Abstract
Background
The impact of traction direction in traction-assisted gastric endoscopic submucosal dissection (ESD) has not been adequately investigated. A clip with line (CWL) is a classical single-directional traction device. In contrast, a spring and loop with clip (SLC; S–O clip) is a newly developed multidirectional traction device.
Aims
To investigate the impact of traction direction in gastric ESD by comparing the procedure-related outcomes of CWL-assisted ESD (CWL-ESD) and SLC-assisted ESD (SLC-ESD).
Methods
We retrospectively examined 140 patients with superficial gastric neoplasms who underwent SLC-ESD or CWL-ESD by a single ESD expert during November 2017–September 2020. The traction direction was classified based on the endoscopic finding in the following five categories: proximal, diagonally proximal, vertical, diagonally distal, and distal. In SLC-ESD, we set vertical traction, using the multidirectional traction function. Propensity score matching was conducted to compensate for the differences in lesion size, injection function of electrosurgical knife, ulcerative lesion, lesion location, and lesion position. The primary outcome was gastric ESD procedure time.
Results
Propensity score matching created 42 pairs. The median gastric ESD procedure time in the SLC-ESD group was significantly shorter than that in the CWL-ESD group (28.3 min vs. 51.0 min, P = 0.022). All traction direction in the SLC-ESD group was vertical, while only 16.7% in the CWL-ESD group. En bloc resection was attained without perforation in all the patients in both groups.
Conclusion
Our findings suggest that SLC can provide vertical traction, which reduces the gastric ESD procedure time.
Graphical Abstract
Multidirectional traction devices can provide vertical traction in most cases of gastric ESD, unlike single-directional traction devices. Vertical traction may reduce the gastric ESD procedure time.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-023-07870-z.
Keywords: Endoscopic submucosal dissection, Gastric ESD, Traction-assisted ESD, Clip with line, Spring and loop with clip, S–O clip
Introduction
Endoscopic submucosal dissection (ESD) is a minimally invasive therapy for superficial gastric neoplasms with little metastatic potential [1, 2], which facilitates en bloc resection of lesions that are hard to resect in en bloc by endoscopic mucosal resection (EMR), aiding precise pathological diagnosis and a lower risk of local recurrence [3]. Nevertheless, the gastric ESD procedure has a longer procedure time and higher perforation rate than EMR owing to technical complications, including a poor field of vision and inadequate tension for the submucosal dissection plane [4, 5]. To date, various traction methods have been developed to provide a good field of vision and adequate tension for the submucosal dissection plane, making the gastric ESD procedure easier [6–10]. However, the traction direction is limited in most traction methods, leading to an unsatisfactory effect in some cases.
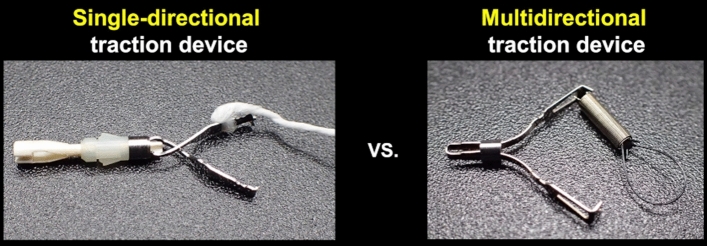
The clip with line (CWL; Fig. 1) could be the first traction device, which can provide per-oral and single-directional traction in the direction in which the line is pulled [6, 11]. In gastric ESD, the traction direction in CWL-assisted ESD (CWL-ESD) changes per the lesion site because the direction in which the line is pulled is limited to the cardia; this could cause a poor field of vision and inadequate tension for the submucosal dissection plane in some cases, resulting in an unsatisfactory effect. Indeed, a multicenter prospective randomized controlled trial (RCT) comparing the conventional ESD and CWL-ESD demonstrated that CWL-ESD did not decrease the gastric ESD procedure time [12]. Conversely, the spring and loop with clip (SLC; S–O clip; Zeon Medical, Tokyo, Japan; Fig. 2) can provide multidirectional traction [13, 14]. Based on the result of a single-center RCT, we reported that SLC-assisted ESD (SLC-ESD) decreased the procedure time of gastric ESD compared with conventional ESD [15]. In this single-center RCT, traction vertical to the gastric wall (vertical traction) was selected for SLC-ESD using its multidirectional traction function. Although the outcomes of both RCTs mentioned above indicated that the efficiency of the traction method varying per the traction direction and vertical traction is optimal in gastric ESD, limited research has been conducted to explore the optimal traction direction in traction-assisted gastric ESD. We hypothesized that SLC-ESD is more effective than CWL-ESD for treating superficial gastric neoplasms because SLC could provide vertical traction for lesions at any site by multidirectional traction function. Hence, this study aims to investigate the impact of traction direction in gastric ESD by comparing the procedure-related outcomes of CWL-ESD and SLC-ESD while setting the vertical traction in SLC-ESD.
Fig. 1.

A clip with line (CWL) can be made by tying commercially available dental floss to the arm part of the clip
Fig. 2.
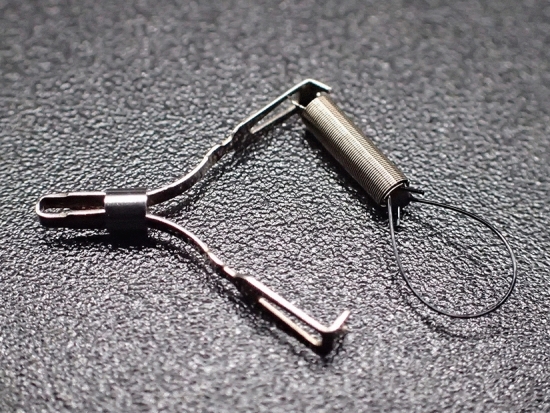
A spring and loop with clip (SLC; S–O clip; Zeon Medical, Tokyo, Japan) has a 5-mm-long spring and a 4-mm-long nylon loop at one side of the clip claws
Methods
Study Design and Study Sample
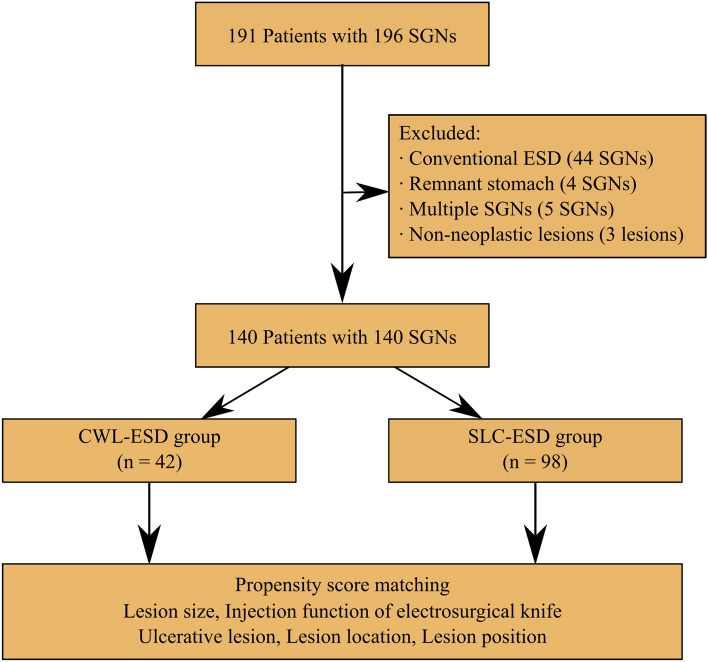
This retrospective, single-center, observational study evaluated the procedure-related outcomes between CWL-ESD and SLC-ESD for superficial gastric neoplasms. Figure 3 shows the patients’ enrollment process. We enrolled that the consecutive cases of gastric ESD performed at the Department of Endoscopy, Shonan Fujisawa Tokushukai Hospital (Fujisawa, Kanagawa, Japan) from November 2017 (introduction of SLC for gastric ESD at our hospital) to September 2020. During this period, 191 patients with 196 superficial gastric neoplasms (early gastric cancer or gastric adenoma) underwent ESD by a single endoscopist (M.N.), who had the experience of > 500 ESD procedures, including > 30 CWL-ESD procedures at the beginning of the study period. Of these, the following were excluded: lesions resected by conventional ESD (n = 44); lesions of the remnant stomach after gastrectomy because the ESD procedure in the remnant stomach is more difficult than in the stomach without gastrectomy (n = 4) [16]; in cases that underwent gastric ESD for ≥ 2 gastric lesions on the same day, only the first lesion was included, and other lesions were excluded (n = 5); and lesions suspected of superficial gastric neoplasms preoperatively but diagnosed as non-neoplastic lesions pathologically after ESD (n = 3). Finally, 140 lesions from 140 patients who underwent CWL-ESD or SLC-ESD were extracted. Based on the treatment approach adopted, all patients were categorized into two groups—CWL-ESD group (n = 42) and SLC-ESD group (n = 98).
