Abstract
Background:
The role of inflammatory cytokines, such as TNF-α and IL-8, in gastric carcinogenesis has been investigated, but their impact remains to be further elucidated.
Methods:
In this study, we measured the serum concentrations of these cytokines and H. pylori serostatus in dyspeptic patients, presenting with normal mucosa (NM = 53), chronic gastritis (CG = 94), and gastric cancer (GC = 82), by ELISA.
Results:
Moderate levels of TNF-α were detected in the NM group (19.9 ± 19.5 pg/ml), which were nearly doubled in patients with CG (35.7 ± 28.0 pg/ml) and drastically declined in GC patients (1.8 ± 5.9 pg/ml). The serum levels of IL-8, however, were not statistically different amongst these three groups.
Conclusion:
TNF-α serum concentration seemed to undergo up- and downregulation, when moving from NM to CG and from CG to GC, respectively. If confirmed in a prospective study, this cytokine can behave as a serum indicator of gastric inflammation and malignant transformation.
Key Words: Inflammation, Interleukin-8, cytokines, Tumor necrosis factor-alpha
INTRODUCTION
Gastric cancer was recognized as the fourth worldwide cause of cancer death in 2020. Among the different types of GC, the intestinal type is the most prevalent one[1]. This GC subtype is highly affected by environmental and genetic factors and is preceded by a cascade of precancerous lesions, as previously described by Correa and Piazuelo[2]. According to Correa’s model, primarily, the normal mucosa of the stomach becomes inflamed, otherwise known as gastritis. Gastric inflammation is initiated by several factors, including H. pylori infection, high-salt diet, nitrate intake, etc. Gastritis may then develop into atrophic gastritis, intestinal metaplasia, dysplasia, and ultimately gastric cancer[2].
TNF-α is a critical cytokine, which is mainly produced by macrophages and is involved in many biological processes, such as acute and chronic inflammation. In the process of carcinogenesis, it has a dual role by which it causes tumor cell death and apoptosis, on the one hand, and potentiates tumorigenesis and progression, on the other hand[3]. IL-8 is another pro-inflammatory chemokine with varying sizes (reviewed in[4]). The activated form of IL-8 is induced in the tumor microenvironment, including cancer cells, macrophages, endothelial cells, and neutrophils and impacts several intracellular signaling cascades via binding to its G protein-coupled receptors (reviewed in[4]). It is involved in carcinogenesis by activating different pathways, including serine/ threonine kinases and protein tyrosine kinases and affects the transcriptome and proteome profile (reviewed in[4]).
While alterations in serum concentration of TNF-α and IL-8 have been investigated in several cancers, including GC, the results remain controversial, and the potential of the mentioned cytokines as serum indicators of GC is under debate. In this study, we have evaluated the serum levels of TNF-α and IL-8 and H. pylori serostatus in three dyspeptic groups, with varying histopathologic status, including those with NM, CG, and GC. We have also investigated the association of other modifying factors, such as age, gender, ethnicity, family history of GC with the serum levels of IL-8 and TNF-α in general and in each group separately.
MATERIALS AND METHODS
Study population
The study population included histologically confirmed GC (n = 82) patients who attended Imam Khomeini Hospital, Tehran, Iran, from 2012 to 2017, as well as dyspeptic patients who were referred to the Gastroenterology Department of Amir-Alam Hospital, Tehran, Iran, at the same time period and were diagnosed as either NM (n = 53) or CG (n = 94) following histopathologic analysis. The interview questionnaire comprised of demographic characteristics, including age, gender, ethnicity, smoking habits, FHGC, and prior history of chemotherapy (Table 1). Of the 82 GC patients, 30 subjects had received chemotherapy, prior to blood sampling.
Table 1.
