Abstract
Introduction:
Extracellular vesicles (EV) released constitutively or following external stimuli from structural and immune cells are now recognized as important mediators of cell-to-cell communication and are involved in the pathogenesis of pneumonia and sepsis, the leading causes of acute respiratory distress syndrome (ARDS) where mortality rates remain up to 40%. Multiple investigators have demonstrated that one of the underlying mechanisms of the effects of EVs are through the transfer of EV content to host cells, resulting in apoptosis, inflammation, and permeability in target organs.
Areas covered:
The current review focuses on preclinical research examining the role of EVs released into the plasma and injured alveolus during pneumonia and sepsis.
Expert opinion:
Inflammation is associated with elevated levels of circulating EVs which are released by activated structural and immune cells and can have significant proinflammatory, procoagulant, and pro-permeability effects in critically ill patients with pneumonia and/or sepsis. However, translation of the use of EVs as biomarkers or potential therapeutic targets clinically may be limited by current methodologies used to identify and quantify EVs accurately (whether from host cells or infecting organisms) and lack of understanding of the role of EVs in the reparative phase during recovery from pneumonia and/or sepsis.
Keywords: Acute Lung Injury, Acute Respiratory Distress Syndrome, Exosomes, Extracellular Vesicles, Microvesicles, Sepsis
1. INTRODUCTION
Extracellular vesicles (EV) are a heterogeneous group of vesicles released from cell membranes or from intracellular sources following various physiologic, pathologic, and/or external stimuli. Once considered cellular debris, EVs are now recognized as important mediators of cell-to-cell communication and regulators of cellular phenotype or behavior[1,2]. The content of EVs, such as nucleic acids, proteins, lipids, and organelles, can influence multiple biological processes of recipient cells. Therefore, EVs can contribute to physiological homeostasis as well as the pathogenesis of diseases or syndromes, depending on the cellular sources of the EVs and following the specific stimulus. Consequently, a growing number of research studies have focused on the roles of EVs in various fields including immunology, oncology, and inflammatory diseases[3–5].
Sepsis is defined as life-threatening end organ dysfunctions resulting from dysregulated host responses to infection. It is characterized by the hyperactivation of the coagulation system and up-regulation of the inflammatory responses, leading eventually to disseminated intravascular coagulation (DIC) and vascular hypo-reactivity. Sepsis is among the leading causes of mortality in intensive care units, and pneumonia is the most common cause of sepsis. The incidence of sepsis is 270 per 100,000/year, with a death rate of approximately 26%[6,7]. Despite decades of pre-clinical studies and clinical trials, sepsis has remained a fatal disease due to lack of sensitive and specific diagnostic tools and/or treatment guidelines. However, progress has been made in patient management as reflected from the Survival Sepsis Campaign and the recent, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[8]. However, there remains an increasing need for new diagnostic biomarkers and therapeutic strategies[9].
Recent promising studies have focused on the nature of inflammation at the level of cellular communication. These studies demonstrated the critical role of plasma EVs and inflammation, specifically that inflammation was associated with elevated blood levels of EVs[10,11]. EVs released from platelets, endothelial cells, and granulocytes were found to be increased in septic patients[12] and were inflammatory, activated endothelial cells, and had procoagulant effects[12–14]. These studies demonstrated that plasma EVs can affect the pathologic progression during inflammation via cell-to-cell interactions. As a result, EVs have emerged as a new possible area of study with diagnostic, prognostic, and/or therapeutic importance.
In this review, we provide the basic concept of EV and its role in severe pneumonia and sepsis. We also discuss the potential role of EVs as diagnostic markers or as therapeutic targets, focusing on acute lung injury (ALI) from pneumonia and sepsis.
2. CHARACTERIZATION OF EXTRACELLULAR VESICLES
2.1. EV Classification
EVs are spherical anuclear microparticles consisting of a phospholipid bilayer and containing various mRNAs, microRNAs, proteins/receptors, DNA and organelles. Although there are significant overlap, EVs are commonly classified into three major groups based on its size, biogenesis, and compositions: exosomes, microvesicles (MV), and apoptotic bodies (AB). In addition, due to the differences in biogenesis, each subgroup has its own characteristic surface and intracellular markers[15]. Exosomes are the smallest subgroup in size (range from 30 to 100 nm) and are produced from endocytic compartments, known as multi-vesicular bodies (MVB). Inward invagination of endosomal membranes produces intracellular MVBs, which contain intraluminal vesicles. MVBs subsequently fuse with the plasma membrane and release their intraluminal vesicles as exosomes[16]. From their endosomal origins, exosomes are enriched in mRNAs, microRNAs, DNA, and proteins that are specific to its cell of origin[17]. Exosomes contain an enriched amount of surface markers from endosomal origin, such as tetraspanins (CD9, CD63, CD81), heat shock protein, ALG-2 interacting protein X, tumor susceptibility gene 101, and MHC class I/II. They are also enriched in lipid rafts due to the process of organelle maturation. MVs had been referred as shedding vesicles, ectosomes, microparticles, or nanoparticles. They are the second largest vesicles (range from 100 to 1000 nm) and are released by direct budding and shedding off plasma membranes. Therefore, the surface markers of MVs vary depending on the composition of the cellular plasma membrane upon release[18]. MVs express abundant phosphatidylserine, cholesterol, ceramide, sphingomyelin, and cell surface-specific molecules such as receptors. They also contain cytoplasmic proteins like exosomes. ABs have the largest particle size amongst the three subgroups, with a diameter range of 100 to 5000 nm. Unlike exosomes and MVs which are released from living cells, ABs are formed by plasma membrane blebbing during the process of apoptosis. ABs display phosphatidylserine on its surface and contain potentially immunogenic cellular components such as fragmented DNA, non-coding RNAs, histones, and cell organelles[19].
In the past, there were no standard method or specific markers used to distinguish EV subgroups despite extensive research. Consequently, the International Society for Extracellular Vesicles (ISEV) was formed to generate some basic guidelines on the minimal information needed to interpret pre-clinical studies: EVs were now considered an umbrella term for all types of vesicles found in body fluids and research samples including exosomes, MVs, and ABs. The society recommended characterizing EVs by physical characteristics (size, density, etc.), isolation or separation techniques, biochemical compositions, biological functions, and description of the cells of origin[20].
2.2. EV Detection & Measurement
Methods of EV isolation and analyses are critical in the study of its function in any pathophysiologic state. Although the ISEV has provided technical protocols and recommendations, a standardized procedure for EV detection and measurements have not yet been established. The recent ISEV statement recommends evaluation of at least three proteins (transmembrane, lipid-bound protein, and cytosolic protein) and the use of two different but complementary techniques for EV analyses.
For EV isolation from biofluids or research samples, sequential centrifugation, density gradient centrifugation, filtration, and chromatography are often used[21,22]. Sequential ultracentrifugation is the most preferred method because it is convenient, inexpensive, and capable of processing large volumes of samples. However, EVs may be influenced by contamination from cellular debris/protein complexes and length of isolation time, and high centrifugation speed which may lead to EV breakdown. Therefore, combination of other techniques have been recommended to improve isolation quality. EVs can be characterized using numerous techniques such as flow cytometry, enzyme-linked immunosorbent assay, electron microscopy, and dynamic light scattering/nanoparticle tracking analysis (NTA)[21,22]. Among them, flow cytometry is the most commonly used technique for EV analysis and can detect particle size, concentration, and some cellular structures such as membrane proteins, but the detection limit may be insufficient for very small exosomes. Fluorescence flow cytometry can allow investigators to distinguish the cellular origin of EV based on the surface antigens. However, identifying the cellular origin of exosomes which are derived from MVBs are limited by the lack of a specific cellular marker. Electron microscopy can assess size, morphology, and specific markers but cannot quantitate the total count of EVs. NTA are often used to corroborate flow cytometry results in terms of size and number of EVs.
2.3. Interactions of EVs with Target cells
EVs are released by somatic cells constitutively or under stimuli and have important effects on cellular processes by interacting and/or transferring its contents to target cells. Some EVs decompose shortly after secretion, resulting in a rapid response on adjacent cells[23,24]. Whereas, other EVs move passively or traffic to target cells with its structure intact. EVs from multiple cellular origins are commonly found in blood and other body fluids including the urine, sputum, pleural effusion, ascites, cerebrospinal fluid, saliva, synovial fluid, semen, and breast milk[25].
EVs act on target cells through surface receptor-ligand interactions, direct membrane fusion, or through uptake by endocytosis. This interaction results in direct stimulation of target cells or transfer of various vesicular contents including genetic information[25,26]. Based on the stimuli and cell of origin, EVs may contain specific substances which are transferred preferentially to certain cell types. For example, EVs from bone marrow dendritic cells were internalized by splenic dendritic cells, and to a lesser extent by B lymphocytes and macrophages, but not by splenic T cells[27]. This specificity was mediated by protein surface receptors and adhesion molecules on EVs and target cells.
3. EXTRACELLULAR VESICLES IN PLASMA
EVs released into the plasma normally contribute to physiological homeostasis but also have profound inflammatory roles during pneumonia and sepsis. EV concentration in plasma is determined by a dynamic balance between its formation and elimination in the liver and spleen. EVs in normal individuals exist at low levels. During injury, increased concentration of EVs derived from platelets, endothelial and epithelial cells, and leukocytes are generated via inflammation[11,28,29]. Most of the circulating EVs in the plasma are platelet-derived EVs (PEV) in both physiologic and inflammatory conditions[30]. PEVs can serve as catalysts for inflammation by stimulating leukocytes, endothelial cells, and even platelets themselves. Endothelial cell-derived EVs (EEV) and leukocyte-derived EVs (LEV) also play critical roles in inflammation. EVs from inflammatory cells promote synthesis of inflammatory cytokines/chemokines, expression of cell adhesion molecules, production of lipid mediators such as lysophosphatidic acid and arachidonic acid (AA) in recipient cells[31]. In a positive feeback loop, inflammatory cytokines can stimulate additional EV secretion from target cells[32]. The following is a summary of the role of EVs derived from each cell type involved in inflammation.
3.1. Platelet Derived EVs
PEVs acts on leukocytes and activated endothelial cells at the site of vascular injury. These EVs contribute to the activation of the immune system by facilitating cell aggregation, cell-to-cell interactions, and the release of cytokines[33–35]. PEVs induce platelet aggregation and enhances leukocytes recruitment via chemokine release as well as the recruitment of other immune cells such as monocytes, T- and B- lymphocytes, and natural killer cells. The binding of PEVs to P-selectin and P-selectin glycoprotein ligand 1 enhances leukocyte-leukocyte interactions and monocyte-endothelial cell interactions[36,37]. PEVs increase the expression of cyclooxygenase-2 in monocytes, which converts AA into prostacyclin. Prostacyclin causes vasodilation that allows inflammatory cells to reach the site of infection[38]. PEVs can stimulate production of pro-inflammatory cytokines including IL-1, IL-6, IL-8, TNF-α, and monocyte chemoattractant protein 1 (MCP-1). These cytokines can activate inflammatory cells to release additional EVs, further aggravating the injury[39]. For example, in transfusion-related ALI, a high concentration of the ligand CD40L/CD154 on PEVs were correlated with antigen-specific adverse reactions[40].
