Abstract
An extensive computer search (from January 2020 to January 2023) was conducted including literature from the PubMed, Scopus, MEDLINE, Web of Science, and EMBASE databases. According to preset criteria, a total of 58 articles were included in this review article. Generally, any patient who becomes infected with COVID-19 can develop post-COVID-19 conditions. The course of COVID-19 is divided into three main stages: acute COVID-19 (up to 4 weeks), post-acute COVID-19 (from 4 to 12 weeks), and post-COVID (from 12 weeks to 6 months). If a more protracted course of COVID (over 6 months) is demonstrated, the term “long-COVID” is used. Although the acute stage of COVID-19 infection most commonly manifests with acute respiratory symptoms, one very common symptom of the disease is pain, while the most common symptoms of post-COVID syndrome are shortness of breath, dry cough, fatigue, loss of olfactory and gustatory function, tightness and chest pain, sleep and mood disturbances, body aches, muscle and joint pain, sore throat, fever, and persistent headaches. All observations demonstrated a high incidence of chronic pain syndromes of various localization in the post- and long-COVID period. Post-COVID chronic pain might include a newly developed chronic pain as a part of post-viral syndrome; worsening of preexisting chronic pain due to the associated changes in the medical services, or a de novo chronic pain in healthy individuals who are not infected with COVID. Chronic pain during and post-COVID-19 pandemic is an important health issue due to the significant impacts of pain on the patients, health care systems, and society as well. Therefore, it is important that patients with chronic pain receive effective treatment according to their specific needs. Accordingly, the main goal of this review article is to provide a broad description about the post-COVID pain and to explore the impact of long COVID-19 on chronic pain patients, and also to give brief reports about the prevalence, risk factors, possible mechanisms, different presentations, and the management tools through a systematic approach.
Keywords: COVID-19, Long COVID, Post-COVID pain, Post-COVID pain syndromes, Post-COVID chronic pain, Post-COVID neuropathic pain, Post-COVID musculoskeletal pain, Post-COVID headache, Telemedicine
Key Summary Points
| Why carry out this study? |
| Post-COVID-19 pain is prevalent and can develop into more challenging and persistent pain. Accordingly, the main objectives of this review are: |
| To give a brief report about the challenges facing the chronic pain management during post-COVID-19. |
| To describe the prevalence, risk factors, and possible mechanisms of chronic pain conditions associated with long COVID-19. |
| To focus on the strategies to overcome the limitations in healthcare delivery and providing the appropriate management for chronic pain patients. |
| To explore the practical tips for the management of post-COVID chronic pain. |
| What was learned from the study? |
| The post-COVID era represents a great challenge to the health care services and has changed our approaches to medicine. |
| All observations demonstrated a high incidence of chronic pain syndromes of various localization in the post- and long-COVID period. |
| COVID-19 is having a profound effect on patients with pain. Delaying, or stopping, treatment will have negative consequences on chronic pain patients. |
| Evidence is promising that new tools such as telemedicine and mobile opioid treatment programs can help to provide ongoing services to chronic pain patients. |
Introduction
Health care systems worldwide are facing extraordinary challenges since the COVID-19 pandemic. Globally, with the end of 2022 and the beginning of a new year, the COVID-19 epidemiological update showed that there have been 657,977,736 confirmed cases of COVID-19, including 6,681,433 deaths globally. This number should be taken with caution, as many countries have changed the practice of routine COVID-19 testing, resulting in underestimations of the actual numbers [1].
The COVID-19 pandemic has changed our approaches to medicine and created a whole new generation of people who have chronic pain. Many pending answers on COVID-19 and its sequelae remain unclear and will remain a challenge for the foreseeable future [2, 3]. The COVID-19 pandemic has drawn attention to the weaknesses of health systems around the world [4].
A significant proportion of patients with COVID-19 experienced long-term and persistent symptoms. Published reports indicate that approximately 10–20% of COVID-19 patients experience persistent long COVID symptoms from a few weeks to a few months following acute infection [5]. This syndrome is characterized by a wide range of health problems including “brain fog” with cognitive disturbances, fatigue, dyspnea, myalgia and muscle weakness, depression, and persistent headaches [6]. Furthermore, a recent comprehensive systematic review and meta-analysis estimated the prevalence of long COVID, and showed that 45% of COVID-19 survivors were experiencing a wide range of unresolved symptoms for at least 4 months after a confirmed COVID-19 infection [7].
Chronic pain is an important health issue and is the most common reason to seek medical care. It ranks among the ten most prevalent diseases worldwide and years lost to disability. For this reason, chronic pain should be properly managed to avoid further complications [8]. COVID-19 is having a profound effect on patients with chronic pain. Delaying or stopping treatment for chronic pain patients will have negative consequences, including increases in pain, disability, and depression. The management of chronic pain during the COVID-19 pandemic is a challenging process, especially with growing evidence that COVID-19 infection is associated with persistent myalgias, referred pain, and widespread hyperalgesia [9].
Methods
An extensive computer search was conducted including literature from the PubMed, Scopus, MEDLINE, Web of Science, and EMBASE databases. Manual screening of references was also conducted, and additional references were added from sites for pain organizations, e.g., International Association for the Study of Pain (IASP) and the World Health Organization (WHO). Relevant guidelines from the American Society of Anesthesiologists (ASA), American Society of Regional Anesthesia (ASRA), American Society of Interventional Pain Physicians, and American Academy of Physical Medicine and Rehabilitation, European Pain Federations, and The WHO database on COVID-19 were screened for relevant publications. The search strategy was restricted to articles that were published between January 2020 and January 2023. The following related keywords were used for the search (“COVID-19”, “coronavirus and SARS-CoV-2”, “post-COVID pain”, “post-COVID pain syndromes”, “post-COVID headache”, “post-COVID chronic pain” “post-COVID neuropathic pain” and “post-COVID musculoskeletal pain”). Articles that met the inclusion criteria, such as articles relevant to the condition and presented information on the post-COVID pain conditions, articles published in English language and involving adult humans were included. The search included observational study, cross-sectional study, cohort study, case–control study, longitudinal study, systematic reviews, and meta-analysis. The exclusion criteria included non-English-language articles, failure to get the full articles, post-COVID pain in children, case report, editorials, or expert opinions. The selected articles for inclusion were screened by two independent reviewers using the same method of evaluation. The final reviewing strategy of the literature search results in a total of 58 articles in this review (Fig. 1) [10]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Fig. 1.
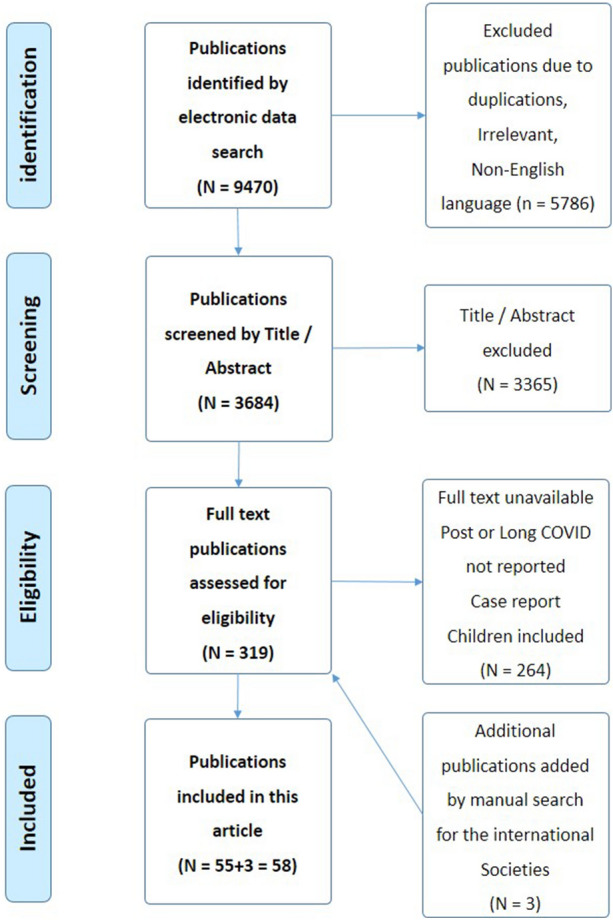
Flow chart of inclusion of studies (PRISMA, 2009) [10]
Definitions
Various definitions have been developed to define different stages of COVID-19 based on the durations and clinical presentations. Standardized definitions are important for the proper diagnosis and management of those patients. The following definitions can be used to differentiate different stages of both ongoing or post-COVID-19 signs and symptoms [1, 11, 12].
Acute COVID-19 infection: Signs and symptoms of COVID-19 for up to 4 weeks [1].
Ongoing symptomatic COVID-19: Signs and symptoms of COVID-19 from 4 weeks up to 12 weeks [1].
Post-COVID-19 syndrome: Signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks to 6 months and are not explained by an alternative diagnosis. It usually presents with clusters of symptoms, often overlapping, which can fluctuate and change over time and can affect any system in the body. Post-COVID-19 syndrome may be considered before 12 weeks while the possibility of an alternative underlying disease is also being assessed [1, 11].
- Long COVID: there are different definitions with more or less similar meanings.
-
oAccording to the National Institute for Health and Care Excellence (NICE) guidelines, long COVID is commonly used to describe signs and symptoms that continue or develop after acute infection consistent with COVID-19 and persist longer than 4 weeks. It includes both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more). If a more protracted course of COVID (over 6 months) is discussed, the term “long-COVID” is used [11, 12].
-
oCenters for Disease Control and Prevention (CDC, 2021): “Wide range of new, returning, or ongoing health problems people can experience 4 or more weeks after first being infected with the virus that causes COVID-19” [13].
