Abstract
Background
A plasma-based clot lysis time (CLT) assay is an established research test to assess plasma fibrinolytic potential, with application in hyperfibrinolytic or hypofibrinolytic conditions. Interprotocol variations make comparisons between laboratories challenging. The aim of this study was to compare the results of 2 different CLT assays performed by 2 distinct research laboratories by using their own protocol.
Methods
We evaluated fibrinolysis in the plasma of 60 patients undergoing hepatobiliary surgery and in plasma from a healthy donor that was spiked with commonly used anticoagulant drugs (enoxaparin, dabigatran, and rivaroxaban) in 2 distinct laboratories (Aarhus and Groningen) by using 2 different assays that differ, among others, in tissue plasminogen activator (tPA) concentration.
Results
Overall conclusions on fibrinolytic potential in patients undergoing hepatobiliary surgery were similar between the 2 CLT assays, with hyperfibrinolytic and hypofibrinolytic profiles identified at the same time points during and after surgery. Severe hypofibrinolysis was less commonly reported in the Aarhus assay (36/319 samples; 11%) than in the Groningen assay (55/319 samples; 17%). No clot formation was observed in 31 of 319 samples in the Aarhus assay vs 0 of 319 samples in the Groningen assay. Clotting times increased much more profoundly on the addition of all 3 anticoagulants in the Aarhus assay.
Conclusions
Despite the differences in laboratory, protocol, reagents, operator, data processing, and analysis, overall conclusions on fibrinolytic capacity are similar between the 2 laboratories. With a higher concentration of tPA in the Aarhus assay, the test becomes less sensitive for the detection of hypofibrinolysis and is more sensitive to the addition of anticoagulants.
Keywords: blood coagulation tests, comparative study, fibrinolysis, fibrin clot lysis time, tissue plasminogen activator
Essentials
-
•
An imbalance in the fibrinolytic process may contribute to bleeding or thrombotic complications.
-
•
Global fibrinolysis assay protocol variations make comparison between laboratories challenging.
-
•
A common difference between protocols is the tissue-type plasminogen activator concentration.
-
•
Overall conclusions on hyperfibrinolysis and hypofibrinolysis are similar between 2 distinct assays.
1. Introduction
Fibrinolysis is the process of enzymatic degradation of a fibrin clot. This process is tightly regulated by activators and inhibitors and is aimed at not only dissolving fibrin clots following hemostasis but also clears intravascular thrombi. The central step of the fibrinolytic system involves the conversion of plasminogen to active plasmin, which then cleaves fibrin to fibrin degradation products. An imbalance in the fibrinolytic process may cause bleeding in the case of hyperfibrinolysis or contribute to the development of thrombosis in the case of hypofibrinolysis. For example, patients with congenital deficiencies in fibrinolysis inhibitors (α2-antiplasmin, plasminogen activator inhibitor-1 [PAI-1]) or patients with excessive release of tissue-type plasminogen activator (tPA) such as following trauma or postpartum may develop hyperfibrinolysis-related bleeding [[1], [2], [3]]. On the contrary, if the balance is shifted toward a hypofibrinolytic state, which may be related to the elevated plasma levels of PAI-1 and increased levels of thrombin-activatable fibrinolysis inhibitor (TAFI) are reported to be associated with an increased risk of venous thrombosis [4]. PAI-1 or TAFI-related hypofibrinolysis may also contribute to thrombosis in patients with type 2 diabetes, acute liver failure, or cancer [[5], [6], [7]]. In addition, sepsis-related disseminated intravascular coagulation is also associated with suppressed fibrinolysis [8].