3.2. Endothelial Cell-Derived EVs
EEVs stimulated by endotoxin or inflammatory cytokines carry tissue factor with procoagulant properties[41,42]. Therefore, they can bind coagulation factors and promote thrombin generation. In addition, EEVs can bind to undamaged and inactivated endothelial cells, increasing expression of intercellular adhesion molecule 1 (ICAM-1) and enhancing monocyte-endothelial cell interactions. These EVs also promote endothelial proliferation, angiogenesis, and cell invasion. EEVs bound to monocytes facilitates migration of monocytes through the endothelium[39].
3.3. Leukocyte Cell-Derived EVs
LEVs secreted by activated polymorphonuclear leukocytes promote release of inflammatory cytokines and can stimulate other regulatory inflammatory molecules like PEVs[39]. These EVs can also stimulate angiogenesis by up-regulation of proangiogenic chemokines[43]. Each LEV has a similar phenotype with the leukocyte from which it is derived. For example, neutrophil-derived EVs (NEV) have an autocrine effect increasing neutrophil chemotaxis. Monocyte-derived EVs (MEV) have been reported to stimulate expression of ICAM-1 by activation of nuclear factor(NF)-κB translocation to the nucleus. T-cell-derived EVs stimulate production of TNF-α and IL-1β by monocytes[43].
4. EXTRACELLULAR VESICLES IN PNEUMONIA
The critical role of EVs in the pathogenesis of ALI is now being recognized. Recent studies have demonstrated that EVs in the plasma as well as alveolar space during the exudative phase of ALI are involved in inflammation and endothelial protein permeability/pulmonary edema, similar to the role played by its parent cells from which they are derived (Figure 1).
Figure 1. Extracellular Vesicles in the Pathogenesis of Acute Lung Injury.
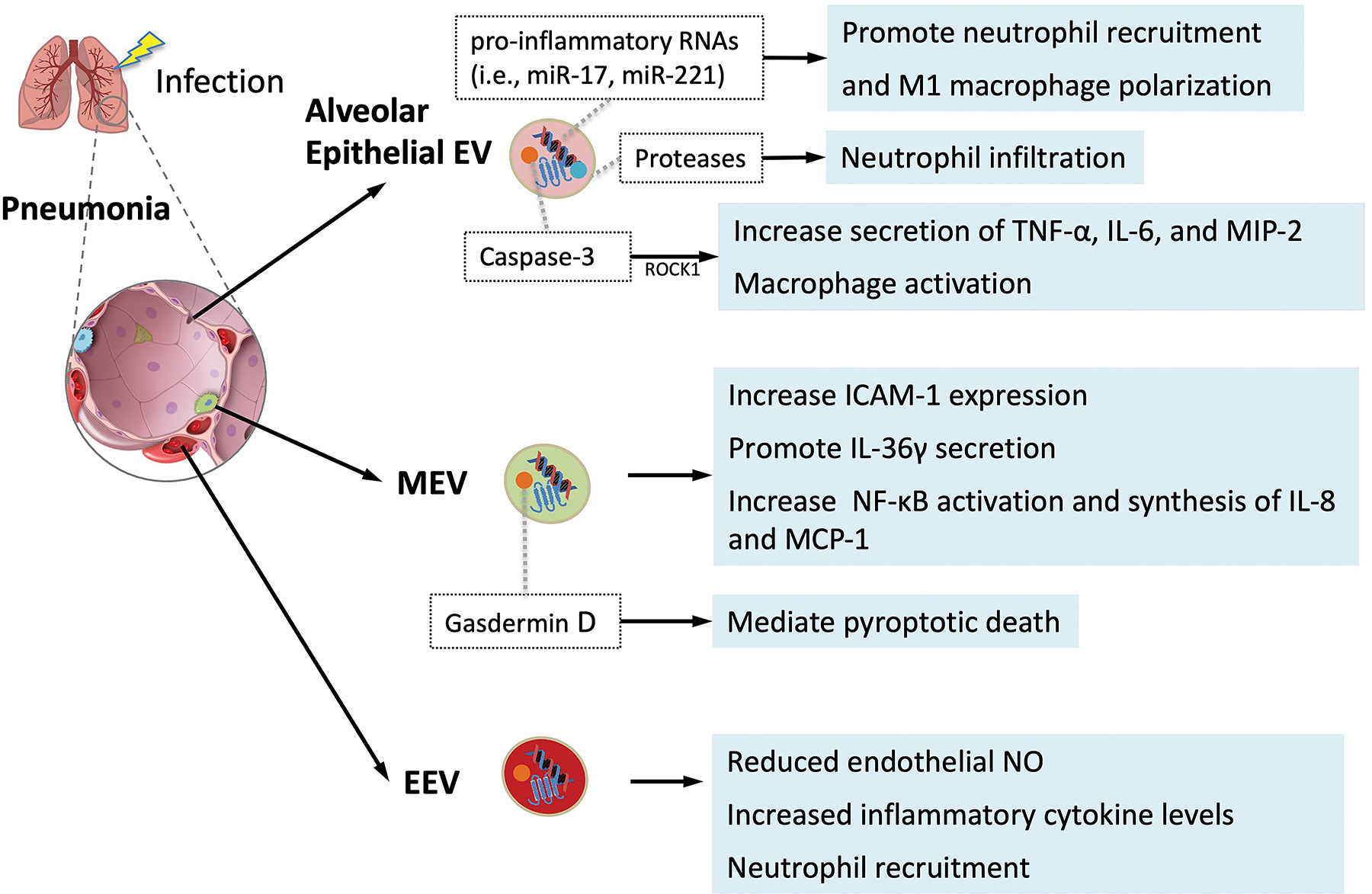
Schematic of potential mechanisms of EVs in the pathogenesis of acute lung injury following pneumonia. The role of alveolar epithelial cell, monocyte/macrophage, and endothelial cell derived EVs are presented based on pre-clinical investigations with a focus on inflammation and endothelial and epithelial cells apoptosis leading to lung inflammation and protein permeability and pulmonary edema. EEV, endothelial cell derived EVs; MEV, monocyte/macrophage derived EVs; MIP-2, macrophage inflammatory protein 2; NFκβ, Nuclear Factor kappa-light-chain-enhancer of activated B cells; ROCKI, Rho-associated coiled-coil kinase I; ICAM-1, intercellular adhesion molecule 1; MCP 1, monocyte chemoattractant protein 1; NO, nitric oxide, TNF-α, tumor necrosis factor alpha.
The concentration of alveolar epithelial cell-derived EVs can increase with any inflammatory injury such as with lipopolysaccharide (LPS) induced activation of alveolar epithelial cells via Toll-like receptor (TLR)-4[44]. Theses epithelial EVs can induce or aggravate pulmonary inflammation by macrophage activation. Macrophages incubated with these EVs have increased secretion of TNF-α, IL-6, and macrophage inflammatory protein 2 (MIP-2). In addition, these epithelial EVs contain caspase-3, a proapoptotic factor, which can activate macrophages via the Rho-associated coiled-coil kinase I pathway. Capase-3-deficient EVs have less lung injury in vivo compared to control epithelial EVs[45]. LPS-induced epithelial EVs also contain proteases that act as neutrophil-chemoattractant, leading to increased neutrophil infiltration[44]. In mice injured with alveolar epithelial EVs, the bronchoalveolar lavage fluid (BALF) contained increased number of macrophages and neutrophils[46]. In contrast, inhibition of EV release was associated with reduce airway inflammation[47]. In terms of mechanisms, these EVs contain abundant levels of pro-inflammatory RNAs including miR-17 and miR-221. The transfer of these miRNAs from alveolar epithelial cells to macrophages can induce up-regulation of integrin β1 and promote neutrophil recruitment and M1-macrophage polarization[48,49]. Interestingly, EVs enriched for miR-17–5p increased viral replication in influenza A patients[50]. Alveolar epithelial EVs also have potent procoagulant effects because they are enriched for tissue factors[51]. All these properties suggest that alveolar epithelial EVs may be a therapeutic target in pneumonia and ARDS.
Multiple recent studies have demonstrated that intra-tracheal LPS induced the release of alveolar macrophage-derived EVs (MEV) into the injured alveolus[52,53] which subsequently triggered EV release from alveolar epithelial cells and neutrophils. LPS induced increased alveolar macrophage-derived MVs led to increased ICAM-1 expression, resulting in increased influx of neutrophils and proteins into the injured alveolus, possibly due to elevated levels of TNF-α in the EVs[53]. Both gram-negative and gram-positive bacteria promoted IL-36γ secretion in alveolar MEVs, causing lung injury[54]. Human bronchial epithelial cells treated with monocyte/macrophage-derived MVs led to an increase in NF-κB activation and synthesis of IL-8 and MCP-1[55]. The plasma of patients with sepsis and ARDS contained MEVs with elevated gasdermin D which is known to mediate pyroptotic death. Incubation with LPS-induced MEVs induced human alveolar endothelial cell death[56]. These studies suggest that alveolar MEVs, containing inflammatory and pro-apoptotic mediators, may play an important role in initiating an acute inflammatory response in the injured alveolus. However, Zhu et al. also found that LPS-stimulation significantly increased miR-221 and miR-222 levels in alveolar macrophage-derived ABs, which targeted cyclin-dependent kinase inhibitor 1B gene and promoted alveolar epithelial cell growth, possibly as a reparative mechanism[52].
Alveolar endothelial cells release EVs in response to various inflammatory stimuli such as LPS, plasminogen activated inhibitor-1, and mechanical stretch[57,58]. Alveolar EEVs can contribute to the inflammatory response in an autocrine fashion; these EVs reduced endothelial nitric oxide (NO) production which was followed by arteriolar vasodilation and pulmonary edema in vivo[59,60]. EEVs also induced alveolar neutrophil recruitment, increased inflammatory cytokine production including TNF-α, IL-1β, and myeloperoxidase, and damaged the alveolar barrier inducing ALI. Other studies suggested that the initial endothelial injury induced the release of alveolar EEVs, which primed the lung to produce a more robust inflammatory response when exposed to subsequent infectious stimuli[61]. A recent study with an ex vivo perfused human lung model also suggested the critical role of alveolar EEVs in bacterial pneumonia[62]. In this study, intrabronchial E.coli bacteria instillation induced the release of alveolar EVs, mainly EEVs and PEVs as identified by flow cytometry, into the perfusate. These pathogenic EVs resulted in lung injury which were similar to E.coli bacteria pneumonia when administered intra-bronchial or intravenous into naïve perfused human lungs. These EVs contained high concentrations of TNF-α and IL-6. When incubated with monocytes, these EVs led to increased secretion of inflammatory cytokines.