Baseline characteristics and serum levels of IL-8 and TNF-α in the studied groups
| Numbers (%) | TNF-α (mean ± SD) | IL-8 (mean ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables |
GC
(n = 82) |
CG
(n = 94) |
NM
(n = 53) |
GC | CG | NM | All | GC | CG | NM | All | ||
| Serum cytokine levels | - | - | - | 1.8 ± 5.9c | 35.7 ± 28.0 a | 19.9 ± 19.5 b | 20.1 ± 25.6 | 21.7± 56.8b | 27.8 ± 51.7a | 25.6 ± 35.9a | 24.3 ± 49.7 | ||
| Age | b | a | a | ||||||||||
| < 60 | 31 (38) | 66 (70) | 41 (77) | 1.0 ± 3.0c | 35.3 ± 29.0a | A 23.1 ± 20.2b | A24.4 ± 26.6 | 14.6 ± 27.9 | 21.1 ± 41.5 | 21.5 ± 26.7 | 19.5 ± 34.6 | ||
| ≥ 60 | 51 (64) | 28 (30) | 12 (23) | 2.3 ± 7.1 c | 31.5 ± 22.9 a | B 9.4 ± 12.7 b | B12.8 ± 19.9 | 25.5 ± 68.0 | 38.0 ± 65.0 | 39.7 ± 56.8 | 30.1 ± 64.6 | ||
| Gender | b | a | a | ||||||||||
| Female | 20 (24) | 52 (55) | 25 (47) | 3.1 ± 8.5 c | 36.2 ± 31.4 a | 17.2 ± 22.2 b | A 24.5 ± 28.3 | 15.9 ± 32.7c | 19.1 ± 25.5bc | 29.7 ± 30.8a | 21.1± 28.7 | ||
| Male | 62 (76) | 42 (45) | 28 (53) | 1.3 ± 4.5 c | 33.4 ± 24.1 a | 17.3 ± 21.3 b | B 16.8 ± 22.7 | 23.3 ± 62.6 | 37.2 ± 69.6 | 22.0 ± 40.1 | 26.6 ± 60.2 | ||
| Ethnicity | b | a | a | ||||||||||
| Fars | 10 (12) | 43 (46) | 18 (34) | 2.7 ± 4.4b | AB34.9 ± 26.2a | 25.5 ± 24.5a | 28.0 ± 26.1 | 11.8 ± 11.8 | 25.9 ± 46.1 | 15.1 ± 13.4 | 21.2 ± 37.0 | ||
| Turk/Turkman | 38 (46) | 24 (26) | 20 (38) | 1.5 ± 6.3c | AB 32.4 ± 28.8a | 17.5 ± 14.8b | 14.8 ± 22.1 | 21.3 ± 31.8 | 31.3 ± 65.8 | 33.9 ± 48.8 | 27.3 ± 47.8 | ||
| Kurd/Lor/Lak | 21 (26) | 14 (15) | 5 (9) | 2.7 ± 7.6b | AB 31.7 ± 25.7a | 9.9 ± 13.6a | 15.0 ± 21.9 | 33.3 ± 104.0 | 15.5 ± 20.0 | 39.4 ± 51.2 | 27.9 ± 77.7 | ||
| Gilak/Mazani/Taleshi | 10 (12) | 4 (4) | 7 (13) | 0.9 ± 2.9 | B 24.7 ± 33.2 | 26.1 ± 19.2 | 13.9 ± 21.0 | 11.1 ± 10.3 | 15.7 ± 22.3 | 23.9 ± 23.1 | 16.2 ± 17.8 | ||
| Other ethnicities | 3 (4) | 9 (10) | 3 (6) | 0.0b | A 59.3 ± 30.2a | 3.7 ± 6.4b | 36.3 ± 37.1 | 12.4 ± 7.4 | 51.6 ± 74.8 | 14.9 ± 15.0 | 36.4 ± 60.1 | ||
| Smoking habits | b | a | b | ||||||||||
| Never | 46 (57) | 71 (75) | 36 (68) | 2.4 ± 7.3 c | 36.9 ± 29.4 a | 20.0 ± 17.4 b | A 23.2 ± 26.7 | 26.7 ± 74.0 | 27.6 ± 52.4 | 21.1 ± 20.7 | 25.1 ± 54.1 | ||
| Ever | 35 (43) | 24 (25) | 17 (32) | 1.1 ± 3.3 c | 26.1 ± 17.9 a | 19.7 ± 23.8ab | B 13.2 ± 19.2 | 15.5 ± 18.8 | 21.5 ± 41.8 | 35.4 ± 55.7 | 21.6 ± 37.5 | ||
| Family history of GC | |||||||||||||
| No | 70 (86) | 78 (88) | 44 (85) | 1.7 ± 5.3 c | A 36.8 ± 27.7 a | 20.7 ± 19.7 b | A 21.0 ± 25.8 | 23.0 ± 61.3 | 29.0 ± 53.6 | 23.6 ± 35.4 | 25.1 ± 52.6 | ||
