Abstract
Stenotrophomonas maltophilia, an environmental aerobic non-fermentative Gram-negative bacilli, has gained attention in many nosocomial outbreaks. COVID-19 patients in intensive care unit have extended hospital stay and are severely immunosuppressed. This study aimed to determine the prevalence and risk factors of S. maltophilia pneumonia in critical COVID-19 patients. A total of 123 COVID-19 patients in ICU admitted between March 2020 and March 2021 were identified from the authors’ institutional database and assessed for nosocomial pneumonia. Demographic data and factors predisposing to S. maltophilia pneumonia were collected and analyzed. The mean age was 66 ± 13 years and 74% were males. Median APACHE and SOFA scores were 13 (IQR = 8–19) and 4 (3–6), respectively. The Median NEWS2 score was 6 (Q1 = 5; Q3 = 8). The Median ICU stay was 12 (Q1 = 7; Q3 = 22) days. S. maltophilia was found in 16.3% of pneumonia patients, leading to a lengthier hospital stay (34 vs. 20 days; p < 0.001). Risk factors for S. maltophilia pneumonia included previous treatment with meropenem (p < 0.01), thrombopenia (p = 0.034), endotracheal intubation (p < 0.001), foley catheter (p = 0.009) and central venous catheter insertion (p = 0.016). S. maltophilia nosocomial pneumonia is frequent in critical COVID-19 patients. Many significant risk factors should be addressed to reduce its prevalence and negative impact on outcomes.
Subject terms: Microbiology, Infection
Introduction
Stenotrophomonas maltophilia are Gram-negative bacilli1 involved in nosocomial infections, notably hospital-acquired pneumonia (HAP) in the intensive care unit (ICU) setting2. S. maltophilia is known to exhibit intrinsic resistance to several antibiotic classes, including beta-lactams such as carbapenems, as well as aminoglycosides, thus limiting therapeutic options and making S. maltophilia-related infections hard to manage3.
Potential risk factors for acquiring S. maltophilia infections were attributed to procedures frequently performed in the ICU3, such as arterial and central venous catheters, mechanical ventilation, tracheostomy, Foley catheters, aerosol therapy and the use of active or passive humidifiers4,5. Trimethoprim-sulfamethoxazole (TMP-SMX) is the first-line treatment for S. maltophilia infections, with levofloxacin being the alternative when resistance to trimethoprim-sulfamethoxazole occurs. Other therapeutic options include ceftazidime, ticarcillin-clavulanic acid, tigecycline, and colistin. However, previous exposure to antibiotics could promote the emergence of resistant strains6.
Bacterial superinfection occurs in approximately 20% of coronavirus disease of 2019 (COVID-19) patients within two days of intubation and can progress to ventilator-associated pneumonia (VAP)7. A recent study showed that COVID-19 patients requiring mechanical ventilation (MV) were significantly more likely to develop VAP than patients requiring MV for other reasons, with an incidence density of 28/1000 ventilator days versus 13/1000 (p-value = 0,009), respectively, thus increasing the mortality rate8. A meta-analysis confirmed that around half of mechanically ventilated COVID-19 patients would develop VAP, with high mortality rates9. VAP in COVID-19 patients can be polymicrobial, spread to the bloodstream and lead to septic shock10.
Data on bacterial superinfection in COVID-19 are scarce or disparate11. A limited number of trials have investigated risk factors in ICU patients4,12, however, none has focused on S. maltophilia infections in COVID-19 patients. In fact, there are few publications on the impact of superinfection in patients with COVID-19. To our knowledge, no solid data analyzing the high prevalence of Stenotrophomonas infection were reported in this population apart from isolated case reports13,14.
Therefore, this study aimed to determine risk factors for acquiring S. maltophilia pneumonia in COVID-19 ICU patients, also to compare mortality rates and outcomes between COVID-19 ICU patients with and without superimposed S. maltophilia infection.
Materials and methods
Study design and participants
We conducted a retrospective study at Hôtel-Dieu de France (HDF) university hospital (Beirut, Lebanon) between March 2020 till March 2021, based on the COVID-19 patients’ database. We included all adult patients admitted for at least 48 h to the ICU for COVID-19 infection. Data including patients’ demographic characteristics, clinical history, laboratory results, physiological exams, imaging results, undertaken treatments, and factors predisposing to S. maltophilia pneumonia were collected. The clinical information was obtained from patient medical charts. The demographic data included age, gender, blood group system ABO and rhesus groups. Comorbidities, such as cardiovascular risk factors, arterial hypertension, diabetes mellitus, immunosuppression, and chronic kidney disease, were also captured. Laboratory exams included : complete blood count, coagulation profile, renal and liver function tests, creatine kinase, lactate dehydrogenase, electrolytes, cardiac enzymes, serum ferritin, C-reactive protein (CRP) and D-dimer. Types of mechanical ventilation and humidification applied were collected.
Stenotrophomonas isolation was performed on quantitative sputum culture in non-intubated patients or on quantitative tracheal aspiration or bronchoalveolar lavage (BAL) in intubated patients.
Data were analysed by physicians from three different departments (pulmonary and critical care, anesthesiology and critical care, and infectious diseases) to resolve any discrepancy in interpretation.
Ethical considerations
This study was approved and written informed consent was waived in the context of the COVID-19 pandemic by the ethics committee of Hotel-Dieu de France Hospital affiliated to Saint Joseph University (approval number: CEHDF-1630). This study was performed in accordance with the Declaration of Helsinki.
Microbiology
Respiratory samples, included sputum culture and tracheal aspirates when on mechanical ventilation were collected. They were inoculated, first directly, then after a 100-fold dilution, onto Columbia agar + 5% sheep blood, Chocolate blood agar and 2 additional selective media; MacConkey agar and Columbia agar + 5% sheep blood + Colistin + Nalidixic acid.
S. maltophilia species were identified using the BD phoenix automated microbiology system (Becton Dickinson, Sparks, Md., USA). S. maltophilia species counts were determined after 24 h of growth at 37 °C and expressed as colony-forming unit per milliliter (CFU/mL). Antimicrobial susceptibility testing technique consisted of a determination of the minium inhibitory concentrations (MICs) to TMP-SMX, levofloxacin, ceftazidime and ticarcillin-clavulanic acid, using the same BD phoenix automated system.
Diagnostic criteria
Sequential Organ Failure Assessment (SOFA)15, Acute Physiology and Chronic Health Evaluation II (APACHE II)16, and National Early Warning Score (NEWS2)17 scores were calculated for each patient upon ICU admission. Invasive procedures during ICU stay, including central venous catheter, urinary catheter, tracheostomy, mechanical ventilation, duration of mechanical ventilation, and duration of ICU stay, were recorded.
The patients were described as acquiring primary SM pneumonia (first pneumonia acquired during ICU stay) or secondary pneumonia (defined as a SM pneumonia acquired after previous pneumonia caused by another pathogen and a recurrence of symptoms and signs after an initial improvement of the patient). Scanographic, baseline laboratory and ICU parameters in the 2 groups without and with Stenotrophomonas infection are summarized in Table 1.
Table 1.