In contrast, EVs released from leukocytes (LEV) or neutrophils (NEV) may have a protective effect in lung injury. In a clinical trial in patients with early ARDS, Guervilly et al. found that elevated BALF and plasma levels of LEVs were correlated with increased survival rate and ventilator-free days[63]. Similarly, Lashin et al. demonstrated that high levels of circulating LEVs with α2-macroglobulin was associated with improved survival rates in critically ill patients with pulmonary sepsis, but not with fecal peritonitis[64]. In vitro, treatment with α2-macroglobulin expressing LEVs attenuated endothelial permeability and potentiated neutrophil phagocytosis. Therefore, LEVs may have a protective effect and be considered as a prognostic marker in ARDS patients. Neutrophil has been known to play a major role in the development of inflammatory lung injury; alveolar infiltration of neutrophils is a characteristic feature of ARDS. Multiple investigators found that intratracheal or intravenous administration of plasma EVs from LPS-treated rats resulted in ALI and a significant increase in inflammatory mediators in both the BALF and plasma when instilled into normal rats[65], suggesting that EVs were inflammatory and NEVs were a major component of these EVs. However, recent studies have also demonstrated that NEVs may have anti-inflammatory effects on macrophages and alveolar epithelial cells. NEVs bound to Mer tyrosine kinase receptor on macrophages, promoting secretion of transforming growth factor-β and inhibited secretion of TNF-α and IL-8[66,67]. NEVs also contained miR-223 that suppress inflammatory properties of alveolar epithelial cells[68]. In this study, Neudecker et al. found that NEVs suppressed inflammatory cytokine release and pulmonary vascular permeability, reducing ventilator induced lung injury in mice. Part of the anti-inflammatory effect of NEVs may be derived from its bacteriostatic properties. Timar et al. found that NEVs released from neutrophils exposed to opsonized bacteria aggregated both nonopsonized and opsonized bacteria, leading to reduced bacterial growth; this effect was dependent on β2 integrin function, actin remodeling and on the glucose supply[69]. Further research is needed to determine how the environment effects the phenotype of NEVs (i.e., pro-inflammatory vs. anti-inflammatory). Recently, Kolonics et al. found that EVs released from resting neutrophils had anti-inflammatory effects on other neutrophils. Whereas, EVs released from neutrophils stimulated with opsonized particles were inflammatory, increasing the production of reactive oxygen species and cytokines from other neutrophils[70]. And perhaps importantly, although the assumption is that EVs retain the phenotype of the parent cell, future research is needed to determine if EVs can have properties opposite of its parent cell in ALI or sepsis.
4.1. Effect of Mechanical Ventilation on EV Release and Acute Lung Injury
Endotracheal intubation and mechanical ventilation is the primary supportive therapy for patients who develop respiratory failure from pneumonia to maintain oxygenation and ventilation. For patients who progresses to ARDS, lower tidal volume ventilation is essential to prevent further aggravation of lung injury and reduce mortality[71]. EVs are now recognized as playing a significant role in ventilation induced lung injury (VILI) resulting from inappropriate higher tidal volume ventilation. Various groups have described a marked increase of circulating endothelial-derived microparticles or EEVs following VILI which was capable of causing significant lung inflammation and injury when administered to naïve mice[72,73]. Interestingly, adoptive transfer of circulating EVs from preterm rats with VILI led to neuroinflammation and microglial activation in the brain in normal newborn rats[74]; among survivors, patients with ARDS had significant neurocognitive morbidity and decreased quality of life that persisted following hospital discharge[75].
5. EXTRACELLULAR VESICLES IN SEPSIS
During sepsis, the plasma levels of EVs are significantly increased, potentially spreading to distance organs through the systemic circulation[76,77] (Figure 2). Mortaza et al. found that EVs derived from septic rats reproduced hemodynamic, inflammatory, and oxidative stress patterns of sepsis in healthy rats[78]. In these experiments, EVs from septic rat increased superoxide anion production, NF-κB activation, nitric oxide synthase (NOS)-2 expression, and NO overproduction. Similarly, Mastronardi et al. found that circulating EVs from septic patients exerted tissue-selective expression of pro-inflammatory proteins in rat, which was not caused by EVs from healthy patients[79]. In this study, rats exposed to septic EVs had increased oxidative and/or nitrate stress, which was expressed in multiple organs to varying degrees. These findings suggested distant dissemination of circulating plasma EVs and its role in the pathogenesis of multiorgan dysfunction in septic shock. EVs from the plasma extracted from septic patients promoted oxidative stress and apoptosis of endothelial cells in vitro through a nicotinamide adenine dinucleotide phosphate oxidase-dependent pathway[12]. Therefore, EVs can play a critical role in both the initiation and propagation of sepsis. In addition to the lungs, EVs from septic shock patients had inflammatory effects on other organs in the mice[79]. In the heart, there was increased reactive oxygen species levels with increased expression of iNOs, COX-2, and NF-kB. Increased oxidative stress was also found in the liver. This was consistent with clinical findings of multiorgan failure in early septic shock, suggesting a potential role of EV dissemination in multiorgan failure. PEVs induced and exacerbated cardiac dysfunction in a NO-dependent manner in isolated rabbit hearts and papillary muscles[80]. PEVs also facilitated endothelial cell apoptosis via NO synthesis, suggesting the possibility of PEV as a target in treating vascular dysfunction in sepsis[81]. In addition, macrophages pretreated with GW4869 (inhibitor of EV synthesis and release) attenuated cardiac dysfunction and improved survival in LPS-induced and cecal ligation and puncture models of sepsis[80]. Choroid plexus epithelial (CPE) cell-derived or circulating plasma EVs are the main source of brain parenchymal inflammation in sepsis[82,83]. The concentrations of CPE-EVs and its pro-inflammatory miRNAs are increased in sepsis. CPE-EVs might transfer pro-inflammatory miRNAs to the brain parenchyma, resulting in neuroinflammation and cognitive dysfunction associated with sepsis[84].
Figure 2. Extracellular Vesicles in the Pathogenesis of Sepsis.
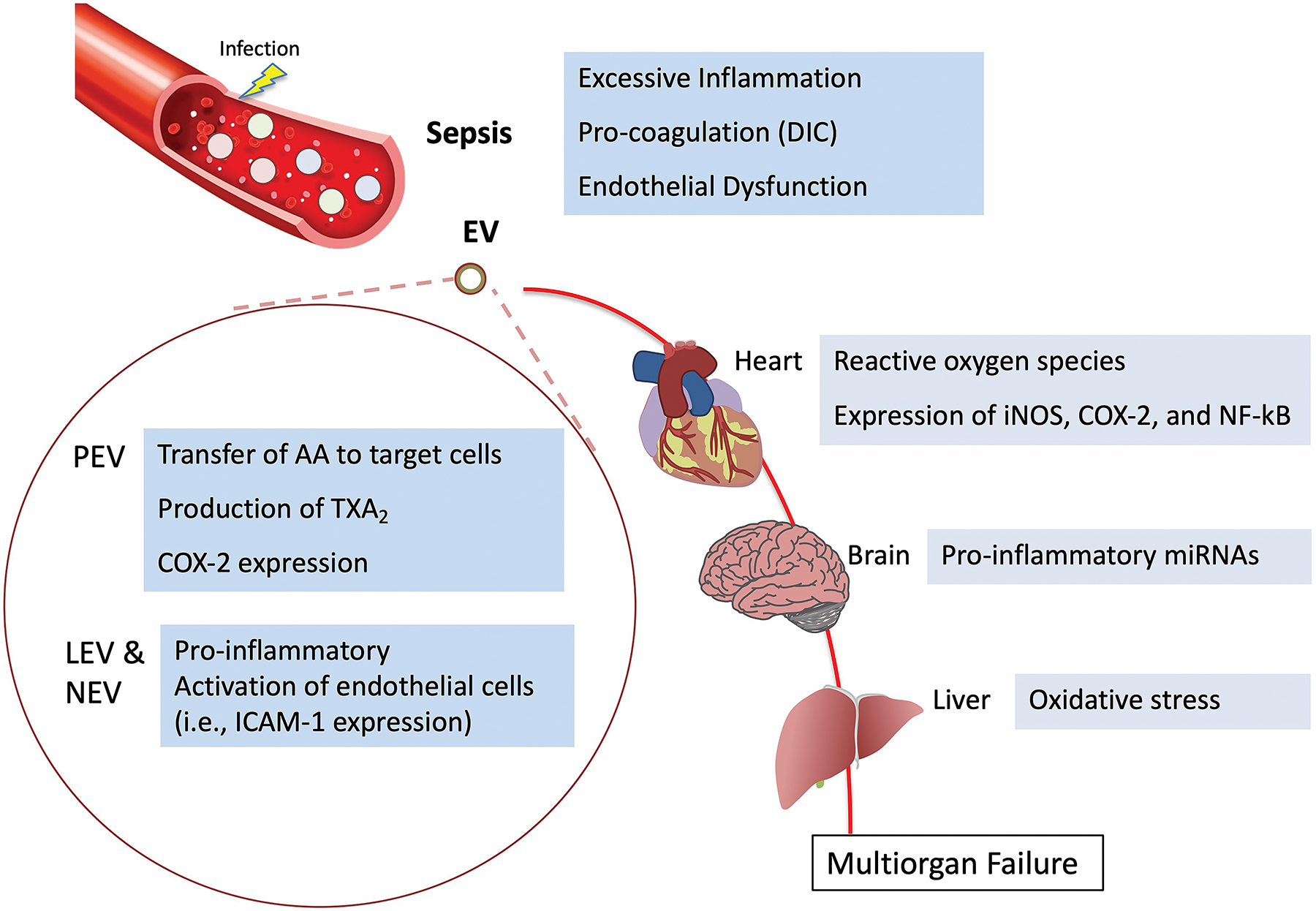
Schematic of the critical role of EVs in the pathogenesis of sepsis leading to multiorgan dysfunction and failure. The role of platelet, leukocyte and neutrophil derived EVs are presented based on pre-clinical studies with a focus on inflammation, coagulation, and endothelial barrier dysfunction. AA, arachidonic acid; COX-2; cyclooxygenase-2; DIC, Disseminated intravascular coagulation; iNOS, inducible nitric oxide synthase; LEV, leukocyte derived EVs; NEV, neutrophil-derived EVs; PEV, platelet derived EVs; TXA2, Thromboxane A2.
Immune cell-derived EVs are known to stimulate cytokine release, monocyte chemotaxis, and tissue factor induction. They also modulate NO and prostacyclin production in endothelial cells[77,85]. Therefore, they have pro-inflammatory and prothrombic properties as well as can induce endothelial dysfunction, which may contribute to multiorgan failure and septic shock[11,86]. We will now explore the properties of EVs derived from each immune or inflammatory cells in terms of inflammatory response, coagulation cascade, and endothelial dysfunction.