-
oWorld Health Organization (WHO, 2021): “Illness that occurs in people who have a history of probable or confirmed SARS-CoV-2 infection, usually within 3 months from the onset of COVID-19, with symptoms and effect that last for at least 2 months, that cannot be explained by an alternative diagnosis” [1].
- o
-
o
Post-COVID-19 condition is defined as the illness that occurs in individuals with a history of probable or confirmed SARS CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction, but also others, and generally have an impact on everyday functioning. Symptoms may be new-onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time [13].
Post-COVID headache: The International Classification of Headache disorders uses a headache duration of more than 3 months after the acute infection for the diagnosis of “Chronic headache attributed to systemic viral infection” [15].
Chronic pain: chronic pain is defined from the International Association for the Study of Pain (IASP) as persistent or recurrent pain lasting more than 3 months or beyond the normal tissue healing [16].
Nociplastic pain: the IASP defines nociplastic pain that ‘arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain’ [17].
Musculoskeletal pain: The Pain Task Force of the (IASP), defines Chronic Primary Musculoskeletal Pain (CPMP) as “chronic pain in the muscles, bones, joints, or tendons that is characterized by significant emotional distress (i.e., anxiety, anger, frustration, and depressed mood) or functional disability” [9, 18].
Challenges Facing Post-COVID-19 Chronic Pain Management
Chronic pain patients may experience additional potential risk of functional and emotional deterioration during a pandemic, which can increase the long-term health burden [19, 20].
The ongoing and long COVID-19 pandemic are associated with new problems affecting chronic pain management. Difficult access to health care facilities, a lack of resources, burdened health care services, mental health problems, and a patient’s associated comorbidities may add more burden to the chronic pain patients [9, 21]. All of these factors contribute to making the delivery of effective pain management more challenging.
- Problems related to the pandemic: [19, 20, 22].
-
oLockdown, travel restrictions, social and physical distances, and isolation.
-
oFear of infection or the health care facilities get infected.
-
oDecrease the risk of exposure of the health care workers to severe infection overtly burdened health care system.
-
oAll elective consultations and interventions are cancelled or postponed.
-
oInterrupted care due to isolations and closing many services such as physiotherapy & supportive services.
-
o
- Problems related to the overstretched health care systems: [9, 23]
-
oIncreased demand on the health care systems, health care workers, and facilities.
-
oThe overuse of imaging as a result of the pandemic and its sequel. Around 69% of general practitioners would refer patients for radiography at first presentation, despite routine use discouraged due to a poor relationship of imaging findings with symptoms.
-
oThe rate of some surgical procedures, e.g., orthopedic and spine surgeries, has increased markedly in recent years.
-
o
- Patient-related factors: [18, 24, 25]
-
oFailure to provide education or advice.
-
oLimited access to the health care facilities.
-
oDifficulty to get refill of pain medications, especially for controlled medications and opioids.
-
oSignificant number of patients are elderly with many comorbidities and multiple medications.
-
oThey are more susceptible to morbidity and mortality from COVID-19.
-
oPossible immune suppression, fatigue, weakness, and associated comorbidities.
-
o
- Mental health problems: [24, 25].
-
oOnset of new or exacerbation of mental health concerns, including anxiety, stress, depression, and post-traumatic stress disorder, have become significant concerns.
-
oLess access to treatment facilities due to isolation, social distancing, and fear of infection, lifting opioid tolerant patients struggling with addiction.
-
oThis interruption has had serious consequences, as it has led to an increase in chronic pain, psychological worsening, and decrease in the quality of life.
-
o
- Interaction between treatment of chronic pain and COVID-19 pandemic: [16, 26]
-
oPainkillers such as NSAIDs and paracetamol may mask the symptoms of COVID-19 infection, e.g., fever and myalgias.
-
oPain medications may interact with the immune system or mask the signs or symptoms of COVID-19 infection.
-
oChronic opioid therapy with high doses may induce immunosuppression.
-
oOral or injectable steroids (e.g., used for interventional pain procedures) are immunosuppressive.
-
oSteroid injections for pain managements may affect the efficacy of COVID-19 vaccines.
-
o
Prevalence of Post-COVID-19 Pain
Approximately 10–20% of acute infection with COVID-19 patients go on to develop prolonged symptoms that may be post-COVID-19 condition [1]. In the United States, there are more than 80 million patients and survivors of COVID-19, which is the highest number in the world [27]. A recent comprehensive systematic review and meta-analysis estimated the prevalence of long COVID, regardless of hospitalization status. A total of 194 studies including 735,006 participants worldwide were included in the analysis. Results showed that 45% of COVID-19 survivors experienced a wide range of unresolved symptoms for at least 4 months after COVID-19 infection [7].
Pain can be an early symptom of acute COVID-19 infection, including sore throat, myalgias, back pain, and headache [28]. It seems that no relationship exists between the initial severity of COVID-19 infection and the likelihood of developing post-COVID-19 conditions(5). Chronic pain might affect up to 50% of the general population, while the prevalence of post-COVID-19 chronic pain was estimated to be 63.3% [29].
Patients with chronic pain infected with COVID-19 are at higher risk for exacerbation of their symptoms, and this is attributed to many factors including social threats, discontinuation of therapy, reduced access to treatments, or associated mental health problems and concerns about health outcomes [25, 30, 31]. COVID-19 may exacerbate preexisting pain or be associated with the appearance of new pain. Another study compared two groups of patients, one group admitted to the hospital due to COVID-19 infection and the other group admitted due to other causes. Results showed that COVID-19 infection was associated with a significantly higher prevalence of de novo chronic pain, chronic daily headache, and new-onset pain in general, which was associated with persistent anosmia [32].
Chronic pain has a positive relationship to viral infection, psychological stress, and consequences of admission to the hospital or intensive care unit (ICU). Post-COVID-19 chronic pain may include either regional or widespread pain [33, 34]. It often causes peripheral or central neurological complications, either through direct invasion of the nervous system or through immune reactions (35, 36).
Prevalence of chronic pain according to the demographics: A cross-sectional study showed that more than three out of five COVID-19 survivors experience chronic pain. Increasing age and female sex correlated with the presence of chronic pain in this population [37].
Prevalence of chronic pain according to the site of pain: COVID-19 pain was more frequently located in the head/neck and lower limbs (p < 0.05), followed by joint pain. New-onset fatigue was more common in COVID-19 survivors necessitating inpatient hospital care. The presence of insomnia in COVID-19 patients correlates with the presence of more new-onset pain (83.3%) compared to those who did not (48.0%, p = 0.024) [32, 38].
Prevalence of chronic pain according to the pathophysiological type of pain: Post-COVID chronic pain exhibits both musculoskeletal and neuropathic pain features. The discrimination between nociceptive, neuropathic, and nociplastic pain represents a current challenge for clinicians [9]. The prevalence of neuropathic pain was estimated to be 24.4% [29]. Preliminary evidence suggests the presence of neuropathic pain in individuals exhibiting post-COVID pain. The neuropathic pain symptoms was positively associated with the duration of post-COVID pain, anxiety levels, and kinesiophobia level. It was found that almost 25% of previously hospitalized COVID-19 survivors with “de novo” post-COVID pain reported a neuropathic pain component [30, 31].
Prevalence in non-hospitalized patients: Few reports that included long-term follow-up in non-admitted patients suggest that (31–53%) still have one or several persistent painful symptoms 1 year after COVID-19 infection, which would translate to a significant number of people worldwide [21, 39, 40]. A recent meta-analysis has revealed that more than 60% of patients exhibited at least one post-COVID-19 symptom. The most prevalent post-COVID-19 symptoms experienced by both hospitalized and non-hospitalized patients were fatigue and dyspnea were. The other symptoms including headache, anosmia, chest pain, or joint pain was lower and more variable [41]. In non-hospitalized patients, the most frequent symptoms were fatigue (34.8), breathlessness (20.4%), muscle pain/myalgia (17.0%), impaired sleep (15.3%), and loss of sense of smell (12.7%) [7].
Prevalence in hospitalized patients: The reported prevalence of musculoskeletal pain post-COVID-19 in previously hospitalized patients ranged from (11–45%) at 6 months or more after discharge [42]. Patients with post-COVID musculoskeletal pain showed a greater number of COVID-19 symptoms at hospital admission, with a greater prevalence of myalgia and headache, longer stay of hospitalization, and higher incidence of ICU admission than those not reporting long-term musculoskeletal post-COVID pain [43]. In hospitalized patients, the five most prevalent symptoms reported were fatigue (28.4%), pain/discomfort (27.9%), impaired sleep (23.5%), breathlessness (22.6%), and impaired usual activity (22.3%) [7].
Risk Factors for Post-COVID-19 Chronic Pain
General risk factors: it is clear that patients with chronic pain infected with COVID-19 sometimes experience exacerbation of their symptoms, which may be due to multiple factors including social threats, discontinuation of therapy, reduced access to treatments, or associated mental health problems and concerns about health outcomes [30, 31].
-
Risk factors in (non-hospitalized) COVID-19 patients: COVID-19 itself is associated with painful symptoms, including myalgia, arthralgia, abdominal pain, headache, and chest pain, and even those not admitted to critical care environments may have pain requiring opioids for symptom management [21, 44]. Post-COVID headache was relatively higher in patients managed in an outpatient setting [45]. These persistent symptoms, which can change over time, confirm that post-COVID-19 chronic pain has a multi-systemic involvement even after mild infection in healthy younger individuals. It has been reported that the risk factors for persistent symptoms 12 months after COVID-19 infection include lower physical fitness, low physical activity, obesity (body mass index > 25 kg/m2), associated co-morbidities (particularly hypertension and chronic pain), and having more than seven of the general COVID-19 symptoms at the onset [44, 45].