Scanographic, baseline laboratory and ICU parameters in the 2 groups without and with Stenotrophomonas infection.
| Statistic | Stenotrophomonas | Test | OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No (103 patients) | Yes (20 patients) | |||||
| Scanographic parameters | ||||||
| @ Baseline | ||||||
| GGO (%) | m ± sd | 30.8 ± 18.6 | 42.1 ± 23.8 | T | 0.032 | |
| Lobar condensation | N (%) | 12 (13.5%) | 2 (11.8%) | Chi2 | 0.86 (0.17–4.22) | 0.848 |
| Pneumomediastinum | N (%) | 1 (1.1%) | 0 (0%) | F | 0.840 | |
| @ 1st Follow-up | ||||||
| GGO (%) | m ± sd | 53 ± 24.3 | 71.7 ± 22.4 | T | 0.009 | |
| Lobar condensation | N (%) | 13 (22.4%) | 6 (40%) | Chi2 | 2.31 (0.69–7.69) | 0.166 |
| Pneumomediastinum | N (%) | 3 (5.2%) | 6 (40%) | Chi2 | 12.2 (2.58–57.9) | 0.000 |
| Pulmonary embolism | N (%) | 7 (12.1%) | 0 (0%) | Chi2 | 0.157 | |
| Baseline laboratory parameters | ||||||
| Leucocytes (× 103) | Me [IQR] | 8.4 [5.8–11.8] | 8.8 [6.4–10.5] | MWU | 0.696 | |
| Neutrophiles (× 103) | Me [IQR] | 6.6 [4.4–10.2] | 7.7 [4.9–9.2] | MWU | 0.417 | |
| Lymphocytes (× 103) | Me [IQR] | .069 [0.48–1.06] | 0.73 [0.58–0.82] | MWU | 0.869 | |
| Lowest platelets level (× 103) | Me [IQR] | 141 [100–196] | 79 [46–101] | MWU | 0.001 | |
| Ferritine | Me [IQR] | 629 [420–1229] | 1239 [360–1739] | MWU | 0.280 | |
| LDH | Me [IQR] | 409 [318–508] | 395 [244–707] | MWU | 0.867 | |
| D-dimers | Me [IQR] | 0.89 [0.41–2.01] | 0.98 [0.76–3.36] | MWU | 0.283 | |
| CRP | Me [IQR] | 120 [59–186] | 147 [95.3–202] | MWU | 0.292 | |
| Procalcitonine | Me [IQR] | 0.26 [0.14–0.56] | 0.25 [0.22–0.8] | MWU | 0.548 | |
| HDL | Me [IQR] | 1.01 [0.71–1.25] | 1.185 [1.02–1.45] | MWU | 0.279 | |
| LDL | Me [IQR] | 2.67 [1.97–3.35] | 2.37 [1.8–3] | MWU | 0.593 | |
| Triglycerides | Me [IQR] | 1.86 [1.30–2.48] | 1.96 [1.47–2.20] | MWU | 0.976 | |
| Serum Creatinin | Me [IQR] | 76 [63–111] | 94 [79–167] | MWU | 0.052 | |
| Follow-up ICU parameters | ||||||
| Highest PEEP | Me [IQR] | 8 [3–12] | 12 [10–14] | MWU | 0.004 | |
| Highest FiO2 | Me [IQR] | 1 [0.7–1] | 1 [0.8–1] | MWU | 0.552 | |
| Humid | N (%) | 84 (93.3%) | 17 (100%) | Chi2 | 0.273 | |
| KTA | N (%) | 92 (89.3%) | 19 (100%) | Chi2 | 0.135 | |
| KTC (binary) | N (%) | 78 (75.7%) | 19 (100%) | Chi2 | 0.016 | |
| KTC (quantitative) | Me [IQR] | 1 [0–1] | 3 [2–3] | MWU | 0.000 | |
| Number of vascular catheters | Me [IQR] | 2 [2–3] | 6 [3–6] | MWU | 0.000 | |
| Foley catheter | N (%) | 68 (66%) | 19 (95%) | Chi2 | 9.78 (1.26–76.1) | 0.009 |
| News 2 Score | Me [IQR] | 6 [5–8] | 6 [4.5–8] | MWU | 0.594 | |
| SOFA | Me [IQR] | 4 [3–7] | 3 [3–5] | MWU | 0.162 | |
| APACHE | Me [IQR] | 13 [8–18] | 15 [12–21] | MWU | 0.439 | |
| ICU transfer day | Me [IQR] | 3 [1–6] | 3 [1–7] | MWU | 0.961 | |
| ICU length of stay | Me [IQR] | 12 [6–18] | 32.5 [20.5–43.5] | MWU | 0.000 | |
| Intubation day | Me [IQR] | 5 [3–10] | 8 [3–12] | MWU | 0.181 | |
| Extubation day | Me [IQR] | 13 [11–19] | 24 [11–29] | MWU | 0.226 | |
Categorical data are presented as frequencies and percentages (N (%)); Continuous data not departing from normality assumptions are presented as mean and standard deviation (m ± sd). Continuous data departing from normality assumptions and ordinal data are presented as Median with its interquartile range (1st quartile–3rd quartile) (Me [IQR]). Chi2: Chi square test; MWU: Mann–Whitney U test. For binary data, the odds ratio with its 95% confidence interval were calculated [OR (95% CI)]. F: Fisher exact test. T: independent samples T test.
Treatment received in the last three months before admission and during hospital stay were noted, including agents used for COVID-19 treatment (hydroxychloroquine, azithromycin, ivermectin, remdesivir, lopinavir, glucocorticoids, baricitinib, and tocilizumab) as well as anti-platelets therapy, anti-coagulants therapy, and antibiotics. A high-resolution CT scan was performed on admission day and repeated when indicated. Chest CT severity score (CT-SS) is used to evaluate the severity of pulmonary involvement18. Ct-SS was validated using the Cohen’s κ and intraclass correlation coefficient with a strong relationship with the Modified Early Warning Score19.
Nosocomial pneumonia was diagnosed using the criteria proposed by the Infectious Diseases Society of America and the American Thoracic Society20 and the International guidelines of the ERS/ESICM/ESCMID/ALAT societies21. SM-associated pneumonia was diagnosed by a positive microbiologic culture from BAL (> 104 CFU/mL) or tracheal aspirates (> 105 CFU/mL) accompanied by radiographic signs of pulmonary infection (presence of new or increasing infiltrates on chest radiograph) and at least two of the following clinical criteria of pulmonary infection: abnormal temperature, abnormal leucocyte counts or macroscopically purulent tracheal secretions. When confirmed, patients diagnosed with SM pneumonia were treated for 10–14 days with systemic SM/TMP. The control group consisted of all other critically ill patients with COVID-19 but without S. maltophilia pneumonia. The control group includes patients without pneumonia as well as those with pneumonia due to another pathogen than SM.
Definition of outcomes
All-cause and ICU mortality, defined as a death event occurring in patients who stayed in the ICU, were recorded. ICU outcomes also included the length of stay, needs for intubation and invasive mechanical ventilation. Cultures and antibiotic sensitivity were analyzed. Dependency outcomes were analyzed using the post-COVID-19 functional status (PCFS)22. Safety outcomes were collected and graded according to the “Common Terminology Criteria for Adverse Events” (CTCAE) v5.023. Inflammatory markers were also monitored. The H-score assessed the risk for any reactive hemophagocytic syndrome every 48 h.