PEVs induce an inflammatory response through intercellular transfer of AA to target cells, which can lead to the production of TXA2 and COX-2 expression in endothelial cells[38,87]. Transferred AA from EVs triggers platelet aggregation, monocyte-endothelial cell interaction, and monocyte chemotaxis[87,88]. The binding of PEVs to leukocytes facilitate leukocyte aggregation and phagocytosis[89]. Lehner et al. reported that the count of PEVs, not EEVs, was significantly correlated with mortality in septic shock patients[90]. In addition, Ohuchi et al. reported that the PEV/platelet ratio was associated with hospital mortality and coagulopathy in critically ill patients[91]. LEVs also can promote an inflammatory response by stimulating endothelial cells via cytokine expression[92]. Activated LEVs contain overexpressed adhesion molecules on the surface[28]. Interestingly, EVs can have anti-inflammatory properties as well, perhaps contributing to the immunomodulation seen in sepsis. PEVs contain not only inflammatory TXA2 but also lipoxin A4 (LXA4). The administration of PEVs and LXA4 attenuated the inflammatory responses in a murine colitis model[93]. A recent study demonstrated that PEVs isolated from stored human platelets reduced the release of TNF-α and IL-10 by macrophages[94]. These PEVs also inhibited the differentiation of monocytes into immature dendritic cells and maturation of immature dendritic cells by LPS. Immature dendritic cells had partially impaired phagocytic activities. In an in vitro study, NEVs inhibited neutrophil chemotaxis response which was mediated by annexin I, which binds to phosphatidylserine on the EV surface[95]. Other studies demonstrated that NEVs contained precursors for lipid mediators such as LXA4, resolvins and protectins[96]. These findings suggest that EVs may have a role in the immunosuppression found in later stages of sepsis.
EVs can be procoagulant in part due to tissue factor and phosphatidylserine on its surfaces. Tissue factor is a transmembrane receptor for Factor VII/VIIa, the primary initiator of the extrinsic coagulation cascade. Tissue factor on the EV surface is not normally expressed on the plasma membrane under physiologic conditions. However, it is present in the plasma membrane during an infectious injury, perhaps as a kind of host defense mechanism against infection. However, excessive expression can induce sepsis-induced coagulopathy. Woei et al. found that the activity of tissue factor on circulating EVs was correlated with disease severity in patients with E.coli urinary tract infection[97]. Circulating tissue factors are mainly from MEV as well as LEV, EEV, and PEVs. On the surface of MEVs, thrombomodulin and tissue factor coexist in a physiologic state. However, tissue factor activity becomes dominant with LPS stimulation[98]. These EVs can lead to endothelial cell apoptosis, resulting in the loss of the anti-coagulant properties of the endothelium[99]. Tissue factors on LEVs are targeted to the site of endothelial injury though interaction of P-selectin on platelets and PSGL-1 on leukocytes[100,101]. EEVs also have procoagulant activities with expression of von Willebrand factor and coagulation factor binding sites[102,103]. Both CD-105 and CD-31 are expressed on endothelial cells as growth factor receptor and platelet adhesion molecule respectively[104,105]. In a cohort study, Delabranche et al. demonstrated that increase in CD-105 labeled EVs and decrease in CD-31 labeled EVs were strongly correlated with early DIC in septic shock[106]. Therefore, these markers may be useful to predict early vascular injury in septic patients. Tissue factors on PEVs interact with macrophages, endothelial cells, or other platelets[107]. Phosphatidylserine from PEV, LEV, and EEV also contribute to coagulopathy in sepsis. The plasma level of phosphatidylserine was higher in septic patients, and endothelial cells treated with septic EVs expressed more phosphatidylserine on the surface compared to EVs from healthy controls[108]. Tripisciano et al. showed that PEVs added to EV-free human plasma resulted in thrombin formation[109]. Thrombin formation was inhibited by annexin V (phosphatidylserine antagonist), but not by tissue factor antibodies, suggesting that thrombin is primarily generated by the exposure of phosphatidylserine on PEV surfaces. Phosphatidylserine on PEVs can enhances factor Va, Xa, and tissue factor activity[110]. PEVs themselves express binding sites for factor IXa, Xa, and VIII. They attach to the subendothelial matrix, increasing the aggregation of more platelets[111]. In addition, PEVs had increased nicotinamide adenine dinucleotide phosphate hydrogen oxidase activity, which can causes oxidative stress and apoptosis of endothelial cells[112].
In addition to its role in inflammation, EVs can contribute to endothelial cell dysfunction with increased endothelial permeability and reduced vascular reactivity to NO. In vitro, EEVs can impair normal endothelial functions by increased oxidative stress in a dose-dependent manner[113]. EEVs attenuate NO release and endothelial NOS (eNOS) phosphorylation in both animal and ex vivo human studies[60]. In a clinical trial, Forest et al. found high levels of EEVs were related with vascular dysfunction in elderly patients, suggesting its role in tissue hypoperfusion and organ dysfunction in septic shock[114]. EVs released from T- lymphocyte also reduced eNOS expression and NO production[115]. These EVs triggered vascular hypo-reactivity through NF-κB activation, increased superoxide anion production, overproduction of iNOS, and reduced activity of eNOS[78,116]. In contrast, PEVs were found to transport AA to epithelial cells, leading to the metabolism to TXA2 and resulting in vascular hyperreactivity. Mostefai et al. found that sensitivity to serotonin and contractility of mouse aorta was increased with EVs from septic patients[117]. This finding suggested that EVs from septic patients might have a protective effect against vascular hypo-reactivity associated with hypotension in septic shock. However, this may be compensatory mechanism to the vascular hypo reactivity during the early phase of septic shock[118].
6. EXTRACELLULAR VESICLES IN COVID-19
Recent publications have suggested that EVs may play a significant role in the pathogenesis of viral infections such as COVID-19 from SARS-CoV-2[119]. Several potential mechanisms are proposed: 1) Cells that express ACE2, which is critical for binding and entry of SARS-CoV2 into target cells, and CD9, which may work with transmembrane protease serine 2 to cleave the ACE2 receptor and facilitate viral entry, can transfer these viral receptors to other cells via EVs, making recipient cells more susceptible for SARS-CoV2 infection[120,121]; 2) And once infected by SARS-CoV2, cells can release EVs containing viral particles (i.e., viral RNA and proteins)[122], which can accelerate and spread the infection. For example, Kwon et al. found that EVs released by A549 lung epithelial cells overexpressing SARS-CoV2 genes, contained viral RNA, and stem cell derived cardiomyocytes incubated with these EVs contained viral genes[123]; aside from alveolar epithelial cells, ACE2 is expressed in cardiomyocytes which may account for the myocardial injury seen with COVID-19 infection. Similar to bacterial pneumonia and sepsis, these studies demonstrate the potential use of EVs released during COVID-19 infection for diagnostic purposes and as therapeutic targets.
7. POTENTIAL DIAGNOSTIC USE OF EV
Guervilly et. al found that higher levels of LEVs in the blood and BALF were associated with improved survival in patients with early-stage ARDS[63]. Another recent study found that the level of circulating EVs was inversely correlated with risk of ARDS in critically ill patients[124]. Both studies demonstrated that EVs may be a prognostic biomarker of ARDS. Researchers are now investigating whether EV content can indicate lung damage. Sphingosin-1 phosphate receptor-3 (S1PR3) in EVs is emerging as a potential diagnostic marker for the severity and outcome associated with inflammatory diseases such as ALI[125]. In vitro, LPS injured endothelial cells released S1PR3-containg EVs, and elevated concentrations of circulating S1PR3-containg EEVs were associated with higher mortality in septic patients. Dakhlallah et al. found that EVs containing DNA methyltransferase, when coupled with total plasma EV number, may be a novel method to diagnose septic shock in patients upon ICU admittance[126]. All these studies demonstrate potential therapeutic targets or offer opportunities to more precisely intervene during sepsis with standard management to regulate EV number or content and potentially patient outcomes (Figure 3)
Figure 3. Potential Use of Extracellular Vesicles as Biomarkers, Therapeutic Targets or as Therapeutic Agents.
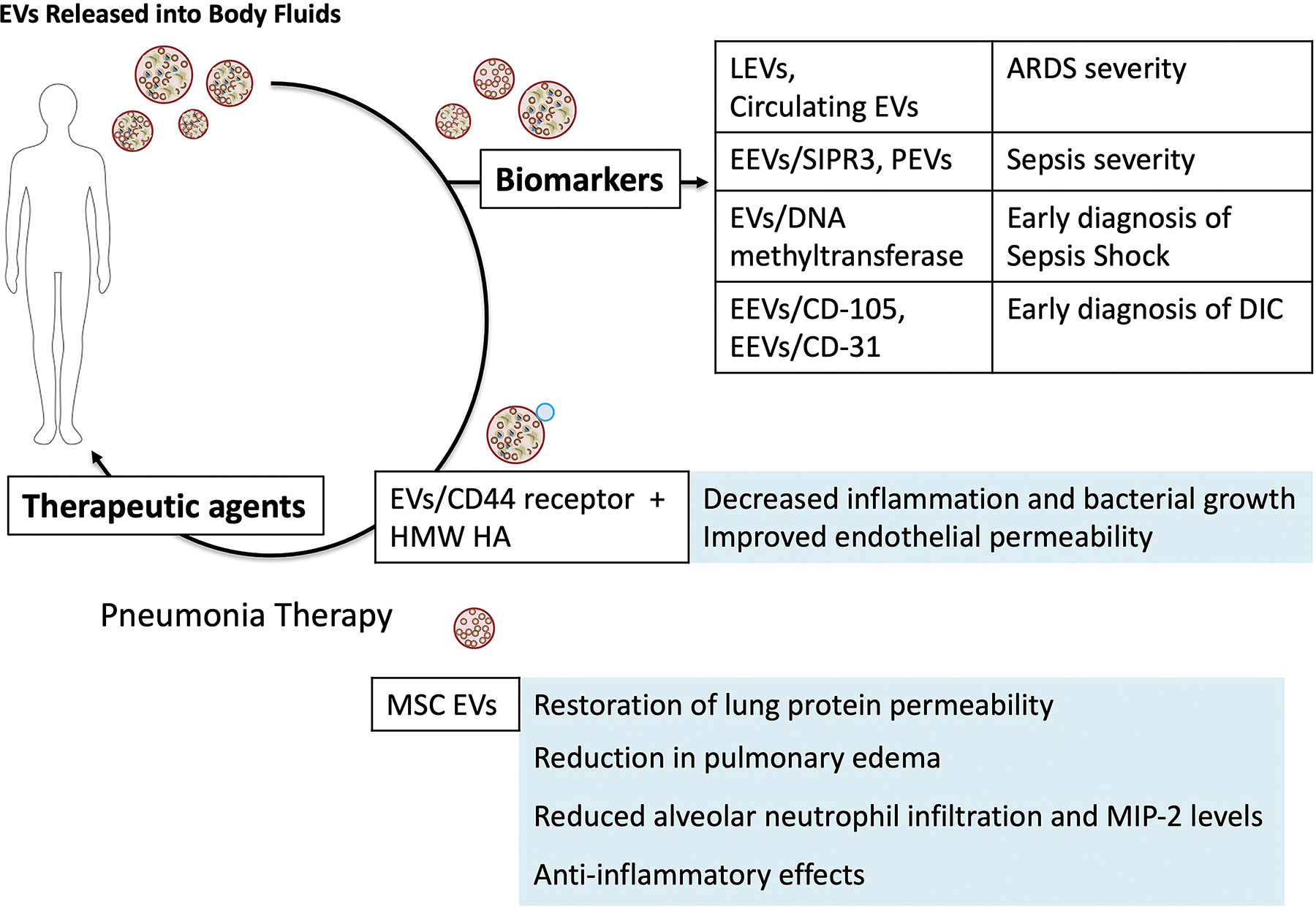
Schematic of the potential use of EVs clinically based on pre-clinical research. Understanding the importance of EVs in the pathogenesis of diseases and syndromes have provided new insight into onset and progression of injury, paving the way for the use of EVs as potential diagnostic markers in syndromes such as ARDS and sepsis. In addition, attempts have been made by multiple investigators to suppress or inhibit plasma EVs to prevent the progression of injury. For example, administration of HMW HA reduced inflammation, endothelial permeability, and bacterial growth in ex vivo perfused human lungs injured with severe bacterial pneumonia in part by binding to inflammatory plasma EVs. Lastly, new therapeutics are being developed in the field of cell based therapy for ARDS or sepsis to take advantage of the properties of EVs released by these stem or progenitor cells (similar phenotype as parent cells, small size, ease of storage increasing availability, and ability to traffic to the site of inflammation). S1PR3, Sphingosin-1 phosphate receptor-3; DIC, disseminated intravascular coagulation; HMW HA, high molecular weight hyaluronic acid; MSC: Mesenchymal stromal or stem cell.