It is commonly understood that long-term symptoms can occur regardless of acute infection severity. However, acute phase severity, hospitalization, greater age, female sex, high body mass index (BMI), and any chronic diseases are factors associated with post-COVID-19 [37, 46]. Several features such as social distancing and isolation at home in addition to the mental health specific problems such as depression, anxiety, post-traumatic stress disorder (PTSD), and cognitive impairment, have well-recognized with chronic pain [25].
Risk factors in (hospitalized) COVID-19 patients: risk factors for the development of persistent and chronic pain post-COVID-19 in hospitalized patients and their mechanisms have been identified. In addition to the general risk factors such as being elderly, having a high body mass index (BMI), and associated comorbidities, potential risk factors for chronic pain include pre-existing painful conditions, acute pain, length of hospital stay, immobility, illness severity such as length of stays in ICU, and number of days on mechanical ventilation, neuromuscular blockade, repeating proning, and neurological insult [35, 47, 48]. Patient weakness may contribute to rapid deconditioning and joint-related pain. Other risk factors include social isolation during hospital admission and post discharge. Pandemic-specific psychological and mental health burden [49–52].
Risk factors due to ICU sitting: unfortunately, pain has received low priority, poor assessment, and management for patients admitted to the ICU during the pandemic. This is attributed to the associated heavy workload by the exhausted health workers [21, 41]. COVID-19 patients are likely to have sustained a prolonged period of ICU admission with immobilization, sedation, and mechanical ventilation. The ICU management protocols add additional risk factors such as the use of neuromuscular block, corticosteroids and the risks of procedural pain such as intubation, tracheostomy, suction, cannulations, sampling, and catheterization. The presence of sepsis, neuro-immune response to infection, painful neurological sequelae, e.g., stroke and multi-organ dysfunction, may worsen the situation. This sitting in the ICU puts patients at high risk of muscle weakness, joint stiffness, myopathy, polyneuropathy, and muscle atrophy. Complications associated with proning sedated patients include brachial plexopathy, joint subluxation, and soft tissue damage. These have the potential to result in persistent neuropathic and musculoskeletal pain after ICU discharge. Patient weakness may contribute to rapid deconditioning and joint-related pain, which may help to explain why chronic shoulder pain has been particularly prevalent in patients who were seen in the ICU for coronavirus treatment [53, 54].
Possible Pathophysiology and Mechanisms
Possible Etiology of Post-COVID Chronic Pain
Post-COVID chronic pain can be associated with any type of pain; it can be nociplastic, neuropathic, or nociceptive. Possible causes may include genetic factors, previous pain experience, and traumatic events that could be physical or emotional [55]. Post-COVID chronic pain might include: a newly developed chronic pain which is a part of post-viral syndrome due to organ damage; exacerbation of preexisting chronic pain due to the abrupt changes, limited access to medical services and the associated mental health problems; or newly developed chronic pain in healthy individuals who are not infected with COVID due to associated risk factors (e.g., poor sleep, inactivity, fear of infection, anxiety, and depression) [30]. Chronic pain conditions can be triggered by psychosocial stressors or organ-specific biological factors. Post-COVID-19 pandemic has many characteristics that could potentially increase the prevalence of chronic pain, especially with stressors extending over many months [25, 30, 55].
Possible Mechanisms/Pathophysiology of Long COVID Pain
The exact mechanisms causing post-COVID pain remain unclear. Recent findings indicated that there were four pathophysiological categories involved: virus-specific pathophysiological variations, oxidative stress, immunologic abnormalities, and inflammatory damage [56–60]. However, the following proposed mechanisms may be responsible for post-COVID pain:
The virus may directly attack multiple tissue types including nerves, the spinal cord, and brain with the associated encephalopathy and structural changes [33, 34].
Another proposed mechanism was the direct viral entry of cells of the musculoskeletal and nervous systems mediated by angiotensin-converting enzyme 2 (ACE2) receptor [42, 61, 62].
The association of persistent symptoms such as fatigue, diffuse myalgia, and joint and musculoskeletal pain are all linked to mitochondrial dysfunction, oxidative stress, and reduced antioxidants [56].
A phenomenon of protracted immunosuppression, known as PICS (persistent inflammation, immunosuppression, and catabolism syndrome), has been presented as a potential major contributing factor for the presentation of post-COVID symptoms [63].
The inflammatory cascades may over-activate and attack the body’s tissues and organs.
The neurotrophism of COVID-19 infection could cause neurodegenerative problems with an inflammatory base [56, 57, 61].
Bradykinins contribute to pro-inflammatory state and also sensitize the sensitive fibers, leading to hyperalgesia [56, 57, 61, 64].
The excessive blood clotting triggered by the virus may lead to symptoms such as phantom limb pain [56, 57].
The potential contribution of psychosocial factors and mental health problems [25, 65].
Characteristic Symptoms of Post-COVID-19 Syndrome
The initial symptoms of acute COVID-19 infection are mainly fever, dry cough or dyspnea, although pain has also been an early symptom such as sore throat, myalgia, low back pain, and headache [24, 28]. Post-COVID chronic pain is the result of the interaction of biological, psychological, and social factors. Clinical studies showed that at least 50% of patients who have been infected with and survived COVID-19 will continue to suffer from symptoms for 6 months or longer [66]. A recent comprehensive systematic review and meta-analysis estimated the prevalence of long COVID, regardless of hospitalization status, and showed that the ten most frequent symptoms are fatigue/weakness, breathlessness, impaired usual activities, taste, smell, depression, muscle pain/myalgia, joint pain, affected sleep, and gastrointestinal symptoms [7]. It appears from the previous publications that post-COVID pain symptoms are fixed and presented (≥ 50%) among the top ten post-COVID-19 symptoms.
Post-COVID-19 Painful Conditions
Acute pain associated with viral infection is common in the early stages of acute COVID-19. The situation is worsened due to additional procedural pain, lack of resources, and overstretched health care services making low priority for symptomatic management of pain [21], while long COVID-19 is associated with an increased number of chronic pain patients either due to worsening of preexisting chronic pain or appearance of new painful conditions. The most common symptoms of people suffering from long COVID-19 painful conditions include generalized body pain, headache, muscle and joint tenderness, and pain due to increased levels of physical or mental stress with painful levels of anxiety or depression [21, 67].
Headache
Headache is one of the most common symptoms during infection, and post-COVID. It has been shown to be a potential long-term problem as a part of the long COVID syndrome [9]. According to The International Classification of Headache Disorders, a headache duration longer than 3 months following the acute infection is used for the diagnosis of “Chronic headache attributed to systemic viral infection” [27, 68, 69].
In a meta-analysis that evaluated 35 studies, accounting for 28,348 COVID-19 survivors, the prevalence of post-COVID headache was higher in patients that were managed in an outpatient setting during the acute phase [45]. It affects between 14 and 60% of patients during the acute COVID-19 phase [70, 71]. Persistent headache in patients with long COVID has a prevalence of 18%, is more prevalent in middle-aged women, and began 2 weeks after the subsiding of respiratory symptoms [27, 69].
Headache is one of the most disabling symptoms of long COVID and may manifest alone or in combination with other symptoms such as muscle weakness, dizziness, and vertigo as well as insomnia or other sleep impairments that may occur with long COVID-19 [67].
Post-COVID headache can present in the form of worsening of a preexisting primary headache or de novo daily headache. Headache may be manifested with a migraine or more frequently, with a tension-type-like phenotype. The intensity of headache ranged between moderate and severe headache and involves the upper part of the head [27].
The pathogenesis of persistent headache may be attributed to cytokine storm with persistent activation of the immune system as demonstrated by the evidence of altered blood levels of cytokines and interleukins. Persistent glial activation and trigeminal-vascular activation are thought to play a role [72, 73].
Effective treatment of post-COVID headache should take into consideration the type of headache (migrainous vs. tension-type-like), comorbidities, and if present, additional post-COVID-19 symptoms such as insomnia, mood disorders, and cognitive difficulties [15, 74]. There are many proposed modalities for the treatment of long-term headaches associated with COVID-19 [24, 35, 60, 75].
Non-pharmacological treatment for post-COVID-19 headache includes patient education with recommendations for lifestyle changes, physical therapy, psychological therapy, and the management of pre-existing comorbidities [62, 76].
Pharmacological treatment in the form of prophylactic treatment for tension-type headache and this includes the tricyclic antidepressant amitriptyline is considered the drug of choice, followed by venlafaxine or mirtazapine [72].
Treatment guidelines recommend simple analgesics (e.g., paracetamol) and non-steroidal anti-inflammatory drugs (NSAIDs) as the first choice for acute treatment, followed by combination preparations that include caffeine. Triptans have been considered as acute therapeutic options [72, 74]. Some data report benefits of glucocorticoids for the treatment of long COVID headache, in terms of reduction of headache frequency and symptom intensity [77, 78].
Neuropathic Pain
COVID-19 often causes peripheral or central neurological complications and induces post-viral immune syndrome. Accordingly, it is anticipated that a considerable number of the chronic pain complications of COVID-19 will be neuropathic in character [79]. The most common peripheral lesions responsible for neuropathic pain include acute or chronic polyneuropathy, Guillain–Barre’ syndrome, chronic inflammatory demyelinating polyneuropathy, or ganglionopathy, while, central nervous system lesions responsible for neuropathic pain include transverse myelitis, encephalomyelitis, and stroke [80].
