Version Changes
Revised. Amendments from Version 1
The revised version of this manuscript states the study objectives more clearly in the introduction. In response to reviewers, the authors also provide additional information regarding how the subset of participants tested for sexually transmitted infection were chosen and the standard protocol the midwives used to capture cervicovaginal images using hand-held colposcopy.
Abstract
Background: Female genital schistosomiasis (FGS) can occur in S. haematobium infection and is caused by egg deposition in the genital tract. Confirming a diagnosis of FGS is challenging due to the lack of a diagnostic reference standard. A 2010 expert-led consensus meeting proposed visual inspection of the cervicovaginal mucosa as an adequate reference standard for FGS diagnosis. The agreement of expert human reviewers for visual-FGS has not been previously described.
Methods: In two Zambian communities, non-menstruating, non-pregnant, sexually-active women aged 18-31 years participating in the HPTN 071 (PopART) Population-Cohort were enrolled in a cross-sectional study. Self-collected genital swabs and a urine specimen were collected at a home visit; trained midwives performed cervicovaginal lavage (CVL) and hand-held colposcopy at a clinic visit. S. haematobium eggs and circulating anodic antigen (CAA) were detected from urine. Two senior physicians served as expert reviewers and independently diagnosed visual-FGS as the presence of sandy patches, rubbery papules or abnormal blood vessels in cervicovaginal images obtained by hand-held colposcopy. PCR-FGS was defined as Schistosoma DNA detected by real-time PCR in any genital specimen (CVL or genital swab).
Results: Of 527 women with cervicovaginal colposcopic images, 468/527 (88.8%) were deemed interpretable by Reviewer 1 and 417/527 (79.1%) by Reviewer 2. Visual-FGS was detected in 35.3% (165/468) of participants by expert review of colposcopic images by Reviewer 1 and in 63.6% (265/417) by Reviewer 2. Cohen’s kappa statistic for agreement between the two reviewers was 0.16, corresponding to "slight" agreement. The reviewers made concordant diagnoses in 38.7% (204/527) participants (100 negative, 104 positive) and discordant diagnoses in 31.8% (168/527) participants.
Conclusions: The unexpectedly low level of correlation between expert reviewers highlights the imperfect nature of visual diagnosis for FGS based on cervicovaginal images. This finding is a call to action for improved point-of-care diagnostics for female genital schistosomiasis.
Keywords: Female genital schistosomiasis, Schistosoma haematobium, hand-held colposcopy
Introduction
Female genital schistosomiasis (FGS), primarily caused by S. haematobium infection, is a neglected tropical disease associated with poverty, inadequate sanitation, and limited access to safe drinking water 1, 2 . FGS occurs when schistosome eggs destined for excretion via the urinary bladder are deposited in the female genital tract. These tissue-deposited eggs can be associated with characteristic genital mucosal lesions and can present with genitopelvic findings including contact bleeding 3 , abnormal vaginal discharge 4 , and in some cases, infertility 5 . Visual-FGS refers to the identification of these characteristic mucosal changes, such as sandy patches (grainy and homogeneous), rubbery papules, and abnormal blood vessels by visual inspection of the cervicovaginal mucosa 3 . The visual detection of FGS-associated lesions requires the insertion of a vaginal speculum, a good light source, and a lens providing adequate magnification 6 . A standard colposcope has traditionally been used in research settings for visual-FGS diagnosis, but the bilharzia and HIV (BILHIV) study demonstrated recently that hand-held colposcopy could also be used to decentralize colposcopy services 6– 9 .
Confirming a diagnosis of FGS is challenging as there is not a widely accepted diagnostic reference standard for research, diagnosis, and screening 2 . A 2010 expert-led consensus meeting proposed visual inspection of the cervicovaginal mucosa as an adequate reference standard for FGS diagnosis 10 . However, the mucosal changes in visual-FGS are non-specific and have also been associated with herpes simplex virus, human papillomavirus (HPV) infection, and cervical precancer 3 . Diagnostic methods that are not adequately specific for FGS diagnosis may lead to over-treatment with praziquantel and may overlook the diagnosis and treatment of sexually transmitted infection (STI) and cervical cancer. Although there is little evidence of praziquantel resistance in humans 11 , indiscriminate treatment may theoretically increase the risk of the development of praziquantel resistance 12 . Since cervicovaginal visualization is widely promoted 13 for FGS screening and diagnosis, we aimed to use BILHIV study data to further evaluate the agreement of human expert reviewers for the diagnosis of visual-FGS. Secondary aims were to evaluate the association between visual-FGS and abdominal, genitourinary, and reproductive manifestations as well as evaluating Schistosoma diagnostic methods for their association with the presence of visual-FGS.
Methods
Study setting and participants
The cross-sectional bilharzia and HIV (BILHIV) study 9 was nested in the HPTN 071 (PopART) cluster randomized trial in Zambia 14 . The S. haematobium is endemic in Zambia, and while more data are needed, prevalences ranging between 14 – 76% were reported in a recent systematic review 15 . The HPTN 071 (PopART) trial was a cluster randomized trial assessing the impact of an HIV-1 combination prevention package including “universal test and treat” 14 . As previously described, after the 36-month HPTN 071 (PopART) visit, community workers made home visits to women expressing interest in the BILHIV study 9 . Between January and August 2018, eligible women who were 18–31 years old, not pregnant, sexually active, and resident in one of two urban communities that participated in HPTN 071 (PopART) in Livingstone, Zambia were enrolled in the BILHIV study. The primary aim of the BILHIV study was to compare the performance of genital self-sampling (cervical and vaginal swabs) to clinic-based cervicovaginal lavage (CVL) for the detection of Schistosoma DNA by quantitative PCR (qPCR) as previously described 9 . A specific pre-specified BILHIV study objective (the subject of the current manuscript) was to compare agreement of expert review of images obtained through hand-held colposcopy for the diagnosis of visual-FGS.