Fig. 3.
Flowchart of the study sample. SGN, superficial gastric neoplasm; ESD, endoscopic submucosal dissection; CWL-ESD, clip with line-assisted endoscopic submucosal dissection; SLC-ESD; spring and loop with clip-assisted endoscopic submucosal dissection
Ethics
The study protocol was approved by the Institutional Review Board (Mirai Iryo Research Center; Tokyo, Japan), registered in the University Hospital Medical Information Network (registration number: UMIN 000047922, registry URL: https://www.umin.ac.jp/), and conducted per the tenets of the Declaration of Helsinki. Furthermore, written informed consent was obtained from all patients before ESD after explaining the potential adverse events of the ESD procedure.
Indications for Gastric ESD
All lesions were assessed histologically by forceps biopsy preoperatively. Per the Japanese guidelines, the indications of ESD for early gastric cancers were the lesions with little lymph node metastasis [17, 18]: (i) clinically intramucosal (cT1a)-differentiated carcinomas of any size, without ulcer findings; (ii) cT1a-differentiated carcinomas (size: ≤ 30 mm) with ulcer findings; and (iii) cT1a-undifferentiated carcinomas (size: ≤ 20 mm) without ulcer findings. Gastric adenomas were included in indications for ESD owing to a potential risk of canceration.
Outcomes
The primary outcome was the gastric ESD procedure time. The secondary outcomes were as follows: dissection speed, en bloc resection rate, complete resection rate, perforation rate, traction device attachment time, number of traction device applications, rate of traction device slip-off, traction direction, conversion rate to conventional ESD, and gastric ESD procedure time or dissection speed based on the lesion location and lesion size.
In the SLC-ESD group, the learning curve for SLC attachment was evaluated as this procedure could be potentially more complicated than that for the CWL attachment. The SLC-ESD group (n = 98) was divided into the first half and the second half, and median SLC attachment time was compared between the two subgroups.
Definitions
The gastric ESD procedure time (min) was defined as the time from the first injection to the completion of submucosal dissection, including the traction device attachment time. Dissection speed (mm2/min) was defined as the specimen area divided by the gastric ESD procedure time. The resected specimen was pinned on the board, and the length (mm) of the longer axis and the shorter axis of the resected specimen were measured immediately after ESD. Specimen size was defined as the longest axis length of the resected specimen. Specimen area (mm2) was calculated using the ellipse formula: specimen area = [(shorter axis length)/2] × [(longer axis length)/2] × 3.14. Lesion size was measured pathologically after resection and defined as the long axis of the lesion. Lesion location (i.e., upper-, middle-, or lower-third of the stomach) and lesion position (i.e., greater curvature, lesser curvature, posterior wall, or anterior wall of the stomach) were defined per the Japanese classification of gastric carcinoma [19]. En bloc resection was defined as the removal of the neoplastic area in a single piece by an electrosurgical knife, without using a snare. Complete resection was defined as en bloc resection with pathologically negative margins at both the horizontal and vertical cut ends. Perforation was diagnosed by endoscopy or the presence of free air on a scheduled chest X-ray in the standing position a day after ESD. Post-ESD bleeding was defined as that requiring endoscopic intervention to attain hemostasis.
The specific traction device-related factors were defined as follows. The SLC attachment time was defined as the time from starting to insert the clip applicator into the accessory channel to completing the anchoring of the SLC loop on the gastric wall. The CWL attachment time was defined as the time from starting to pull out the endoscope to completing the CWL attachment on the lesion. If traction device reattachment was performed, the time needed for reattachment was included in the traction device attachment time. Traction device-related damage to the specimen was defined as any tear or split of the specimen due to the traction force. In SLC-ESD, removal of the anchor clip was considered successful when it had been pulled from the gastric wall and extracted out from the body.
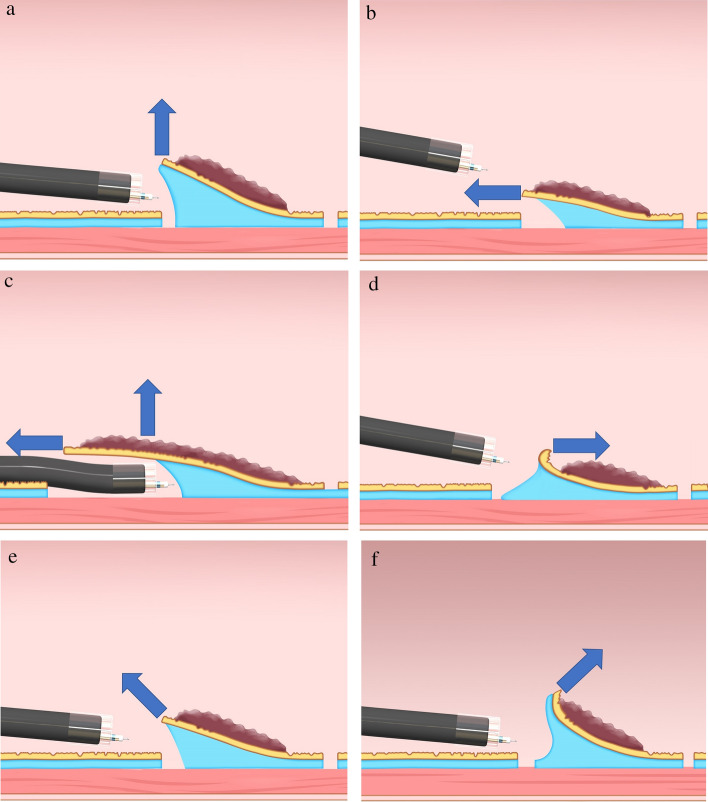
Based on the relationship between the endoscope tip and the gastrointestinal wall, the traction direction can be divided into the following five categories: proximal, diagonally proximal, vertical, diagonally distal, and distal (Fig. 4) [20]. Vertical traction was defined as traction applied at a right angle to the gastric wall, where the gastric wall around the lesion was assumed to be in the horizontal plane. Other traction directions were defined using the same approach. The traction direction of each case was classified as one of these categorizations by endoscopic findings. If ≥ 2 traction devices were used in one case, the traction direction was determined per the use for the longest time.