A recent meta-analysis estimated that the frequency of post-COVID neuropathic pain ranged between 0.4 and 25% [81]. Another study reported the prevalence of “de novo” post-COVID neuropathic pain in almost 25% of previously hospitalized COVID-19 survivors. The presence of neuropathic pain was associated with more anxiety, kinesiophobia, and the duration of post-COVID pain [82].
There is preliminary evidence supporting that neuropathic pain at early post-COVID can be associated with serum levels of neurofilament light chain (NFL) as a potential biomarker [83], while secondary analysis found no association between serological biomarkers at the acute phase of COVID-19 and the development of long COVID neuropathic pain symptoms at 6 months and 1 year after infection [84, 85].
The high expression of angiotensin-converting enzyme-2 (ACE2) receptors within nervous system cells such as neurons and microglia of the spinal cord could explain the neuro-invasive potential of the COVID-19-associated neuropathic symptoms [86].
Neuropathic pain as a complication of COVID-19 is difficult to treat. The mainstay of treatment is represented by gabapentoids, antidepressants, tramadol, and topical agents (lidocaine plasters, capsaicin patches or botulinum toxin). Strong opioids may be considered in refractory cases. Non-pharmacological treatments include invasive or noninvasive neuro-stimulation techniques [87, 88].
Musculoskeletal Pain
All types of pain may occur after COVID-19, such as nociceptive, neuropathic, and nociplastic pain—especially in critical care survivors [37]. Nociceptive pain is more prevalent than neuropathic pain. Musculoskeletal pains have been noticed to be a prominent complaint among COVID-19 patients (30%) and other musculoskeletal complaints have been described in 15–36% of cases [89–91]. The prevalence of musculoskeletal pain syndromes among post-COVID-19 patients was also reported in a meta-analysis that included over 25,000 patients (outpatients and previously hospitalized patients) at 4 weeks, and persistent musculoskeletal symptoms were present, including myalgia in 5.7%, arthralgia in 4.6%, and chest pain in 7.9% of patients. The prevalence of post-COVID musculoskeletal pain increased at 60 days, but decreased later on after 180 days [42, 67, 92].
Post-COVID musculoskeletal pain includes a higher prevalence of a generalized widespread pain as well as localized pain syndromes such as cervical pain and lower extremity pain, followed by lumbar spine and upper extremities. A higher prevalence of musculoskeletal pain was also reported in non-hospitalized patients than hospitalized patients [61, 93]. After COVID-19 infection, there are four patterns of musculoskeletal involvement, including myalgia 37.5%, arthralgia 5.7%, new-onset backache 6.8%, and generalized body ache 50%. Musculoskeletal pain may occur three different ways: first, de novo musculoskeletal pain following COVID-19; second, exacerbation of preexisting musculoskeletal pain after COVID-19 infection; third, increasing musculoskeletal pain in non-infected individuals as a result of COVID-19-associated factors, e.g., lockdown, isolation, unreachable medical services [94].
Chest Pain
Persistent chest pain is one of the most common symptoms among patients with long COVID-19. A huge number of patients were seeking medical advice because of chest pain [95]. Chest pain persists in 12–22% of patients for few months after acute COVID-19 infection [96–98]. The prevalence of chest pain in non-hospitalized patients was 14.7% compared to 9.1% in hospitalized patients 99 (104). Patients with long COVID-19 present with a wide range of symptoms, ranging from mild to severe chest pain and tenderness. In some patients, it may be so severe that it significantly impairs the ability to perform everyday activities. In post-COVID patients, detailed history-taking and investigations, including blood testing, CT scan, and MRI, were essentially needed to differentiate between cardiac and pulmonary sources of chest pain [96–98].
Following COVID-19 infection, chest pain may be due to underlying cardiac causes such as myocardial injury, coronary artery disease, or myocarditis [100]. Coronary micro-vascular ischemia could be the mechanism of persistent chest pain in patients that have recovered from COVID-19 [101]. Those patients require cardiac referral, proper evaluation, and urgent interventions in other cases [100].
Patients who present with post-COVID persistent chest pain should be thoroughly investigated for pulmonary emboli. It has also been proposed as a potential mechanism for post-COVID chest pain, particularly when accompanied by shortness of breath [102]. Mechanisms of micro-vascular disease in COVID-19 include endothelial injury with endothelial dysfunction and micro-vascular inflammation, and thrombosis [103, 104].
Mild cases of chest pain may resolve following recovery from acute COVID-19 and not requiring further treatment. For persistent chest pain, a short course of non-steroidal anti-inflammatory drugs or paracetamol may be required. Chest tightness and bronchospasm can be treated by inhaled bronchodilators.
Myalgia
Muscle pain is one of the most common complaints during both the acute stage and post COVID-19. It has been reported in 21–62.5% of the patients according to different meta-analysis studies [67, 105, 106]. The prevalence of myalgia was higher in hospitalized patients (22.7%) compared to in non-hospitalized patients (16.8%). Sex differences were not consistent among different reports. Some studies showed a higher prevalence of both myalgia and arthralgia in males compared to females [12], while a significant number of studies showed the opposite [107, 108]. Myalgia was commonly experienced at the acute phase and persists as a component of long COVID in some patients [61, 109]. In addition to the widespread viral-induced myalgias, the most common areas for myalgia are the lower leg, arm, and shoulder girdle [43]. Weakness of the lower limbs has also been reported as suggestive of a motor peripheral neuropathy in post-COVID-19 infection [110, 111].
Myositis is muscle inflammation caused by metabolic abnormalities, which may be triggered by COVID-19 infection. Painful myositis numbers are escalating in long-COVID-19. The affected patients complain of muscle pain, tenderness, fatigue, and weakness [43, 67, 110, 111].
Arthralgia
Arthralgia is pain in one or more of a person’s joints. COVID-19 is considered as a current trigger in some patients. Viral arthritis is the inflammation of the joints caused by a viral infection. The most common regional areas for arthralgia are the knee joint, ankle joint, and shoulder joint [12]. Although arthralgia is less common compared to myalgia, which is more commonly described, arthralgia is associated with more severe pain [9, 67, 89]. COVID-19- associated viral arthralgia was a novel clinical entity that did not appear to be typical of a viral prodromal or of a reactive arthropathy, and had distinct characteristics from the other musculoskeletal presentations of COVID-19 [89, 90].
Fibromyalgia
Fibromyalgia consists of widespread pain and tenderness on palpation at well-defined locations on the neck, trunk, and extremities. Characteristics that occur in more than 75% of fibromyalgia patients include muscle tenderness, chronic fatigue, stiffness, headaches, and sleep disturbance. Fibromyalgia has been suggested to be related to deficient immune regulatory mechanisms and this indicates a prolonged immune system impact in patients with long-COVID-19 [67, 112]. There is an association between chronic pain comorbidities and psychiatric disorders with fibromyalgia [113].
Fatigue
Chronic fatigue syndrome is a medical condition that lasts at least 6 months or more. This syndrome may impair a person’s ability to perform daily activities and is associated with sleep disorders. Fatigue is one of the most major symptoms associated with COVID-19 infection [114]. It may resolve after the acute phase of COVID-19. However, fatigue and weakness can persist for a few months or longer, particularly among ICU patients. Fatigue is most commonly prevalent among women of middle age and older patients [115]. Long COVID-19 syndrome with the associated psychological and immune stresses may affect the underlying nervous system negatively, leading to worsening symptoms in persons with chronic fatigue syndrome, myofascial pain, and fibromyalgia [67, 92, 115].
How COVID-19 Changed the Management of Chronic Pain
The unprecedented pandemic has created a new “face” of chronic pain post COVID. It leads to rapid and significant changes in the management of chronic pain and the medical practice in general. The COVID-19 pandemic not only had negative effects on medical health systems but also make changes and created new services in the medical practices.
The “COVID lifestyle” created what is called “the lockdown lifestyle”. People stopped exercising, getting fresh air and sunshine, and socializing, which led to anxiety, depression, isolation, and fearfulness. Vitamin D deficiency is pretty widespread and was made worse during the lockdowns. All of these things exacerbate chronic pain.
More emphasis on program-directed self-management, rehabilitation, and physical therapy.
Increased awareness by the pandemic, methods of infection control for the general populations.
Increased awareness of health care providers by the infection control, use of PPE.
Triaging of the patients according to the urgency of the medical condition, severity of pain, and the infectious status.
The development of telemedicine, eHealth, app-based solutions, and remote care.
Rapid growth of telemedicine and eHealth for effective communications, evaluation, assessment, as well as management of the chronic pain.
New methods for drug prescription, refill of medications and delivery of controlled medications such as “mobile opioid clinics”.
Warning the health care services by the weaknesses and deficiencies during the hard times such as the pandemic and how to prioritize the services according to the available resources.
Development of new clinical practice guidelines for the diagnosis, management, medical and interventional pain therapy.
Expansion of the pain procedures that exclude steroids due to their immune-suppressant effects such as radiofrequency ablations, regenerative injections (e.g., platelets-rich plasma “PRP”, bone marrow extracts and stem cells injections). Also, the injections of high volumes with lower concentrations of local anesthetics only without steroids.
Proper utilization of the opioids depending on those with the lowest immune-suppressant effects.
Telemedicine
Telemedicine, or eHealth, has emerged as a unique technology to facilitate efficient communication to provide essential health care services during the pandemic. It is the most immediate way to enable physicians to continue treatment of patients. The role of telemedicine has declined after the pandemic but is still used by some health institutes for selected patients [9, 116].
The International Association for the Study of Pain (IASP) recommended the rapid introduction of eHealth services for chronic pain patients during the COVID-19 pandemic [3].