| Yes | 11 (14) | 11 (12) | 8 (15) | 2.4 ± 8.4 b | B 12.2 ± 11.4 a | 13.1 ± 18.3 a | B 8.6 ± 13.1 | 14.0 ± 8.1 | 9.7 ± 10.5 | 35.0 ± 41.4 | 17.3 ± 23.7 | ||
| H. pylori serostatus | c | b | a | ||||||||||
| Negative | 37 (46) | 29 (31) | 36 (68) | 1.3 ± 3.2 b | 30.3 ± 24.6 a | 18.3 ± 19.4 a | B 15.6 ± 21.1 | 18.6 ± 30.7 | 22.1 ± 51.4 | 28.0 ± 41.0 | 22.2 ± 40.4 | ||
| Positive | 44 (54) | 64 (69) | 17 (32) | 2.0 ± 7.5 c | 37.1 ± 29.8 a | 23.5 ± 20.0 b | A 24.2 ± 28.4 | 25.5 ± 73.9 | 29.5 ± 51.7 | 20.6 ± 21.2 | 26.5 ± 56.9 | ||
| Chemotherapy | |||||||||||||
| Yes | 30 (36) | - | - | 1.3 ± 6.0B | - | - | 8.5 ± 10.3B | - | - | ||||
| No | 40 (48) | - | - | 3.5 ± 6.7A | - | - | 28.8 ± 78.8A | - | - | ||||
| ND | 12 (16) | - | - | 0.3 ± 1.1C | - | - | 28.7 ± 26.0A | - | - | ||||
| Tumor subsite | |||||||||||||
| Cardia | 19 (24) | - | - | 3.2 ± 4.6 | - | - | 11 ± 17.1 | - | - | ||||
| Non-cardia | 54 (66) | - | - | 1.5 ± 6.6 | - | - | 26.8 ± 68.8 | - | - | ||||
| Mixed | 7 (10) | - | - | 1.3 ± 3.3 | - | - | 12.9 ± 7.7 | - | - | ||||
| IA | 4 (5) | - | - | 0 | - | - | 3.7 ± 3.8 | - | - | ||||
| IB | 13 (17) | - | - | 4.7 ± 9.4 | - | - | 12.1 ± 8.4 | - | - | ||||
| II | 19 (25) | - | - | 1.0 ± 2.5 | - | - | 24.8 ± 38.8 | - | - | ||||
| IIIA | 17 (22) | - | - | 3.4 ± 9.5 | - | - | 39.1 ± 115.9 | - | - | ||||
| IIIB | 13 (17) | - | - | 0 | - | - | 17.2 ± 19.3 | - | - | ||||
| IV | 10 (13) | - | - | 1.7 ± 4.3 | - | - | 21.7 ± 20.4 | - | - | ||||
| Tumor grade | |||||||||||||
| Poorly differentiated | 26 (32) | - | - | 2.0 ± 7.4 | - | - | 17.5 ± 20.6 | - | - | ||||
| Moderately Differentiated | 28 (34) | - | - | 1.5 ± 5.8 | - | - | 39.2 ± 93.1 | - | - | ||||
| Well-differentiated | 11 (13) | - | - | 0.4 ± 1.2 | - | - | 7.2 ± 6.5 | - | - | ||||
| Unknown | 17 (21) | - | - | 2.6 ± 4.8 | - | - | 8.4 ± 10.4 | - | - | ||||
| Tumor subtype | |||||||||||||
| Intestinal | 24 (48) | - | - | 0.9 ± 2.3 | - | - | 42.3 ± 100.6 | - | - | ||||
| Diffuse | 5 (10) | - | - | 7.2 ± 16.0 | - | - | 16.3 ± 13.4 | - | - | ||||
| Signet ring cell | 11 (22) | - | - | 3.8 ± 9.4 | - | - | 4.6 ± 4.5 | - | - | ||||
| Mixed | 6 (12) | - | - | 2.2 ± 5.3 | - | - | 30.7 ± 23.6 | - | - | ||||
| Other types | 4 (8) | - | - | 3.0 ± 1.7 | - | - | 8.6 ± 6.5 | - | - | ||||