Statistical analyses
Categorical variables were expressed as frequencies and percentages. Continuous variables not departing from normality assumptions (Quartile-Quartile plots, Shapiro–Wilk test) were expressed as mean ± standard deviation, whereas continuous variables with skewed distribution and ordinal variables were expressed as median with interquartile range (1st quartile–3rd quartile). The univariate odds ratio (OR) with its 95% confidence interval (95%CI) was calculated for Boolean categorical variables. Only exposure to potential risk factors before S. maltophilia acquisition was considered. Chi-squared, Fisher’s exact, and Fisher–Halton–Freeman tests were applied to analyze categorical, as appropriate. The Mann–Whitney U test and the independent-samples T-test were used to compare continuous variables, as appropriate. The analysis was performed on SPSS v26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Results
Patients’ clinical characteristics
A total of 123 COVID-19 ICU patients (74% men) were enrolled between March 2020 and March 2021, with a mean age of 66.5 ± 13.5 years. The median APACHE score at ICU admission was 13 (Q1 = 8; Q3 = 19), and the SOFA score was 4 (Q1 = 3; Q3 = 6). The median ICU transfer day was three days (Q1 = 1; Q3 = 6), and ICU median duration of stay was 12 days (Q1 = 7; Q3 = 22). Blood type distribution was A group (45.1%), O group (35.3%), B group (12.7%) and AB group (6.9%), with Rhesus positive predominance (92.2%). The most prevalent comorbidities reported were arterial hypertension (67.5%), diabetes mellitus (45.5%), cardiovascular disease (34.1%), renal failure (18.7%) and chronic immunosuppression (8.9%). Protocolized treatment received before ICU admission included systemic steroids (90.2%), ivermectin (77.2%), tocilizumab (32.5%), remdesivir (16.3%), hydroxychloroquine (16.3%), baricitinib (11.4%) and lopinavir/ritonavir (2.4%). The median corticoid dose was equivalent to 20 mg per day of dexamethasone (Q1 = 20; Q3 = 20). The chest CT performed at admission showed important lung involvement with ground-glass opacities (SS-CT 33% ± 20) and lobar consolidation in 13.2% of the patients. Blood exams confirmed high inflammatory markers with a ferritin median of 679 ng/mL (Q1 = 408; Q3 = 1316), LDH of 408 UI/mL (Q1 = 298; Q3 = 515) and lymphopenia of 690 cells/µL (Q1 = 480; Q3 = 970). The median NEWS2 score was 6 (Q1 = 5; Q3 = 8). The oxygen needs on admission in more than 4 l/min and on nonrebreathing mask/Optiflow were 27.6% and 44.7%, respectively.
Description of SM pneumonia outbreak
A total of 42 nosocomial pneumonia events (27.5%) were diagnosed. The most common isolated pathogens were S. maltophilia in 20 patients (16.2%), Pseudomonas spp. and Enterobacterales species in 16 patients (6%) and 4 patients (9.5%), respectively. Staphylococcus aureus and Aspergillus spp. were rarely reported (2 patients (4.8%) and 4 patients (9.5%), respectively) (Fig. 1). Patients with S. maltophilia pneumonia were more prone to a second S. maltophilia pneumonia in 13.8%. Out of the 20 SM pneumonias acquired, 5 were primary infections (25%), while 15 were secondary infections (75%). Demographic data and patient characteristics were divided into two groups, without and with S. maltophilia infection (Table 2).
Figure 1.
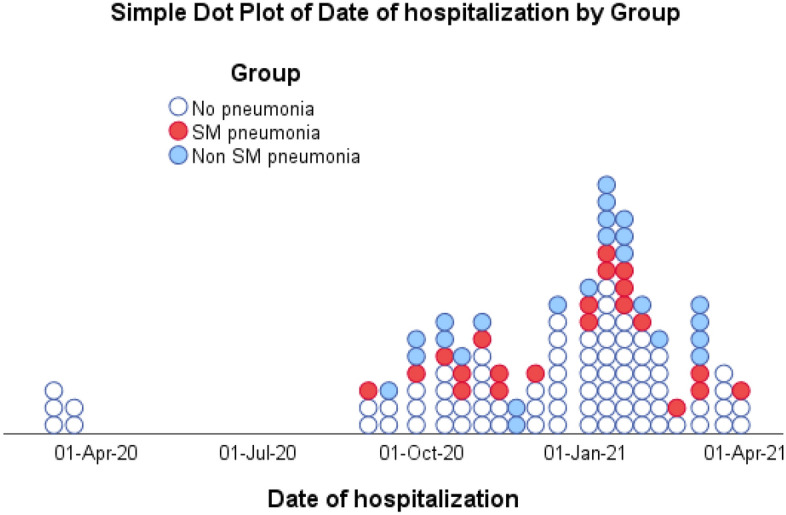
Distribution of critical COVID-19 patients (123 patients) all along the year from March 2020 till March 2021. White dot: Critical COVID-19; Blue dot: non-SM HAP; Red dot: SM HAP.
Table 2.
Baseline clinical characteristics, medical history and risk factors in the 2 groups without and with Stenotrophomonas infection.