Several forms of eHealth services have been rapidly promoted during this crisis, with differing levels of effectiveness [116]. Also, the Medical Council of India along with National Institution for Transforming India (NITI Aayog) released “Telemedicine Practice Guidelines” enabling registered medical practitioners to provide healthcare using telemedicine [22].
The long-term benefits of telemedicine have been evaluated after 1 year post-COVID. The study evaluated the impact of a completely digital program in patients with chronic musculoskeletal pain. Results showed that participants included in the program reported significantly higher improvements in pain and function in comparison to the control group of non-starters at 1-year follow-up [117].
Modalities of telemedicine: different modalities of telemedicine have been introduced including virtual visits via video, phone, or chat, as well as remote patient monitoring and technology-enabled modalities such as using smartphone apps to manage disease [22, 118].
Benefits
Telemedicine plays an important role in consulting physicians and health care providers without unnecessary exposure [9, 16].
Telemedicine can decrease the risk of exposure to COVID-19 for both chronic pain patients as well as HCWs “health care workers” [9, 16].
Improved access to care even for patients living in areas remote from the clinic through saving the resources and reducing costs at all levels by minimizing the use of PPE, transportation, and traveling [16, 22].
It facilitates the communications with those coming from long distances, physically unfit patients with multiple comorbidities, or already-infected patients [22, 117].
Some non-pharmacological and physical tools such as patient’s educations, psychological support, medical instructions, exercises, and posture or lifestyle changes can be easily implemented through telemedicine [22, 117].
Telemedicine can ease the workload on the already-burdened health care system and HCWs [16, 116].
Scope of the Services by Telemedicine
To triage the cases according to the urgency of the medical condition [9, 16].
To triage the cases according to the risk of infection [9, 16].
To evaluate patients, assess pain, and plan treatment of chronic pain [30].
To prescribe and refill pain medications including opioids [60].
To resolve patient concern and offer patient’s education [16, 22].
To assess and treat emotional distress of chronic pain patients [22, 117].
Limitations
No updated clinical practice guidelines to accommodate the rapid changes of the health care services in response to the pandemic [16].
The medico-legal issues for the use of telemedicine such as description of controlled medications, refill of opioids and identification of the patient or caregiver, as well as obtaining consent [22, 117, 118].
Not suitable in some areas, such as rural areas and developing countries with restricted facilities [9, 30].
Telemedicine is not suitable for patients with advanced diseases or low level in using technology [9, 30].
Telemedicine does not replace clinical practice and the need of face-to-face consultations and patient’s examination, especially for new patients, rapid changes of the patient’s condition, or those with associated multiple comorbidities [22, 60, 117].
Telemedicine is potentially less accurate in evaluation of the patient’s condition compared to the conventional in-person visit [16, 22].
Future Plan of Telemedicine
The use of telemedicine may be declining after the pandemic, with a return to ‘normal life and improved access to care even for patients living in areas remote from the clinic. However, the pandemic time has created a new window for the introduction of such new services to reduce the risk of exposure and facilitate easy communications after the pandemic [16, 60].
Changing the practice from face-to-face consultations to telemedicine or mixed services needs more comprehensive work and evidence before replacing the current practices [22, 117].
Telemedicine technology is a promising tool of communications when used in selected patients under certain conditions, such as post-COVID-19 pandemic [116, 117].
This newly introduced communication technology needs comprehensive program-directed education and training for both the HCWs and the patients to develop the competences needed to engage with digital tools [116, 117].
Long-term effects, comparison with face-to-face visits, implementations in normal situations after the pandemics and patient’s satisfaction all still lacking evidence and need further evaluation [117].
Program-directed training for self-management, rehabilitation, and physical therapy should be created and available via video tutorials and applications for smartphones [116–118].
An updated pain assessment tools including simple pain scales, neuropathic pain scales, and the Pain Catastrophizing Scale (PCS) should be developed and validated to be implemented for the virtual consultation setting [116, 117].
Build new hybrid, integrated models for chronic pain management to ensure that patients receive the right care at the right time in the best format to meet their clinical needs.
The financial costs for both systems should be compared and addressed thoroughly [18, 116].
Mobile Opioid Treatment Program
Mobile opioid treatment programs are designed to make the treatment of patients with opioid use disorder as easy and accessible as possible, even for the marginalized, who lack reliable transportation, live in chaotic situations, rural communities, and hard-to-reach populations [119]. For decades, mobile methadone clinics have used vans or other vehicles to bring methadone maintenance programs into the community. They may offer the opioid agonists methadone or buprenorphine treatment [120].
The mobile narcotic program uses technology, such as smartphone apps or online resources, and may allow mobile patients to benefit from counseling as well. The programs have policies and procedures to store, transport, deliver, account for, reconcile, and dispose of opioid waste and would be subject to audit. A mobile opioid program is an important service of particular value to underserved communities [120]. This program can be updated and used in hard times such as the pandemics to make treatment available and beneficial for such people during COVID as well as post-COVID era.
Practical Tips for the Management of Post-COVID Chronic Pain
Management of post-COVID chronic pain should be directed to involve post-COVID pain syndromes, persistent pain and discomfort, pain-associated treatment, intermittent procedural pain and tenderness from multiple types of pain conditions, as well as preexisting chronic pain issues [67, 121].
General Management
Continuous monitoring and evaluations are essential for every patient before the management of post-COVID chronic pain and should be performed regularly [7, 16].
Patients who are recovering from COVID-19 require proper assessment to determine the most vulnerable group and investigate the most suitable treatment for such patients [7, 18].
The presence of psychiatric conditions, mental health problems, and occupational and social situations should be taken into consideration during the management of post-COVID pain [25].
Mild-to-moderate pain associated with post-COVID symptoms can be relieved with simple analgesics such as acetaminophen and NSAIDs [9, 16].
Patients with moderate-to-severe pain, opioids with minimal immune-suppression effects (e.g., buprenorphine, tramadol, or oxycodone) are recommended. For neuropathic pain symptoms, gabapentoids are suitable options [9, 121].
Infection-Control Guidelines Should be Followed
The infection-control precautions according to the WHO recommendations should be followed (5).
When patient visits are required, patients and their caregivers should be screened for symptoms of COVID-19, according to available screening tools and practice [7].
Patient’s triaging according to the risk of COVID-19 infection with social distancing and isolations should be applied when required [16, 121].
Personal protection measures such as hand hygiene, face mask, and gloves during patient care, and cleaning of surfaces in the patient care environment should be taken according to the local regulations by healthcare authorities [16, 121].
Pain Management and COVID-19
Medications that reduce post-COVID-19 syndrome: A warning by a European agency that NSAIDs can mask the symptoms and signs of COVID-19 infection, and this may delay the diagnosis of the disease [7, 56].
Medications and immune system: Medications used to relieve pain can depress the immune system. Some opioids may cause immunosuppression while corticosteroids may induce secondary adrenal failure in addition to the immunosuppressant effect [24, 60, 75].
- Corticosteroids:
- o
- o
-
oIn immune-compromised patients, epidural injection with the lowest dose of steroids or without steroids should be considered.
- o
-
oRandomized controlled trials (RCT) have shown that epidural steroid injection doses exceeding 40 mg methylprednisolone, 20 mg triamcinolone, and 10 mg dexamethasone provide no recognizable pain relief difference compared to lower doses. No additional benefits for doses greater than 10 mg triamcinolone or 4 mg dexamethasone were observed [122, 123].
-
oThere is a strong correlation between the epidural volume and pain relief irrespective of the steroid dose [124].
-
oThere are publications reporting that radiofrequency denervation is a safe practice in the treatment of interventional pain during the pandemic [125].
- o
- Opioids:
- o
- o
- o
- o
-
oOpioids decrease the natural killer cells, a dose-dependent effect, and interfere in the cellular response by acting directly on the hypothalamic–pituitary–adrenal axis (producing corticosteroids) or in the sympathetic system (producing adrenaline). Both act on lymphocytes by negatively modulating the response of natural killer cells.
-
oVarious opioids differ in their effects on the immune system, with morphine and fentanyl having the greatest immunosuppressive action [126].
- o
-
oDose escalation and before increasing the dose, it is important to differentiate between disease progression from other opioid drawbacks, e.g., tolerance and hyperalgesia.
-
oSpecial precautions for the transdermal opioids formula, the elevated temperature associated with COVID-19, may increase absorption from transdermal patches and could increase opioid side effects [9].
Interactions Between Opioids and Antiviral Treatment
Interactions between opioids and antiviral treatments may interfere with the treatment outcomes through different mechanisms, e.g.,
Decreased metabolic pathways: Anti-viral medications, e.g., lopinavir/ritonavir inhibiting CYP3A4, and this may inhibit the metabolic pathway of some opioids (e.g., oxycodone) resulting in increased plasma levels, with possible increasing the risk of overdose and respiratory depression [126, 128, 129].
Increased metabolic pathway: the concomitant use of lopinavir/ritonavir with methadone may significantly decrease the plasma levels of methadone, possibly due to an induction of methadone metabolic clearance, involving either or both (CP450 3A and CYP450 2D6) [129, 130].
Enzyme inducers: Induction of other enzymes, such as intestinal glycoprotein P‐450, could also contribute to decreases in drug levels, with possible precipitation of withdrawal symptoms [130].
Medications not affected by the antiviral medications: Morphine, buprenorphine, and tapentadol are not dependent on CYP450 enzymatic activity and can be used safely with antiviral therapy [130, 131].
Pain Medications and COVID-19 Vaccine
Glucocorticoid injections for pain procedures and musculoskeletal pain may interfere with the potency and efficiency of COVID-19 vaccines. They are generally accepted at 1 week before and after COVID-19 vaccine administration, considering the duration of action, during COVID-19 vaccine administration [26, 75].