Laboratory assessment of a patients’ fibrinolytic state by the measurement of individual fibrinolytic factors is not very informative except in the very rare conditions, in which a single deficiency or excess is present. However, in many clinical conditions, such as liver failure or sepsis, plasma concentrations of multiple profibrinolytic and antifibrinolytic proteins are altered simultaneously, and therefore, global fibrinolysis tests will provide a clinically more relevant assessment of the patient’s fibrinolytic capacity. However, global tests of fibrinolysis are not commonly used in the clinical setting. Some clinical and research laboratories use the euglobulin clot lysis time (CLT) [9], which uses the euglobulin precipitate and is therefore per definition not a global test. A patient’s global fibrinolytic state can be assessed by using viscoelastic tests with whole blood, such as rotational thromboelastometry (ROTEM) or thromboelastography (TEG). However, standard ROTEM or TEG protocols are not sensitive to detect (mild) hyperfibrinolysis or hypofibrinolysis [10,11]. A plasma-based CLT assay, where exogenous tissue factor, calcium, phospholipids, and tPA are added to induce clotting and fibrinolysis, is an accepted research test for plasma fibrinolytic potential, with application in conditions expected to be associated with hyperfibrinolytic or hypofibrinolytic changes [12]. Several variations of global fibrinolysis potential tests exist, eg, overall hemostatic potential assays and the Nijmegen hemostasis assay, which directly measures thrombin and plasmin generation [13,14]. The clotting and fibrinolysis assay or the CLT assay has been widely used, with different laboratories using distinct protocols [12,[15], [16], [17], [18], [19], [20], [21], [22], [23]]. The most important differences include the concentration of tPA and tissue factor, dilution of plasma, and differences in data analysis and reporting [16,24]. These interprotocol variations make comparisons between laboratories challenging. For example, in a study with plasma from 80 healthy individuals, it was demonstrated that determinants of 3 distinct clot lysis assays, executed in a single laboratory, were not identical [25]. In addition, even with the same protocol, substantial interlaboratory variation exists [16]. The aim of this study was to compare the results of 2 different CLT assays performed by 2 distinct research laboratories by using their own protocol. We tested the clinical samples of patients undergoing hepatobiliary surgery, as we previously demonstrated for normo-, hyperfibrinolysis, and hypofibrinolytic profiles under these conditions [26,27].
2. Methods
Citrated plasma samples were obtained from patients undergoing orthotopic liver transplantation (OLT) (n = 20), partial hepatectomy (n = 20), or pylorus-preserving pancreatico-duodenectomy (PPPD) (n = 20) at King’s College Hospital, London, United Kingdom, between September 2017 and December 2017. Patient details have been previously published [28]. In short, blood samples were taken into 3.2% sodium citrate tubes and were centrifuged at 18 °C for 10 minutes at 2000 g and subsequently for 10 minutes at 10,000 g within 30 minutes after blood collection. For patients undergoing OLT, plasma samples were drawn at the following time points: after induction of anesthesia, 30 minutes after the start of the anhepatic phase, 30 minutes after reperfusion, at the end of the surgery, and on postoperative days 1, 3, and 6. Plasma samples from patients undergoing partial hepatectomy or PPPD were drawn at the following time points: after induction of anesthesia, at the end of the surgery, and on postoperative days 1, 3, and 6. The results of healthy controls were included as a reference range for both assays. Analysis of the effects of anticoagulants on the CLT assays, to study the sensitivity of both assays to the addition of anticoagulants, was performed with plasma from a healthy donor that was spiked with a range of concentrations of enoxaparin (Clexane, Sanofi; 0-2 U/mL), enoxaparin neutralized with polybrene, the direct thrombin-inhibitor dabigatran (Pradaxa, Boehringer Ingelheim; 0-200 ng/mL), or the direct factor Xa-inhibitor rivaroxaban (Xarelto, Bayer; 0-300 ng/mL).
The study was approved by the National Research Ethic Service Committee London—Westminster, Study Number 17/LO/0527. All patients and healthy donors gave written informed consent. Plasma samples from patients and from the healthy donor spiked with anticoagulants were transported from Groningen to Aarhus on dry ice and stored at −80 °C until analysis. Intralaboratory variability was determined by including a normal plasma sample in each measurement. The time between measurements in both laboratories was less than 1 month to avoid the influence of storage time.
CLT assays were performed as described before [12,15] and are summarized in the Table. The following parameters were derived in the Aarhus assay: time to initial fibrin formation (lag phase) and time from maximum absorbance to 50% lysis (50% lysis time). The following parameters were derived in the Groningen assay: time where 50% of fibrin formation has occurred (t1/2 coagulation), time where 50% of fibrinolysis has occurred (t1/2 fibrinolysis), and the time between t1/2coagulation and t1/2fibrinolysis (CLT). Samples without complete lysis were arbitrarily assigned a CLT of 45 minutes for the Aarhus assay and 180 minutes for the Groningen assay.
Table.