| Statistic | Stenotrophomonas | Test | OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No (103 patients) | Yes (20 patients) | |||||
| Age | m ± sd | 65.5 ± 13.3 | 68.2 ± 7.8 | T | 0.384 | |
| Male gender | N (%) | 75 (72.8%) | 16 (80%) | Chi2 | 0.67 (0.21–2.18) | 0.503 |
| Blood type | ||||||
| O | N (%) | 33 (38.8%) | 3 (17.6%) | Chi2 | 0.015 | |
| A | N (%) | 39 (45.9%) | 7 (41.2%) | Chi2 | ||
| B | N (%) | 10 (11.8%) | 3 (17.6%) | Chi2 | ||
| AB | N (%) | 3 (3.5%) | 4 (23.5%) | Chi2 | ||
| Positive Rhesus antigen | N (%) | 77 (90.6%) | 17 (100%) | Chi2 | 0.188 | |
| O2 needs at admission | ||||||
| No O2 | N (%) | 10 (9.7%) | 1 (5%) | MWU | 0.161 | |
| O2 < 4 L/min | N (%) | 18 (17.5%) | 4 (20%) | |||
| O2 4–8 L/min | N (%) | 32 (31.1%) | 2 (10%) | |||
| MHC/Optiflow | N (%) | 42 (40.8%) | 13 (65%) | |||
| Endotracheal tube | N (%) | 1 (1%) | 0 (0%) | |||
| Initial ICU admission | N (%) | 51 (49.5%) | 9 (45%) | Chi2 | 0.83 (0.32–2.18) | 0.712 |
| Day symptoms started | m ± sd | − 6.9 ± 5.8 | − 6.1 ± 4.4 | T | 0.559 | |
| 1st Ct value | m ± sd | 22.8 ± 5.6 | 20.2 ± 4.4 | T | 0.113 | |
| Weight in kg | m ± sd | 84.6 ± 15.3 | 84.3 ± 18.3 | T | 0.944 | |
| Hypertension | N (%) | 71 (68.9%) | 12 (60%) | Chi2 | 0.68 (0.25–1.81) | 0.435 |
| Diabetes mellitus | N (%) | 45 (43.7%) | 11 (55%) | Chi2 | 1.58 (0.60–4.13) | 0.353 |
| Cardiovascular disease | N (%) | 37 (35.9%) | 5 (25%) | Chi2 | 0.59 (0.20–1.77) | 0.346 |
| Chronic renal failure | N (%) | 19 (18.4%) | 4 (20%) | Chi2 | 1.11 (0.33–3.68) | 0.870 |
| Heart failure | N (%) | 14 (14%) | 4 (20%) | Chi2 | 1.54 (0.45–5.27) | 0.493 |
| Immunosuppression | N (%) | 10 (10%) | 3 (15%) | Chi2 | 1.59 (0.40–6.38) | 0.511 |
| Obstructive lung disease | N (%) | 26 (26%) | 5 (25%) | Chi2 | 0.95 (0.31–2.87) | 0.926 |
| Previous treatment | ||||||
| Previous pathogen | N (%) | 5 (5%) | 2 (10%) | Chi2 | 2.11 (0.38–11.74) | 0.384 |
| Treatment with meropenem | N (%) | 4 (4%) | 6 (30%) | Chi2 | 10.39 (2.60–41.5) | 0.000 |
| Treatment with quinolone | N (%) | 6 (5.9%) | 1 (5%) | Chi2 | 0.83 (0.09–7.32) | 0.869 |
| Treatment with piperacillin/tazobactam | N (%) | 8 (8%) | 4 (20%) | Chi2 | 2.88 (0.77–10.7) | 0.102 |
Categorical data are presented as frequencies and percentages (N (%)); Continuous data not departing from normality assumptions are presented as mean and standard deviation (m ± sd). Chi2: Chi square test; MWU: Mann–Whitney U test. For binary data, the odds ratio with its 95% confidence interval were calculated [OR (95% CI)]. F: Fisher exact test. T: independent samples T test.
Risk factors associated with S. maltophilia pneumonia
S. maltophilia pneumonia was encountered more frequently in group B (17%) and group AB (23.5%) blood types compared to 11.8% and 3.5% in the control group, respectively (p = 0.015). Previous antibiotic administration in the last three months was a risk factor for the development of S. maltophilia pneumonia, especially with meropenem, 30% vs. 4%, respectively, with OR = 10.4 (95% CI 2.6–41.5). Furthermore, antibiotic treatment during the current hospital stay increased this risk, especially carbapenems, 75% vs. 46.6% in the control group, with OR = 3.4 (95% CI 1.16–10.16), and aminoglycosides, 75% vs. 27.2% in the control group, with OR = 8 (95% CI 2.67–24.17) (Table 3). SS-CT with ground-glass opacities in S. maltophilia pneumonia was 42% ± 24% compared to 31% ± 19% in the control group (p = 0.062). On follow-up, S. maltophilia pneumonia was related to the development of pneumomediastinum in 60% vs. 5.2% in the control group (OR = 12.2; 95% CI 2.58–57.86) and internal hemorrhage in 30% vs. 11.7% in the control group (OR = 3.3; 95% CI 1.05–10.06).
Table 3.
Treatment characteristics in the 2 groups without and with Stenotrophomonas infection.
| Statistic | Stenotrophomonas | Test | OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No (103 patients) | Yes (20 patients) | |||||
| Hydroxychloroquine | N (%) | 18 (17.5%) | 2 (10%) | Chi2 | 0.52 (0.11–2.46) | 0.407 |
| Azithromycine | N (%) | 34 (33%) | 6 (30%) | Chi2 | 0.87 (0.31–2.46) | 0.793 |
| Ivermectine | N (%) | 22 (21.4%) | 6 (30%) | Chi2 | 1.58 (0.54–4.58) | 0.399 |
| Lopinavir/Ritonavir | N (%) | 3 (2.9%) | 0 (0%) | F | 0.584 | |
| Remsidevir | N (%) | 17 (16.5%) | 3 (15%) | Chi2 | 0.89 (0.24–3.39) | 0.867 |
| Glucocorticoids | N (%) | 91 (88.3%) | 20 (100%) | Chi2 | 0.108 | |
| Baricitinib | N (%) | 12 (11.7%) | 2 (10%) | Chi2 | 0.84 (0.17–4.09) | 0.832 |
| Tocilizumab | N (%) | 32 (31.1%) | 8 (40%) | Chi2 | 1.48 (0.55–3.97) | 0.435 |
| IL6 assay results | Me [IQR] | 47 [28.7–122.2] | 42.2 [24–55] | MWU | 0.000 | |
| Antibiotics start day | Me [IQR] | 1[1–2] | 1 [1–1.5] | MWU | 0.605 | |
| Targeted antibiotics | N (%) | 25 (26.3%) | 5 (25%) | Chi2 | 0.93 (0.31–2.83) | 0.903 |
| Antibiotics association | N (%) | 60 (65.2%) | 17 (89.5%) | Chi2 | 4.53 (0.98–20.87) | 0.053 |
| Penicillin | N (%) | 51 (49.5%) | 13 (65%) | Chi2 | 1.89 (0.7–5.13) | 0.205 |
| Cephalosporin | N (%) | 49 (47.6%) | 11 (55%) | Chi2 | 1.35 (0.51–3.53) | 0.543 |
| Carbapenem | N (%) | 48 (46.6%) | 15 (75%) | Chi2 | 3.44 (1.16–10.2) | 0.020 |
| Aminosid | N (%) | 28 (27.2%) | 15 (75%) | Chi2 | 8.04 (2.67–24.2) | 0.000 |
| Quinolone | N (%) | 28 (27.2%) | 9 (45%) | Chi2 | 2.19 (0.82–5.85) | 0.112 |
| Glycopeptide | N (%) | 41 (39.8%) | 12 (60%) | Chi2 | 2.27 (0.85–6.03) | 0.095 |
| Trimethoprim-sulfamethoxazole | N (%) | 7 (6.8%) | 14 (70%) | Chi2 | 32 (9.39–109.1) | 0.000 |
Categorical data are presented as frequencies and percentages (N (%)); Continuous data departing from normality assumptions and ordinal data are presented as Median with its interquartile range (1st quartile–3rd quartile) (Me [IQR]). Chi2: Chi square test; MWU: Mann–Whitney U test. For binary data, the odds ratio with its 95% confidence interval were calculated [OR (95% CI)]. F: Fisher exact test. T: independent samples T test.