Pain Interventions
- Triage the pain procedures: [7, 9, 16]
-
oElective: Patient normally could wait more than 4 weeks and no significant harm is anticipated with postponement of the procedure.
-
oSemi-urgent: Where a delay of the procedure for more than a few weeks could potentially lead to worsening of the patient’s condition.
-
oUrgent: These procedures are time-sensitive; a delay in proceeding would result in significant exacerbation and worsening of the condition.
-
o
Perform urgent procedures with the minimal number of personnel, to minimize the risk of exposures.
It is recommended to avoid deep sedation that requires airway support or manipulation.
Procedures should be limited to urgent cases.
The procedure should be conducted in a negative pressure room.
Physicians should be adequately protected and PPE is highly considered.
After the procedure, the patient should be monitored in the same room
Then, they can be transferred to an appropriate isolation area.
Hospital Visit Versus Telemedicine
Patient’s triaging the according to the type and severity of pain may be helpful in differentiating those who may be adequately treated by telemedicine from those who need face-to-face consultations [7, 11, 19, 41].
Stable opioid-tolerant patients have permitted opioid prescriptions via telemedicine to reduce the risk of withdrawal [11, 16].
Patients at risk of opioid withdrawal should be scheduled for an in-patient visit [16, 19].
Patients with severe exacerbation of chronic pain: a short-term electronic prescription after evaluation via telemedicine is reasonable. Then arrange for a visit to the pain clinic [22, 41, 60].
Patients need opioids for longer durations: an inpatient visit is recommended to identify patients who might be candidates for opioids or other interventions [7, 41].
For implantable intrathecal pumps, an in-patient or clinic appointment is required for refill of opioids [11, 16].
Future Directions
Continuation of pain management protocols is highly recommended to avoid the negative impacts on the patients with more suffering, disability, and psychological stresses. Several researches are focused on prevention and treatment interventions for post-COVID-19 syndrome. The following examples are based on exercise, antioxidant supplements, and other pharmacological approaches. An exercise-based rehabilitation program showed change of maximum oxygen uptake [56], while hyperbaric oxygen treatment patients will be subjected to 100% oxygen by mask for 90 min with 5-min air. It showed improvements in memory, attention, and information process with post-COVID-19 symptom. Clinical findings assessed the role of vitamin D2 and vitamin D3 supplementation and showed significantly reduced risk of COVID-19 infection and death within 30 days. Therefore, the researchers believe vitamin D3 supplementation could be a valuable strategy for limiting the spread of COVID-19 infection and related death and racial differences in COVID-19 outcomes [132].
For specific post-COVID symptoms, a low-dose of naltrexone and NAD “nicotinamide adenine dinucleotide” is used for one group of patients compared to a corresponding placebo tablet and patch for 12 weeks. Results showed improvements of fatigue, well-being, and quality of life [133]. Another technique by using transcutaneous vagus nerve stimulation “TVNS” in the treatment of long COVID chronic fatigue syndrome. The study results suggested that non-invasive stimulation of the auricular branch of the vagus nerve is a possible therapeutic modality for treating long COVID with at least a third of the patients showing improvement, although it is possible that the positive result was simply a placebo response to treatment in the absence of a control group for comparison [134]. There are many trials with the main goals to optimize the patient’s symptoms, improve the function, and enhance the quality of life. Furthermore, any successful treatment protocol should include a clear plan based on the patient’s symptoms, underlying cause, and associated comorbidities. Physical fitness, rehabilitation programs, and mental health care should be taken into considerations when needed. Continuity of treatment with regular follow-up is essential for post-COVID chronic pain [9, 122].
Summary and Conclusions
The COVID-19 pandemic has had unforeseen impacts on the health care services. It has changed our lives and our approach to medicine. COVID-19 is also having a profound effect on chronic pain patients. A significant number of patients infected with COVED-19 developed post- or long COVID-19 symptoms with more burden on patients with chronic pain. Post-COVID-19 is associated with worsening of previous pain or appearance of de novo pain. Delaying or stopping treatment for patients who are suffering from severe pain will have negative consequences, including increases in pain, disability, and depression.
The management of chronic pain associated with long COVID seems easier compared to that during COVID pandemic with less barriers or restrictions and moving to near-normal life. The post-COVID era is characterized by increased awareness of the infection-control guidelines. Availability of screening tests as well as different vaccinations with millions of people became vaccinated. The use of new technology such as telemedicine showed great advances, more orientation, specifically oriented tools for the assessment and management of chronic pain, as well as published guidelines for the use of telemedicine in pain management. A huge number of publications covering all aspects are now available. Many evidence-based guidelines by different international pain societies with a clear plan for the management of different types of chronic pain were created. However, more research is needed to understand the actual problem of post-COVID pain, the possible pathophysiological mechanisms, and the target-directed prevention and management of post-COVID chronic pain.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author’s Contributions
Salah N. El-Tallawy (Corresponding Author): concept and design, writing, searching, supervision for all steps. Joseph V. Perglozzi: design, editing, revision of final draft. Rania S. Ahmed: searching, study screening, editing. Abdullah M. Kaki: revision of final draft, editing. Mohamed S. Nagiub: searching, study screening, editing. JoAnn K LeQuang: design, editing, revision of final draft. Mamdouh M.M. Haddarah: revision of the final draft.
Disclosure
All authors declare no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Salah N. El-Tallawy, Email: salaheltallawy@yahoo.com
Joseph V. Perglozzi, Email: jpergolizzi@adminnemaresearchcom.onmicrosoft.com
Rania S. Ahmed, Email: raniasalah246890@gmail.com
Abdullah M. Kaki, Email: amkaki@yahoo.com
Mohamed S. Nagiub, Email: hamadasalah13579@gmail.com
JoAnn K. LeQuang, Email: joannlequang@gmail.com
Mamdouh M. Hadarah, Email: hadmaa@icloud.com
References
- 1.World Health Organization: COVID-19 Weekly epidemiological update on COVID-19 - 4 January 2023. Edition 124. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---4-january-2023
- 2.Muller JE, Nathan DG. COVID-19, nuclear war, and global warming: lessons for our vulnerable world. Lancet. 2020;395(10242):1967–1968. doi: 10.1016/S0140-6736(20)31379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton A, Olliaro P, Sigfrid L, Carson G, Hastie C, Kaushic C, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis. 2021;21(5):601–602. doi: 10.1016/S1473-3099(21)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger Z, Evans N, Phelan A, Silverman R. COVID-19: control measures must be equitable and inclusive. BMJ. 2020 doi: 10.1136/bmj.m1141. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization World Health Statistics, COVID-19. Geneva 2021. https://www.who.int/data/gho/publications/world-health-statistics. Accessed 31 Aug 2021.
- 6.Martelletti P, Bentivegna E, Spuntarelli V, Luciani M. Long-COVID headache. SN Compr. Clin Med. 2021;3(8):1704–1706. doi: 10.1007/s42399-021-00964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of Long COVID among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. Clin Med. 2023;55:101762. doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global, regional, and national disability-adjusted life years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–922. [DOI] [PMC free article] [PubMed]
- 9.El-Tallawy SN, Nalamasu R, Pergolizzi JV, Gharibo C. Pain management during the COVID-19 pandemic. Pain Ther. 2020;9:453–466. doi: 10.1007/s40122-020-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence, Practitioners RC of G, Scotland HI. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guidel; 2020:1–35. Available from: https://www.nice.org.uk/guidance/ng188/resources/COVID19-rapid-guideline-managing-thelongterm-effects-of-COVID19-pdf-51035515742. Accessed Jun 9, 2022. [PubMed]
- 12.Khoja O, Passadouro BS, Mulvey M, Delis I, Astill S, Tan AL, Sivan M. Clinical characteristics and mechanisms of musculoskeletal pain in long COVID. J Pain Res. 2022;15:1729–1748. doi: 10.2147/JPR.S365026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emergency use ICD codes for COVID-19 disease outbreak. Found in: International definitions of Diseases 11th Revision ICD-11 (who.int) https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-COVID-19-disease-outbreak.
- 14.NHS England and NHS Improvement website information on Long COVID. https://www.england.nhs.uk/coronavirus/post-COVID-syndrome-long-COVID/
- 15.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1–211 [DOI] [PubMed]
- 16.Puntillo F, Giglio M, Brienza N, Viswanath O, Urits I, Kaye AD, Pergolizzi J, Paladini A, Varrassi G. Impact of COVID-19 pandemic on chronic pain management: looking for the best way to deliver care. Best Pract Res Clin Anaesthesiol. 2020;34:529–537. doi: 10.1016/j.bpa.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosek E, Cohen M, Baron R, et al. Do we need a third mechanistic descriptor for chronic pain states. Pain. 2016;157:1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 18.Marinangeli F, Giarratano A, Petrini F. Chronic pain and COVID-19: pathophysiological, clinical and organizational issues. Minerva Anestesiol. 2021;87:828–832. doi: 10.23736/S0375-9393.20.15029-6. [DOI] [PubMed] [Google Scholar]
- 19.Pan American Health Organization. Framework for the Implementation of a Telemedicine Service. Washington DC, PAHO 2016. Available at: https://iris.paho.org/bitstream/handle/10665.2/28414/9789275119037_eng.pdf?sequence=6&isllowed=y
- 20.Wadehra S. COVID long haulers and the new chronic pain profile. Pract Pain Manag. 2022;22(1).