Fig. 4.
Classification of the traction direction. A Vertical traction; B proximal traction; C proximal traction combined with hood traction; D distal traction; E diagonally proximal traction; and F diagonally distal traction (Nagata [20])
Propensity Score Matching Analysis
Propensity score matching analysis was conducted to minimize the sampling bias and confounding. Reportedly, the gastric ESD procedure time can be prolonged by technical complications associated with lesions located at the upper- and middle-third of the stomach, lesions positioned at the greater curvature of the upper- and middle-third of the stomach, ulcerative lesions, and lesion size [4, 5, 12, 21, 22]. Moreover, the injection function of an electrosurgical knife could shorten the gastric ESD procedure time [23]. Thus, the propensity score was calculated using a logistic regression model with the traction method for gastric ESD (CWL-ESD group or SLC-ESD group) as an objective variable. The above-mentioned factors were included for propensity score matching analysis as explanatory variables. The CWL-ESD and SLC-ESD groups were matched according to the propensity score using the following algorithm: one to one, nearest neighbor within a caliper of 0.2, and without replacement.
Subgroup Analysis
The subgroup analyses for gastric ESD procedure time and dissection speed by the lesion size and location after propensity score matching were performed for the statistical adjustment of potential confounding.
Setting of Gastric ESD
All patients were hospitalized and underwent ESD under intravenous sedation. Blood tests and chest X-ray in the standing position were performed routinely on the day after ESD to diagnose adverse events. The ESD procedure was performed using a single-channel endoscope with a water supply function (GIF-Q260J; Olympus, Tokyo, Japan) and a straight transparent hood (D-201-11804; Olympus). A multibending endoscope (GIF-2TQ260M; Olympus), which has two bent sites at its tip, was used only when it was hard to access the lesion with a GIF-Q260J owing to anatomic reasons [24]. A straight needle-type electrosurgical knife (i.e., DualKnife; KD-650L; Olympus) or a straight needle-type electrosurgical knife with an injection function (i.e., DualKnifeJ [KD-655L; Olympus] or FlushKnifeBT-S [DK2620JI-B20; Fujifilm, Tokyo, Japan]) was used to perform mucosal incision and submucosal dissection with an electrosurgical generator (VIO300D; ERBE Elektromedizin GmbH, Tübingen, Germany). Endoscopic hemostasis was attained using an electrosurgical knife or hemostatic forceps (FD-412LR, CoagrasperG; Olympus). A mixture of 0.4% hyaluronic acid (MucoUp; Boston Scientific, Marlborough, Massachusetts, USA) and saline solution in a 1:1 ratio was injected into the submucosa. The stomach was extended using carbon dioxide (CO2) insufflation. An overtube (TOP, Tokyo, Japan) was used to decrease the risk of aspiration. If the distended stomach was difficult to maintain because of belching, a leak cutter (TOP) was attached to the overtube.
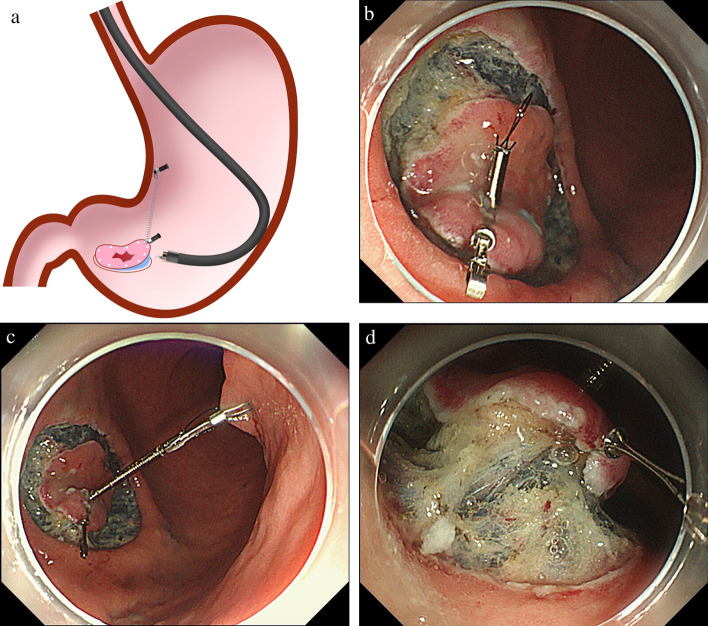
CWL-ESD Procedure (Video 1)
The CWL-ESD procedure did not include the traction method other than CWL-ESD, underwater techniques [25–27], the pocket creation method [28], the endoscopic submucosal tunnel dissection method [29], or any other special technique. The endoscope was withdrawn after a circumferential mucosal incision. The clip applicator was inserted into the accessory channel of the endoscope. The CWL, the clip (HX-610-090; Olympus) with a commercially available waxed nylon dental floss tied to its arm, was attached to the clip applicator (Fig. 5A). Then, the endoscope was reinserted through the overtube, and the CWL was attached to the oral or anal edge of the lesion (Fig. 5B). These steps ensured that the line came out of the mouth without passing through the accessory channel of the endoscope, while avoiding the interference between the devices positioned from the accessory channel and the line. Finally, pulling the line provided per-oral traction (Fig. 5C). To sustain the traction force, a sinker (~ 10 g) was attached to the line. After resection, the specimen with the CWL was extracted from the body.
Fig. 5.