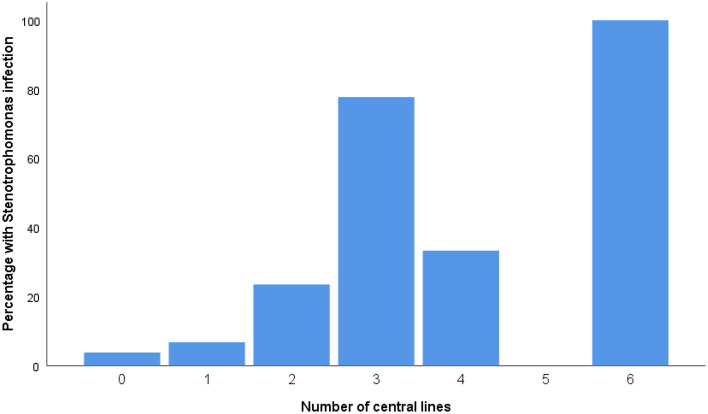
Hospital stay in S. maltophilia pneumonia was 34 days (Q1 = 22; Q3 = 83) compared to 20 days (Q1 = 13; Q3 = 29) in the control group (p = 0.0001). ICU stay in S. maltophilia pneumonia was 33 days (Q1 = 21; Q3 = 44) vs. 12 days (Q1 = 6; Q3 = 18) in the control group (p = 0.0001). S. maltophilia infection showed to be proportional to the number of central lines placed during the ICU stay (p = 0.016) (Fig. 2). This finding is also applicable to other installed invasive devices like the urine Foley (OR = 9.8; 95% CI 1.26–76.1) and endotracheal intubation (OR = 16.6; 95% CI 2.14–128.52). On the other hand, factors including the use of active humidifiers or having chronic obstructive pulmonary disease (COPD) were not associated with a higher incidence of SM infection.
Figure 2.
Relation between the probability of acquiring S. maltophilia pneumonia and the number of central lines installed during ICU stay.
Antimicrobial susceptibility of S. maltophilia strains
In vitro analysis of TMP-SMZ sensitivity was high in the first nosocomial pneumonia (95%) and persisted in the subsequent infection (92.3%). In contrast, levofloxacin sensitivity dropped significantly from 65% at the first infection to 46.2% during subsequent infection (Fig. 3). All S. maltophilia strains remained sensitive to minocycline and colistin in vitro.
Figure 3.
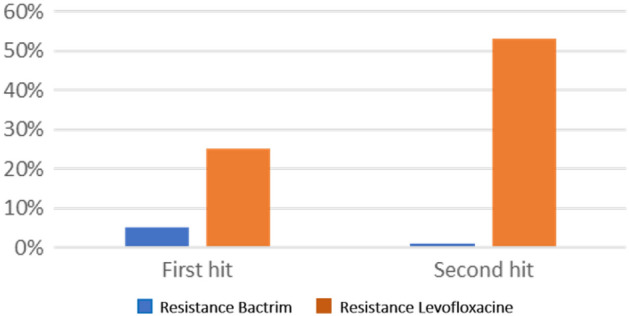
In Vitro resistance of S. maltophilia pathogen to antibiotics (Trimethoprim-sulfamethoxazole and Levofloxacine) during first and second hit.
As to the treatment received by the patients, 10 patients received a monotherapy with TMP-SMZ, 6 received a monotherapy with Levofloxacin, while 2 received a combination theraoy with TMP-SMZ and levofloxacine and 2 patients passed away before initiating treatment.
Outcomes
Clinical and functional outcomes in the 2 groups without and with Stenotrophomonas infection are presented in Table 4. The mortality rate of ICU COVID-19 patients with S. maltophilia pneumonia was 60% vs. 40% in the control group (p 0.161). Lengh of ICU stay for COVID-19 patients with S. maltophilia pneumonia was prolonged, with a median of 34 days (Q1 = 22; Q3 = 83) vs. 20 days (Q1 = 13; Q3 = 29) (p < 0.001).
Table 4.
Clinical and functional outcomes in the 2 groups without and with Stenotrophomonas infection.
| Statistic | Stenotrophomonas | Test | OR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No (103 patients) | Yes (20 patients) | |||||
| Mortality | ||||||
| N | N (%) | 62 (60.2%) | 8 (40%) | FFH | 0.161 | |
| COVID-19-related | N (%) | 37 (35.9%) | 11 (55%) | |||
| Not COVID-19-related | N (%) | 4 (3.9%) | 1 (5%) | |||
| Length of stay | Me [IQR] | 20 [13–29] | 34 [22–82.5] | MWU | 0.000 | |
| Prone position | N (%) | 53 (52.5%) | 13 (68.4%) | Chi2 | 1.96 (0.69–5.57) | 0.200 |
| Thromboembolic event | N (%) | 12 (11.8%) | 3 (15%) | Chi2 | 1.32 (0.34–5.19) | 0.687 |
| Hemorrhagic event | N (%) | 12 (11.7%) | 6 (30%) | Chi2 | 3.25 (1.05–10.06) | 0.034 |
| Day of Hemorrhagic event | Me [IQR] | 17.5 [10.5–21] | 20 [14–31] | MWU | 0.511 | |
| Intubation | N (%) | 55 (53.4%) | 19 (95%) | Chi2 | 16.6 (2.14–128.5) | 0.001 |
| Discharge status | ||||||
| Autonomous | N (%) | 31 (49.2%) | 1 (14.3%) | MWU | 0.038 | |
| O2 | N (%) | 27 (42.9%) | 4 (57.1%) | |||
| Ventilation | N (%) | 2 (3.2%) | 0 (0%) | |||
| Tracheostomy | N (%) | 3 (4.8%) | 2 (28.6%) | |||
| PCFS at discharge | ||||||
| 0 | N (%) | 10 (14.7%) | 7 (50%) | MWU | 0.242 | |
| 1 | N (%) | 7 (10.3%) | 0 (0%) | |||
| 2 | N (%) | 19 (27.9%) | 1 (7.1%) | |||
| 3 | N (%) | 14 (20.6%) | 2 (14.3%) | |||
| 4 | N (%) | 18 (26.5%) | 4 (28.6%) | |||
| 2-month post-discharge status | ||||||
| Autonomous | N (%) | 47 (81%) | 2 (28.6%) | MWU | 0.001 | |
| O2 | N (%) | 8 (13.8%) | 2 (28.6%) | |||
| Ventilation | N (%) | 2 (3.4%) | 1 (14.3%) | |||
| Tracheostomy | N (%) | 1 (1.7%) | 2 (28.6%) | |||
| PCFS @ 2-Month post discharge | ||||||
| 0 | N (%) | 24 (36.9%) | 7 (50%) | MWU | 0.710 | |
| 1 | N (%) | 24 (36.9%) | 1 (7.1%) | |||
| 2 | N (%) | 9 (13.8%) | 1 (7.1%) | |||
| 3 | N (%) | 2 (3.1%) | 0 (0%) | |||
| 4 | N (%) | 6 (9.2%) | 5 (35.7%) | |||
Categorical data are presented as frequencies and percentages (N (%)); Continuous data departing from normality assumptions and ordinal data are presented as Median with its interquartile range (1st quartile–3rd quartile) (Me [IQR]). Chi2: Chi square test; MWU: Mann–Whitney U test; PCFS: Post-COVID-19 Functional Status. For binary data, the odds ratio with its 95% confidence interval were calculated [OR (95% CI)]. FFH: Fisher–Freeman–Halton exact test. T: independent samples T test.
Survivors with S. maltophilia pneumonia had much higher long-term complication rates, i.e., 28.6% tracheostomy, 57% oxygen dependence, and 28.6% ventilation vs. 1.7%, 13.8%, and 3.4%, respectively, in the control group (p < 0.001) (Fig. 4).
Figure 4.