Investigators are now exploring the use of EVs from pleural fluids/effusions as diagnostic biomarkers[127]. In patients with lung cancer, Roman-Canal et al. identified miRNA-1–3p, miRNA-144–5p and miRNA 150–5p as promising biomarkers of lung cancer diagnosis[128]. In patients with ALI, Lin et al. found elevated EV levels of miR-205–5p and miR-200b in patients with pneumonia compared to EVs from patients with pulmonary tuberculosis or lung cancer[129].
8. USE OF EV AS THERAPEUTIC TARGETS OR AS THERAPEUTIC AGENTS
Given the inflammatory nature of EVs released into the plasma during ALI or sepsis, attempts have been made to suppress the biological effects of these EVs. For example, Liu et al. found that EVs released into the plasma during E.coli pneumonia in ex vivo perfused human lungs induced ALI when administered intravenously into naïve lungs, and intravenous administration of high molecular weight hyaluronic acid (HMW HA) as therapy neutralized these inflammatory EVs by binding to CD44 receptors on the EVs and diminishing its effect[62]. The implication of these studies may be significant in further understanding the pathogenesis of ARDS from pneumonia. Similar to the role of EVs in establishing the tumor microenvironment or the pre-metastatic niche[130], circulating EVs during pneumonia may migrate/traffic easily into the injured alveolus due to its small size and interact with target cells (i.e., alveolar epithelial, endothelial and macrophages and the extracellular matrix) to prepare for the migration of professional phagocytes into the lung. Targeting circulating EVs during the exudative phase may be one method to prevent or dampen the “cytokine storm” in ARDS. Hyaluronan or hyaluronic acid, a non-sulfated glycosaminoglycan composed of repeating polymeric disaccharides, D-glucuronic acid and N-acetyl-D-glucosamine, is one of the chief component of the extracellular matrix essential for maintaining the normal structure of alveolar air-blood barrier[131]. During lung injury and diseases, HMW HA (> 1000 kDa), the predominant form of HA in health, undergoes degradation by lysosomal hyaluronidases, reactive oxygen and nitrogen species, and inflammatory mediators[132] into low-molecular weight HA (< 500 kDa). Surprisingly, in contrast to HMW HA, exogenous administration of LMW HA can decrease endothelial cell barrier function and induce inflammation[133] in part by binding to cell surface receptors such as CD44, TLR2 and 4, HABP2 or RHAMM[134]. Investigators have attempted to take advantage of these opposing properties of HA for therapy. Singleton et al. found that intravenous administration of HMW HA 4 h following LPS induced ALI improved endothelial permeability[135] via activation of sphingosine 1 phosphate, Akt and Rac[136]. Muto et al. found that mice exposed to HMW HA prior to LPS induced sepsis had reduced serum IL-6 and TNFα levels; however, the protective effect of HMW HA in sepsis was lost in CD44(−/−) mice[137]; CD44 is commonly expressed on innate immune cells and its released EVs which may be critical for cell or EV trafficking to sites of injury[138]. In addition, Lee et al. recently found that incubation of RAW264.7 cells with HMW HA increased phosphorylation of ezrin/radixin/moesin, a known downstream target of CD44, and increased E.coli bacterial phagocytosis by the mouse macrophage cell line[139].
Mesenchymal stromal or stem cells (MSC) are multipotent adult cells derived from a variety of tissues (i.e., bone marrow, fat, umbilical cord, etc.) with regenerative and immunomodulatory properties[140]. Based on many pre-clinical studies demonstrating therapeutic efficacy in lung injury/diseases, multiple clinical trials have been completed or are underway with the use of MSC in ARDS or sepsis including from COVID-19 with some biological effects seen. However, several long term safety concerns are present with MSC administration: 1) Risk of malignant differentiation, 2) Difficulty and complexity of storage (i.e., DMSO and storage in bone marrow transplant facility) for clinical use, and 3) Respiratory and hemodynamic effect of transfusion due to the size of MSC[141]. Therefore, many investigator have studied the therapeutic use of MSC-derived EVs as a safe alternative or as a “cell-free therapy” for both ARDS and sepsis. MSC EV provide several advantages compared to MSC: 1) Because MSC EV are not self-replicating, the risk of malignant differentiation is significantly reduced; 2) MSC EVs can be stored in a −80°C freezer without preservatives and still maintain biological activity; 3) And due to their size, MSC EV infusion do not cause significant respiratory or hemodynamic effects, allowing multiple administrations. In preclinical small animal studies, MSC EV have showed therapeutic benefits in both sterile and pathogen-induced lung injury models similar to the parent cells. (A thorough review of the therapeutic use of MSC EV as well as EV from other progenitor or stem cells in ARDS and sepsis can be found by Liu et al[142]). The mechanisms proposed were primarily focused on the transfer of the content of the EVs to target immune and endothelial/epithelial cells in the injured alveolus: Similar to the parent cells, MSC EVs were found to modulate immune cells to suppress inflammation possibly via transfer of IDO or TGF-β1 and increased IL-10 expression by target cells. EVs miR-181c and miR-146a downregulated TLR4 signaling pathway and enhanced M2 macrophages polarization[143,144]. In addition, investigators found that MSC EVs inhibited B cell proliferation and differentiation in a dose-dependent manner and regulated the activation of T cells[145,146]. Specifically, MSC EVs preferentially induced the conversion of T helper (Th) cells toward Th type 2 phenotype, suppressed differentiation of interleukin 17-producing effector T cells and increased the number of regulatory T cells[147]. EV mRNAs and proteins such as keratinocyte and hepatocyte growth factors were shown to be involved in increasing alveolar fluid clearance or the absorption of pulmonary edema fluid. EV mRNAs and proteins such as angiopoietin-1 were shown to suppress lung protein permeability whether through prevention of “actin stress fiber” formation or increased angiogenesis[142]. In studying pre-clinical models of myocardial injury, Bian et al. found that exogenous MSC EVs, which contained the pro-angiogenic factors PDGF-D, EGF, FGF, VEGF and SCF, induced the proliferation, migration, and tube formation of the endothelial cells in vitro[148]. Wang et al. found that miR-210 enriched in MSC EVs was critical for increased angiogenesis[149]. EV miRNAs such as miR145 as well as organelles such as mitochondria transfer were shown to increase the bioenergetics of target cells such as macrophages and increase phagocytosis of bacteria[150]. MSC EV were also found to have antiviral activity. Qian et al. found that MSC exosomes inhibited HCV viral replication through transfer of microRNAs including let-7f, miR-145, miR-199a, and miR-221 from MSC exosomes to target cells[151]. Surprisingly, several groups found that even apoptotic MSCs possessed immunosuppressive effects. Sung et al. found that apoptotic MSCs reduced mortality in rats after sepsis induction via CLP perhaps by causing a modulation of the local immune response with a down-regulation of the innate and adaptive immunity[152,153]. Currently, despite the promising preclinical studies, the major limitation to the clinical use of MSC EV is the lack of potency of the EV compared to the parent cell which may make the production cost prohibitive for clinical trial. Studies are on-going to address this limitation (Figure 3).
One promising alternative approach to using circulating EVs as a therapeutic comes from the cancer literature: EVs are being explored as drug delivery vehicles[130]. Whether through passive or active means (i.e., electroporation), EVs are being loaded with miRNAs, mRNAs, proteins, peptides and synthetic drugs with anti-tumor activity in pre-clinical models and early clinical trials. The advantages of using circulating EVs are its biocompatibility, low immunogenicity and ability to traffic and interact with specific target cells (i.e., alveolar epithelial and endothelial cells in ARDS) as a smart drug. The disadvantage, aside from the difficulty in manufacturing at a large-scale while maintaining the phenotype of the EVs, is identifying the dominant cellular sources of EVs during each phase of the disease or syndrome. However, one can imagine potentially isolating circulating EVs from patients with ARDS or sepsis, loading the vesicles with therapeutic agents and re-administering the EVs to target specific cells in the injured organs.
CONCLUSIONS
Inflammation is associated with elevated levels of circulating EVs. EVs are released by activated structural and immune cells and can have significant proinflammatory, procoagulant, and pro-permeability effects in critically ill patients with pneumonia leading to ARDS and/or sepsis. However, depending on the cellular origin, external stimuli, and internal cargos (i.e., mRNA, microRNA, protein, organelle and receptor), EVs can also promote or mitigate inflammatory injury depending on the injury milieu. Therefore, future studies are needed to determine whether EVs or EV content can be a diagnostic biomarker or therapeutic target to suppress end organ injury.
9. EXPERT OPINION
Despite decades of research, morbidity and mortality associated with ARDS or septic shock has not changed dramatically. Understanding the role of EVs in the pathogenesis of severe pneumonia and/or sepsis may offer new therapeutic insights or targets to suppress inflammation, protein permeability and bacterial growth which may prevent end organ damage/dysfunction. However, multiple questions remain which may prevent translation of EV directed therapy from pre-clinical models of ARDS/sepsis to clinical trials.
9.1. Phases of Sepsis and EVs
Similar to the exudative, proliferative, and fibrotic phases of ARDS, the immune system plays a significant role in the progression of sepsis from severe sepsis to septic shock with immunoparalysis and death or recovery[154]. In the early phase following exposure to pathogen-derived molecular patterns (i.e,, endotoxin) or endogenous host-derived danger signals (i.e., damage-associated molecular patterns), the innate immune system responds by the release of inflammatory cytokines/chemokines and activation of coagulation, complement and phagocytes. With progression to septic shock, the adaptive immune system becomes dominant with the activation of B cells, neutrophils and myeloid suppressor cells and the release of immunoglobulins. Regulatory T cells may be critical for recovery but may also participate in profound immunosuppression which reduces survival[155,156]. All these changing dynamics in the cellular response to infection can have profound effects on the cellular sources and the phenotypes of EVs (inflammatory vs. anti-inflammatory). Hence, it is critical to understand the phases of syndromes such as ARDS or sepsis when interpreting studies analyzing circulating EVs in both pre-clinical models and human samples.