Basic principles of the 2 clot lysis time assays.
| Aarhus assay [12] | Groningen assay [15] | |
|---|---|---|
| Material | Citrated plasma | Citrated plasma |
| Coagulation trigger | Tissue Factor (Dade Innovin, Siemens Healthcare), 1:5000 dilution | Tissue Factor (Dade Innovin, Siemens Healthcare), 1:1000 dilution |
| Calcium chloride | 26.7 mM | 17 mM |
| Phospholipids | 4 μM (Rossix) | 10 μM (Avanti Polar Lipids) |
| Fibrinolysis activator | tPA (Calbiochem, Sigma-Aldrich, Merck), 116 ng/mL. Activity: ≥300,000 IU/mg protein | tPA, (Actilyse, Boehringer Ingelheim), 56 ng/mL. Activity: 522,000 to 696,000 IU/mg protein |
| Buffer | HEPES buffer 20 mM, NaCl 150 mM, pH 7.4 (Ampliqon) | HEPES buffer 25 mM, 137 mM NaCl, 3.5 mM KCl, 0.1% BSA, pH 7.4 |
| Maximum assay time | 80 min | 180 min |
| Plasma dilution (plasma:mix) | 1:1.15 | 1:1 |
| Reader and software | Victor Reader X4; 2030 Work Out and WorkOut 2.5 (Perkin Elmer) | Spectramax 340 kinetic microplate reader and SoftMax Pro (Molecular Devices Corporation), LysisAnalyzer version 4.0.0.0 |
| Parameters | Time from max absorbance to 50% lysis (s); lag phase (s); peak absorbance (AU); time to peak absorbance (s); area under curve (AU×s) | CLT (min); t1/2 coagulation (min); t1/2 fibrinolysis (min); OD max (OD); time to OD max (min); slope ½ coagulation; slope ½ fibrinolysis |
Concentration and dilution are the final concentration or dilution in the 96-well microtiter plate.
AU, absorbance units; BSA, bovine serum albumin; CLT, clot lysis time; HEPES, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); OD, optical density; tPA, tissue plasminogen activator.
Anti-factor Xa (anti-Xa) activity and the endogenous thrombin potential (ETP) were measured in plasma samples from OLT patients at time points 5, 6, and 7. Anti-Xa activity was measured on an automated coagulation analyzer (ACL 300 TOP) by using Heparin LRT (Hyphen Biomed). The ETP was measured by using calibrated automated thrombography, with a protocol and reagents from Thrombinoscope BV, Maastricht, the Netherlands, as described before [28]. Coagulation was activated with recombinant tissue factor (final concentration 5 pM) and phospholipids (final concentration 4 μM).
3. Results
We compared the results of 2 different CLT assays, of which typical CLT curves are shown in Figure 1. Both laboratories used an internal control sample of pooled plasma in each experiment. The Aarhus control sample had a mean lysis time of 12 ± 2 minutes (or a coefficient of variation of 16%). The Groningen control sample had a mean CLT of 72 ± 3 minutes (coefficient of variation of 4%). Figure 2 shows the lysis times from samples that were taken at multiple time points from patients who underwent hepatobiliary surgery and were measured with the 2 assays by 2 distinct laboratories. The samples that were measured are very heterogeneous and include samples with very short lysis times and samples where no lysis occurs within the time that the assay runs. The conclusions from both assays are overall very similar. For example, in both assays, mean CLTs were decreased compared with control values in samples taken during OLT with the development of a hypofibrinolytic state at the end of OLT and in the postoperative period. However, the mean CLT values normalized over time in the Aarhus assay, whereas in the Groningen assay, sustained hypofibrinolysis was observed. A sustained postoperative hypofibrinolytic state after partial hepatectomy and pancreas resection was observed in both assays. However, in patients undergoing a pancreas resection, the Aarhus assay appeared to normalize more rapidly compared with the Groningen assay.
Figure 1.
Typical examples of a clot lysis curve from 2 CLT assay variants. (A) Clot lysis curve from Aarhus. (B) Clot lysis curve from Groningen.
Figure 2.