Home and clinic-based sample collection
As previously described, the home visit included written informed consent, a questionnaire, genital self-sampling (cervical and vaginal), and collection of a urine specimen 9 . There were no restrictions on the timing of urine self-sample collection, and 69.5% (419/603) of the total BILHIV study samples were performed between 9:00 and 14:00 9 . Enrolled women who were not currently menstruating were then invited to attend Livingstone Central Hospital cervical cancer clinic, where midwives collected CVL. After speculum insertion, normal saline (10 mL) was flushed across the cervix and vaginal walls for one minute with a bulb syringe and CVL fluid was collected from the posterior fornices.
Hand-held colposcopy and image review
At the clinic, cervicovaginal images were captured with a portable colposcope (EVA System, MobileODT, Tel Aviv, Israel) according to a standardized protocol. Per the protocol, trained midwives evaluated the cervix, anterior fornix, posterior fornix, left and right lateral cervix and vaginal walls and captured images of each location using the zoom and lighting functions in the Mobile ODT colposcope. Two senior physicians who have training and expertise in colposcopy and FGS served as expert reviewers. Digital images were independently evaluated by the expert reviewers for any of the four recognized FGS cervicovaginal manifestations: grainy sandy patches, homogenous yellow sandy patches, rubbery papules, and abnormal blood vessels 16 . At their discretion, expert reviewers could exclude images that they felt could not be evaluated due to technical issues, image quality, or limited cervical visualization. If any of the four recognized FGS cervicovaginal manifestations was present, the participant was categorized as “visual-FGS”. If none of the four cervicovaginal manifestations were present the participant was categorized as “visual-FGS not detected” 16 . The expert reviewers were both senior practicing physicians at the Professor level, who have training and expertise in standard colposcopy. Reviewer 1 (EFK) is full-time FGS researcher and an infectious diseases physician and Reviewer 2 (BV) is an obstetrician and gynecologist who regularly analyses images for cervical cancer. Both reviewers have extensive practical and research-based expertise in evaluating and diagnosing FGS in endemic settings. Additionally, both reviewers contributed as authors of the 2015 WHO FGS Pocket Atlas 16 . Each reviewer was informed of the study setting and methods, but both were blinded to the study participants’ FGS and Schistosoma status.
Women with at least one of the visual manifestations of FGS 3, 16 or with any positive urine or genital Schistosoma diagnostic result were treated free-of-charge with 40 mg/kg praziquantel. Testing for STIs was not performed at the point-of-care and participants with suspected STIs were offered syndromic management, as per local guidelines 17 . In line with national and local clinic protocols adapted to real-world resource limitations, human papillomavirus (HPV) testing was not performed.
In parallel with BILHIV study procedures, participants could choose to engage in free cervical cancer screening using the visual inspection with acetic acid (VIA) technique. In the subset of women who engaged in cervical cancer screening, midwives applied 3-5% acetic acid to the cervix after CVL collection, as previously described 18 . An opaque white reaction was classified as positive and no change as negative 19 . Images for FGS analyses were taken before application of acetic acid. Images for cervical cancer screeing were taken after application of acetic acid.
Urine microscopy, and circulating anodic antigen
Up to 60mL of fresh urine was centrifuged and examined by microscopy for S. haematobium eggs. The participant was considered to have urinary schistosomiasis if a pellet contained at least one S. haematobium egg 9 . All study specimens were stored at -80°C. A lateral flow assay utilizing up-converting reporter particles for the quantification of circulating anodic antigen (CAA) was performed on urine samples, as previously described 9, 20 . Analyzing the equivalent of 417 μL urine (wet reagent, UCAA hT 417), a test result indicating a CAA value >0.6 pg/mL was considered positive 21 .
qPCR for detection of Schistosoma DNA
DNA extraction, amplification and detection of the Schistosoma-specific internal-transcribed-spacer-2 (ITS-2) target by real-time (qPCR) was performed at Leiden University Medical Center, as previously described, using 200 µL of CVL, cervical or vaginal swab fluid 9, 22 .
Other infections
Due to budgetary constraints, a subset of participants was evaluated for STI. As previously described, all participants with FGS and all participants with probable FGS were selected for characterization of the cervicovaginal microbiota and STI by qPCR on cervical swabs 23 . Three FGS-negative participants were selected for every FGS and probable FGS participant using a random number generator. The FGS-negative participants were frequency-matched by age to participants with FGS 23 . Laboratory-based fourth-generation HIV-1 testing (Abbott Architect HIV Ag/Ab Combo Assay) was performed for HPTN 071 (PopART) Population Cohort participants at each study visit 14 . STIs were quantified among a subset of participants by qPCR using the S-DiaCTNG TM (for C. trachomatis and N. gonorrhea) and S-DiaMGTV TM kits (for M. genitalium and T. vaginalis) (Diagenode Diagnostics, Seraing, Belgium) on DNA from cervical swabs at Ghent University (Ghent, Belgium) according to the manufacturer’s instructions.
Consent
The study was approved by the University of Zambia Biomedical Research Ethics Committee (011-08-17), the Zambia National Health Research Authority and the London School of Hygiene and Tropical Medicine Ethics Committee (14506). Permission to conduct the study was given by Livingstone District Health Office and the Livingstone Central Hospital superintendent.