9.2. Reparative Role of Human EVs
As suggested by the immunosuppressive properties of EVs released from multiple stem and progenitor cells, not all EVs released into the plasma during bacterial pneumonia and/or sepsis are inflammatory. Many EVs may be immunomodulatory as well, especially during the different phases of the syndrome. For example, activated and recruited macrophages (M1) are critical for the exudative phase of ARDS where the cells are primarily inflammatory whereas similar macrophages are shifted to the (M2) phenotype during the proliferative phase and are involved eliminating apoptotic cells and participating in fibrosis[157]. Multiple laboratories have demonstrated that EVs released by stimulated or activated endothelial cells or macrophages in vitro or EVs released into the plasma in LPS injured rats can induce ALI when administered into naïve animals whether through intra-tracheal or intravenous routes[53,61,65]. Whereas, Yang et al. found that EVs isolated from M2 macrophages (subtype b) significantly attenuated the severity of DSS-induced colitis in mice which was associated with increased regulatory T (Treg) cells in the spleens and levels of IL-4[158]. Similar to the role of Treg cells in the maintenance of immune tolerance against self and foreign antigens through the control of harmful inflammation, investigators have also speculated that EVs released by Treg cells may contribute to infectious tolerance by intervening with CD4+ T cells differentiation and/or stability[159]. In the proliferative phase of ARDS, regulatory T-cells promote pulmonary repair by modulating T helper cell immune responses[160]. Thus, targeting all EVs to suppress the “cytokine storm” associated with ARDS or septic shock may be appealing but may not be successful[161], similar to the largely equivocal or negative results from previous clinical trials using anti-inflammatory drugs (i.e., corticosteroids) in ARDS[162] and anti-TNF in sepsis shock[163,164]. Regardless, one definite conclusion that can be drawn from these studies is that the timing of sample collection for analyses for EV content is critical for correctly characterizing the phenotype of these EVs. One wonders about the phenotype of the EVs in the study by Guervilly et al. that found that higher levels of LEVs in the blood and BALF were associated with improved survival in patients with early-stage ARDS[63].
9.3. Role of EV Released by Pathogens
Gram positive and negative bacteria and viruses release EVs[165,166] that contain specific cargo molecules such as nucleic acids, virulence factors, and antibiotic resistance components which are biologically active[167,168]. These EVs, which range in size from 20 to 400 nm, were first discovered to originate from controlled blebbing of the outer membrane of Gram-negative bacteria and were often referred to as outer-membrane vesicles (OMV). However, in addition to membrane blebbing, EVs can also be formed by endolysin-triggered cell lysis[165]. More importantly, these EVs can incorporate bacterial virulence factors into human cells[169]. For example, in cystic fibrosis, many investigators have found these bacterial EVs can induce apoptosis in the host cell, both stimulate and suppress the immune system, contribute to biofilm production, and prevent the effect of antibiotics[170]. Similar to human host EVs, flow cytometry can be used to detect and semi-quantitate bacterial EVs. Recently, Volgers et al. used bead-based flow-cytometry (4-μM-sized latex beads coated with antibodies directed against specific marker proteins) to distinguish bacterial and host-cell vesicle populations using human cells infected with bacterial in vitro[171]. However, many vesicles, especially exosomes which are released from MVBs or following endolysin-triggered cell lysis do not contain cell or bacterial specific markers which prevents accurate identification and quantification. And, perhaps more importantly, the role of EVs in the pathogenesis of bacterial infection is complex, often overlapping with role of the parent bacteria. There are subtle differences in how bacteria use EVs in the pathogenesis of pneumonia. For example, OMVs released by Streptococcus pneumonia, the most commonly identified community acquired pneumonia pathogen, contributes to pathogenicity by transporting virulence factor such as pneumolysin, MalX, and PspA[172]. OMVs released by Pseudomonas aeruginosa participates in the formation of biofilms and the adherence of the bacteria on the biofilm facilitating the propagation of the infection[173]. OMVs released by Haemophilus influenzae protect bacteria from complement lysis[174]. However, regardless of the organisms, the RNA and protein content of host EVs from the blood and injured alveolus are altered and is often associated with disease severity. Future research is critically needed in developing a more sensitive and specific technique to identify and quantitate bacterial EVs in complex human specimens such as plasma or BALF from patients injured with severe pneumonia.
9.4. Future Directions
The potential to target EVs released into the plasma or injured alveolus during bacterial pneumonia and/or sepsis is very promising given the critical role of inflammatory EVs in the pathogenesis of the syndrome as demonstrated by multiple investigators. However, EVs are also released from bacteria, and not all EVs are inflammatory. In addition, the current methodology to identify and quantitate EVs such as using flow cytometry needs to be more sensitive and specific. Thus, prior to recommending a non-specific therapeutic such as high molecular hyaluronic acid to bind and sequester these plasma EVs for elimination, further research is needed to better understand the role of EV in syndrome pathogenesis.
ARTICLE HIGHLIGHTS.
Multiple preclinical studies of acute lung injury (ALI) or sepsis have demonstrated that circulating EVs whether from endothelial, epithelial, or immune cells have significant proinflammatory, procoagulant, and pro-permeability effects in target cells which can result in end organ damage.
Given the critical role of EVs in the pathogenesis of diseases or syndromes, EVs are now studied as potential biomarkers and therapeutic targets to suppress the inflammatory properties of these EVs. For example, investigators have demonstrated that instillation of high molecular weight hyaluronic acid can bind plasma EVs and suppress the injurious properties of these EVs in perfused human lungs injured with severe bacterial pneumonia. In addition, EVs derived from stem or progenitors cells such as mesenchymal stem or stromal cells represent an attractive area of research for treating inflammatory lung diseases, including ALI or sepsis, in part due to the mechanisms underlying their therapeutic effects: the transfer of mRNAs, microRNAs, proteins, receptors, and possibly organelles from the EVs to the injured tissue.
However, current methodologies such as flow cytometry to identify and quantify EVs may not be sensitive or specific enough given that a significant portion of EVs are derived from intracellular vesicles where cell specific markers are not present. In addition, the phenotype of EVs derived from immune cells may change during the progression of the disease or syndrome. For example, EVs released from macrophages or regulatory T cells during the proliferative phase of ARDS may have significant immunomodulatory properties.
Thus, further research is required to better understand the role of EVs in the pathogenesis of diseases or syndromes prior to any clinical translation. A better understanding may prevent applying a therapeutic where the phenotype of the target (i.e., EVs) changes dramatically during the progression of the disease or syndrome. This may prevent the largely equivocal or negative results from previous clinical trials using anti-inflammatory drugs (i.e., corticosteroids) in ARDS and anti-TNF inhibitors in sepsis shock.
Abbreviations Used:
- AA
Arachidonic acid
- AB
Apoptotic bodies
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- CD
Cluster of differentiation
- COX-2
Cyclooxygenase-2
- DIC
Disseminated intravascular coagulation
- DMSO
Dimethyl sulfoxide
- EEV
Endothelial cell derived EVs
- EV
Extracellular vesicles
- HMWHA
High molecular weight hyaluronic acid
- LEV
Leukocyte derived EVs
- IL
Interleukin
- LPS
Lipopolysaccharide
- MEV
Monocyte/macrophage derived EVs
- mRNA
Messenger RNA
- miRNA
Micro RNA
- MV
Microvesicles
- MVB
Multi-vesicular bodies
- NEV
Neutrophil derived EVs
- NFκβ
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- NO
Nitric Oxide
- NTA
Nanoparticle tracking analysis
- OMV
Outer member vesicles
- PEV
Platelet derived EVs
- RNA
Ribonucleotide
- TNF
Tumor necrosis factor
- TLR
Toll like receptor
- Treg
Regulatory T cells
Footnotes
Declaration of Interests: This work was supported by the National Institute of Health National Heart, Lung, and Blood Institute grant number HL 113022 and 148781 for Dr. JW Lee. Drs. Hwang and Shimizu have nothing to declare.
BIBLIOGRAPHY
- 1.Iraci N, Leonardi T, Gessler F, et al. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int J Mol Sci. 2016. Feb 6;17(2):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013. Feb 18;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lane RE, Korbie D, Hill MM, et al. Extracellular vesicles as circulating cancer biomarkers: opportunities and challenges. Clin Transl Med. 2018. May 31;7(1):14. * A review on EVs in cancer.
- 4.Morel O, Morel N, Jesel L, et al. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol. 2011. Sep;33(5):469–86. [DOI] [PubMed] [Google Scholar]
- 5.Groot Kormelink T, Mol S, de Jong EC, et al. The role of extracellular vesicles when innate meets adaptive. Semin Immunopathol. 2018. Sep;40(5):439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016. Feb 1;193(3):259–72. [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016. Feb 23;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017. Mar;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Teixeira L. Sepsis biomarkers. Value and limitations. Am J Respir Crit Care Med. 2014. Nov 15;190(10):1081–2. [DOI] [PubMed] [Google Scholar]
- 10.Distler JH, Huber LC, Gay S, et al. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006. Dec;39(8):683–90. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000. Feb 1;95(3):930–5. [PubMed] [Google Scholar]
- 12.Reid VL, Webster NR. Role of microparticles in sepsis. Br J Anaesth. 2012. Oct;109(4):503–13. [DOI] [PubMed] [Google Scholar]
- 13.Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1beta-rich microparticles. J Immunol. 2011. May 1;186(9):5489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004. Jun 15;103(12):4545–53. [DOI] [PubMed] [Google Scholar]
- 15.Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012. Oct 15;21(R1):R125–34. [DOI] [PubMed] [Google Scholar]
- 17.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009. Aug;9(8):581–93. [DOI] [PubMed] [Google Scholar]
- 18.Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013. May;113(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008. Feb;15(2):243–50. [DOI] [PubMed] [Google Scholar]
- 20. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. ** The consensus guidelines by the ISEV for the characterization of EVs in pre-clinical studies of injury.