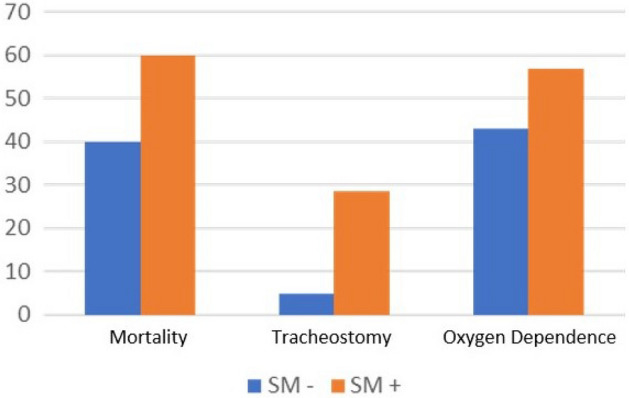
Difference in clinical outcome (in terms of mortality, need for tracheostomy and oxygen dependence) between ICU COVID patients with and without S. maltophilia superinfection.
Discussion
In this retrospective observational study involving 123 patients with severe COVID-19 infections, 20 (16.3%) were found to have S. maltophilia-associated nosocomial pneumonia. The increasing prevalence of S. maltophilia pneumonia has been extensively studied. The ubiquitous nature and intrinsic multi-drug resistance of S. maltophilia, coupled with its ability to rapidly colonize surfaces, such as endotracheal tubes, central venous lines and foley catheters, and form biofilms; a paramount virulence factor rendering it hard to eradicate; contributed to the increasing incidence of nosocomial S. maltophilia infections, mainly among immunocompromised individuals1,24. Surveys from several continents document an increasing isolation rate for S. maltophilia over the years without reaching rates as high as ours. In England and Wales, the annual number of S. maltophilia strains isolated from blood samples increased by 93% between 2000 and 2006. Also, a Taiwanese tertiary-care hospital reported an increase of 83% in the prevelance of S. maltophilia strains recovered from (blood samples?) between 1999 to 20041. However, S. maltophilia’s prevalence in our study is higher compared to available literature. One multicenter study, conducted among cystic fibrosis patients, showed comparable high rates. The authors found that S. maltophilia strains have been more frequently isolated from patients’ respiratory samples, noting a considerable variability between centers, with a mean of 4–6% and peaks of 10–25%25,26. This significant difference can be attributable to many risk factors that have been linked to the increase of S. maltophilia pneumonia over the years found in our population, similar to previous findings27,28. This high prevalence of S. maltophilia nosocomial pneumonia observed in our institution can be attributed to known risk factors previously identified that happened to cluster among our patients27,29. It is well-established that patients with a severe illness requiring intensive care and taking treatment involving immunosuppressive agents, indwelling devices (e.g., catheters and ventilation tubes), a higher frequency of catheters manipulation, a prolonged and escalating broad-spectrum antibiotic therapy, prolonged intubation, and extended hospital stays are at a greater risk for acquiring S. maltophilia infections, highly increasing among critically ill populations27,28.
Most of these risk factors were present in our sample, explaining the high incidence of S. maltophilia pneumonia. The development of a biofilm is probably a considerable virulence factor associated with S. maltophilia infection. It cancolonize surfaces rapidly becoming hard to eradicate24. In this study, endotracheal intubation, central venous catheter, urine Foley, and prolonged ICU stay were associated with S. maltophilia infection. Among immunocompromised COVID-19 patients, S. maltophilia pneumonia was associated with the presence of a central venous catheter and a Foley catheter. Moreover, the more the catheter was changed, the higher the risk of infection. However, arterial catheters were not associated with a higher risk of infection. Endotracheal tubes are also easily colonized by the pathogen, and infection is associated with a longer duration of intubation and mechanical ventilation1. Management of the environment, hospital water supply, and proper handling of medical supplies and equipment play a prominent role in the prevention of S. maltophilia1. Many outbreaks of S. maltophilia in ICU were reported to emerge from water faucets in contact with respiratory tract and ventilator circuits30. In our study, the use of active humidifiers was not associated with more infection than passive humidifiers, and all water faucets specimens in the ICU setting were negative. S. maltophilia pneumonia occurs in high-risk individuals such as those who had prolonged ICU stays and those who have been on mechanical ventilation for a long time, strongly confirmed by our findings, where extended stays were associated with a higher prevalence of infection. Furthermore, S. maltophilia infections are a factor for prolonged ICU stay.
In addition to extrinsic risk factors predisposing for S. maltophilia nosocomial infections, other critical factors intrinsic to the host can impact both the risk of getting infected with S. maltophilia and outcomes of associated infections. Chronic obstructive pulmonary disease (COPD) has been previously found to be an independent risk factor for ICU-acquired S. maltophilia31. However, COPD did not show to be a significant risk factor in our sample. The proportion of blood group A Rh + in our hospitalized ICU patients with COVID-19 was 45.1%. This was higher than encountered in hospitalized Covid-19 on ward 39.9% (CI 35.2–44.7%), as outpatients 44.8% (CI 39.8–49.9%) in the same period of the pandemic and as controls in the general population 32.3%32,33. This is in contrast with the increased risk of S. maltophilia pneumonia encountered more frequently in our study in group B and group AB blood types. Other thoracic or systemic complications were found to be significantly related to S. maltophilia pneumonia. Systemic hemorrhage was an independent significant risk factor for S. maltophilia secondary infection in 30% of the patients (p value < 0.05). To our knowledge, this study is the first that establishes a correlation between acute alveolar hemorrhage and S. maltophilia infection among COVID-19 patients with no hematologic malignancies or hematopoietic stem cell transplants. This rapid alveolar progressive hemorrhage syndrome associated with S. maltophilia infection has only been reported in patients with hematologic malignancies34–37. Whether the hemorrhage precedes S. maltophilia infection or the other way around is yet to be determined. It may reflect the severity of the alveolar damage and vascular disruption.
Pneumothorax was another thoracic complication related to S. maltophilia pneumonia among critically-ill COVID-19 patients38,39. In our study, six out of fifteen patients (40%) diagnosed with S. maltophilia pneumonia had a follow-up CT scan that showed a pneumothorax compared to three out of the 58 patients without S. maltophilia pneumonia (5.2%). This study is the first to report such a significant association with COVID-19 infected patients, and our literature review only revealed few case reports.
In our study, broad-spectrum antibiotics exposure appeared to be a relevant risk factor for S. maltophilia pneumonia in ICU admitted patients40. Meropenem, aminoglycosides, and TMP-SMX were highly associated with S. maltophilia infections. Moreover, patients who developed S. maltophilia pneumonia have been more frequently exposed to antibiotics combination therapy during their ICU stay than the control group (89.5% vs. 65.2%; p = 0.037), with higher use of quinolones (45% vs. 27.2%; p = ns), carbapenems (75% vs. 46.6%; p = 0.02), and aminoglycosides (75% vs. 27.2%; p < 0.001).