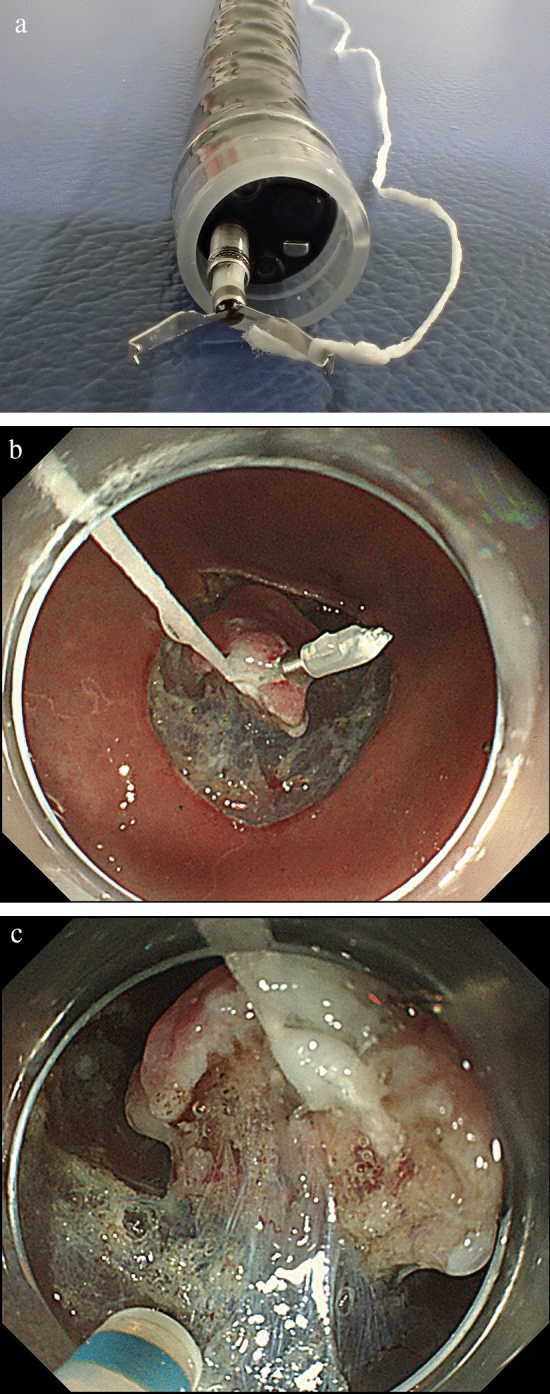
The procedure of clip with line-assisted endoscopic submucosal dissection (CWL-ESD). A The CWL was attached to the clip applicator. B The CWL was attached to the edge of the lesion. C Pulling the line from outside the body provided a good field of vision and sufficient tension for the dissection plane
SLC-ESD Procedure (Video 2)
All procedures not specific to the SLC were performed as described in the CWL-ESD procedure. If both the forward and retroflexed endoscopic positions had similar degrees of difficulty performing submucosal dissection, the forward endoscopic position was prioritized, as it was less likely to cause interference between the endoscope and the spring (Figs. 6A). First, the SLC was attached to the oral or anal edge of the lesion after a circumferential mucosal incision (Fig. 6B). Second, the regular clip captured the loop part of the SLC and then anchored it to the gastric wall (Fig. 6C). Finally, the spring extension provided traction for the lesion, providing a good field of vision and adequate tension for the dissection plane (Fig. 6D). A noteworthy advantage of the SLC is that its traction direction can be controlled in any direction by the anchor site. We set traction vertical to the gastric wall (vertical traction) as much as possible, using the multidirectional traction function of the SLC. Contrary to the CWL-ESD procedure, SLC and anchor clip could be delivered to the stomach through the accessory channel of the endoscope without the endoscope withdrawal during SLC attachment. For the SLC-ESD procedure, a modified attachment method was used to streamline the attachment procedure and avoid interference between the endoscope and the spring part of the SLC [15, 30, 31]. After resection, the anchor clip was removed from the gastric wall with forceps, and the specimen, SLC, and anchor clip were extracted from the body.
Fig. 6.
Spring and loop with clip (SLC; S–O clip; Zeon Medical, Tokyo, Japan)-assisted endoscopic submucosal dissection (SLC-ESD). A Interference between the endoscope and spring part of the SLC during submucosal dissection rarely occurs. B After circumferential mucosal incision, the SLC is delivered via the accessory channel of the endoscope and attached to the lesion; C the regular clip captures the loop part of the SLC and then anchors it on the gastric wall; D the traction provides a good field of vision and sufficient tension for the dissection plane (Nagata [31])
Statistical Analysis
All statistical analyses were performed in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were analyzed using the Fisher exact test and were presented as plain numbers and percentages. The normality of the distribution of continuous variables was evaluated by the Kolmogorov–Smirnov test. Then, continuous variables were reported as medians (interquartile range), and differences between the groups were analyzed using the Mann–Whitney U test. In this study, all tests were two-sided, and differences between variables were considered statistically significant for P < 0.05.
Results
Baseline Characteristics, Procedure-Related Outcomes, and Traction Device-Related Factors Before Propensity Score Matching
The baseline characteristics revealed no significant differences (Table 1), but some differences were found in the procedure-related outcomes and traction device-related factors (Table 2). Although not statistically significant, the median gastric ESD procedure time in the SLC-ESD group was shorter than in the CWL-ESD group (35.6 min vs. 51.0 min, P = 0.213). In addition, the median dissection speed in the SLC-ESD group was significantly faster than in the CWL-ESD group (22.4 mm2/min vs. 17.1 mm2/min, P = 0.002). The median specimen size in the SLC-ESD group was significantly larger than in the CWL-ESD group (36.0 mm vs. 31.0 mm, P = 0.045). The median specimen area in the SLC-ESD group was significantly larger than in the CWL-ESD group (825.4 mm2 vs. 647.2 mm2, P = 0.031). Furthermore, en bloc resection was attained without perforation in all the cases in both groups. The traction direction significantly differed between the SLC-ESD and CWL-ESD groups (P < 0.001). Although almost traction direction in the SLC-ESD group was vertical (95.9%), it was only 16.7% in the CWL-ESD group. Moreover, the median attachment time in the SLC-ESD group was significantly shorter than in the CWL-ESD group (100 s vs. 115 s, P = 0.002). In the SLC-ESD group, the median SLC attachment time was not significantly different between the first half and the second half (100 s vs. 97.5 s, P = 0.973). Although not statistically significant, slip-off occurred more frequently in the CWL-ESD group than in the SLC-ESD group (9.5% vs. 3.1%, P = 0.197). While no damage to the specimen was observed in the SLC-ESD group, it was significantly more in the CWL-ESD group by 11.9% (P = 0.002). While there was no case in which the traction method (CWL-ESD or SLC-ESD) was switched to the other traction method, conversion to conventional ESD was needed in some cases. Notably, the conversion rate to conventional ESD was significantly lower in the SLC-ESD group than in the CWL-ESD group (1.0% vs. 11.9%, P = 0.009).
Table 1.
Baseline characteristics of patients who underwent CWL-ESD or SLC-ESD for superficial gastric neoplasms before propensity score matching
| CWL-ESD n = 42 |
SLC-ESD n = 98 |
P | |
|---|---|---|---|
| Age, years, | |||
| Median (IQR) | 75 (69–80) | 74 (68–81) | 0.731 |
| Sex, male/female | 30 (71.4)/12 (28.6) | 70 (71.4)/28 (28.6) | 1.000 |
| Lesion size, mm | |||
| Median (IQR) | 12 (8–20) | 14 (9–21) | 0.341 |
| Range | 1–70 | 2–57 | |
| Lesion location | 0.970 | ||
| Upper | 8 (19.0) | 17 (17.3) | |
| Middle | 21 (50.0) | 49 (50.0) | |
| Lower | 13 (31.0) | 32 (32.7) | |
| Lesion position | 0.653 | ||
| Greater curvature | 8 (19.0) | 25 (25.5) | |
| Lesser curvature | 15 (35.7) | 39 (39.8) | |
| Anterior wall | 8 (19.0) | 16 (16.3) | |
| Posterior wall | 11 (26.2) | 18 (18.4) | |
| Morphology | 0.075 | ||
| Depressed (0–IIc, 0–III) | 17 (40.5) | 57 (58.2) | |
| Flat (0–IIb) | 2 (4.8) | 1 (1.0) | |
| Protruded (0–I, 0–IIa) | 23 (54.8) | 40 (40.8) | |
| Histology | 0.367 | ||
| Adenoma | 8 (19.0) | 14 (14.3) | |
| Differentiated adenocarcinoma | 31 (73.8) | 81 (82.7) | |
| Undifferentiated adenocarcinoma | 3 (7.1) | 3 (3.1) |
Values are n (%) unless otherwise indicated
CWL-ESD clip with line-assisted endoscopic submucosal dissection, IQR interquartile range, SLC-ESD spring and loop with clip-assisted endoscopic submucosal dissection
Table 2.