Fibrinolysis times in plasma from patients who underwent hepatobiliary surgery, as measured by 2 laboratories with different protocols. The Aarhus lab reported 50% lysis time, the Groningen lab reported CLT. (A) Results from patients who underwent liver transplantation. Samples were taken at the following time points: 1, after induction of anesthesia; 2, 30 minutes after the start of the anhepatic phase; 3, 30 minutes after reperfusion; 4, at the end of the surgery; 5, postoperative day 1; 6, postoperative day 3; 7, postoperative day 6. (B) Results from patients who underwent partial hepatectomy. (C) Results from patients who underwent PPPD. Plasma samples from patients who underwent partial hepatectomy or PPPD were taken at the following time points: 1, after the induction of anesthesia; 2, at the end of the surgery; 3, postoperative day 1; 4, postoperative day 3; 5, postoperative day 6. Dashed area between the dotted lines indicates range of normal values from 120 healthy subjects in the Aarhus assay [30]. Gray area between the dotted lines indicates range of normal values from 35 healthy subjects in the Groningen assay. CLT, clot lysis time; PPPD, pylorus preserving pancreaticoduodenectomy.
When analyzing individual samples, a severe hypofibrinolytic state (defined by incomplete lysis during the time the assay is run) was less frequently reported by the Aarhus assay. Specifically, severe hypofibrinolysis was reported in 36 of 319 samples (11%) in the Aarhus assay and in 55 of 319 samples (17%) in the Groningen assay. On the contrary, the Aarhus assay reported many samples where no coagulation was observed, which were categorized as ‘flat curves’. In particular, samples from patients who underwent OLT had many flat curves: 26 of 138 samples showed a flat curve, whereas 2 of 88 samples from patients who underwent partial hepatectomy and 3 of 93 samples from patients who underwent PPPD had a flat curve. No flat curves were reported in the results from the Groningen assay. To correlate the results from both assays, linear regression was calculated for all 3 patient groups combined. The R2 was 0.54. The R2 from the data without the flat curves and no lysis values was 0.36 (Figure 3).
Figure 3.
Linear regression of CLT values between the 2 contributing laboratories. (A) Linear regression of all the data combined. (B) Linear regression of the data where samples where no coagulation or no fibrinolysis was observed were excluded.
Because the release of endogenous heparinoids is a well-known phenomenon in liver transplantation [31], we hypothesized that the flat curves in the Aarhus assay may be related to heparinoids in the samples. Therefore, we measured anti-Xa activity in these samples, which was present in 10 of 21 (48%) of the samples where a flat curve was observed and in 9 of 36 (25%) of the samples where no flat curve was observed. We also measured ETP in these samples, which was 449 nM IIa × minute in samples with a flat curve where anti-Xa activity was measured. In samples where no anti-Xa activity was measured, the ETP was 856 nM IIa × minute.
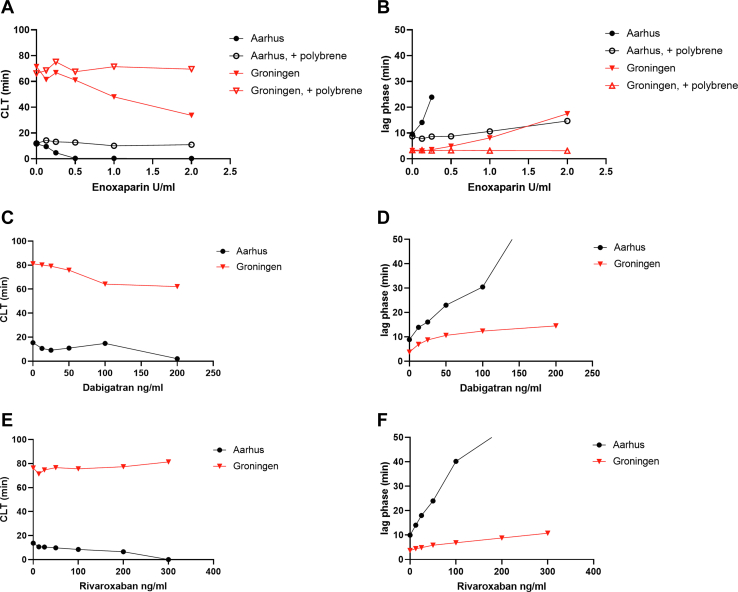
We studied the effects of the addition of heparin and other clinically used anticoagulants to plasma on the results of both CLT assays to investigate the sensitivity of both assays to the addition of anticoagulants. Plasma from a healthy donor was spiked with enoxaparin, enoxaparin neutralized with polybrene, dabigatran, and rivaroxaban, in a range of concentrations. Figure 4 shows the (clot) lysis times and lag phase from both assays. Addition of enoxaparin, dabigatran, and rivaroxaban dose dependently decreased the clot lysis times in both assays. The enoxaparin-induced decrease of CLT was completely reversed by polybrene in both assays. However, all 3 drugs at clinically relevant concentrations led to flat curves in the Aarhus assay, whereas in the Groningen assay, all concentrations tested resulted in measurable CLTs. Indeed, clotting times increased much more profoundly on the addition of all 3 anticoagulants in the Aarhus assay (Figure 4).