For baseline characteristics, the frequencies were analyzed using chi-squared method. For categories 2-7, the frequencies were analyzed among the studied groups (GC, CG, and NM), and for categories 8-12, the frequencies were analyzed in each category in GC patients. The serum concentration of TNF-α and IL-8 were compared among the studied groups in the mentioned categories (1-11). For this comparison, in each row, variables without a common superscript letter on the right side of the numbers are statistically different (p < 0.05). For simplicity, the superscript letters were used only for the rows with a significant difference. Also, the concentrations were compared in each category within each studied group individually. For this comparison, in each column, variables without a common capital superscript letter on the left side of the numbers are statistically different (p < 0.05). For simplicity, the capital letters were used only for the columns with a significant difference.
Serum analysis
For each patient, 2.5 ml of whole blood was collected. The sera were isolated and kept at -70°C until further analysis. A commercial anti-H. pylori IgG ELISA kit (SERION Diagnostics, Germany) was used to determine the H. pylori serostatus. To quantify IL-8 and TNF-α serum concentrations, R&D ELISA kit (DuoSet ELISA, USA) was used according to the manufacturer’s instructions.
Statistical analysis
The mean serum concentrations of TNF-α and IL-8 were compared amongst different patient groups, using the Kruskal-Wallis test, followed by multiple comparisons using Dunn's multiple comparisons test. Chi-square test was used for the analysis of baseline characteristics in Table 1. Data were analyzed in SPSS statistics software (version 24). Graphs were illustrated using Graphpad prism (version 8). The p values less than 0.05 were considered as statistically significant.
RESULTS AND DISCUSSION
Study population
Analysis of the demographic factors among the patient groups indicated that GC patients were significantly older than the non-GC (CG and NM) subjects (63 ± 11 y vs. 54 ± 11 y and 55 ± 8 y; p < 0.001) and were predominantly male (p < 0.001). Most of the GC patients were of Turk/Turkman (46%), and Kurd/Lor/Lak (26%) ethnicities, whereas in CG patients, Fars (46%) and Turk/Turkman (26%) ethnicities, and in NM patients Turk/Turkman (38%), and Fars (34%) ethnicities predominated the population. The majority of GC tumors were of the noncardia subsite (66%) and intestinal subtype (48%).
H. pylori serostatus
The NM group showed the lowest percentage of H. pylori seropositivity (32%), compared to CG (69%), and GC (54%) (p < 0.001). The H. pylori serostatus did not indicate any significant association with age, gender, ethnicity, FHGC, and smoking habits.