Comparison of procedure-related outcomes and traction device-related factors with CWL-ESD and SLC-ESD for superficial gastric neoplasms before propensity score matching
| CWL-ESD n = 42 |
SLC-ESD n = 98 |
P | |
|---|---|---|---|
| Gastric ESD procedure time, min | |||
| Median (IQR) | 51.0 (25.9–81.3) | 35.6 (22.8–67.1) | 0.213 |
| Range | 12.8–294.4 | 10.0–168.0 | |
| Dissection speed, mm2/min | |||
| Median (IQR) | 17.1 (9.3–23.2) | 22.4 (15.3–32.3) | 0.002† |
| Range | 3.3–46.4 | 6.2–84.7 | |
| Specimen size, mm | |||
| Median (IQR) | 31.0 (26.0–40.3) | 36.0 (29.3–44.5) | 0.045† |
| Range | 16.0–90.0 | 21.0–86.0 | |
| Specimen area, mm2 | |||
| Median (IQR) | 647.2 (482.2–931.4) | 825.4 (593.5–1206.7) | 0.031† |
| Range | 201.0–4874.9 | 296.7–3843.4 | |
| Depth | 0.925 | ||
| Mucosa* | 36 (85.7) | 84 (85.7) | |
| Submucosa (< 500 μm) | 2 (4.8) | 6 (6.1) | |
| Submucosa (≥ 500 μm) | 4 (9.5) | 8 (8.2) | |
| Presence of ulcer findings | 5 (11.9) | 15 (15.3) | 0.793 |
| En bloc resection | 42 (100) | 98 (100) | NA |
| Complete resection | 39 (92.9) | 94 (95.9) | 0.428 |
| Adverse events | |||
| Post-ESD bleeding | 0 (0) | 3 (3.1) | 0.554 |
| Perforation | 0 (0) | 0 (0) | NA |
| Use of a multibending endoscope | 3 (7.1) | 0 (0) | 0.026† |
| Use of an electrosurgical knife with an injection function | 0 (0) | 40 (40.8) | < 0.001† |
| Traction device-related factors | |||
| Traction direction | < 0.001† | ||
| Vertical | 7 (16.7) | 94 (95.9) | |
| Proximal | 13 (31.0) | 0 (0.0) | |
| Diagonally proximal | 2 (4.8) | 4 (4.1) | |
| Distal | 16 (38.1) | 0 (0.0) | |
| Diagonally distal | 4 (9.5) | 0 (0.0) | |
| Attachment time, second | |||
| Median (IQR) | 115 (99–140) | 100 (82–119) | 0.002† |
| Range | 61–288 | 53–281 | |
| Application, n | |||
| Median | 1 | 1 | 0.860 |
| Range | 1–4 | 1–2 | |
| Reattachment | 6 (14.3) | 15 (15.3) | 1.000 |
| Slip-off | 4 (9.5) | 3 (3.1) | 0.197 |
| Damage to the specimen | 5 (11.9) | 0 (0) | 0.002† |
| Successful removal of anchor clip | NA | 94 (95.9) | NA |
| Conversion to conventional ESD | 5 (11.9) | 1 (1.0) | 0.009† |
Values are n (%) unless otherwise indicated
CWL-ESD clip with line-assisted endoscopic submucosal dissection, ESD endoscopic submucosal dissection, IQR interquartile range, NA not applicable, SLC-ESD spring and loop with clip-assisted endoscopic submucosal dissection
*Intramucosal cancers and adenomas are included in this category
†P < 0.05
Matching Factors, Procedure-Related Outcomes, and Traction Device-Related Factors After Propensity Score Matching
This propensity score model was reliable (Likelihood Ratio Test; P < 0.001) and demonstrated acceptable discrimination between the two groups (c statistic = 0.725). Multicollinearity was less probable because all the variance inflation factors of explanatory variables were < 1.102. Propensity score matching generated 42 pairs. Table 3 shows matching factors, procedure-related outcomes, and traction device-related factors after propensity score matching. No significant differences were observed in matching factors, specimen size, and specimen area between the two groups. The median gastric ESD procedure time in the SLC-ESD group was significantly shorter than that in the CWL-ESD group (28.3 min vs. 51.0 min, P = 0.022). The median dissection speed in the SLC-ESD was significantly faster than that in the CWL-ESD (24.8 mm2/min vs. 17.1 mm2/min, P = 0.001). All traction direction was vertical in the SLC-ESD group, while only 16.7% in the CWL-ESD group. Moreover, the median attachment time in the SLC-ESD group was significantly shorter than in the CWL-ESD group (100 s vs. 115 s, P = 0.002). Damage to the specimen and conversion to conventional ESD occurred more frequently in the CWL-ESD group than in the SLC-ESD group, although not statistically significant (7.1% vs. 0%, P = 0.241 and 11.9% vs. 0%, P = 0.055, respectively).
Table 3.