Statistical methods
The planned sample size of the BILHIV study was based on calculations related to the primary BILHIV study objective, as previously described 9 . Participant characteristics were summarized by median and interquartile range (IQR) for continuous variables, and by frequency and percentage for categorical variables. Participants missing data for a specific variable were excluded from analysis involving that variable. The primary analysis evaluated the agreement between the two expert reviewers using Cohen’s kappa statistic. A secondary analysis evaluated the association between visual-FGS (exposure) and abdominal, genitourinary, and reproductive manifestations (outcomes). Crude associations were evaluated using chi-squared tests, and logistic regression was used to calculate crude and adjusted odds ratios (OR) for the association of visual-FGS with clinical manifestations; this was done separately for each expert reviewer’s diagnosis of visual-FGS. In this study we employed various diagnostic tests to evaluate urinary Schistosoma infection (CAA and urine microscopy), and FGS (portable colposcopy, and Schistosoma DNA on CVL and genital swabs) as previously described 23– 25 . Another secondary analysis evaluated each diagnostic method for its association with the presence of visual-FGS, separately for each expert reviewer. Due to small numbers, for evaluating the association of visual-FGS with PCR-FGS, we used a composite definition of PCR-FGS or “any positive genital PCR”, defined as any positive cervical or vaginal swab or CVL specimen. Chi-squared tests were used to assess crude associations, and logistic regression was used to calculate crude and age-adjusted odds ratio (OR) of the various Schistosoma and FGS diagnostics with the presence or absence of visual-FGS. We were unable to adjust for other potential confounders due to small numbers, particularly for STI and cervical pre-cancer status which were collected on a sub-set of participants. For both secondary analyses, exact logistic regression was used for analyses where 5 or fewer participants in a particular exposure category had the outcome. Due to the exploratory nature of these analyses, we did not adjust for multiple comparisons. Data were analyzed using STATA 15.1 (Stata Corporation, College Station, TX).
Results
Baseline characteristics and demographics
The BILHIV study enrolled 603 eligible women, 527 (87.4%) of whom had cervicovaginal images captured by portable colposcopy. Of the 527 women with images, 468 (88.8%) were deemed interpretable by Reviewer 1 and 418 (79.3%) by Reviewer 2 ( Figure 1). Each reviewer designated a proportion of images uninterpretable, leading to differences in denominators. The median age of the participants was 24 years (range 22 – 28) and 323 (61.3%) had attended some secondary school ( Table 1). The majority of participants were married, had previously been pregnant, and had been sexually active within the last six months. There was no association between visual FGS, as identified by any expert review, and current or childhood water contact.
Figure 1. Flowsheet of cervicovaginal image review after hand-held colposcopy in BILHIV study participants.
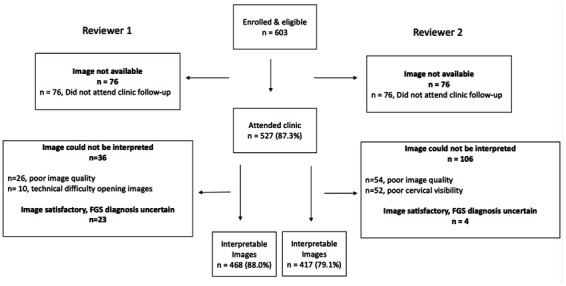
Table 1. Demographics and reproductive health characteristics of the BILHIV study participants who underwent portable colposcopy (n=527).
| Participant Characteristics | Study population (n=527) | |
|---|---|---|
| Age in years – Median (IQR) | 24 (22 – 28) | |
| Marital status | Single | 213 (40.4) |
| Married or cohabitating | 292 (55.4) | |
| Divorced or separated | 22 (4.2) | |
| Education (highest level) | None or any primary school | 155 (29.4) |
| Any secondary school | 323 (61.3) | |
| Trade, degree or higher | 49 (9.3) | |
| District | Community A | 290 (55.0) |
| Community B | 237 (45.0) | |
| Household members | 1-3 | 162 (30.7) |
| 4-5 | 210 (39.9) | |
| 6+ | 155 (29.4) | |
| Employment status | Unemployed | 363 (68.9) |
| Employed | 164 (31.1) | |
| Current water contact | None | 447 (84.8) |
| Any | 80 (15.2) | |
| Childhood water contact | None | 151 (28.7) |
| Any | 376 (71.3) | |
| Reproductive Health Characteristics | ||
| Age at sexual debut (years) * | 8-16 | 221 (42.0) |
| 17-19 | 228 (43.4) | |
| 20-24 | 77 (14.6) | |
| Lifetime sexual partners | 1 | 145 (27.5) |
| 2 | 134 (25.4) | |
| 3 | 108 (20.5) | |
| 4+ | 140 (26.6) | |
| Prior pregnancy * | No | 74 (14.1) |
| Yes | 452 (85.9) | |
| Currently sexually active ** | No | 65 (12.4) |
| Yes | 460 (87.6) | |
| Condom use with last sex † | No | 381 (73.7) |
| Yes | 136 (26.3) | |
| HIV-1 infection †† | Not detected | 407 (77.8) |
| Detected | 116 (22.2) | |
| Any STI ∼ | Not detected | 138 (63.8) |
| Detected | 73 (34.6) | |
| Any Hormonal Contraception ∼∼ | No | 201 (38.1) |
| Yes | 326 (61.9) | |
| VIA ‡ | Negative | 213 (89.9) |
| Positive | 24 (10.1) |
*Participants who responded with “no answer” (n=1) are not shown in the table
**Defined as any sexual activity in the last 6 months; Participants who responded with “no answer” (n=2) are not shown in the table
†Participants who responded with “no answer” (n=10) are not shown in the table
††Participants with missing data (n=4) are not shown in the table
∼STI were evaluated in a sub-set of women from the BILHIV study, missing values (n=316) are not shown in the table)
∼∼Any hormonal contraception is defined as use of injectable agents, implants, or oral contraceptive pills
‡VIA results were not collected in the BILHIV study and were not available for all participants, participants with missing data (n=366) are not shown in the table
Prevalence of visual-FGS and interrater agreement
Visual-FGS was detected in 35.3% (165/468) of participants by expert review of digital images from hand-held colposcopy by Reviewer 1 and in 63.6% (265/417) by Reviewer 2. The Cohen’s kappa statistic for interrater agreement between the two expert reviewers was 0.16, corresponding to "slight" agreement ( Table 2). The reviewers made concordant diagnoses in 38.7% (204/527) participants (100 concordant negative, 104 concordant positive) and discordant diagnoses in 31.8% (168/527) cases (reviewer 1 positive, reviewer 2 negative in 32; reviewer 2 positive and reviewer 1 negative in 136). Both reviewers agreed 14 images were unevaluable. A further 26.7% (141/527) images were discordant in evaluability by the expert reviewers (Reviewer 1, n=45; Reviewer 2, n=96).