- 21.Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesthesia. 2020;125(4):436–449. doi: 10.1016/j.bja.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghai B, Malhotra N, Bajwa SJ. Telemedicine for chronic pain management during COVID-19 pandemic. Indian J Anaesth. 2020;64:456–462. doi: 10.4103/ija.IJA_652_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin I, Wiles L, Waller R, Goucke R, Nagree Y, Gibberd M, Straker L, Maher C, O’Sulliva P. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: systematic review. Br J Sports Med. 2020;54:79–86. doi: 10.1136/bjsports-2018-099878. [DOI] [PubMed] [Google Scholar]
- 24.Pascarella G, Strumia A, Piliego C, Bruno F, del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Tallawy SN, Titi MA, Ejaz AA, Abdulmomen A, Elmorshedy H, Aldammas F, Baaj J, Alharbi M, Alqatari A. Prevalence and risk factors associated with mental health symptoms among anesthetists in Saudi Arabia during the COVID-19 pandemic. Int J Ment Health. 2022;51(4):448–469. doi: 10.1080/00207411.2022.2035905. [DOI] [Google Scholar]
- 26.Hong SM, Park YW, Choi EJ. Steroid injections in pain management: influence on coronavirus disease 2019 vaccines. Korean J Pain. 2022;35(1):14–21. doi: 10.3344/kjp.2022.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tana C, Bentivegna E, Cho SJ, et al. Long COVID headache. J Headache Pain. 2022;23:93. doi: 10.1186/s10194-022-01450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song XJ, Xiong DL, Wang ZY, et al. Pain management during the COVID-19 pandemic in China: lessons learned. Pain Med. 2020;21(7):1319–1323. doi: 10.1093/pm/pnaa143.pnaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zis P, Ioannou C, Artemiadis A, Christodoulou K, Kalampokini S, Hadjigeorgiou GM. Prevalence and determinants of chronic pain post-COVID; cross-sectional study. J Clin Med. 2022;11:5569. doi: 10.3390/jcm11195569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161:1694–1697. doi: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karos K, McParland JL, Bunzli S, Devan H, Hirsh A, Kapos FP, Keogh E, Moore D, Tracy LM, Ashton-James CE. The social threats of COVID-19 for people with chronic pain. Pain. 2020;161:2229–2235. doi: 10.1097/j.pain.0000000000002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares FHC, Kubota GT, Fernandes AM, et al. Prevalence and characteristics of new-onset pain in COVID-19 survivors, a controlled study. Eur J Pain. 2021;25:1342–1354. doi: 10.1002/ejp.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp HI, Laycock H, Costello A, Brett SJ. Chronic pain in critical care survivors: a narrative review. Br J Anaesthesia. 2019;123(2):e372–e384. doi: 10.1016/j.bja.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zis P, Loannou C, Artemiadis A, Christodoulou K, Kalampokini S, Hadjigeorgiou GM. Prevalence and determinants of chronic pain post-COVID; Cross-sectional study. J Clin Med. 2022;11:5569. doi: 10.3390/jcm11195569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntillo KA, Max A, Chaize M, Chanques G, Azoulay E. Patient recollection of ICU procedural pain and post ICU burden: the memory study. Crit Care Med. 2016;44:1988–1995. doi: 10.1097/CCM.0000000000001875. [DOI] [PubMed] [Google Scholar]
- 41.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-de-las-Peñas C, Navarro-Santana M, Plaza-Manzano G, Palacios-Ceña, Arendt-Nielsen L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta-analysis. Pain. 2022;163:1220–1231. doi: 10.1097/j.pain.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 43.Fernándezdelas-Peñas C, de-la-Llave-Rincóna A, Ortega-Santiagoa R, et al. Prevalence and risk factors of musculoskeletal pain symptoms as long-term post-COVID sequelae in hospitalized COVID-19 survivors: a multicenter study. Pain. 2022;163:e989–e996. doi: 10.1097/j.pain.0000000000002564. [DOI] [PubMed] [Google Scholar]
- 44.Lovell N, Maddocks M, Etkind SN, et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage. 2020;60(1):E77–E81. doi: 10.1016/j.jpainsymman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-de-Las-Penas C, Navarro-Santana M, Gomez-Mayordomo V, Cuadrado ML, Garcia-Azorin D, Arendt-Nielsen L, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a metaanalysis of the current literature. Eur J Neurol. 2021;28(11):3820–3825. doi: 10.1111/ene.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kisiela MA, Janols H, Nordqvist T, Bergquist J, Hagfeldt S, Malinovschi A, Svartengren M. Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Upsala J Med Sci. 2022;127:e8794. doi: 10.48101/ujms.v127.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth. 2020;125(4):440–443. doi: 10.1016/j.bja.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JK, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021;10:181–209. doi: 10.1007/s40122-021-00235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gustafson OD, Rowland MJ, Watkinson PJ, McKechnie S, Igo S. Shoulder impairment following critical illness: a prospective cohort study. Crit Care Med. 2018;46(11):1769–1774. doi: 10.1097/CCM.0000000000003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, Reid TR. A review of persistent post-COVID syndrome (PPCS) Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arca KN, Starling AJ. Treatment-refractory headache in the setting of COVID-19 pneumonia: migraine or meningoencephalitis? Case report. SN Comprehensive Clin Med. 2020;2(8):1200–1203. doi: 10.1007/s42399-020-00369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goettler CE, Pryor JP, Reilly PM. Brachial plexopathy after prone positioning. Crit Care. 2002;6:540–542. doi: 10.1186/cc1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fletcher SN, Kennedy DD, Ghosh IR, et al. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- 55.Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76:2–11. doi: 10.1097/PSY.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierce JD, Shen Q, Cintron SA, Hiebert JP. Post-COVID-19 syndrome. Nurs Res. 2022;71(2):164–174. doi: 10.1097/NNR.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 57.Wadehra S. COVID long haulers and the new chronic pain profile. Pract Pain Manag. 2022;22(1).
- 58.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly-Davies G. Why COVID infections leave some patients in chronic pain. Pain News Network. 2021.
- 60.Martín MTF, Solórzano EO. COVID-19 and pain: what we know so far. Multidisciplinary Pain J. 2021;1:36–44. [Google Scholar]
- 61.Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. 2022;41(1):289–296. doi: 10.1007/s10067-021-05942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, Reid TR. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. 2021:1–9. [DOI] [PMC free article] [PubMed]
- 64.McFarland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Report. 2021;6:e885. doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandez-de-Las-Penas C, Rodriguez-Jimenez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by SARS-CoV-2 infection is associated to persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain. 2021 doi: 10.1097/j.pain.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 66.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fricton J. COVID-19 long-haulers trigger an increase in pain management needs. Available in: https://mhnpc.com/2021/05/18/COVID-triggers-increased-pain-management-needs/
- 68.Headache Classification Committee of the International Headache Society The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 69.Mutiawati E, Kusuma HI, Fahriani M, Harapan H, Syahrul S, Musadir N. Headache in post-COVID-19 patients: its characteristics and relationship with the quality of life. Medicina. 2022;58:1500. doi: 10.3390/medicina58101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martelletti P, Bentivegna E, Luciani M, Spuntarelli V. Headache as a prognostic factor for COVID-19. Time to re-evaluate. SN Compr Clin Med. 2020;2(12):2509–2510. doi: 10.1007/s42399-020-00657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pullen MF, Skipper CP, Hullsiek KH, Bangdiwala AS, Pastick KA, Okafor EC, Lofgren SM, Rajasingham R, Engen NW, Galdys A, Williams DA, Abassi M, Boulware DR. Symptoms of COVID-19 outpatients in the United States. Open Forum Infect Dis. 2020;7(7):ofaa271. doi: 10.1093/ofid/ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caronna E, Ballve A, Llaurado A, Gallardo VJ, Ariton DM, Lallana S, Lopez Maza S, Olive Gadea M, Quibus L, Restrepo JL, Rodrigo-Gisbert M, Vilaseca A, Hernandez Gonzalez M, Martinez Gallo M, Alpuente A, Torres-Ferrus M, Pujol Borrell R, Alvarez-Sabin J, Pozo-Rosich P. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trigo J, Garcia-Azorin D, Planchuelo-Gomez A, Martinez-Pias E, Talavera B, Hernandez-Perez I, Valle-Penacoba G, Simon-Campo P, de Lera M, Chavarria-Miranda A, Lopez-Sanz C, Gutierrez-Sanchez M, Martinez- Velasco E, Pedraza M, Sierra A, Gomez-Vicente B, Arenillas JF, Guerrero AL. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21(1):94. doi: 10.1186/s10194-020-01165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caronna E, Pozo-Rosich P. Headache as a symptom of COVID-19: narrative review of 1-year research. Curr Pain Headache Rep. 2021;25(11):73. doi: 10.1007/s11916-021-00987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ooi EE, Dhar A, Petruschke R, et al. Use of analgesics/antipyretics in the management of symptoms associated with COVID-19 vaccination. NPJ Vaccines. 2022;7:31. doi: 10.1038/s41541-022-00453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, Haroon S, Price G, Davies EH, Nirantharakumar K, Sapey E, Calvert MJ, TLC Study Group Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prakash S, Shah ND. Post-infectious new daily persistent headache may respond to intravenous methylprednisolone. J Headache Pain. 2010;11(1):59–66. doi: 10.1007/s10194-009-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dono F, Consoli S, Evangelista G, D’Apolito M, Russo M, Carrarini C, et al. New daily persistent headache after SARS-CoV-2 infection: a report of two cases. Neurol Sci. 2021;42(10):3965–3968. doi: 10.1007/s10072-021-05444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Attala N, Martineza V, Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Report. 2021;9(6):e884. doi: 10.1097/PR9.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez Y, Vatti N, Ramírez-Santana C, Chang C, Mancera-Páez O, Gershwin ME, Anaya JM. Chronic inflammatory demyelinating polyneuropathy as an autoimmune disease. J Autoimmun. 2019;102:8–37. doi: 10.1016/j.jaut.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 81.Stefano GD, Falco P, Galosi E, Di Pietro G, Leone C, Truini A. A systematic review and meta-analysis of neuropathic pain associated with coronavirus disease 2019. EJP. 2023;27(1):44–53. doi: 10.1002/ejp.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hruschak V, Flowers KM, Azizoddin DR, Jamison RN, Edwards RR, Schreiber KI. Cross-sectional study of psychosocial and pain-related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. Pain. 2021;162(2):619–629. doi: 10.1097/j.pain.0000000000002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bouhassira D, Chassany O, Gaillat J, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 2012;153:342–349. doi: 10.1016/j.pain.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 85.Ferreira ML, Albuquerque MFP, de Brito CAA, et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study. Lancet Neurol. 2020;19:826–839. doi: 10.1016/S1474-4422(20)30232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cherry CL, Wadley AL, Kamerman PR. Painful HIV-associated sensory neuropathy. Pain Manag. 2012;2:543–552. doi: 10.2217/pmt.12.67. [DOI] [PubMed] [Google Scholar]