Comparison of matching factors, procedure-related outcomes, and traction device-related factors with CWL-ESD and SLC-ESD for superficial gastric neoplasms after propensity score matching
| CWL-ESD n = 42 |
SLC-ESD n = 42 |
P | |
|---|---|---|---|
| Matching factors | |||
| Lesion size, mm | |||
| Median (IQR) | 12 (8–20) | 13 (8–19) | 0.911 |
| Range | 1–70 | 5–48 | |
| Lesion position (GC of U and M) | 5 (11.9) | 5 (11.9) | 1.000 |
| Lesion location (U and M) | 28 (66.7) | 30 (71.4) | 0.814 |
| Presence of ulcer findings | 5 (11.9) | 5 (11.9) | 1.000 |
| Use of electrosurgical knife with an injection function | 0 (0) | 0 (0) | NA |
| Procedure-related outcomes | |||
| Gastric ESD procedure time, min | |||
| Median (IQR) | 51.0 (25.9–81.3) | 28.3 (18.0–52.9) | 0.022† |
| Range | 12.8–294.4 | 11.3–105.3 | |
| Dissection speed, mm2/min | |||
| Median (IQR) | 17.1 (9.3–23.2) | 24.8 (18.0–34.2) | 0.001† |
| Range | 3.3–46.4 | 9.0–66.6 | |
| Specimen size, mm | |||
| Median (IQR) | 31.0 (26.0–40.3) | 34.5 (30.0–38.8) | 0.254 |
| Range | 16.0–90.0 | 22.0–61.0 | |
| Specimen area, mm2 | |||
| Median (IQR) | 647.2 (482.2–931.4) | 752.8 (632.7–983.8) | 0.222 |
| Range | 201.0–4874.9 | 345.4–2684.7 | |
| Depth | 1.000 | ||
| Mucosa* | 36 (85.7) | 37 (88.1) | |
| Submucosa (< 500 μm) | 2 (4.8) | 2 (4.8) | |
| Submucosa (≥ 500 μm) | 4 (9.5) | 3 (7.1) | |
| En bloc resection | 42 (100) | 42 (100) | NA |
| Complete resection | 39 (92.9) | 37 (88.1) | 1.000 |
| Adverse events | |||
| Post-ESD bleeding | 0 (0) | 2 (4.8) | 0.494 |
| Perforation | 0 (0) | 0 (0) | NA |
| Use of a multibending endoscope | 3 (7.1) | 0 (0) | 0.241 |
| Traction device-related factors | |||
| Traction direction | < 0.001† | ||
| Vertical | 7 (16.7) | 42 (100) | |
| Proximal | 13 (31.0) | 0 (0.0) | |
| Diagonally proximal | 2 (4.8) | 0 (0.0) | |
| Distal | 16 (38.1) | 0 (0.0) | |
| Diagonally distal | 4 (9.5) | 0 (0.0) | |
| Attachment time, second | |||
| Median (IQR) | 115 (99–140) | 100 (82–121) | 0.020† |
| Range | 61–288 | 59–281 | |
| Application, n | |||
| Median | 1 | 1 | 0.726 |
| Range | 1–2 | 1–2 | |
| Reattachment | 4 (9.5) | 5 (11.9) | 1.000 |
| Slip-off | 2 (4.8) | 1 (2.4) | 1.000 |
| Damage to the specimen | 3 (7.1) | 0 (0) | 0.241 |
| Successful removal of anchor clip | NA | 42 (100) | NA |
| Conversion to conventional ESD | 5 (11.9) | 0 (0.0) | 0.055 |
Values are n (%) unless otherwise indicated
CWL-ESD clip with line-assisted endoscopic submucosal dissection, ESD endoscopic submucosal dissection, IQR interquartile range, NA not applicable, SLC-ESD spring and loop with clip-assisted endoscopic submucosal dissection, GC greater curvature of the stomach, M middle-third of the stomach, U upper-third of the stomach
*Intramucosal cancers and adenomas are included in this category
†P < 0.05
Endoscopic Position During Submucosal Dissection
Table 4 presents the endoscopic position during submucosal dissection after propensity score matching. Overall, we observed a trend for selecting the forward endoscopic position in the SLC-ESD group than in the CWL-ESD group, although the difference was not statistically significant. In both groups, the selection of the forward endoscopic position tended to be in the lesions located at the upper- and lower-third of the stomach, and more selection of the retroflexed endoscopic position in the middle-third of the stomach, although the difference was not statistically significant.
Table 4.
Endoscopic position during submucosal dissection after propensity score matching
| CWL-ESD | SLC-ESD | P | |
|---|---|---|---|
| Total | n = 42 | n = 42 | 0.266 |
| Retroflexed endoscopic position | 19 (45.2) | 14 (33.3) | |
| Forward endoscopic position | 23 (54.8) | 28 (66.7) | |
| Lesion location | |||
| Upper | n = 8 | n = 9 | 1.000 |
| Retroflexed endoscopic position | 3 (37.5) | 3 (33.3) | |
| Forward endoscopic position | 5 (62.5) | 6 (66.7) | |
| Middle | n = 21 | n = 21 | 0.536 |
| Retroflexed endoscopic position | 13 (61.9) | 10 (47.6) | |
| Forward endoscopic position | 8 (38.1) | 11 (52.4) | |
| Lower | n = 13 | n = 12 | 0.593 |
| Retroflexed endoscopic position | 3 (23.1) | 1 (8.3) | |
| Forward endoscopic position | 10 (76.9) | 11 (91.7) |
Values are n (%) unless otherwise indicated
CWL-ESD clip with line-assisted endoscopic submucosal dissection, SLC-ESD spring and loop with clip-assisted endoscopic submucosal dissection
Subgroup Analysis
Table 5 presents the results of the subgroup analysis for the gastric ESD procedure time and dissection speed by lesion location and lesion size after propensity score matching. The median gastric ESD procedure time in the SLC-ESD group was shorter than in the CWL-ESD group in any subgroups although not statistically significant. The median dissection speed for lesions at the upper-, middle-, and lower-third of the stomach, and for those measuring ≤ 20 mm in size, was significantly faster in the SLC-ESD group than in the CWL-ESD group.
Table 5.
Subgroup analysis comparing the gastric ESD procedure time and dissection speed by lesion location and lesion size after propensity score matching
| CWL-ESD | SLC-ESD | P | |
|---|---|---|---|
| n = 42 | n = 42 | ||
| ESD procedure time, min (IQR) | |||
| Lesion location | |||
| n = 28 | n = 30 | ||
| Upper, middle | 53.1 (34.8–85.9) | 41.9 (26.0–64.3) | 0.050 |
| n = 14 | n = 12 | ||
| Lower | 23.8 (18.5–54.3) | 17.2 (15.0–24.2) | 0.100 |
| Lesion size | |||
| n = 32 | n = 35 | ||
| ≤ 20 mm | 35.2 (21.8–57.2) | 26.5 (16.9–44.7) | 0.100 |
| n = 10 | n = 7 | ||
| > 20 mm | 111.1 (80.5–145.8) | 71.0 (39.9–91.8) | 0.051 |
| Dissection speed, mm2/min (IQR) | |||
| Lesion location | |||
| n = 28 | n = 30 | ||
| Upper, middle | 15.4 (9.0–19.9) | 20.7 (17.0–27.3) | 0.002 † |
| n = 14 | n = 12 | ||
| Lower | 23.9 (16.7–31.4) | 41.1 (33.0–44.2) | 0.005 † |
| Lesion size | |||
| n = 32 | n = 35 | ||
| ≤ 20 mm | 17.7 (11.3–22.9) | 25.4 (19.0–35.9) | 0.002 † |
| n = 10 | n = 7 | ||
| > 20 mm | 13.8 (8.4–22.9) | 21.4 (15.1–28.2) | 0.143 |
CWL-ESD clip with line-assisted endoscopic submucosal dissection, ESD endoscopic submucosal dissection, IQR interquartile range, SLC-ESD spring and loop with clip-assisted endoscopic submucosal dissection
†P < 0.05
Discussion
This study was conducted to investigate the impact of traction direction in gastric ESD by comparing the procedure-related outcomes of CWL-ESD and SLC-ESD. Propensity score matching analysis revealed that SLC-ESD was significantly associated with shorter gastric ESD procedure time and higher dissection speed. Traction direction was significantly different between the two groups, and all traction direction was vertical in the SLC-ESD group and was only 16.7% in the CWL-ESD group. These findings indicate that vertical traction shortens the gastric ESD procedure time and increases the dissection speed compared with other traction directions.