Table 2. Agreement of expert reviewers for the presence or absence of visual-FGS.
| Reviewer 1 | FGS not
detected |
Visual
FGS |
Not
evaluable |
Total | |||
|---|---|---|---|---|---|---|---|
| Reviewer 2 | Cohen’s Kappa
(+ SE) ** |
Interpretation | |||||
| Visual FGS not
detected |
100 (19.0) | 32 (6.1) | 20 (3.8) | 152 (28.8) * | |||
| Visual FGS | 136 (25.8) | 104 (19.7) | 25 (4.7) | 265 (50.3) * | 0.16 (0.04) | None to slight | |
| Not evaluable | 67 (12.7) | 29 (5.5) | 14 (2.7) | 110 (20.9) | |||
| Total | 303 (57.5) * | 165 (31.3) * | 59 (11.2) | 527 (100.0) |
FGS – female genital schistosomiasis, SE – standard error
*total evaluable for Reviewer 1: visual FGS not detected 303/468 (64.7), visual FGS detected 165/468 (35.3); total evaluable for Reviewer 2: visual FGS not detected 152/417 (36.5), visual FGS detected 265/417 (63.5);
**Cohen’s kappa is restricted to those participants where both Reviewer 1 and Reviewer 2 provided a diagnosis
Visual FGS and Schistosoma laboratory tests
Of the 527 participants, 6.1% (32/527) had urinary S. haematobium infection, as diagnosed by urine microscopy, and 14.9% (78/525) had a detectable urine CAA. There was no association between S. haematobium egg-positive urine microscopy or urine CAA and visual-FGS, as defined by Reviewer 1 or Reviewer 2’s assessmen ( Table 3).
Table 3. Associations of Schistosoma diagnostics with visual-FGS.
| Participant
Characteristics |
Visual FGS
not detected |
Visual FGS
detected |
Crude OR | P-
value * |
Adjusted OR ** | P-
value † |
|
|---|---|---|---|---|---|---|---|
|
Schistosoma diagnostics
(Reviewer 1) |
n=303 | n=165 | |||||
| Eggs on urine microscopy | Not detected | 287 (94.7) | 151 (91.5) | reference | 0.3 | reference | 0.2 |
| Detected | 16 (5.3) | 14 (8.5) | 1.66 (0.73 – 3.74) | 1.68 (0.74 – 3.81) | |||
| Circulating anodic antigen ~ | Not detected | 261 (86.1) | 131 (80.4) | reference | 0.1 | reference | 0.1 |
| Detected | 42 (13.9) | 32 (19.6) | 1.52 (0.92 – 2.52) | 1.49 (0.91 – 2.51) | |||
| Any positive genital PCR | DNA not
detected |
288 (95.1) | 155 (93.9) | reference | 0.8 | reference | 0.7 |
| DNA detected | 15 (4.9) | 10 (6.0) | 1.24 (0.54 – 2.82) | 1.27 (0.55 – 2.94) | |||
|
Schistosoma Diagnostics
(Reviewer 2) |
n=152 | n=265 | |||||
| Eggs on urine microscopy †† | Not detected | 147 (96.7) | 243 (91.7) | reference | 0.06 | reference | 0.07 |
| Detected | 5 (3.3) | 22 (8.3) | 2.65 (0.95 – 9.17) | 2.65 (0.95 – 9.19) | |||
| Circulating anodic antigen ~ | Not detected | 131 (86.7) | 215 (81.4) | reference | 0.2 | reference | 0.2 |
| Detected | 20 (13.3) | 49 (18.6) | 1.49 (0.85 – 2.62) | 1.49 (0.85 – 2.63) | |||
| Any positive genital PCR | DNA not
detected |
146 (96.1) | 248 (93.6) | reference | 0.3 | reference | 0.3 |
| DNA detected | 6 (3.9) | 17 (6.4) | 1.69 (0.64 – 4.33) | 1.67 (0.64 – 4.36) |
*Chi squared p-value unless otherwise noted
**Adjusted for age
†Likelihood ratio test p-value
Missing values not included in the table: ~ (n=2)
††Odds Ratios and p-values obtained through exact logistic regression in both crude and adjusted analyses
PCR-FGS, defined as any positive Schistosoma qPCR from a genital sample, was diagnosed in 5.0% (30/603) of participants [3.4% (18/527) cervical swab, 2.7% (14/527) vaginal swab, and 2.7% (14/527) CVL]. Further details regarding the operating characteristics of these tests have previously been described 9 . There was no association between visual-FGS and PCR-FGS, 10 of the 165 women (6.0%) identified by Reviewer 1 as having visual-FGS had PCR-FGS and 17 of the 265 women (6.4%) identified by Reviewer 2 as having visual-FGS had PCR-FGS, compared to 4.9% and 3.9% among women identified by Reviewer 1 and 2 as not having visual-FGS respectively ( Table 3).
Symptoms
The association between abdominal, genitourinary, and reproductive manifestations and visual-FGS is shown in Table 4. Neither vaginal discharge, vaginal bleeding after sex, the presence of external genital sores, dysuria nor abdominal pain was associated with the presence of visual FGS as diagnosed by either expert reviewer ( Table 4). Self-reported delay in conception was associated with the presence of visual-FGS, as assessed by Reviewer 1, both in crude analysis and after adjusting for age and community of residence (aOR 2.74 [1.29 – 5.83], p<0.01). Visual-FGS, as defined by Reviewer 2’s assessment of hand-held colposcopy images, was associated with hematuria (aOR 4.44 [1.00 – 40.63] p=0.05) and dyspareunia (aOR 1.71 [0.99 – 2.95], p=0.05), albeit with weak evidence of an association ( Table 4).