Alterations in serum TNF-α levels
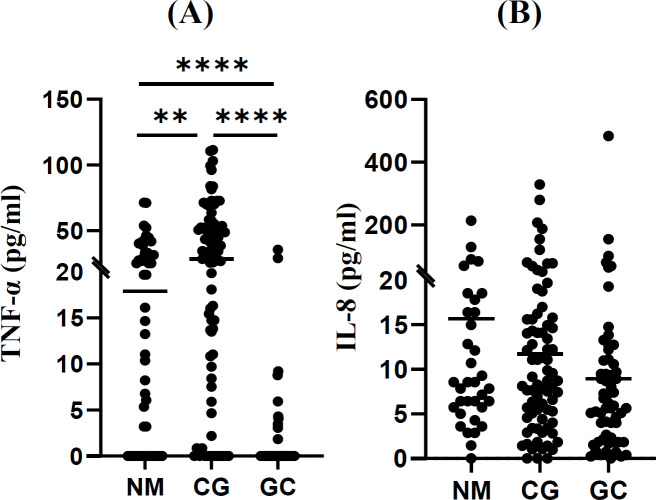
The serum concentration of TNF-α was significantly higher in the CG patients, as compared to the NM group (35.7 ± 28.0 vs. 19.9 ± 19.5 pg/ml; p = 0.0087; Fig. 1A and Table 1). TNF-α concentration was higher in GC patients, who had received chemotherapy (n = 30; 3.5 ± 6.7), as compared to those who did not (n = 53; mean ± SD: 1.3 ± 6.0 pg/ml; p = 0.0012). The maximum concentration of TNF-α was 71.5, 111.4, and 35.8 pg/ml, in the NM, CG, and GC groups, respectively. Combining the three groups showed that the TNF-α concentration was significantly higher in the following subjects: (1) aged <60 vs. those above (p = 0.001), (2) females vs. males (p = 0.027), (3) never vs. ever smokers (p = 0.009), (4) No FHGC vs. those with FHCG (p = 0.010), and (5) H. pylori seropositive vs. negative (p = 0.045) (Table 1). In the NM group, individuals older than 60 years of age, had significantly higher TNF-α levels, as compared to those younger (23.1 ± 20.2 vs. 9.4 ± 12.7 pg/ml; p = 0.033). Moreover, the TNF-α levels were not significantly different between Hp-seronegative NM and CG patients. Interestingly, the serum levels of TNF-α in CG patients were significantly lower in subjects with FHGC, as compared to those without (12.2 ± 11.4 vs. 36.8 ± 27.7; p = 0.003). Combining the NM and CG groups also revealed a lower TNF-α level in patients with FHGC than those without (12.5 ± 14.2 vs. 31.0 ± 36.3 pg/ml; p = 0.003). According to the herein performed extensive literature review (Supplementary Table 1), our results are not in line with many studies[5–7] which have reported relatively higher serum levels of TNF-α in GC patients, as compared to healthy controls. However, our results are consistent with two other studies indicating a lower concentration of TNF-α in GC patients[8,9]. Moreover, similar to our experiment, there are several studies in which TNF-α could not be detected in the serum of all or most of the studied patients including GC and healthy individuals[10–12]. The absence of TNF-α production by various tumor cells, including cell lines derived from T-lymphocytes, colon and breast cancers, has also been documented[13]. On the other hand, in a comparative study, serum TNF-α was detectable in patients with ovarian and breast tumors, but not in GC cases[14]. In other studies, TNF-α serum concentrations in GC patients was similar to the controls[15,16]. Absence or low level of TNF-α in the serum of GC patients can have various reasons. For instance, Ohno et al[11] have suggested that TNF-α is not always secreted, even when its mRNA is detected. These authors showed no expression of TNF-α in GC cell lines, including KATO-III and MKN-45. It has also been hypothesized that a decrease in TNF-α expression might be due to immune suppression in GC patients[9]. Moreover, it has been reported that the half-life of TNF-α is very short, making it difficult to detect, which leads to variable reported levels[17]. Another possible reason for TNF-α suppression in GC patients might be T cell exhaustion. It is well known that the effector T cells are exhausted in chronic infections and cancer and might lose their normal capacity in cytokine production[18]. Accordingly, TNF-α is highly suppressed in the exhausted CD8+ effector T cells[19]. Thus, to gain more information about TNF-α expression in blood cells and tumor-infiltrating immune cells in GC patients, we analyzed the open source high-throughput data from Gene Expression Omnibus (GEO) repository at the transcript level. It should be noted that expression of TNF-α at transcript level does not necessarily reflect its expression at protein level. Supporting the T-cell exhaustion hypothesis, a recent single-cell RNA-seq of both tumor-infiltrating and peripheral blood cells of GC patients showed a marked downregulation of genes involved in the cytokine production of exhausted CD8+ effector T cells[20]. However, TNF-α secretion is not limited to the effector T cells, but is also produced by other cell types