- 21.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011. Mar;105(3):396–408. [DOI] [PubMed] [Google Scholar]
- 22.Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011. Dec;7(6):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012. Nov;14(11):1697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Cicero A, Schiera G, Proia P, et al. Oligodendroglioma cells shed microvesicles which contain TRAIL as well as molecular chaperones and induce cell death in astrocytes. Int J Oncol. 2011. Dec;39(6):1353–7. [DOI] [PubMed] [Google Scholar]
- 25.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015. Jun;25(6):364–72. [DOI] [PubMed] [Google Scholar]
- 26.Tetta C, Bruno S, Fonsato V, et al. The role of microvesicles in tissue repair. Organogenesis. 2011. Apr-Jun;7(2):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012. Jan 19;119(3):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimi S, Ogura H, Tanaka H, et al. Activated polymorphonuclear leukocytes enhance production of leukocyte microparticles with increased adhesion molecules in patients with sepsis. J Trauma. 2002. Mar;52(3):443–8. [DOI] [PubMed] [Google Scholar]
- 29.Burger D, Schock S, Thompson CS, et al. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013. Apr;124(7):423–41. [DOI] [PubMed] [Google Scholar]
- 30.Diamant M, Tushuizen ME, Sturk A, et al. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004. Jun;34(6):392–401. [DOI] [PubMed] [Google Scholar]
- 31.Wu ZH, Ji CL, Li H, et al. Membrane microparticles and diseases. Eur Rev Med Pharmacol Sci. 2013. Sep;17(18):2420–7. [PubMed] [Google Scholar]
- 32.Cloutier N, Tan S, Boudreau LH, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013. Feb;5(2):235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinkla S, van Cranenbroek B, van der Heijden WA, et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016. Apr 21;127(16):1976–86. [DOI] [PubMed] [Google Scholar]
- 34.Aatonen M, Gronholm M, Siljander PR. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost. 2012. Feb;38(1):102–13. [DOI] [PubMed] [Google Scholar]
- 35.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010. Jan 29;327(5965):580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibikova M, Zivny J, Janota J. Cell Membrane-Derived Microvesicles in Systemic Inflammatory Response. Folia Biol (Praha). 2018;64(4):113–124. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Okamae F, Abe M, et al. Platelets expressing P-selectin and platelet-derived microparticles in stored platelet concentrates bind to PSGL-1 on filtrated leukocytes. Clin Appl Thromb Hemost. 2000. Oct;6(4):213–21. [DOI] [PubMed] [Google Scholar]
- 38.Barry OP, Kazanietz MG, Pratico D, et al. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999. Mar 12;274(11):7545–56. [DOI] [PubMed] [Google Scholar]
- 39.Cognasse F, Hamzeh-Cognasse H, Laradi S, et al. The role of microparticles in inflammation and transfusion: A concise review. Transfus Apher Sci. 2015. Oct;53(2):159–67. [DOI] [PubMed] [Google Scholar]
- 40.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006. Oct 1;108(7):2455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 42.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Distler JH, Pisetsky DS, Huber LC, et al. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005. Nov;52(11):3337–48. [DOI] [PubMed] [Google Scholar]
- 44.Szul T, Bratcher PE, Fraser KB, et al. Toll-Like Receptor 4 Engagement Mediates Prolyl Endopeptidase Release from Airway Epithelia via Exosomes. Am J Respir Cell Mol Biol. 2016. Mar;54(3):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon HG, Cao Y, Yang J, et al. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015. Dec 10;6:e2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H, Zhang D, Zhu Z, et al. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016. Oct 12;6:35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulshreshtha A, Ahmad T, Agrawal A, et al. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013. Apr;131(4):1194–203, 1203 e1–14. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Groot M, Pinilla-Vera M, et al. Identification of miRNA-rich vesicles in bronchoalveolar lavage fluid: Insights into the function and heterogeneity of extracellular vesicles. J Control Release. 2019. Jan 28;294:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H, Zhang D, Wu J, et al. Lung Epithelial Cell-Derived Microvesicles Regulate Macrophage Migration via MicroRNA-17/221-Induced Integrin beta1 Recycling. J Immunol. 2017. Aug 15;199(4):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheller N, Herold S, Kellner R, et al. Proviral MicroRNAs Detected in Extracellular Vesicles From Bronchoalveolar Lavage Fluid of Patients With Influenza Virus-Induced Acute Respiratory Distress Syndrome. J Infect Dis. 2019. Jan 29;219(4):540–543. [DOI] [PubMed] [Google Scholar]
- 51.Bastarache JA, Fremont RD, Kropski JA, et al. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009. Dec;297(6):L1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Z, Zhang D, Lee H, et al. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J Leukoc Biol. 2017. Jun;101(6):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Soni S, Wilson MR, O’Dea KP, et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016. Nov;71(11):1020–1029. ** An early study demonstrating the inflammatory properties of macrophage derived EVs in acute lung injury in mice.
- 54.Kovach MA, Singer BH, Newstead MW, et al. IL-36gamma is secreted in microparticles and exosomes by lung macrophages in response to bacteria and bacterial components. J Leukoc Biol. 2016. Aug;100(2):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neri T, Armani C, Pegoli A, et al. Role of NF-kappaB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur Respir J. 2011. Jun;37(6):1494–502. [DOI] [PubMed] [Google Scholar]
- 56.Mitra S, Exline M, Habyarimana F, et al. Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury. Am J Respir Cell Mol Biol. 2018. Jul;59(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Meng X, Gao Y, et al. Isolation and phenotypic characteristics of microparticles in acute respiratory distress syndrome. Int J Clin Exp Pathol. 2015;8(2):1640–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews AM, Rizzo V. Microparticle-Induced Activation of the Vascular Endothelium Requires Caveolin-1/Caveolae. PLoS One. 2016;11(2):e0149272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Densmore JC, Signorino PR, Ou J, et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006. Nov;26(5):464–71. [DOI] [PubMed] [Google Scholar]
- 61.Buesing KL, Densmore JC, Kaul S, et al. Endothelial microparticles induce inflammation in acute lung injury. J Surg Res. 2011. Mar;166(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu A, Park JH, Zhang X, et al. Therapeutic Effects of Hyaluronic Acid in Bacterial Pneumonia in Ex Vivo Perfused Human Lungs. Am J Respir Crit Care Med. 2019. Nov 15;200(10):1234–1245. ** A recent study demonstrating that high molecular weight hyaluronic acid can bind EVs in the plasma and reduce parameters of acute lung injury following severe bacterial pneumonia.
- 63. Guervilly C, Lacroix R, Forel JM, et al. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit Care. 2011;15(1):R31. * An early study demonstrating that leukocyte derived EVs can be used as a biomarker for ARDS.
- 64.Lashin HMS, Nadkarni S, Oggero S, et al. Microvesicle Subsets in Sepsis Due to Community Acquired Pneumonia Compared to Faecal Peritonitis. Shock. 2018. Apr;49(4):393–401. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Meng X, Liang X, et al. Administration of microparticles from blood of the lipopolysaccharide-treated rats serves to induce pathologic changes of acute respiratory distress syndrome. Exp Biol Med (Maywood). 2015. Dec;240(12):1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eken C, Martin PJ, Sadallah S, et al. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J Biol Chem. 2010. Dec 17;285(51):39914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004. Oct 15;104(8):2543–8. [DOI] [PubMed] [Google Scholar]
- 68. Neudecker V, Brodsky KS, Clambey ET, et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med. 2017. Sep 20;9(408). * A recent study demonstrating that EVs derived from granulocytes can have immunomodulatory properties via tranfer of miRNA.
- 69. Timar CI, Lorincz AM, Csepanyi-Komi R, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013. Jan 17;121(3):510–8. * An early study demonstrating that EVs derived from granulocytes can have immunomodulatory properties.
- 70.Kolonics F, Szeifert V, Timar CI, et al. The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell. Cells. 2020. Dec 18;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. May 4;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 72.Letsiou E, Sammani S, Zhang W, et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol. 2015. Feb;52(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cabrera-Benitez NE, Valladares F, Garcia-Hernandez S, et al. Altered Profile of Circulating Endothelial-Derived Microparticles in Ventilator-Induced Lung Injury. Crit Care Med. 2015. Dec;43(12):e551–9. [DOI] [PubMed] [Google Scholar]
- 74.Chavez L, Meguro J, Chen S, et al. Circulating extracellular vesicles activate the pyroptosis pathway in the brain following ventilation-induced lung injury. J Neuroinflammation. 2021. Dec 29;18(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005. Feb 15;171(4):340–7. [DOI] [PubMed] [Google Scholar]
- 76.Zafrani L, Gerotziafas G, Byrnes C, et al. Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am J Respir Crit Care Med. 2012. Apr 1;185(7):744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meziani F, Delabranche X, Asfar P, et al. Bench-to-bedside review: circulating microparticles--a new player in sepsis? Crit Care. 2010;14(5):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mortaza S, Martinez MC, Baron-Menguy C, et al. Detrimental hemodynamic and inflammatory effects of microparticles originating from septic rats. Crit Care Med. 2009. Jun;37(6):2045–50. [DOI] [PubMed] [Google Scholar]
- 79.Mastronardi ML, Mostefai HA, Meziani F, et al. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med. 2011. Jul;39(7):1739–48. [DOI] [PubMed] [Google Scholar]
- 80.Essandoh K, Yang L, Wang X, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015. Nov;1852(11):2362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azevedo LC, Janiszewski M, Pontieri V, et al. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care. 2007;11(6):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li JJ, Wang B, Kodali MC, et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation. 2018. Jan 8;15(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balusu S, Van Wonterghem E, De Rycke R, et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. 2016. Oct;8(10):1162–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010. Oct 27;304(16):1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puddu P, Puddu GM, Cravero E, et al. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can J Cardiol. 2010. Apr;26(4):140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogura H, Kawasaki T, Tanaka H, et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001. May;50(5):801–9. [DOI] [PubMed] [Google Scholar]
- 87. Barry OP, Pratico D, Lawson JA, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997. May 1;99(9):2118–27. ** An early study demonstrating the critical role of platelet derived EVs in propogating an inflammatory response.
- 88.Barry OP, Pratico D, Savani RC, et al. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998. Jul 1;102(1):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jy W, Mao WW, Horstman L, et al. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol Dis. 1995;21(3):217–31; discussion 231a. [DOI] [PubMed] [Google Scholar]
- 90.Lehner GF, Harler U, Haller VM, et al. Characterization of Microvesicles in Septic Shock Using High-Sensitivity Flow Cytometry. Shock. 2016. Oct;46(4):373–81. [DOI] [PubMed] [Google Scholar]
- 91. Ohuchi M, Fujino K, Kishimoto T, et al. Association of the Plasma Platelet-Derived Microparticles to Platelet Count Ratio with Hospital Mortality and Disseminated Intravascular Coagulopathy in Critically Ill Patients. J Atheroscler Thromb. 2015. Aug 26;22(8):773–82. * A recent study looking at the use of PEVs as a potential biomarker for DIC in critically ill patients.