Table 4. Associations of genitourinary and abdominal symptoms with visual-FGS.
| Participant
Characteristics |
Visual
FGS not detected |
Visual
FGS detected |
P-value * | Crude OR | P-value | Adjusted OR ** | P-
value † |
|
|---|---|---|---|---|---|---|---|---|
|
Signs & Symptoms
(Reviewer 1) |
n= 303 | n=165 | ||||||
| Vaginal discharge | Not present | 273 (90.1) | 139 (84.2) | 0.06 | reference | 0.06 | reference | 0.1 |
| Present | 30 (9.9) | 26 (15.8) | 1.70 (0.97 – 2.99) | 1.58 (0.90 – 2.80) | ||||
| Dyspareunia | Not present | 239 (78.9) | 139 (84.2) | 0.2 | reference | 0.2 | reference | 0.1 |
| Present | 64 (21.1) | 26 (15.8) | 0.70 (0.42 – 1.15) | 0.68 (0.41 – 1.12) | ||||
| Vaginal bleeding after
sex |
Not present | 289 (95.4) | 153 (92.7) | 0.2 | reference | 0.2 | reference | 0.3 |
| Present | 14 (4.6) | 12 (7.3) | 1.62 (0.73 – 3.59) | 1.48 (0.66 – 3.31) | ||||
| Vaginal sores | Not present | 280 (92.4) | 154 (93.3) | 0.7 | reference | 0.7 | reference | 0.6 |
| Present | 23 (7.6) | 11 (6.7) | 0.87 (0.41 – 1.83) | 0.84 (0.40 – 1.77) | ||||
| Dysuria | Not present | 259 (85.5) | 140 (84.9) | 0.9 | reference | 0.9 | reference | 0.8 |
| Present | 44 (14.5) | 25 (15.1) | 1.05 (0.62 – 1.79) | 0.92 (0.53 – 1.59) | ||||
| Hematuria | Not present | 295 (97.4) | 155 (93.9) | 0.07 | reference | 0.07 | reference | 0.1 |
| Present | 8 (2.6) | 10 (6.1) | 2.38 (0.92 – 6.20) | 2.18 (0.84 – 5.67) | ||||
| Abdominal pain | Not present | 217 (71.6) | 123 (74.5) | 0.5 | reference | 0.5 | reference | 0.4 |
| Present | 86 (28.4) | 42 (25.5) | 0.86 (0.56 – 1.33) | 0.82 (0.53 – 1.26) | ||||
| Delay in conception †† | No | 235 (94.4) | 111 (86.7) | 0.01 | reference | 0.01 | reference | <0.01 |
| Yes | 14 (5.6) | 17 (13.3) | 2.57 (1.22 – 5.40) | 2.74 (1.29 – 5.83) | ||||
|
Signs & Symptoms
(Reviewer 2) |
n=152 | n=265 | ||||||
| Vaginal discharge | Not present | 133 (87.5) | 236 (89.1) | 0.6 | reference | 0.6 | reference | 0.6 |
| Present | 19 (12.5) | 29 (10.9) | 0.86 (0.46 – 1.60) | 0.84 (0.45 – 1.57) | ||||
| Dyspareunia | Not present | 131 (86.2) | 208 (78.5) | 0.05 | reference | 0.05 | reference | 0.05 |
| Present | 21 (13.8) | 57 (21.5) | 1.71 (0.99 – 2.95) | 1.71 (0.99 – 2.95) | ||||
| Vaginal bleeding after
sex |
Not present | 144 (94.7) | 251 (94.7) | 1.0 | reference | 1.0 | reference | 1.0 |
| Present | 8 (5.3) | 14 (5.3) | 1.00 (0.41 – 2.45) | 0.99 (0.40 – 2.42) | ||||
| Vaginal sores | Not present | 146 (96.1) | 244 (92.1) | 0.1 | reference | 0.1 | reference | 0.1 |
| Present | 6 (3.9) | 21 (7.9) | 2.09 (0.83 – 5.31) | 2.10 (0.83 – 5.31) | ||||
| Dysuria | Not present | 132 (86.8) | 231 (87.2) | 0.9 | reference | 0.9 | reference | 0.9 |
| Present | 20 (13.2) | 34 (12.8) | 0.97 (0.54 – 1.76) | 0.95 (0.52 – 1.75) | ||||
| Hematuria ††† | Not present | 150 (98.7) | 250 (94.3) | 0.03 | reference | 0.05 | reference | 0.05 |
| Present | 2 (1.3) | 15 (5.7) | 4.49 (1.02 – 40.99) | 4.44 (1.00 – 40.63) | ||||
| Abdominal pain | Not present | 108 (71.0) | 192 (72.5) | 0.8 | reference | 0.8 | reference | 0.7 |
| Present | 44 (28.9) | 73 (27.5) | 0.93 (0.60 – 1.45) | 0.92 (0.59 – 1.44) | ||||
| Delay in conception ‡ | No | 112 (91.8) | 193 (91.9) | 1.0 | reference | 1.0 | reference | 1.0 |
| Yes | 10 (8.2) | 17 (8.1) | 0.99 (0.44 – 2.22) | 1.01 (0.45 – 2.92) |
*Chi squared p-value
**Adjusted for age and district of residence
†Likelihood ratio test p-value
††(Reviewer 1) Declined to answer (n=33) and not applicable (n=95) are not included in the table (54 visual FGS not detected; 37 FGS; 37 missing)
†††Odds Ratios and p-values obtained through exact logistic regression in both crude and adjusted analyses
‡(Reviewer 2) Declined to answer (n=33) and missing (n=95) are not included in the table; (30 visual FGS not detected; 55 FGS detected; 43 missing)
Discussion
Diagnostics for neglected tropical diseases should be accurate, accessible, and affordable, with specimen collection that is easy 26 . Making a diagnosis of FGS is challenging as there is currently not a widely accessible, sensitive and non-invasive reference standard for either diagnosis or screening which confirms Schistosoma genital involvement at the point-of-care. In a 2010 expert-led consensus meeting, visual imaging of the vagina and cervix with photocolposcopic methods was proposed as an adequate reference standard for FGS visual diagnosis 27 . Imaging is currently the only widely available point-of-care diagnostic tool for FGS diagnosis outside of the research setting and the BILHIV study sought to use hand-held colposcopy to enable community-based FGS diagnosis 9 . Visual imaging can be useful in the assessment of Schistosoma-related morbidity, praziquantel treatment response, and defining the natural history of visual-FGS. Additionally, hand-held and traditional colposcopy have the logistical advantage that they can be integrated with existing cervical cancer screening programmes 28 . However, visual imaging has important limitations. Firstly, interpretation of visual imaging is subjective. Secondly, visual imaging lacks specificity as the characteristic sandy patches can also be associated with STI and the abnormal blood vessels can also be associated with cervical precancer 3 . This study shows “slight” agreement between senior, highly experienced expert reviewers, highlighting the imperfect nature of human expert review of images for FGS.