Figure 4.
CLT (Figures A, C, and E) and lag phase (Figures B, D, and F) in plasma from a healthy donor that was spiked with a range of concentrations of anticoagulants, measured in 2 laboratories with different protocols.
4. Discussion
In this study, we compared the results of 2 CLT assays that are used to assess the global plasma fibrinolytic potential. We showed that the 2 CLT assays have a reasonable correlation and give similar conclusions on fibrinolysis capacity in plasma samples from patients who underwent hepatobiliary surgery. However, the Aarhus variant of the CLT assay uses a much higher tPA concentration and therefore requires less time to perform the test. The Aarhus test less frequently indicates extreme hypofibrinolysis compared with the Groningen assay and had more results where no coagulation is observed. In addition, the Aarhus test results in a flat curve when clinically relevant doses of low molecular weight heparin (LMWH) and direct oral anticoagulants (DOACs) are present in the sample, and this phenomenon is not present in the Groningen variant of the CLT assay.
An important difference between these tests is the concentration of tPA added to induce fibrinolysis, which will directly affect the final lysis times [32]. With a higher amount of tPA as used in the Aarhus assay, clot lysis starts immediately following clot formation, whereas in the Groningen assay, consolidation of the clot before lysis is observed (Figure 1). An ideal amount of tPA in the test allows proper clot formation but does not require a very long experiment time. Although the conclusions of both tests are similar, there are caveats in the use of very high tPA levels. High tPA may cause the initiation of fibrinolysis already during clot formation and may in addition cause fibrinogenolysis. These effects may, in part, explain why patients who underwent OLT showed many flat curves. In addition, the presence of heparinoids in the samples in part explain the flat curves in the Aarhus assay, and these flat curves were particularly observed in patients undergoing OLT. Heparinoids may be present in the samples by 2 mechanisms. First, heparin that was administered to the donor before aortic cross-clamping binds to the liver endothelium and is released into the systemic circulation of the recipient after reperfusion. Second, heparin-like substances (glycosaminoglycans; GAGs) such as heparan sulfate are present in the cirrhotic patient as a result of liver disease and infection and may increase during liver transplant surgery because of ischemic reperfusion injury [33]. In addition, a higher amount of tPA makes the test less sensitive to hypofibrinolysis because the high amount of tPA will mask high levels of PAI-1 in plasma. The Groningen assay may be therefore be preferential in suspected hypofibrinolysis samples, such as in samples taken from patients with acute liver failure or diabetes.
A postoperative fibrinolytic shutdown has been attributed to high levels of PAI-1 [34] and has been shown in samples taken after surgery from patients who underwent hepatobiliairy surgery [26]. Indeed, extreme hypofibrinolysis in this study was observed in the samples that were taken at the end of surgery but normalized much quicker in the Aarhus assay. Conversely, high tPA levels may also be beneficial in severe hypofibrinolytic samples because they may better distinguish between different grades of hypofibrinolysis. Higher tPA levels, as used in the Aarhus assay, also mean that results are obtained faster, which will be beneficial if plasma-based clot lysis tests would be used in clinical settings. The distinct advantages and disadvantages of high and low tPA levels in the assay make it difficult to advice which variant to use in a particular setting, and future studies with clinically relevant outcomes are required to provide a definitive answer to this question.
Besides differences in tPA levels, other differences in the protocol including plasma dilution and the concentration and source of tissue factors and lipids, equipment and operator, and data processing and analysis influence the CLT assay results. Recently, Pieters et al. [16] described the variability in the results of a combined turbidity and clot lysis assay, where a significant variability between the 9 laboratories that followed the same protocol with the same samples was shown.