- 92.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998. Oct 15;161(8):4382–7. [PubMed] [Google Scholar]
- 93.Tang K, Liu J, Yang Z, et al. Microparticles mediate enzyme transfer from platelets to mast cells: a new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun. 2010. Sep 24;400(3):432–6. [DOI] [PubMed] [Google Scholar]
- 94.Sadallah S, Eken C, Martin PJ, et al. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011. Jun 1;186(11):6543–52. [DOI] [PubMed] [Google Scholar]
- 95.Dalli J, Norling LV, Renshaw D, et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008. Sep 15;112(6):2512–9. [DOI] [PubMed] [Google Scholar]
- 96.Johnson BL III, Kuethe JW, Caldwell CC. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr Metab Immune Disord Drug Targets. 2014;14(3):210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woei AJFJ, van der Starre WE, Tesselaar ME, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014. May;133(5):799–803. [DOI] [PubMed] [Google Scholar]
- 98.Satta N, Freyssinet JM, Toti F. The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br J Haematol. 1997. Mar;96(3):534–42. [DOI] [PubMed] [Google Scholar]
- 99.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008. Nov;100(5):878–85. [DOI] [PubMed] [Google Scholar]
- 100.Iwamoto S, Kawasaki T, Kambayashi J, et al. Platelet microparticles: a carrier of platelet-activating factor? Biochem Biophys Res Commun. 1996. Jan 26;218(3):940–4. [DOI] [PubMed] [Google Scholar]
- 101.Satta N, Toti F, Feugeas O, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994. Oct 1;153(7):3245–55. [PubMed] [Google Scholar]
- 102.Jy W, Jimenez JJ, Mauro LM, et al. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. J Thromb Haemost. 2005. Jun;3(6):1301–8. [DOI] [PubMed] [Google Scholar]
- 103.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003. Dec;123(5):896–902. [DOI] [PubMed] [Google Scholar]
- 104.Delabranche X, Boisrame-Helms J, Asfar P, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013. Oct;39(10):1695–703. [DOI] [PubMed] [Google Scholar]
- 105.Zafrani L, Ince C, Yuen PS. Microparticles during sepsis: target, canary or cure? Intensive Care Med. 2013. Oct;39(10):1854–6. [DOI] [PubMed] [Google Scholar]
- 106.Delabranche X, Quenot JP, Lavigne T, et al. Early Detection of Disseminated Intravascular Coagulation During Septic Shock: A Multicenter Prospective Study. Crit Care Med. 2016. Oct;44(10):e930–9. [DOI] [PubMed] [Google Scholar]
- 107.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009. Feb;19(2):43–51. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y, Meng H, Ma R, et al. Circulating Microparticles, Blood Cells, and Endothelium Induce Procoagulant Activity in Sepsis through Phosphatidylserine Exposure. Shock. 2016. Mar;45(3):299–307. [DOI] [PubMed] [Google Scholar]
- 109.Tripisciano C, Weiss R, Eichhorn T, et al. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci Rep. 2017. Jul 26;7(1):6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chou J, Mackman N, Merrill-Skoloff G, et al. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004. Nov 15;104(10):3190–7. [DOI] [PubMed] [Google Scholar]
- 111.Holme PA, Solum NO, Brosstad F, et al. Microvesicles bind soluble fibrinogen, adhere to immobilized fibrinogen and coaggregate with platelets. Thromb Haemost. 1998. Feb;79(2):389–94. [PubMed] [Google Scholar]
- 112.Janiszewski M, Do Carmo AO, Pedro MA, et al. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit Care Med. 2004. Mar;32(3):818–25. [DOI] [PubMed] [Google Scholar]
- 113.Brodsky SV, Zhang F, Nasjletti A, et al. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004. May;286(5):H1910–5. [DOI] [PubMed] [Google Scholar]
- 114.Forest A, Pautas E, Ray P, et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci. 2010. Apr;65(4):414–20. [DOI] [PubMed] [Google Scholar]
- 115.Martin S, Tesse A, Hugel B, et al. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004. Apr 6;109(13):1653–9. [DOI] [PubMed] [Google Scholar]
- 116.Tesse A, Martinez MC, Hugel B, et al. Upregulation of proinflammatory proteins through NF-kappaB pathway by shed membrane microparticles results in vascular hyporeactivity. Arterioscler Thromb Vasc Biol. 2005. Dec;25(12):2522–7. [DOI] [PubMed] [Google Scholar]
- 117.Mostefai HA, Andriantsitohaina R, Martinez MC. Plasma membrane microparticles in angiogenesis: role in ischemic diseases and in cancer. Physiol Res. 2008;57(3):311–320. [DOI] [PubMed] [Google Scholar]
- 118.Laher I Microparticles have macro effects in sepsis. Crit Care Med. 2011. Jul;39(7):1842–3. [DOI] [PubMed] [Google Scholar]
- 119. Xia X, Yuan P, Liu Y, et al. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology. 2021. Aug;163(4):416–430. ** EVs in the pathogenesis of COVID-19 pneumonia.
- 120.Inal JM. Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy. Clin Sci (Lond). 2020. Jun 26;134(12):1301–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Earnest JT, Hantak MP, Li K, et al. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017. Jul;13(7):e1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goodlet KJ, Bansal S, Arjuna A, et al. COVID-19 in a lung transplant recipient: Exploring the diagnostic role of circulating exosomes and the clinical impact of advanced immunosuppression. Transpl Infect Dis. 2021. Apr;23(2):e13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwon Y, Nukala SB, Srivastava S, et al. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res Ther. 2020. Nov 30;11(1):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shaver CM, Woods J, Clune JK, et al. Circulating microparticle levels are reduced in patients with ARDS. Crit Care. 2017. May 25;21(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X, Singleton PA, Letsiou E, et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012. Nov;47(5):628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dakhlallah DA, Wisler J, Gencheva M, et al. Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. J Extracell Vesicles. 2019;8(1):1669881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo P, Mao K, Xu J, et al. Metabolic characteristics of large and small extracellular vesicles from pleural effusion reveal biomarker candidates for the diagnosis of tuberculosis and malignancy. J Extracell Vesicles. 2020. Jul 14;9(1):1790158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roman-Canal B, Moiola CP, Gatius S, et al. EV-associated miRNAs from pleural lavage as potential diagnostic biomarkers in lung cancer. Sci Rep. 2019. Oct 21;9(1):15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lin J, Wang Y, Zou YQ, et al. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 2016. Oct 14. * Use of EVs in pleural effusions as a diagnotic biomarker.
- 130. Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018. Oct;15(10):617–638. ** Innovative strategies studied in cancer research to ultilize EVs as a therapeutic.
- 131.Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken). 2010. Jun;293(6):982–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev. 2016. Feb 1;97:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011. Aug;301(2):L137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lesley J, Hascall VC, Tammi M, et al. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000. Sep 1;275(35):26967–75. [DOI] [PubMed] [Google Scholar]
- 135.Singleton PA, Mirzapoiazova T, Guo Y, et al. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010. Nov;299(5):L639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singleton PA, Dudek SM, Ma SF, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006. Nov 10;281(45):34381–93. [DOI] [PubMed] [Google Scholar]
- 137.Muto J, Yamasaki K, Taylor KR, et al. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009. Dec;47(2–3):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khan AI, Kerfoot SM, Heit B, et al. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004. Dec 15;173(12):7594–601. [DOI] [PubMed] [Google Scholar]
- 139.Lee JH, Liu A, Park JH, et al. Therapeutic Effects of Hyaluronic Acid in Peritonitis-Induced Sepsis in Mice. Shock. 2020. Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Volarevic V, Markovic BS, Gazdic M, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu A, Zhang X, He H, et al. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020. Feb;20(2):125–140. **A review summarizing the pre-clinical use of EVs derived from stem or progenitor cells for acute lung injury.
- 143.Li X, Liu L, Yang J, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016. Jun;8:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song Y, Dou H, Li X, et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017. May;35(5):1208–1221. [DOI] [PubMed] [Google Scholar]
- 145.Budoni M, Fierabracci A, Luciano R, et al. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22(2):369–79. [DOI] [PubMed] [Google Scholar]
- 146.Di Trapani M, Bassi G, Midolo M, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016. Apr 13;6:24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016. Aug;64(4):831–40. [DOI] [PubMed] [Google Scholar]
- 148.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014. Apr;92(4):387–97. [DOI] [PubMed] [Google Scholar]
- 149.Wang N, Chen C, Yang D, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017. Aug;1863(8):2085–2092. [DOI] [PubMed] [Google Scholar]
- 150.Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017. Nov 15;196(10):1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qian X, Xu C, Fang S, et al. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl Med. 2016. Sep;5(9):1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sung PH, Chang CL, Tsai TH, et al. Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res Ther. 2013;4(6):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thum T, Bauersachs J, Poole-Wilson PA, et al. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J Am Coll Cardiol. 2005. Nov 15;46(10):1799–802. [DOI] [PubMed] [Google Scholar]
- 154.Jarczak D, Kluge S, Nierhaus A. Sepsis-Pathophysiology and Therapeutic Concepts. Front Med (Lausanne). 2021;8:628302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuhlhorn F, Rath M, Schmoeckel K, et al. Foxp3+ regulatory T cells are required for recovery from severe sepsis. PLoS One. 2013;8(5):e65109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martin MD, Badovinac VP, Griffith TS. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Front Immunol. 2020;11:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang X, Xiu H, Zhang S, et al. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang R, Liao Y, Wang L, et al. Exosomes Derived From M2b Macrophages Attenuate DSS-Induced Colitis. Front Immunol. 2019;10:2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rojas C, Campos-Mora M, Carcamo I, et al. T regulatory cells-derived extracellular vesicles and their contribution to the generation of immune tolerance. J Leukoc Biol. 2020. Sep;108(3):813–824. [DOI] [PubMed] [Google Scholar]
- 160.Tan W, Zhang C, Liu J, et al. Regulatory T-cells promote pulmonary repair by modulating T helper cell immune responses in lipopolysaccharide-induced acute respiratory distress syndrome. Immunology. 2019. Jun;157(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020. May 2;395(10234):1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hensley MK, Sjoding MW, Prescott HC. COUNTERPOINT: Should Corticosteroids Be Routine Treatment in Early ARDS? No. Chest. 2021. Jan;159(1):29–33. [DOI] [PubMed] [Google Scholar]
- 163.Fisher CJ Jr., Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996. Jun 27;334(26):1697–702. [DOI] [PubMed] [Google Scholar]
- 164.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998. Mar 28;351(9107):929–33. [PubMed] [Google Scholar]
- 165.Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019. Jan;17(1):13–24. [DOI] [PubMed] [Google Scholar]
- 166. Jung AL, Schmeck B, Wiegand M, et al. The clinical role of host and bacterial-derived extracellular vesicles in pneumonia. Adv Drug Deliv Rev. 2021. Sep;176:113811. ** Excellent review on the use of EVs by bacteria and viruses to increase infection.
- 167.Bauman SJ, Kuehn MJ. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 2009. Feb 3;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006. Aug;8(9–10):2400–8. * An early study demonstrating the functional properties of bacterial derived EVs in inflammation and pathogenicity of bacteria.
- 169.Toyofuku M Bacterial communication through membrane vesicles. Biosci Biotechnol Biochem. 2019. Sep;83(9):1599–1605. [DOI] [PubMed] [Google Scholar]
- 170.Vitse J, Devreese B. The Contribution of Membrane Vesicles to Bacterial Pathogenicity in Cystic Fibrosis Infections and Healthcare Associated Pneumonia. Front Microbiol. 2020;11:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Volgers C, Benedikter BJ, Grauls GE, et al. Bead-based flow-cytometry for semi-quantitative analysis of complex membrane vesicle populations released by bacteria and host cells. Microbiol Res. 2017. Jul;200:25–32. [DOI] [PubMed] [Google Scholar]
- 172.Olaya-Abril A, Prados-Rosales R, McConnell MJ, et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014. Jun 25;106:46–60. [DOI] [PubMed] [Google Scholar]
- 173.Turnbull L, Toyofuku M, Hynen AL, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016. Apr 14;7:11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paulsson M, Che KF, Ahl J, et al. Bacterial Outer Membrane Vesicles Induce Vitronectin Release Into the Bronchoalveolar Space Conferring Protection From Complement-Mediated Killing. Front Microbiol. 2018;9:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]