Visual FGS-diagnosis is a widely accepted diagnostic tool for evaluating Schistosoma-associated genital morbidity. However, visual-FGS screening is often centralized in settings with access to traditional colposcopy and is invasive, requiring vaginal speculum insertion and trained medical professionals (physicians, nurses, or midwives) to visualize the cervix and vagina at high resolution 9 . Additionally, visual-FGS diagnosis requires a full inspection of the mucosal surfaces of the vagina and cervix. If metal specula are used, post-examination autoclaving and appropriate disinfection further constrains the settings in which this diagnostic strategy can be seamlessly implemented. Disposable specula have risks and benefits. While hygienic and convenient, disposable plastic specula may not be sturdy enough when rotated to inspect the anterior and posterior vaginal walls and may contribute to missed visual-FGS diagnoses 6 . A good light source is needed for optimal cervicovaginal visualization 6 , as well as a device which can provide adequate magnification, ideally a colposcope, hand-held colposcope, or digital camera 8 . Thus, colposcopy, whether hand-held or traditional, for visual-FGS diagnosis is not readily scalable for use as a population-based screening technique.
In this current work, without complete STI and HPV testing or cervicovaginal biopsy on each participant, it is challenging to assess the significance of the sandy patches and abnormal blood vessels identified by the clinical expert reviewers. Notably, researchers in Tanzania performed macroscopic cervicovaginal examinations comparing S. haematobium endemic and non-endemic areas, finding 75% of participants in endemic areas had cervical lesions (including sandy patches, edema, erosions and petechiae) compared with 36% of women in non-endemic areas (although their travel and medical history were not described) 29 . The Tanzanian study illustrates the limited specificity of visual techniques, since one-third of the women had cervical lesions in communities where S. haematobium is not endemic.
Other diagnostic approaches such as PCR-based methods, have been implemented in research settings but are not yet field-deployable 9 . Antigen, antibody, and pathogen-based diagnostics (such as microscopy) are useful diagnostic adjuncts for Schistosoma infection, but do not confirm the involvement of genital tissue. Future diagnostic algorithms may be optimized by first performing a microbiologic S. haematobium diagnosis prior to performing screening for genital involvement 9 . Promising pathogen detection strategies that can be implemented at the point-of-care include isothermal DNA amplification methods 30, 31 . These field-deployable molecular assays should be further developed for use at the point-of-care to identify Schistosoma DNA in self-collected genital swabs 31 .
Our study did not show a consistent association between expert diagnosis of visual-FGS and abdominal, genitourinary and reproductive symptoms. Reviewer 1’s evaluation suggested an association between self-reported delay in conception and visual-FGS and Reviewer 2’s evaluation suggested a weak association with hematuria and dyspareunia in participants with visual-FGS. A retrospective study from Tanzania evaluating histopathology reported tubal schistosomiasis in 4 patients reporting with infertility 32 and a cross-sectional study from Zimbabwe found strong evidence that the presence of S. haematobium in pap smear was associated with infertility in women aged 20 – 49 years, after adjusting for age and HIV status 5 . While alluring to consider the association of delayed conception identified by one reviewer with visual-FGS in isolation, the association would have been strengthened by consistency of the findings across reviewers. Additionally, in interpreting this result, it is important to consider the possibility of a type 1 error when large numbers of statistical tests are performed.
Previous work on visual-FGS has compared visual imaging to other diagnostic standards 33 or have used computerized algorithms 34, 35 , or a combination of human reviewers and a digital gridding technique to evaluate visual-FGS 7 . A recent Madagascan study utilized human reviewers together with a digital image gridding technique to review images of women with known FGS-associated clinical lesions and found a Fleiss kappa of 0.55 (“moderate” agreement) for detecting rubbery papules. Reviewers in that study achieved a higher agreement than that described in our study, potentially by undergoing an initial consensus rating exercise to reach agreement on uniform rating of images. Our approach in the BILHIV study illustrates a real-world scenario where expert reviewers may not necessarily have the opportunity for consensus agreement prior to consultation. This is the first study to assess the agreement of human expert reviewers for diagnosing visual-FGS with hand-held colposcopy, where both reviewers were blinded to the participants’ FGS and Schistosoma diagnostic status. In this study, both expert reviewers are experienced clinical Professors who have expertise in diagnosing FGS in endemic settings and contributed as authors to the 2015 WHO FGS Pocket Atlas 16 .
While our approach is unique, this work has some limitations. The prevalence of urinary schistosomiasis and PCR-FGS were low, thus limiting precision in effect sizes and power to detect association when comparing PCR-FGS and urinary schistosomasis with visual-FGS. Additionally, the urban setting, relatively narrow age range of the participants and low urinary S. haematobium prevalence may limit generalizability. Future additional work in a setting with higher schistosomiasis prevalence would be needed to definitively exclude an association between symptoms, standard Schistosoma, and FGS diagnostics and visual-FGS. To replicate real-world conditions, standardized equipment on which to perform image review was not provided to reviewers. Thus, we cannot exclude that differences in color, brightness, contrast, or saturation of images on the reviewers’ computers contributed to differences between reviewers. Additionally, future work could incorporate artificial intelligence, such as computer algorithms to detect the characteristic color change caused by involvement of the genital mucosa with FGS 35 or the use of digital gridding techniques 7 . Additionally, a initial consensus rating exercise could be incorporated into future work with human expert review for FGS-associated lesions. The presence or absence of the specific FGS lesion (sandy patch, rubbery papule, abnormal blood vessels) was not consistently documented along with the presence or absence of visual-FGS, limiting analysis by lesion type. Study participants self-reported their time-to-conception status, thus results may be subject to recall bias. STI testing was only performed on a subset of the study population and visual inspection with acetic acid data were not obtained within the BILHIV study, thus data on these variables are incomplete 18 . Without complete STI and HPV testing or cervicovaginal biopsy on each participant, it is challenging to assess the significance of the sandy patches and abnormal blood vessels identified by the clinical expert reviewers. Thus, we cannot exclude residual or unmeasured confounding.