The susceptibility of a fibrin clot to fibrinolysis is increased by LMWH and DOACs, which has been shown in experiments with healthy donor plasma that was spiked with anticoagulants and with plasma from patients receiving these agents [17,29,35]. These effects are, at least in part, attributable to the decreased activation of TAFI [[36], [37], [38], [39]]. The increased susceptibility to fibrinolysis of a fibrin clot by LMWH and DOACs suggest that anticoagulants have therapeutic efficacy not only based on inhibiting coagulation but also by enhancing fibrinolysis. We spiked healthy donor plasma with a range of plasma concentrations of enoxaparin, dabigatran, and rivaroxaban with the aim to compare the sensitivity of the 2 assays to the addition of anticoagulants. We found that the clot-formation lag phase was prolonged in a concentration-dependent manner (Figure 4). The effect on lag phase and lysis times is much more pronounced in the Aarhus assay than that in the Groningen assay, which again may be explained not only by the higher levels of tPA but also the lower levels of tissue factor and lipids and the difference in plasma dilution in the Aarhus assay.
In conclusion, this study shows that 2 different protocols for a global test of fibrinolysis provide similar conclusions in samples from patients who underwent hepatobiliary surgery. Despite the differences in laboratory, protocol, reagents, operator, and data processing and analysis, overall conclusions on fibrinolytic capacity are similar between the 2 laboratories. With a higher concentration of tPA, the test becomes less sensitive for the detection of hypofibrinolysis and is more sensitive to the addition of anticoagulants. The results shown may refute the discussion [40] about difficulties in interpretation and variation of the CLT assay results. Although differences between laboratories will exist even with similar protocols, with well-validated tests, good internal control procedures, valid reference intervals, and standardization of reporting, comparison across studies can be optimized.
Acknowledgments
We gratefully acknowledge Bente van den Boom, Tsai-Wing Ow, Andreas Prachalias, Alina Phoolchund, Nurya Dunsire, Zoka Milan, and Nigel Heaton for their assistance with sample collection, and Trang Le, Vivi Bo Mogensen, and Mai Stenulm Veirup for their laboratory assistance.
Author contributions
E.G.D.—performed experiments, analyzed data, and wrote the manuscript; J.B.L.—conceived the project; performed experiments, interpreted data, and revised the manuscript; S.B.—collected the samples and revised the manuscript; W.B.—collected the samples and supervised sample collection and revised the manuscript; A.M.H.—conceived the project, interpreted data, and revised the manuscript; T.L.—conceived the project, supervised experiments, interpreted data, and wrote the manuscript.
Declaration of competing interest statement
The authors have no conflicts of interest pertaining to the present paper. J.B.L. has the following general conflicts of interest: Received speaker’s fees from Bristol-Myers Squibb and travel support from Bayer.
Footnotes
Funding information This study was funded in part by a grant from the Dutch Thrombosis Foundation (2018-02).
Handling Editor: Henri Spronk
References
- 1.Franchini M., Zaffanello M., Mannucci P.M. Bleeding disorders in primary fibrinolysis. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22137027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saes J.L., Schols S.E.M., van Heerde W.L., Nijziel M.R. Hemorrhagic disorders of fibrinolysis: a clinical review. J Thromb Haemost. 2018;16:1498–1509. doi: 10.1111/jth.14160. [DOI] [PubMed] [Google Scholar]
- 3.Kruithof E.K., Tran-Thang C., Gudinchet A., Hauert J., Nicoloso G., Genton C., et al. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- 4.Meltzer M.E., Lisman T., de Groot P.G., Meijers J.C., le Cessie S., Doggen C.J., et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–121. doi: 10.1182/blood-2010-02-267740. [DOI] [PubMed] [Google Scholar]
