review, making it comprehensive. Lastly, heterogeneity sources were explored and addressed in our review, thus improving our estimates’ reliability.
A total of 180 articles were excluded after full-text assessment, where 60% were excluded because they were conducted among the patients. However, considering the scarcity of doctors at the grass-root level, we specifically wanted to assess the accuracy of screening by FHW to derive implementable results for the population-based cancer screening program. Despite the comprehensive search undertaken, only five articles were retrieved for COE. As a result, meta-regression could not be performed to explore the reasons for heterogeneity. However, we did a subgroup analysis to assess the effect of the study site and prevalence of oral cancer and PMD on the estimates of diagnostic accuracy. Besides, we could not find any articles about TBS, OC, and CLI, which could be because no studies were done for population-based mass screening where FHW was involved. Prevalence among the included studies varied widely. However, we tried to address it by sub-group analysis to see the effect of the prevalence of oral cancer and PMD on summary estimates.
Conclusions and Policy recommendations
Conventional Oral Examination by trained frontline health workers had high (>80%) sensitivity and specificity for screening of oral cancers and PMD. The screening techniques TBS, OC, and CLI, were not studied for mass screening by trained FHW.
COE after training FHW should be considered for screening of oral cancer and potentially malignant disorders, especially in resources constrained LMICs. However, the FHW should be sufficiently trained to get the desired benefits of early detection.
Author Contribution Statement
Conceptualization: Ayush, Dahy; Data curation: Dahy, Ayush; Formal analysis: Dahy, Ayush, Rizwan; Investigation: Dahy, Ankita, Pooja, Pankaj, Jyoti; Methodology: Dahy, Ayush, Rizwan; Project administration: Ayush, Ankur; Resources: Ayush, Vijendra; Supervision: Rizwan, Ankur, Vijendra; Validation: Ayush, Pankaj, Jyoti, Rizwan; Visualisation: Ayush, Pankaj, Jyoti, Rizwan; Writing - original draft: Dahy, Ayush; Writing -review and editing: Dahy, Ayush, Rizwan, Ankita, Pooja, Pankaj, Jyoti, Ankur, Vijendra.
Acknowledgments
We are thankful to the authors of the included studies for generating valuable and useful data.
Funding Statement
This study was funded by the Department of Health Research, Ministry of Health & Family Welfare, Government of India.
Approval
This study was not a part of a student thesis. Primary data collection involving human subjects was not required for this study. The study includes the compilation of already published data. The protocol of this study was registered under PROSPERO with registration number CRD42021267620.
Ethical Declaration
This study is exempt from ethical review and approval. It is a meta-analysis aggregating freely available data from published research. The informed consent and all due ethical proceedings were already satisfied by the authors of the individual eligible articles.
Data Availability
The data underlying this article are available in the article and in its online Supplementary Material.
Study Registration
The protocol of this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42021267620.
Conflict of Interest
None.
References
- Al-Abbadi MA. Basics of cytology. Avicenna J Med. 2011;1:18–28. doi: 10.4103/2231-0770.83719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngol Ital. 2009;29:187–90. [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe AA, Usgodaarachchi US, Johnson NW. Evaluation of the utilization of primary healthcare staff for control of oral cancer: A Sri Lankan experience. Transl Res Oral Oncol. 2016;1:1–6. [Google Scholar]
- Babshet M, Nandimath K, Pervatikar S, Naikmasur V. Efficacy of oral brush cytology in the evaluation of the oral premalignant and malignant lesions. J Cytol. 2011;28:165–72. doi: 10.4103/0970-9371.86342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry AMM. Evaluation of efficacy of chemiluminescence for diagnosis of leukoplakia. Oral Sci. 2014;Int:11, 56–9. [Google Scholar]
- Downer MC, Moles DR, Palmer S, Speight PM. A systematic review of test performance in screening for oral cancer and precancer. Oral Oncol. 2004;40:264–73. doi: 10.1016/j.oraloncology.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Freeman SC, Kerby CR, Patel A, et al. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol. 2019;19:81. doi: 10.1186/s12874-019-0724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph BK. Oral cancer: prevention and detection. Med Princ Pract. 2002;11:32–5. doi: 10.1159/000057776. [DOI] [PubMed] [Google Scholar]