In conclusion, with only “slight” agreement between experienced expert reviewers who identified visual-FGS from digital images obtained during point-of-care colposcopy, we suggest caution when visual imaging is used as a stand-alone FGS diagnostic. While we await field-deployable molecular methods to supplement FGS diagnosis, further studies could evaluate if combining colposcopy for visual-FGS with point-of-care STI diagnostics might improve specificity. Our findings highlight the imperfect nature and challenges of visual diagnosis of FGS as a research and clinical endpoint and is a call to action for improved point-of-care diagnostics and diagnostic pathways for female genital schistosomiasis.
Acknowledgements
We wish to recognize the BILHIV study participants, their contribution made this study possible. We thank the BILHIV study community workers: Namakau Chola, Ethel Mwansa, Mwiingana Lukonga, Ruth Mwanza, Mervis Kantukaleza, and Judith Lungu. We recognize Mr. Clement Mwakamui (Zambart) for his enduring administrative support. We also gratefully acknowledge Eric A.T. Brienen (LUMC) for performing the Schistosoma PCR analysis, Pytsje T. Hoekstra (LUMC) and Claudia J. de Dood for performing the CAA analysis.
Funding Statement
Professor A Bustinduy received funding from the Wellcome Trust (Award 205954/Z/17/Z). Professor P Cools received funding from the Research Foundation – Flanders (BOFSTG2019010101) and was financially supported by a Bill and Melinda Gates Foundation Project (OPP1120972). Professor E Webb and Professor R Hayes received funding from MRC Grant Reference MR/R010161/1, and Dr SC Francis received salary from MRC Grant Reference MR/N023692/1. These awards are jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and are also part of the EDCTP2 program supported by the European Union. HPTN 071 (PopART) was supported by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR); the International Initiative for Impact Evaluation with support from the Bill and Melinda Gates Foundation; the NIAID, the National Institute on Drug Abuse, and the National Institute of Mental Health, all part of the National Institutes of Health. Professor Eyrun Kjetland was supported by South-Eastern Regional Health Authority, Norway project #2016055 and from the European Research Council under the European Union's Horizon 2020 /ERC Grant agreement no. 101057853 (DUALSAVE-FGS).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 6 approved]
Author contributions
Amy S. Sturt – data curation, formal analysis, BILHIV project administration, visualization, original manuscript preparation, manuscript editing and revision
Henrietta Bristowe – formal analysis, original manuscript preparation, manuscript editing and revision
Emily L. Webb – data curation, formal analysis, supervision, original manuscript preparation, manuscript editing and revision
Isaiah Hansingo – resources, supervision, writing – review and editing
Comfort R. Phiri – BILHIV project administration, writing – review and editing
Maina Mudenda – investigation, writing – review and editing
Joyce Mapani – investigation, writing – review and editing
Tobias Mweene – investigation, writing – review and editing
Bruno Levecke – resources, manuscript editing and revision
Piet Cools – resources, investigation, manuscript editing and revision
Govert J. van Dam – investigation, writing – review and editing
Paul L.A.M. Corstjens – investigation, writing – review and editing
Helen Ayles – resources, writing – review and editing
Richard J. Hayes – supervision, resources, writing – review and editing
Suzanna C. Francis – supervision, writing – review and editing
Lisette van Lieshout – investigation, writing – review and editing
Bellington Vwalika - investigation, writing – review and editing
Eyrun F. Kjetland – investigation, writing – review and editing
Amaya L. Bustinduy – funding acquisition, supervision, original manuscript preparation, manuscript editing and revision
References
- 1. WHO: Schistosomiasis. Geneva: World Health Organization;2022; [accessed November 10, 2022]. Reference Source [Google Scholar]
- 2. Bustinduy AL, Randriansolo B, Sturt AS, et al. : An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: The time is now. Adv Parasitol. 2022;115:1–44. 10.1016/bs.apar.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 3. Kjetland EF, Ndhlovu PD, Mduluza T, et al. : Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72(3):311–9. 10.4269/ajtmh.2005.72.311 [DOI] [PubMed] [Google Scholar]
- 4. Kjetland EF, Kurewa EN, Ndhlovu PD, et al. : Female genital schistosomiasis--a differential diagnosis to sexually transmitted disease: genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop Med Int Health. 2008;13(12):1509–17. 10.1111/j.1365-3156.2008.02161.x [DOI] [PubMed] [Google Scholar]
- 5. Kjetland EF, Kurewa EN, Mduluza T, et al. : The first community-based report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil Steril. 2010;94(4):1551–3. 10.1016/j.fertnstert.2009.12.050 [DOI] [PubMed] [Google Scholar]
- 6. Norseth HM, Ndhlovu PD, Kleppa E, et al. : The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl Trop Dis. 2014;8(11):e3229. 10.1371/journal.pntd.0003229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arenholt LTS, Aaroe KK, Norderud K, et al. : Cervical lesion proportion measure using a digital gridded imaging technique to assess cervical pathology in women with genital schistosomiasis. PLoS Negl Trop Dis. 2022;16(7): e0009995. 10.1371/journal.pntd.0009995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Søfteland S, Sebitloane MH, Taylor M, et al. : A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynaecol Obstet. 2021;153(2):190–9. 10.1002/ijgo.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sturt AS, Webb EL, Phiri CR, et al. : Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: The BILHIV study. PLoS Negl Trop Dis. 2020;14(7):e0008337. 10.1371/journal.pntd.0008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kjetland EF, Leutscher PD, Ndhlovu PD: A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58–65. 10.1016/j.pt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 11. Tushabe JV, Lubyayi L, Sserubanja J, et al. : Does Intensive Treatment Select for Praziquantel Resistance in High-Transmission Settings? Parasitological Trends and Treatment Efficacy Within a Cluster-Randomized Trial. Open Forum Infect Dis. 2020;7(4):ofaa091. 10.1093/ofid/ofaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Summers S, Bhattacharyya T, Allan F, et al. : A review of the genetic determinants of praziquantel resistance in Schistosoma mansoni: Is praziquantel and intestinal schistosomiasis a perfect match? Front Trop Dis. 2022;3:933097. 10.3389/fitd.2022.933097 [DOI] [Google Scholar]