Trimethoprim-Sulfamethoxazole (TMP-SMZ) and levofloxacin remain the cornerstone of antimicrobial treatment for S. maltophilia infections41. However, the rapid emergence of resistance to these molecules is of concern. They should be initiated once S. maltophilia has been identified as the cause of nosocomial pneumonia, in order to prevent the risk of colonization, which is often challenging41. The molecular mechanisms contributing to its resistance to antibiotics include β-lactamase production, the expression of Qnr genes, and the presence of class 1 integrons and efflux pumps30,42. Our study showed only 5% (1/20) of TMP-SMX resistance among S. maltophilia isolated during the first episode of hospital-acquired pneumonia. This rate did not increase during the second episode (1%). In contrast, 25% (5/20) of S. maltophilia isolated during the first pulmonary infections exhibited resistance to levofloxacin, with an increase to 53.8% (7/13) during the second episode. Previous exposure to fluoroquinolones could have promoted the emergence of resistance to this antibiotic6. Furthermore, COVID-19 ICU patients with S. maltophilia superinfection were more exposed to fluoroquinolones compared to the control group, which can be determinant in the development of acquired resistance. Moxifloxacin is reported to be a little more active than levofloxacin; hence, further exploring its activity could be helpful. Despite colistin susceptibility in vitro, it may not be a very effective drug for many systemic infections, including pneumonia, and would be the last resort drug. While combination therapy has been proposed for S. maltophilia infections, there is no solid evidence that patients receiving combinations would have better outcomes. Cefiderocol, a fourth-generation siderophore cephalosporin, is usually active in vitro against S. maltophilia, including multiresistant isolates. A recent study comparing cefiderocol and high-dose extended-infusion meropenem for nosocomial pneumonia showed non-inferiority between the two drugs, supporting that the drug works well for pneumonia43.
As for the clinical outcomes, the mortality varies significantly between COVID-19 patients with and without S. maltophilia secondary pneumonia. While the median death rate is 40% in ICU COVID-19 patients in the non-S. maltophilia group, this percentage increases to approximately 60% in those with S. maltophilia. The survivors have a much higher long-term complication rate at discharge, with a 28.6% tracheostomy rate and 57% oxygen dependence vs. 4.8 and 42%, respectively. This significant difference persists at two months post-discharge. These numbers matches the literature, where overall mortality following S. maltophilia pneumonia is estimated at 21–69%27,28,35. No previous research has been conducted among COVID-19 patients. In our study, it is hard to delineate directly attributable mortality risk factors to S. maltophilia pneumonia and disease repercussions from those related to the COVID-19 infection itself.
In addition to prompt antibiotic therapy, the implementation of strict measures of infection control, particularly hand hygiene, the use of personal protective equipment, and proper environment cleaning, is critical to reduce the transmission of S. maltophilia-associated nosocomial infections (including ventilator-associated pneumonia) and limit outbreaks3–5,44,45.
If patient-to-patient transmission is suspected, contact isolation precautions for all infected patients on the floor becomes a necessity, in addition to screening stool cultures and/or sputum samples to identify other colonized patients to segregate within the unit46. It is recommended to investigate potential infection sources, such as hospital unit water supply, faucets, ice machines, oxygen humidification reservoirs, and any hospital products used mutually between patients (e.g., dialyzer effluent, disinfectant solutions). Reviewing disinfection protocols is warranted to identify any breaches, such as using non-sterile water during these processes.
This study has two merits. First, and to our best knowledge, it is the only study to focus solely on S. maltophilia infection in COVID-19 patients. While the association of SM with crtitically ill patients is well known, its association with Covid-19 is described for the first time, showing much higher rates than ever seen before. Second, it outlines the most notable risk factor predictors of S. maltophilia nosocomial pneumonia among critically ill COVID-19 patients, the different antibiotic treatment modalities, infection control measures, and the best approach in investigating potential sources. However, our study has some limitations. Since it was an observational retrospective analysis, some variables that could have impacted the clinical course and disease outcomes could not be controlled at baseline. It is noteworthy to acknowledge the difficulty to differentiate with a high level of certainty whether S. maltophilia is the culprit agent causing pneumonia or is merely a colonizing bacteria, especially in the context of polymicrobial growth. It was also hard to discern if S. maltophilia pneumonia is the direct cause of death or just a marker of severe underlying disease mainly driven by comorbidities and complications inherent to a prolonged ICU stay. Thus, death could not be directly linked to S. maltophilia but was rather classified under hospital mortality. Although this study was conducted in two separate critical care units of a single university hospital in Lebanon, our treatment management, further predisposing risk factors, and antibiotic susceptibility for S. maltophilia isolates may differ from those in other hospitals. Therefore, our data may not be extrapolated to sites located in other geographic areas. The main limitation of the study remains the fact that it was conducted in a single center. All studied patients were admitted to Hotel Dieu de France hospital’s ICU which may cause a geographic bias. Second, the small number of cases of SM pneumonia encountered prevented the application of multivariate analysis for risk factors and outcomes. Finally, this study was conducted from March 2020 until March 2021, while the vaccine was still not available in Lebanon. All patients included were not yet vaccinated. These results should be confirmed in a vaccinated population.
Conclusion
S. maltophilia nosocomial pneumonia is a worrisome and frequent complication among patients with severe COVID-19 admitted to the ICU and carries significant morbidity and mortality. Identifying patients at high risk for S. maltophilia nosocomial infection is paramount, as it allows the early recognition and prompt treatment of this infection. The judicious use of antibiotics is crucial, and TMP-SMZ remains the gold standard antimicrobial therapy.
Acknowledgements
This work would not have been possible without the unwavering support of our visionary and humanistic administration, namely the company of Jesus, and all the medical and nursing staff. This policy was at the service of humans and for humans in the image of our noblest principles. Authors would like also to thank Science PRO sarl for conducting critical review and editing of the article.