such as macrophages and natural killer cells[21]. Similar to ELISA test reports on the sera of patients, the results of high-throughput analysis of immune cells in GC patients have been controversial. In the tumor-infiltrating and blood dendritic cells of GC patients, TNF-α mRNA expression is upregulated compared to the adjacent normal tissue and blood of healthy individuals[20]. Moreover, the suppression of several TNF receptor superfamily genes and receptor-associated factors were reported in peripheral blood leukocytes of GC patients, using RNA-seq analysis (GSE49515 dataset). In a single-cell RNA-seq analysis, the authors reported four clusters of macrophages, two of which expressed higher levels of TNF. At the same time, other subclusters had deficient TNF expression[22], indicating a heterogeneous tumor microenvironment. Therefore, the inconsistent reports on TNF-α expression under cancerous conditions might be due to the heterogeneous tumor microenvironment and the functional state of the immune cells in the blood. Of note, there has been a report on the enhanced effects of chemotherapy, when combined with TNF-α treatment. In this approach, TNF-α induces apoptosis in cancer cells[23].
Fig. 1.
Mean serum concentrations of TNF-α (A) and IL-8 (B) in the patient groups. ** and **** show p < 0.01 and p < 0.0001, respectively
Alterations in serum IL-8 levels
When the results from the three studied groups were combined, no association was found between IL-8 concentration and the listed demographic factors (Table 1). The serum IL-8 concentration among the three clinical groups (25.6 ± 35.9 [NM], 27.4 ± 51.2 [CG], and 20.2 ± 55.1 [GC]) showed that the concentration of IL-8 was significantly lower in the GC patients, as compared to the NM group (P = 0.027). On the other hand, in contrast to TNF-α, the serum IL-8 concentration was lower in GC patients, who received chemotherapy prior to blood sampling (8.5 ± 10.3 vs. 28.8 ± 78.8; P = 0.0299). Therefore, we excluded the chemotherapy-treated patients and performed a reanalysis that resulted in the elimination of the statistically significant differences among the studied groups (Fig. 1B). The maximum concentrations of IL-8 in the NM, CG, and GC groups were 213.5, 328.1, and 482.9 pg/ml, respectively. Although the serum levels of IL-8 were not significantly different among the studied groups, following the exclusion of chemotherapy-treated patients, the GC showed the highest maximum serum level of IL-8 compared to the CG and NM groups. Several studies have evaluated the levels of this cytokine in the serum of GC patients (Supplementary Table 2). In most of these studies, higher concentrations of IL-8 have been reported in the serum of GC patients compared to controls[6,24,25]. However, other studies have reported no significant difference between IL-8 concentration in the GC cases vs. controls[15,16]. The single-cell RNA-seq analysis identified a higher expression of IL-8 in the tumor infiltrating B cells of GC patients compared to the adjacent normal tissue. Nevertheless, they detected no significant differences in the IL-8 levels produced by the peripheral blood B cells from GC patients compared to healthy individuals[20]. Similarly, in our study, IL-8 concentrations were similar amongst the NM, CG, and GC groups. Therefore, serum IL-8 levels were unable to differentiate between the studied patient groups.
In conclusion, our findings indicate that serum TNF-α levels are strongly diminished in most GC patients, especially those who did not receive chemotherapy. Further analysis of the role of this cytokine in GC carcinogenesis, its regulating molecules, and its potential as a GC indicator is needed.
DECLARATIONS
Acknowledgments
The authors are thankful to the Pasteur Institute of Iran (Tehran) and Paris for supporting this study.
Ethical statement
The above-mentioned sampling protocols were approved by the Ethics Committee of the Pasteur Institute of Iran, Tehran (ethical code: IR.PII.REC.1394.57). Every patient provided a written informed consent.