- 5.Bryk-Wiązania A.H., Undas A. Hypofibrinolysis in type 2 diabetes and its clinical implications: from mechanisms to pharmacological modulation. Cardiovasc Diabetol. 2021;20:191. doi: 10.1186/s12933-021-01372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronostaj K., Richter P., Nowak W., Undas A. Determinants of hypofibrinolysis in patients with digestive tract cancer. Prz Gastroenterol. 2016;11:104–110. doi: 10.5114/pg.2016.57619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisman T., Bakhtiari K., Adelmeijer J., Meijers J.C.M.C.M., Porte R.J.J., Stravitz R.T.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10:1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. [DOI] [PubMed] [Google Scholar]
- 8.Iba T., Connors J.M., Nagaoka I., Levy J.H. Recent advances in the research and management of sepsis-associated DIC. Int J Hematol. 2021;113:24–33. doi: 10.1007/s12185-020-03053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilich A., Noubouossie D.F., Henderson M., Ellsworth P., Betbadal K.F., Campello E., et al. Development and application of global assays of hyper- and hypofibrinolysis. Res Pract Thromb Haemost. 2019;4:46–53. doi: 10.1002/rth2.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gall L.S., Brohi K., Davenport R.A. Diagnosis and Treatment of Hyperfibrinolysis in Trauma (A European Perspective) Semin Thromb Hemost. 2017;43:224–234. doi: 10.1055/s-0036-1598001. [DOI] [PubMed] [Google Scholar]
- 11.Lisman T. Decreased Plasma Fibrinolytic Potential As a Risk for Venous and Arterial Thrombosis. Semin Thromb Hemost. 2017;43:178–184. doi: 10.1055/s-0036-1585081. [DOI] [PubMed] [Google Scholar]
- 12.Larsen J.B., Hvas A.-M. Fibrin Clot Formation and Lysis in Plasma. Methods Protoc. 2020;3 doi: 10.3390/mps3040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curnow J.L., Morel-Kopp M.-C., Roddie C., Aboud M., Ward C.M. Reduced fibrinolysis and increased fibrin generation can be detected in hypercoagulable patients using the overall hemostatic potential assay. J Thromb Haemost. 2007;5:528–534. doi: 10.1111/j.1538-7836.2007.02362.x. [DOI] [PubMed] [Google Scholar]
- 14.van Geffen M., Loof A., Lap P., Boezeman J., Laros-van Gorkom B.A., Brons P., et al. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematology. 2011;16:327–336. doi: 10.1179/102453311X13085644680348. [DOI] [PubMed] [Google Scholar]
- 15.Lisman T., de Groot P.G., Meijers J.C.M., Rosendaal F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 16.Pieters M., Philippou H., Undas A., de Lange Z., Rijken D.C., Mutch N.J. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1007–1012. doi: 10.1111/jth.14002. [DOI] [PubMed] [Google Scholar]
- 17.Ammollo C.T., Semeraro F., Incampo F., Semeraro N., Colucci M. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. 2010;8:790–798. doi: 10.1111/j.1538-7836.2010.03739.x. [DOI] [PubMed] [Google Scholar]
- 18.Undas A., Celinska-Lowenhoff M., Lowenhoff T., Szczeklik A. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J Thromb Haemost. 2006;4:1029–1036. doi: 10.1111/j.1538-7836.2006.01882.x. [DOI] [PubMed] [Google Scholar]
- 19.Scott D.J.A., Prasad P., Philippou H., Rashid S.T., Sohrabi S., Whalley D., et al. Clot Architecture Is Altered in Abdominal Aortic Aneurysms and Correlates With Aneurysm Size. Arterioscler Thromb Vasc Biol. 2011;31:3004–3010. doi: 10.1161/ATVBAHA.111.236786. [DOI] [PubMed] [Google Scholar]
- 20.Carter A.M., Cymbalista C.M., Spector T.D., Grant P.J. Heritability of Clot Formation, Morphology, and Lysis. Arterioscler Thromb Vasc Biol. 2007;27:2783–2789. doi: 10.1161/ATVBAHA.107.153221. [DOI] [PubMed] [Google Scholar]
- 21.Zucker M., Seligsohn U., Salomon O., Wolberg A.S. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–1130. doi: 10.1111/jth.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutch N.J., Koikkalainen J.S., Fraser S.R., Duthie K.M., Griffin M., Mitchell J., et al. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J Thromb Haemost. 2010;8:2017–2024. doi: 10.1111/j.1538-7836.2010.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim P.Y., Stewart R.J., Lipson S.M., Nesheim M.E. The relative kinetics of clotting and lysis provide a biochemical rationale for the correlation between elevated fibrinogen and cardiovascular disease. J Thromb Haemost. 2007;5:1250–1256. doi: 10.1111/j.1538-7836.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 24.Longstaff C. Development of Shiny app tools to simplify and standardize the analysis of hemostasis assay data: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:1044–1046. doi: 10.1111/jth.13656. [DOI] [PubMed] [Google Scholar]