While surgeons can alter the traction direction for the target lesion manually in general surgery, it is challenging for endoscopists to change that in traction-assisted ESD owing to complex procedures. Thus, it is crucial to examine the optimal traction direction in traction-assisted ESD. Despite limited research focusing on the traction direction, some studies indicated that the efficiency of the traction method depends on the traction direction. A single-center, prospective RCT comparing the conventional ESD and SLC-ESD in gastric ESD confirmed that median gastric ESD procedure time was significantly decreased in SLC-ESD, while the vertical traction was selected for SLC-ESD using the multidirectional traction function of SLC [15]. However, in a multicenter, prospective RCT comparing CWL-ESD and conventional ESD, CWL-ESD did not achieve a significant reduction in the gastric ESD procedure time in the total population, although CWL-ESD was found to shorten the gastric ESD procedure time for lesions at the greater curvature of the upper- and middle-third of the stomach [12]. As the traction direction of the CWL-ESD in the stomach is limited to the cardia, it provides the vertical traction for lesions primarily at the greater curvature of the upper- and middle-third of the stomach. Hence, the two RCTs mentioned above suggest that the most effective traction direction in gastric ESD is vertical to the gastric wall [32]. Traction direction in CWL-ESD can be controlled, and vertical traction can be obtained in combination with the pulley method. Therefore, the pulley method may improve the gastric ESD procedure time and dissection speed in CWL-ESD. However, feasibility and effectiveness should be investigated and compared with conventional ESD in clinical practice because the pulley method in gastric ESD has been mainly reported in a small number of cases or ex vivo studies [33–35].
All subgroup analyses revealed considerably shorter median gastric ESD procedure time in the SLC-ESD than in the CWL-ESD groups although without a statistically significant difference. Furthermore, the SLC-ESD exhibited a significant increase in the dissection speed for lesions regardless of the location. Thus, SLC-ESD could be more effective than CWL-ESD, irrespective of the lesion location. Nevertheless, the SLC-ESD procedure is more expensive than the CWL-ESD procedure, which costs around ¥6000 and ¥1000 (US $46.15 and US $7.69, at an exchange rate of ¥130 = US $1), respectively. Regarding the cost-effectiveness, CWL-ESD should be preferred over SLC-ESD for lesions at the greater curvature where the vertical traction can be attained with both SLC-ESD and CWL-ESD.
Despite limited research on the efficacy of traction directions other than the vertical traction for submucosal dissection, some studies suggested its utility for submucosal dissection in specific conditions. In a multicenter RCT comparing the conventional ESD and CWL-ESD in the esophagus, the CWL-ESD group demonstrated a significant reduction in the procedure time of esophageal ESD than the conventional ESD group [36]. In the esophageal ESD, as the esophagus is a narrow cylindrical lumen, submucosal dissection is performed in the forward endoscopic position and the CWL is attached to the oral side of the lesion, thereby naturally providing the proximal traction. The endoscope tip can approach parallel to the lesion owing to a narrow cylindrical esophageal lumen and can easily access the submucosa without the vertical traction by the CWL. After getting under the mucosa, the vertical traction by the hood and proximal traction using the CWL are combined, permitting strong vertical traction to the submucosa (Fig. 4C). Hence, this multicenter RCT suggested that the proximal traction is effective when the endoscope tip can approach the lesion parallelly.
In CWL-ESD in the stomach, the proximal traction can be used when the forward endoscopic position is selected. Compared with esophageal ESD, the endoscope cannot approach parallel to the lesion in gastric ESD per the lesion location because the stomach lumen is larger and more angulated than the esophagus. If the endoscope cannot approach parallel to the lesion, the mucosal flap falls proximally owing to the proximal traction, resulting in difficulty approaching the submucosa. Hence, the proximal traction might not always be effective in gastric ESD.
Although SLC is a special device and is available in limited countries, internal traction devices, which can provide vertical traction may substitute SLC. An internal traction device is defined as a device in which the device acts only inside the gastrointestinal tract. Such traction devices involve a ring thread [37], multiloop [38], double clip and rubber band [39], clip band [40], and multiloop traction device [41]. However, these devices are made of thread, band, or polyethylene instead of a spring, which may result in a shortage of elasticity for the large lumen of the stomach. If the endoscope interferes with these materials, specimen laceration may be a concern owing to lower elasticity. The modified attachment method we reported to avoid the interference between the endoscope and the spring part of the SLC can be adapted for these traction devices [15, 30, 31]. Nevertheless, most reports on the use of these devices are for colorectal ESD. Therefore, the feasibility of these devices for gastric ESD needs to be assessed.
This study has several limitations worth acknowledging. First, bias cannot be eliminated because of the retrospective study design. Propensity score matching analysis can minimize bias due to observed differences between groups but are still subject to biases due to unobserved differences. Second, the gastric ESD procedure time and dissection speed could be affected by the learning curve of ESD because all ESD procedures were performed by only one operator. The CWL for gastric ESD was introduced before the SLC, and SLC was used instead of CWL after its introduction in our department. However, due to concerns about the supply shortages associated with the Coronavirus Disease 2019 pandemic, the SLC was used only for difficult cases in 2020 during the study period. This approach was adopted because the SLC was a special device that could not be manufactured in-house. Alternatively, the CWL could be manufactured in-house using a regular clip and commercially available dental floss. Hence, due to the ease of procurement, the CWL was used instead of the SLC for standard cases after the Coronavirus Disease 2019 pandemic during the study period and the operator’s proficiency was more advanced in the CWL-ESD group, which might be favorable to the CWL-ESD group. Nevertheless, this study suggested that SLC-ESD was more effective than CWL-ESD in the stomach. Finally, the cases that were converted from CWL-ESD or SLC-ESD to the conventional ESD were included in the analysis. However, the conversion cases were found in the CWL-ESD group considerably more than in the SLC-ESD group, suggesting that SLC-ESD is more effective than CWL-ESD in the clinical practice of gastric ESD.
In conclusion, this study suggests that SLC can provide vertical traction in most cases of gastric ESD, unlike CWL. Vertical traction may reduce the gastric ESD procedure time.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1. Clip-with-line-assisted endoscopic submucosal dissection (CWL-ESD) for a superficial gastric neoplasm (MP4 72527 KB)
Video 2. Spring-and-loop with clip-assisted endoscopic submucosal dissection (SLC-ESD) for a superficial gastric neoplasm (MP4 74928 KB)
Author’s contributions
No additional writing assistance was used for this manuscript. Mitsuru Nagata and Masayuki Namiki contributed to the design of the study. Mitsuru Nagata analyzed the data and drafted the manuscript. All authors critically reviewed and revised the manuscript.