Data availability
The data are available upon request.
Author contributions
MA: carried out the study and wrote and revised manuscript; ME: provided lab assistance; MT: assisted in gastric sampling; MAM: performed gastric surgery; MEH: performed gastric endoscopy, ET: revised the manuscript; MV: revised the manuscript; MM: wrote and revised the manuscript
Conflict of interest
None declared
Funding/support
This project was supported by an ACIP (Pasteur International Concerted Action) grant (ACIP2015-10) from Institut Pasteur Paris; and Grant #833 from Pasteur Institute of Iran, as partial fulfillment of MA Ph.D. dissertation (code: TP-9347).
Supplementary Materials
References
- 1.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przegla̜d gastroenterologiczny. 2019;14(1):26. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P, Piazuelo MB. The gastric precancerous cascade. Journal of digestive diseases. 2012;13(1):2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nature reviews. immunology. 2022;15:1–15. doi: 10.1038/s41577-022-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najdaghi S, Razi S, Rezaei N. An overview of the role of interleukin-8 in colorectal cancer. Cytokine. 2020;135:155205. doi: 10.1016/j.cyto.2020.155205. [DOI] [PubMed] [Google Scholar]
- 5.Bounder G, Jouimyi MR, Boura H, Touati E, Michel V, Badre W, Jouhadi H, Kadi M, Eljihad M, Benomar H, Kettani A, Lebrazi H, Maachi F. Associations of the -238(G/A) and -308(G/A) TNF-α Promoter Polymorphisms and TNF-α Serum Levels with the Susceptibility to Gastric Precancerous Lesions and Gastric Cancer Related to Helicobacter pylori Infection in a Moroccan Population. APJCP. 2020;21(6):1623–1629. doi: 10.31557/APJCP.2020.21.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Xiang C, Wu J, Dong W, Zhan Z, Wang R, Zhang JF. Relationship between serum inflammatory cytokines and lifestyle factors in gastric cancer. Molecular and clinical oncology. 2019;10(3):401–414. doi: 10.3892/mco.2019.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksoy EK, Akpınar MY, Doğan Ö, Göktaş Z, Sapmaz FP, Şimşek GG, Uzman M, Nazlıgül Y. Clinical Significance of Serum Vascular Endothelial Growth Factor, Pigment Epithelium-Derived Factor, Tumor Necrosis Factor Alpha, and Progranulin Levels in Patients with Gastric Cancer and Gastric Precancerous Lesions. Journal of gastrointestinal cancer. 2019;50(3):537–542. doi: 10.1007/s12029-019-00251-8. [DOI] [PubMed] [Google Scholar]
- 8.Kabir S, Grant C, Daar AS. Serum levels of interleukin-1, interleukin-6 and tumour necrosis factor-alpha in patients with gastric carcinoma. Cancer letters. 1995;95(1–2):207–12. doi: 10.1016/0304-3835(95)03895-4. [DOI] [PubMed] [Google Scholar]
- 9.Shimamura H, Iwagaki H, Gouchi A, Morimoto Y, Ariki N, Funaki M, Tanaka N. Autologous serum deprivation restored IL-1 receptor antagonist production by peripheral blood mononuclear cells in patients with gastric cancer. The Journal of international medical research. 2000;28(6):277–287. doi: 10.1177/147323000002800604. [DOI] [PubMed] [Google Scholar]
- 10.Makino T, Noguchi Y, Yoshikawa T, Doi C, Nomura K. Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. The British journal of surgery. 1998;85(12):1658–62. doi: 10.1046/j.1365-2168.1998.00938.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohno M, Kato M, Nakamura T, Saitoh Y. Gene expression for tumor necrosis factor alpha and its production in gastric cancer patients. Japanese journal of cancer research. 1994;85(10):1029–1034. doi: 10.1111/j.1349-7006.1994.tb02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.C W Wu, C W Chi, M C Hsieh, M F Chao, W Y Lui FKP. Serum tumor necrosis factor in patients with gastric cancer. Anticancer research. 1998;18(3A):1597–1599. [PubMed] [Google Scholar]