- 13. Wells N, Chappuis F, Beran D: Spotlight on experiences of medicine unavailability: access to medicines challenges for NCDs and NTDs - the contrasting cases of insulin and praziquantel. Expert Rev Clin Pharmacol. 2020;13(4):341–53. 10.1080/17512433.2020.1740589 [DOI] [PubMed] [Google Scholar]
- 14. Hayes RJ, Donnell D, Floyd S, et al. : Effect of Universal Testing and Treatment on HIV Incidence - HPTN 071 (PopART). N Engl J Med. 2019;381(3):207–18. 10.1056/NEJMoa1814556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalinda C, Chimbari MJ, Mukaratirwa S: Schistosomiasis in Zambia: a systematic review of past and present experiences. Infect Dis Poverty. 2018;7(1):41. 10.1186/s40249-018-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization: Female genital schistosomiasis: a pocket atlas for clinical health-care professionals. World Health Organization,2015; Accessed April 30, 2021. Reference Source [Google Scholar]
- 17. Zambian Ministry of Health: Guidelines for the Etiological and Clinical Management of Sexually Transmitted Infections in Zambia.2017. [Google Scholar]
- 18. Rafferty H, Sturt AS, Phiri CR, et al. : Association between cervical dysplasia and female genital schistosomiasis diagnosed by genital PCR in Zambian women. BMC Infect Dis. 2021;21(1):691. 10.1186/s12879-021-06380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sankaranarayanan R, Wesley R, Thara S, et al. : Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. 2003;106(3):404–8. 10.1002/ijc.11245 [DOI] [PubMed] [Google Scholar]
- 20. Corstjens PL, De Dood CJ, Kornelis D, et al. : Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141(14):1841–55. 10.1017/S0031182014000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corstjens P, de Dood CJ, Knopp S, et al. : Circulating Anodic Antigen (CAA): A Highly Sensitive Diagnostic Biomarker to Detect Active Schistosoma Infections-Improvement and Use during SCORE. Am J Trop Med Hyg. 2020;103(1_Suppl):50–7. 10.4269/ajtmh.19-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obeng BB, Aryeetey YA, de Dood CJ, et al. : Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102(7):625–33. 10.1179/136485908X337490 [DOI] [PubMed] [Google Scholar]
- 23. Sturt AS, Webb EL, Himschoot L, et al. : Association of Female Genital Schistosomiasis With the Cervicovaginal Microbiota and Sexually Transmitted Infections in Zambian Women. Open Forum Infect Dis. 2021;8(9):ofab438. 10.1093/ofid/ofab438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sturt AS, Webb EL, Patterson C, et al. : Cervicovaginal Immune Activation in Zambian Women With Female Genital Schistosomiasis. Front Immunol. 2021;12(181):620657. 10.3389/fimmu.2021.620657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sturt AS, Webb EL, Phiri CR, et al. : Female Genital Schistosomiasis and HIV-1 Incidence in Zambian Women: A Retrospective Cohort Study. Open Forum Infect Dis. 2021;8(7):ofab349. 10.1093/ofid/ofab349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Land KJ, Boeras DI, Chen XS, et al. : REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. 2019;4(1):46–54. 10.1038/s41564-018-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kjetland EF, Norseth HM, Taylor M, et al. : Classification of the lesions observed in female genital schistosomiasis. Int J Gynaecol Obstet. 2014;127(3):227–8. 10.1016/j.ijgo.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 28. Engels D, Hotez PJ, Ducker C, et al. : Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Organ. 2020;98(9):615–24. 10.2471/BLT.20.252270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poggensee G, Kiwelu I, Weger V, et al. : Female genital schistosomiasis of the lower genital tract: prevalence and disease-associated morbidity in northern Tanzania. J Infect Dis. 2000;181(3):1210–3. 10.1086/315345 [DOI] [PubMed] [Google Scholar]
- 30. Gandasegui J, Fernández-Soto P, Carranza-Rodríguez C, et al. : The Rapid-Heat LAMPellet Method: A Potential Diagnostic Method for Human Urogenital Schistosomiasis. PLoS Negl Trop Dis. 2015;9(7):e0003963. 10.1371/journal.pntd.0003963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Archer J, Patwary FK, Sturt AS, et al. : Validation of the isothermal Schistosoma haematobium Recombinase Polymerase Amplification (RPA) assay, coupled with simplified sample preparation, for diagnosing female genital schistosomiasis using cervicovaginal lavage and vaginal self-swab samples. PLoS Negl Trop Dis. 2022;16(3):e0010276. 10.1371/journal.pntd.0010276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swai B, Poggensee G, Mtweve S, et al. : Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect Dis. 2006;6:134. 10.1186/1471-2334-6-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galappaththi-Arachchige HN, Holmen S, Koukounari A, et al. : Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: A cross sectional study in rural South Africa. PLoS One. 2018;13(2): e0191459. 10.1371/journal.pone.0191459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holmen SD, Kleppa E, Lillebø K, et al. : The first step toward diagnosing female genital schistosomiasis by computer image analysis. Am J Trop Med Hyg. 2015;93(1):80–6. 10.4269/ajtmh.15-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holmen SD, Kjetland EF, Taylor M, et al. : Colourimetric image analysis as a diagnostic tool in female genital schistosomiasis. Med Eng Phys. 2015;37(3):309–14. 10.1016/j.medengphy.2014.12.007 [DOI] [PubMed] [Google Scholar]