Funding
This research received no specific grant form any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
Mitsuru Nagata, Masayuki Namiki, Tomoaki Fujikawa, and Hiromi Munakata have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221–226. doi: 10.1055/s-2001-12805. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 4.Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Jin YF, Tomoeda M, et al. Influential factors in procedure time of endoscopic submucosal dissection for gastric cancer with fibrotic change. Dig Endosc. 2011;23:296–301. doi: 10.1111/j.1443-1661.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- 6.Oyama T, Kikuchi Y, Shimaya S, et al. Endoscopic mucosal resection using a hooking knife (hooking EMR) [in Japanese] Stomach Intest. 2002;37:1151–1161. [Google Scholar]
- 7.Imaeda H, Iwao Y, Ogata H, et al. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007–1010. doi: 10.1055/s-2006-925264. [DOI] [PubMed] [Google Scholar]
- 8.Chen PJ, Chu HC, Chang WK, Hsieh TY, Chao YC. Endoscopic submucosal dissection with internal traction for early gastric cancer (with video) Gastrointest Endosc. 2008;67:128–132. doi: 10.1016/j.gie.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos) Gastrointest Endosc. 2009;69:10–15. doi: 10.1016/j.gie.2008.03.1127. [DOI] [PubMed] [Google Scholar]
- 10.Hijikata Y, Ogasawara N, Sasaki M, et al. Endoscopic submucosal dissection with sheath-assisted counter traction for early gastric cancers. Dig Endosc. 2010;22:124–128. doi: 10.1111/j.1443-1661.2010.00948.x. [DOI] [PubMed] [Google Scholar]
- 11.Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375–378. doi: 10.5946/ce.2012.45.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Takizawa K, Suzuki S, et al. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: A multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2018;87:1231–1240. doi: 10.1016/j.gie.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto N, Osada T, Shibuya T, et al. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy. 2008;40:E94–E95. doi: 10.1055/s-2007-995603. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto N, Osada T, Shibuya T, et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video) Gastrointest Endosc. 2009;69:1370–1374. doi: 10.1016/j.gie.2008.12.245. [DOI] [PubMed] [Google Scholar]
- 15.Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: A randomized controlled trial (with videos) Gastrointest Endosc. 2021;93:1097–1109. doi: 10.1016/j.gie.2020.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Nishide N, Ono H, Kakushima N, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in remnant stomach or gastric tube. Endoscopy. 2012;44:577–583. doi: 10.1055/s-0031-1291712. [DOI] [PubMed] [Google Scholar]
- 17.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 18.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition) Dig Endosc. 2021;33:4–20. doi: 10.1111/den.13883. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 20.Nagata M. Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction? World J Gastroenterol. 2022;28:1–22. doi: 10.3748/wjg.v28.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn JY, Choi KD, Choi JY, et al. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: Analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911–916. doi: 10.1016/j.gie.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Ono S, Kato M, Nakagawa M, Imai A, Yamamoto K, Shimizu Y. Outcomes and predictive factors of “not self-completion” in gastric endoscopic submucosal dissection for novice operators. Surg Endosc. 2013;27:3577–3583. doi: 10.1007/s00464-013-2929-0. [DOI] [PubMed] [Google Scholar]
- 23.Esaki M, Ihara E, Esaki M, et al. Comparisons of outcomes between ProKnife injection endoscopic submucosal dissection and conventional endoscopic submucosal dissection for large gastric lesions in ex vivo porcine model study: A randomized controlled trial. DEN open. 2022;2:e91. doi: 10.1002/deo2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada K, Horikawa Y, Koyanagi R, et al. Usefulness of a multibending endoscope in gastric endoscopic submucosal dissection. VideoGIE. 2019;4:577–583. doi: 10.1016/j.vgie.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos) Gastrointest Endosc. 2018;87:1345–1353. doi: 10.1016/j.gie.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Nagata M. Underwater endoscopic submucosal dissection in saline solution using a bent-type knife for duodenal tumor. VideoGIE. 2018;3:375–377. doi: 10.1016/j.vgie.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagata M. Tapered hood with wide holes in its sides for efficient air bubble removal during underwater endoscopic submucosal dissection. Dig Endosc. 2022;34:654. doi: 10.1111/den.14232. [DOI] [PubMed] [Google Scholar]
- 28.Miura Y, Hayashi Y, Lefor AK, Osawa H, Yamamoto H. The pocket-creation method of ESD for gastric neoplasms. Gastrointest Endosc. 2016;83:457–458. doi: 10.1016/j.gie.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 29.Choi HS, Chun HJ, Seo MH, et al. Endoscopic submucosal tunnel dissection salvage technique for ulcerative early gastric cancer. World J Gastroenterol. 2014;20:9210–9214. doi: 10.3748/wjg.v20.i27.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata M. Modified attachment method using an S-O clip for gastric endoscopic submucosal dissection. VideoGIE. 2019;4:151–153. doi: 10.1016/j.vgie.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata M. Internal traction method using a spring-and-loop with clip (S–O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg Endosc. 2020;34:3722–3733. doi: 10.1007/s00464-020-07590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata M. Optimal traction direction in traction-assisted gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2022;14:667–671. doi: 10.4253/wjge.v14.i11.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CH, Chen PJ, Chu HC, et al. Endoscopic submucosal dissection with the pulley method for early-stage gastric cancer (with video) Gastrointest Endosc. 2011;73:163–167. doi: 10.1016/j.gie.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Nomura T, Kamei A, Sugimoto S. Gastric endoscopic submucosal dissection using the “pulley clip with elastic line” method. Endoscopy. 2018;50:E104–e106. doi: 10.1055/s-0043-125358. [DOI] [PubMed] [Google Scholar]
- 35.Ge PS, Thompson CC, Jirapinyo P, Aihara H. Suture pulley countertraction method reduces procedure time and technical demand of endoscopic submucosal dissection among novice endoscopists learning endoscopic submucosal dissection: A prospective randomized ex vivo study. Gastrointest Endosc. 2019;89:177–184. doi: 10.1016/j.gie.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Takizawa K, Nonaka S, et al. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: A multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2020;91:55–65. doi: 10.1016/j.gie.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Masaki T. Novel effective and repeatedly available ring-thread counter traction for safer colorectal endoscopic submucosal dissection. Surg Endosc. 2017;31:3040–3047. doi: 10.1007/s00464-016-5326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki Y, Tanuma T, Nojima M, et al. Multiloop as a novel traction method in accelerating colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;91:185–190. doi: 10.1016/j.gie.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 39.Jacques J, Charissoux A, Bordillon P, et al. High proficiency of colonic endoscopic submucosal dissection in Europe thanks to countertraction strategy using a double clip and rubber band. Endosc Int Open. 2019;7:E1166–e1174. doi: 10.1055/a-0965-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge PS, Aihara H. A novel clip-band traction device to facilitate colorectal endoscopic submucosal dissection and defect closure. VideoGIE. 2020;5:180–186. doi: 10.1016/j.vgie.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui H, Tamai N, Futakuchi T, Kamba S, Dobashi A, Sumiyama K. Multi-loop traction device facilitates gastric endoscopic submucosal dissection: ex vivo pilot study and an inaugural clinical experience. BMC Gastroenterol. 2022;22:10. doi: 10.1186/s12876-021-02085-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Clip-with-line-assisted endoscopic submucosal dissection (CWL-ESD) for a superficial gastric neoplasm (MP4 72527 KB)
Video 2. Spring-and-loop with clip-assisted endoscopic submucosal dissection (SLC-ESD) for a superficial gastric neoplasm (MP4 74928 KB)