- 25.Siudut J., Iwaniec T., Plens K., Pieters M., Undas A. Determinants of plasma fibrin clot lysis measured using three different assays in healthy subjects. Thromb Res. 2021;197:1–7. doi: 10.1016/j.thromres.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Kleiss S.F., Adelmeijer J., Meijers J.C.M., Porte R.J., Lisman T. A sustained decrease in plasma fibrinolytic potential following partial liver resection or pancreas resection. Thromb Res. 2016;140:36–40. doi: 10.1016/j.thromres.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Ruitenbeek K., Meijers J.C.M., Adelmeijer J., Hendriks H.G.D., Porte R.J., Lisman T. Intact thrombomodulin-mediated regulation of fibrinolysis during and after liver transplantation, despite a profoundly defective thrombomodulin-mediated regulation of coagulation. J Thromb Haemost. 2010;8(7):1646–1649. doi: 10.1111/j.1538-7836.2010.03886.x. [DOI] [PubMed] [Google Scholar]
- 28.Bos S., van den Boom B., Ow T.-W., Prachalias A., Adelmeijer J., Phoolchund A., et al. Efficacy of pro- and anticoagulant strategies in plasma of patients undergoing hepatobiliary surgery. J Thromb Haemost. 2020;18:2840–2851. doi: 10.1111/jth.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirienzo L., Vitulli A., Mancazzo F., Ammollo C.T., Dellanoce C., Paoletti O., et al. Differential effect of direct oral anticoagulants on thrombin generation and fibrinolysis in patients with atrial fibrillation and venous thromboembolism. Blood Transfus. 2021 doi: 10.2450/2021.0153-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neergaard-Petersen S., Mogensen V.B., Veirup M.S., Grove E.L., Kristensen S.D., Hvas A.-M. Fibrin clot lysis assay: Establishment of a reference interval. Thromb Res. 2018;167:9–11. doi: 10.1016/j.thromres.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Kettner S.C., Gonano C., Seebach F., Sitzwohl C., Acimovic S., Stark J., et al. Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation. Anesth Analg. 1998;86:691–695. doi: 10.1097/00000539-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 32.He S., Bremme K., Blombäck M. A laboratory method for determination of overall haemostatic potential in plasma. I. Method design and preliminary results. Thromb Res. 1999;96:145–156. doi: 10.1016/s0049-3848(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 33.Senzolo M., Cholongitas E., Thalheimer U., Riddell A., Agarwal S., Mallett S., et al. Heparin-like Effect in Liver Disease and Liver Transplantation. Clin Liver Dis. 2009;13:43–53. doi: 10.1016/j.cld.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Kassis J., Hirsh J., Podor T.J. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood. 1992;80:1758–1764. [PubMed] [Google Scholar]
- 35.Undas A., Zabczyk M. Antithrombotic medications and their impact on fibrin clot structure and function. J Physiol Pharmacol an Off J Polish Physiol Soc. 2018;69 doi: 10.26402/jpp.2018.4.02. [DOI] [PubMed] [Google Scholar]
- 36.Lisman T., De Groot P.G. Rebuttal to: Effect of heparin on TAFI-dependent inhibition of fibrinolysis. J Thromb Haemost. 2003;1:200–201. doi: 10.1046/j.1538-7836.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 37.Mosnier L.O., von dem Borne P.A., Meijers J.C., Bouma B.N. Plasma TAFI levels influence the clot lysis time in healthy individuals in the presence of an intact intrinsic pathway of coagulation. Thromb Haemost. 1998;80:829–835. [PubMed] [Google Scholar]
- 38.Lisman T., Leebeek F.W.G., Mosnier L.O., Bouma B.N., Meijers J.C., Janssen H.L., et al. Thrombin-Activatable Fibrinolysis Inhibitor Deficiency in Cirrhosis Is Not Associated With Increased Plasma Fibrinolysis. Gastroenterology. 2001;121:131–139. doi: 10.1053/gast.2001.25481. [DOI] [PubMed] [Google Scholar]
- 39.Mosnier L.O., Lisman T., van den Berg H.M., Nieuwenhuis H.K., Meijers J.C., Bouma B.N. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost. 2001;86:1035–1039. [PubMed] [Google Scholar]
- 40.Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost. 2018;16:652–662. doi: 10.1111/jth.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]