Author contributions
Conceptualization: M.Ra., M.Ri.; Investigation: M.Ra., M.A.H., R.I., R.R., K.M., M.Ri.; Methodology Resources: M.Ra., M.A.H., R.I., R.R., G.S.; Validation: M.Ri.; Original draft: M.Ra., M.Ri.; Writing—review and editing: M.Ra., M.A.H., R.I., R.R., J.A.J., C.H., E.A., G.S., B.H., G.S., K.M., R.S., M.Ri.; Data curation: G.S.; Formal analysis: G.S.; Software: G.S.; Visualization: G.S.; Project administration: M.Ri.; Supervision: M.Ri.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: An emerging opportunist human pathogen. Lancet Infect. Dis. 2009;9:312–323. doi: 10.1016/S1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 2.AZUREA Research Network et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: A nationwide retrospective study. Crit. Care. 2019;23:371. doi: 10.1186/s13054-019-2649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke JS. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nseir S, et al. Intensive care unit-acquired Stenotrophomonas maltophilia: Incidence, risk factors, and outcome. Crit. Care. 2006;10:R143. doi: 10.1186/cc5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 1998;11:57–80. doi: 10.1128/CMR.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CH, Yu C-M, Hsu S-T, Wu R-X. Levofloxacin-resistant Stenotrophomonas maltophilia: Risk factors and antibiotic susceptibility patterns in hospitalized patients. J. Hosp. Infect. 2020;104:46–52. doi: 10.1016/j.jhin.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Pickens CO, et al. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am. J. Respir. Crit. Care Med. 2021;204(8):921–932. doi: 10.1164/rccm.202106-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes M, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care. 2021;25(1):25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain, S., Khanna, P. & Sarkar, S. Comparative evaluation of ventilator-associated pneumonia in critically ill COVID-19 and patients infected with other corona viruses: a systematic review and meta-analysis. Monaldi Arch Chest Dis. Preprint at (2021). [DOI] [PubMed]
- 10.Rouyer M, et al. Ventilator-associated pneumonia in COVID-19 patients: A retrospective cohort study. Antibiot. Basel. 2021;10(8):988. doi: 10.3390/antibiotics10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Toro MD, et al. Clinical epidemiology of Stenotrophomonas maltophilia colonization and infection: A multicenter study. Medicine (Baltimore) 2002;81:228–239. doi: 10.1097/00005792-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hanes SD, et al. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002;35:228–235. doi: 10.1086/341022. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara M, et al. Stenotrophomonas maltophilia bacteremia associated with severe COVID-19: Successful treatment with appropriate antimicrobial therapy. Cureus. 2022 doi: 10.7759/cureus.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa K, Nakamura T, Kawai F, Uehara Y, Mori N. Stenotrophomonas maltophilia infection associated with COVID-19: A case series and literature review. Am. J. Case Rep. 2022;23:e936889–e936891. doi: 10.12659/AJCR.936889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Smith GB, et al. The national early warning score 2 (NEWS2) Clin. Med. Lond. Engl. 2019;19:260. doi: 10.7861/clinmedicine.19-3-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R, et al. Chest CT severity score: An imaging tool for assessing severe COVID-19. Radiol. Cardiothorac. Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mruk B, Plucińska D, Walecki J, Półtorak-Szymczak G, Sklinda K. Chest computed tomography (CT) severity scales in COVID-19 disease: A validation study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021;27:e931283-1–e931283-6. doi: 10.12659/MSM.931283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalil AC, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres A, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur. Respir. J. 2017;50:3. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 22.Klok FA, et al. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020;56:2001494. doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Common Terminology Criteria for Adverse Events (CTCAE). 147 (2017).
- 24.Flores-Treviño S, et al. Stenotrophomonas maltophilia biofilm: Its role in infectious diseases. Expert Rev. Anti Infect. Ther. 2019;17:877–893. doi: 10.1080/14787210.2019.1685875. [DOI] [PubMed] [Google Scholar]
- 25.Döring G, Hoiby N, Consensus Study Group Early intervention and prevention of lung disease in cystic fibrosis: A European consensus. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2004;3:67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Gr G, Jl B. Factors affecting the incidence of Stenotrophomonas maltophilia isolation in cystic fibrosis. Chest. 2002;121:1754–1760. doi: 10.1378/chest.121.6.1754. [DOI] [PubMed] [Google Scholar]
- 27.Tseng C-C, et al. Risk factors for mortality in patients with nosocomial Stenotrophomonas maltophilia pneumonia. Infect. Control Hosp. Epidemiol. 2009;30:1193–1202. doi: 10.1086/648455. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, et al. Risk factors for predicting mortality of COVID-19 patients: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0243124. doi: 10.1371/journal.pone.0243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graff GR, Burns JL. Factors affecting the incidence of Stenotrophomonas maltophilia isolation in cystic fibrosis. Chest. 2002;121:1754–1760. doi: 10.1378/chest.121.6.1754. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Córdova A, et al. Molecular epidemiology, antibiotic resistance, and virulence traits of stenotrophomonas maltophilia strains associated with an outbreak in a Mexican Tertiary Care Hospital. Front. Cell. Infect. Microbiol. 2020;10:50. doi: 10.3389/fcimb.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saugel B, et al. Stenotrophomonas maltophilia in the respiratory tract of medical intensive care unit patients. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2012;31:1419–1428. doi: 10.1007/s10096-011-1459-8. [DOI] [PubMed] [Google Scholar]
- 32.Baz EM, Mahfouz RA, Tabarani RS, Ayoub TH, Zaatari GS. Lebanese population: Prevalence of the erythrocyte phenotypes. J. Med. Liban. 2001;49:140–142. [PubMed] [Google Scholar]
- 33.Kerbage A, et al. Impact of ABO and Rhesus blood groups on COVID-19 susceptibility and severity: A case-control study. J. Med. Virol. 2022;94:1162–1166. doi: 10.1002/jmv.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dührsen U, Hollwitz B, Kaulfers P-M, Hossfeld DK. Fatal pulmonary hemorrhage in patients with acute leukemia and fulminant pneumonia caused by Stenotrophomonas maltophilia. Ann. Hematol. 1997;74:155–161. doi: 10.1007/s002770050275. [DOI] [PubMed] [Google Scholar]
- 35.Tada K, et al. Stenotrophomonas maltophilia infection in hematopoietic SCT recipients: High mortality due to pulmonary hemorrhage. Bone Marrow Transplant. 2013;48:74–79. doi: 10.1038/bmt.2012.87. [DOI] [PubMed] [Google Scholar]
- 36.Mori M, et al. Life-threatening hemorrhagic pneumonia caused by Stenotrophomonas maltophilia in the treatment of hematologic diseases. Ann. Hematol. 2014;93:901–911. doi: 10.1007/s00277-014-2028-x. [DOI] [PubMed] [Google Scholar]
- 37.Imoto W, et al. Clinical characteristics of rapidly progressive fatal hemorrhagic pneumonia caused by Stenotrophomonas maltophilia. Intern. Med. Tokyo Jpn. 2020;59:193–198. doi: 10.2169/internalmedicine.3358-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zantah M, Dominguez Castillo E, Townsend R, Dikengil F, Criner GJ. Pneumothorax in COVID-19 disease-incidence and clinical characteristics. Respir. Res. 2020;21:236. doi: 10.1186/s12931-020-01504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVID-19 and Pneumothorax: A Multicentre Retrospective Case Series | European Respiratory Society. https://erj.ersjournals.com/content/early/2020/09/03/13993003.02697-2020 (2021). [DOI] [PMC free article] [PubMed]
- 40.Ibn Saied W, et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: Risk factors and outcome. J. Infect. 2020;80:279–285. doi: 10.1016/j.jinf.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Biagi M, et al. Activity of potential alternative treatment agents for Stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or trimethoprim-sulfamethoxazole. J. Clin. Microbiol. 2020;58:e01603–e1619. doi: 10.1128/JCM.01603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang Y-T, Lin C-Y, Chen Y-H, Hsueh P-R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 2015;6:893. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wunderink RG, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021;21:213–225. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 44.Gideskog M, Welander J, Melhus Å. Cluster of S. maltophilia among patients with respiratory tract infections at an intensive care unit. Infect. Prev. Pract. 2020;2:100097. doi: 10.1016/j.infpip.2020.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waite TD, Georgiou A, Abrishami M, Beck CR. Pseudo-outbreaks of Stenotrophomonas maltophilia on an intensive care unit in England. J. Hosp. Infect. 2016;92:392–396. doi: 10.1016/j.jhin.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Apisarnthanarak A, et al. Stenotrophomonas maltophilia intestinal colonization in hospitalized oncology patients with diarrhea. Clin. Infect. Dis. 2003;37:1131–1135. doi: 10.1086/378297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.