- Klongnoi B, Sresumatchai V, Khovidhunkit SP, et al. Pilot model for community based oral cancer screening program: outcome from 4 northeastern provinces in Thailand. Int J Environ Res Public Health. 2021;18:9390. doi: 10.3390/ijerph18179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey R, Walsh T, Brocklehurst P, et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2015;2015:CD010276. doi: 10.1002/14651858.CD010276.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B, Sankaranarayanan R, Sunilkumar KB, et al. Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer. 1997;76:390–4. doi: 10.1038/bjc.1997.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta FS, Gupta PC, Bhonsle RB, et al. Detection of oral cancer using basic health workers in an area of high oral cancer incidence in India. Cancer Detect Prev. 1986;9:219–25. [PubMed] [Google Scholar]
- Moles DR, Downer MC, Speight PM. Meta-analysis of measures of performance reported in oral cancer and precancer screening studies. Br Dent J. 2002;192:340–4. doi: 10.1038/sj.bdj.4801370. [DOI] [PubMed] [Google Scholar]
- Moyer VA. Screening for oral cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:55–60. doi: 10.7326/M13-2568. [DOI] [PubMed] [Google Scholar]
- National Cancer Control Programme. National Guidelines for Management of Oral Potentially Malignant Disorders for Medical and Dental Practitioners. Ministry of Health. 2019;Nutrition and Indigenous Medicine:Sri Lanka. ISBN 978–955-3666-27-7. [Google Scholar]
- O’Donovan J, O’Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: a systematic scoping review of the literature. BMJ Glob Health. 2019;4:e001452. doi: 10.1136/bmjgh-2019-001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip PM, Nayak P, Philip S, et al. Population-based cancer screening through community participation: Outcome of a district wide oral cancer screening program from rural Kannur, Kerala, India. South Asian J Cancer. 2018;7:244–8. doi: 10.4103/sajc.sajc_104_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman P, Anderson BO, Basu P, et al. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015;16:e352–61. doi: 10.1016/S1470-2045(15)00078-9. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- Shashidara R, Sreeshyla HS, Sudheendra US. Chemiluminescence: a diagnostic adjunct in oral precancer and cancer: a review. J Cancer Res Ther. 2014;10:487–91. doi: 10.4103/0973-1482.138215. [DOI] [PubMed] [Google Scholar]
- Sridharan G, Shankar AA. Toluidine blue: A review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16:251–5. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa S, Ono S, Nakano K, et al. Clinical study on primary screening of oral cancer and precancerous lesions by oral cytology. Diagn Pathol. 2020;15:107. doi: 10.1186/s13000-020-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisht N, Ravikiran A, Samatha Y, et al. Chemiluminescence and Toluidine blue as diagnostic tools for detecting early stages of oral cancer: An invivo Study. J Clin Diagn Res. 2014;8:Zc35–8. doi: 10.7860/JCDR/2014/7746.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Liu JL, Brocklehurst P, et al. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev. 2013;2013:CD010173. doi: 10.1002/14651858.CD010173.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya KA, Ekanayake AN, Sivayoham S, et al. Utilization of primary health care workers for early detection of oral cancer and precancer cases in Sri Lanka. Bull World Health Organ. 1984;62:243–50. [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya S, Pindborg JJ. Reliability of oral precancer screening by primary health care workers in Sri Lanka. Community Dent Health. 1990;7:73–9. [PubMed] [Google Scholar]
- Warnakulasuriya KA, Nanayakkara BG. Reproducibility of an oral cancer and precancer detection program using a primary health care model in Sri Lanka. Cancer Detect Prev. 1991;15:331–4. [PubMed] [Google Scholar]
- Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cervical cancer screening in developing countries : report of a WHO consultation. Geneva: World Health Organization; 2002. https://apps.who.int/iris/handle/10665/42544 . [Google Scholar]
- World Health Organization. The world health report: 2006 working together for health. World Health Organization: 2006. https://apps.who.int/iris/handle/10665/43432 . [Google Scholar]
- World Health Organization. The health workforce in India. World Health Organization; https://apps.who.int/iris/handle/10665/250369 . [Google Scholar]
- Yadav S, Rawal G. The current status of dental graduates in India. Pan Afr Med J. 2016;23:22. doi: 10.11604/pamj.2016.23.22.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.