- 87.Finnerup NB, Attal N, Haroutounian S, Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moisset X, Moisset X, Bouhassira D, Avez Couturier J, Alchaar H, Conradi S, Delmotte MH, Lanteri-Minet M, Lefaucheur JP, Mick G, Piano V, Pickering G, Piquet E, Regis C, Salvat E, Attal N. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev Neurol (Paris) 2020;176:325–352. doi: 10.1016/j.neurol.2020.01.361. [DOI] [PubMed] [Google Scholar]
- 89.Hoong CWS, Amin MNME, Tan TC, Lee JE. Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. Int J Infect Dis. 2021;104:363–9. http://creativecommons.org/licenses/by-ncnd/4.0/ [DOI] [PMC free article] [PubMed]
- 90.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li L, Huang T, Wang Y, Wang Z, Liang Y, Huang T, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azadvari M, Haghparast A, Nakhostin-Ansari A, EmamiRazavi SZ, Hosseini M. Musculoskeletal symptoms in patients with long COVID: a cross-sectional study on Iranian patients. Heliyon. 2022;8(8):e10148. doi: 10.1016/j.heliyon.2022.e10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bileviciute-ljungar I, Norrefalk J, Borg K. Pain burden in post-COVID-19 syndrome following mild COVID-19 infection. J Clin Med. 2022;11(3):771. doi: 10.3390/jcm11030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ballering AV, van Zon SKR, Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fiala K, Martens J, Abd-Elsayed A. Post-COVID Pain Syndromes. Curr Pain Headache Reports. 2022;26:379–383. doi: 10.1007/s11916-022-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carfì A, Bernabei R, Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;73(3):e826–e829. doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sardari A, Tabarsi P, Borhany H, et al. Myocarditis detected after COVID-19 recovery. Eur Heart J Cardiovasc Imaging. 2021;22:131. doi: 10.1093/ehjci/jeaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vallejo N, Teis A, Mateu L, Genís AB. Persistent chest pain after recovery of COVID-19: microvascular disease-related angina? Eur Heart J. 2021 doi: 10.1093/ehjcr/ytab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varatharaj A, Thomas N, Ellul MA, Davies NW, Pollak TA, Tenorio EL, Plant G. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lowenstein CJ, Solomon SD. Severe COVID-19 Is a microvascular disease. Circulation. 2020;142:1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Janssens KAM, Rosmalen JGM, Ormel J, van Oort FV, Oldehinkel AJ. Anxiety and depression are risk factors rather than consequences of functional somatic symptoms in a general population of adolescents: the TRAILS study. J Child Psychol Psychiatry. 2010;51:304–312. doi: 10.1111/j.1469-7610.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 106.Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: Lifelines, a three-generation [DOI] [PubMed]
- 107.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):1–22. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021 doi: 10.7759/cureus.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khoja O, Silva Passadouro B, Mulvey M, Delis I, Astill S, Tan AL, Sivan M. Clinical characteristics and mechanisms of musculoskeletal pain in long COVID. J Pain Res. 2022;17(15):1729–1748. doi: 10.2147/JPR.S365026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alonso-Matielo H, da Silva Oliveira VR, de Oliveira VT, Dale CS. Pain in COVID Era. Front Physiol. 2021;12:624154. doi: 10.3389/fphys.2021.624154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdelnour L, Eltahir Abdalla M, Babiker S. COVID-19 infection presenting as motor peripheral neuropathy. J Formos Med Assoc. 2020;119:1119–1120. doi: 10.1016/j.jfma.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryabkova VA, Churilov LP, Shoenfeld Y. Neuroimmunology: what role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int J Mol Sci. 2019;20:5164. doi: 10.3390/ijms20205164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lichtenstein A, Tiosano S, Amital H. The complexities of fibromyalgia and its comorbidities. Curr Opin Rheumatol. 2018;30:94–100. doi: 10.1097/BOR.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 114.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, O’Connor L, Leavy D, O’Brien K, Dowds J, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi: 10.1097/j.pain.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slattery BW, Haugh S, O’Connor L, Francis K, Dwyer CP, O'Higgins S, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res. 2019;21(7):e11086. doi: 10.2196/11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Areias AC, Costa F, Janela D, Molinos M, Moulder RG, Lains J, Scheer JK, Bento V, Yanamadala V, Correia FD. Long-term clinical outcomes of a remote digital musculoskeletal program: an ad hoc analysis from a longitudinal study with a non-participant comparison group. Healthcare. 2022;10:2349. doi: 10.3390/healthcare10122349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kathleen K. Telemedicine for pain management: where does it stand as we head into 2023? Practical Pain Management 2022; Oct 12, Vol 22, 6.
- 119.Saucier R. Lowering the threshold: models of accessible methadone and buprenorphine treatment. (2010). Accessed: May 24, 2021: https://www.opensocietyfoundations.org/publications/lowering-threshold.
- 120.Breve F, Batastini L, LeQuang JK, et al. Mobile narcotic treatment programs: on the road again? Cureus. 2022;14(3):e23221. doi: 10.7759/cureus.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mikkelsen ME, Abramoff B. COVID-19: evaluation and management of adults with persistent symptoms following acute illness ("Long COVID"). UpToDate Dec 2022; Topic 129312 Version 59.0. Available at: https://www.uptodate.com/contents/COVID-19-evaluation-and-management-of-adults-with-persistent-symptoms-following-acute-illness-long-COVID#disclaimerContent.
- 122.Lee JH, Kim DH, Kim DH, et al. Comparison of clinical efficacy of epidural injection with or without steroid in lumbosacral disc herniation: a systematic review and meta-analysis. Pain Phys. 2018;21(5):449468. [PubMed] [Google Scholar]
- 123.Van Boxem K, Rijsdijk M, Hans G, et al. Safe use of epidural corticosteroid injections: recommendations of the WIP Benelux Work Group. Pain Pract. 2019;19:61–92. doi: 10.1111/papr.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rabinovitch DL, Peliowski A, Furlan AD. Influence of lumbar epidural injection volume on pain relief for radicular leg pain and/or low back pain. Spine J. 2009;9:509–517. doi: 10.1016/j.spinee.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 125.Türkyilmaz GG, Rumeli S. Attitude changes toward chronic pain management of pain physicians in Turkey during the COVID-19 pandemic. Agri. 2022;34(2):77–83. doi: 10.14744/agri.2019.01878. [DOI] [PubMed] [Google Scholar]
- 126.Raff M, Belbachir A, El-Tallawy S, Ho KY, Nagtalon E, Salti A, Seo JH, Tantri AR, Wang H, Wang T, Buemio KC, Gutierrez C, Hadjiat Y. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Ther. 2019;8(1):19–39. doi: 10.1007/s40122-019-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Page GG. Immunologic effects of opioids in the presence or absence of pain. J Pain Symptom Manage. 2005;29:S25–31. doi: 10.1016/j.jpainsymman.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 128.Bianco GL, Papa A, Schatman MEA, et al. Practical advices for treating chronic pain in the time of COVID-19: a narrative review focusing on interventional techniques. J Clin Med. 2021;10:2303. doi: 10.3390/jcm10112303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nieminen TH, Hagelberg NM, Saari TI, et al. Oxycodone concentrations are greatly increased by the concomitant use of ritonavir or lopinavir/ritonavir. Eur J Clin Pharmacol. 2010;66:977–985. doi: 10.1007/s00228-010-0879-1. [DOI] [PubMed] [Google Scholar]
- 130.Gudin J. Opioid therapies and cytochrome P450 interactions. J Pain Symptom Manag. 2012;44:S4–S14. doi: 10.1016/j.jpainsymman.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 131.McCance-Katz EF, Rainey PM, Friedland G, Jatlow P. The protease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- 132.Gibbons JB, Norton EC, McCullough JS, et al. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci Rep. 2022;12:19397. doi: 10.1038/s41598-022-24053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Kelly B, Vidal L, McHugh T, Woo J, Avramovic G, Lambert JS. Safety and efficacy of low dose naltrexone in a long COVID cohort; an interventional pre-post study. Brain Behav Immun Health. 2022;24:100485. doi: 10.1016/j.bbih.2022.100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Natelson B, Blate M, Soto T. Transcutaneous vagus nerve stimulation in the treatment of long COVID chronic fatigue syndrome. medRxiv. 2022 doi: 10.1101/2022.11.08.22281807v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