- 13.M Krönke, G Hensel, C Schlüter, P Scheurich, S Schütze KP. Tumor necrosis factor and lymphotoxin gene expression in human tumor cell lines. Cancer research. 1998;48(19):5417–5421. [PubMed] [Google Scholar]
- 14.Balkwill F, Burke F, Talbot D, Tavernier J, Osborne R, Naylor S, Talbot D, Durbin H, Tavernier J, Fiers W. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet (London, England) 1987;2(8570):1229–1232. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Zauco N, Torres J, Gómez A, Camorlinga-Ponce M, Muñoz-Pérez L, Herrera-Goepfert R, Medrano-Guzmán R, Giono-Cerezo S, Maldonado-Bernal C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: a controlled study. BMC Cancer. 2017;17(1) doi: 10.1186/s12885-017-3310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Xu L, Run ZC, Feng W, Liu W, Zhang PJ, Li Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World journal of gastroenterology. 2018;24(21):2269–2278. doi: 10.3748/wjg.v24.i21.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simó R, Barbosa-Desongles A, Lecube A, Hernandez C, Selva DM. Potential role of tumor necrosis factor-α in downregulating sex hormone-binding globulin. Diabetes. 2012;61(2):372–382. doi: 10.2337/db11-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature reviews. Immunology. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M, Kiernan CH, Stairiker CJ, Hope JL, Leon LG, van Meurs M, Brouwers-Haspels I, Boers R, Boers J, Gribnau J, van IJcken WFJ, Bindels EW, Hoogenboezem RM, Erkeland SJ, Mueller YM, Katsikis PD. Rapid in vitro generation of bona fide exhausted CD8+ T cells is accompanied by Tcf7 promotor methylation. PLoS pathogens. 2020;16 doi: 10.1371/journal.ppat.1008555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu K, Hui B, Wang Q, Lu C, Shi W, Zhang Z, Rong D, Zhang B, Tian Z, Tang W, Cao H, Wang X, Chen Z. Single-cell RNA sequencing of immune cells in gastric cancer patients. Aging. 2020;12(3):2747–2763. doi: 10.18632/aging.102774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephs SF, Ichim TE, Prince SM, Kesari S, Marincola FM, Escobedo AR, Jafri A. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. Journal of translational medicine. 2018;16(1):242. doi: 10.1186/s12967-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H, Yu D, Yang P, Guo R, Kong M, Gao Y, Yu X, Lu X, Fan X. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA Sequencing. Clinical and translational medicine. 2022;12(2) doi: 10.1002/ctm2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayasooriya RGPT, Moon DO, Park SR, Choi YH, Asami Y, Kim MO, Jang JH, Yeon Kim B, Seog Ahn J, Kim GY. Combined treatment with verrucarin A and tumor necrosis factor-α sensitizes apoptosis by overexpression of nuclear factor-kappaB-mediated Fas. Environmental toxicology and pharmacology. 2013;36(2):303–310. doi: 10.1016/j.etap.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes J, Michel V, Camorlinga-Ponce M, Gomez A, Maldonado C, De Reuse H, Torres J, Touati E. Circulating mitochondrial DNA level, a noninvasive biomarker for the early detection of gastric cancer. Cancer epidemiology, biomarkers and prevention. 2014;23(11):2430–2438. doi: 10.1158/1055-9965.EPI-14-0471. [DOI] [PubMed] [Google Scholar]
- 25.Konturek SJ, Starzynska T, Konturek PC, Karczewska E, Marlicz K, Lawniczak M, Jaroszewicz-Heigelman H, Bielanski W, Hartwich A, Ziemniak A, Hahn E G. Helicobacter pylori and CagA status, serum gastrin, interleukin-8 and gastric acid secretion in gastric cancer. Scandinavian journal of gastroenterology. 2002;37(8):891–898. doi: 10.1080/003655202760230838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request.