Abstract
Atopic dermatitis is a common inflammatory skin condition with an underlying pathophysiology that may have impact beyond the skin. Previous studies showed a higher prevalence of dental cavities in patients with atopic dermatitis. Our study aimed to establish an association of other dental anomalies in patients with moderate–severe atopic dermatitis. We prospectively recruited 15 patients with moderate–severe atopic dermatitis for a formal dental examination by a paediatric dentist. Hypodontia and microdontia were more prevalent in patients with moderate–severe atopic dermatitis as compared to reference populations and were statistically significant. Dental caries, enamel hypoplasia and agenesis of 3rd molars were also prevalent but did not reach statistical significance. Our study shows a novel finding of higher prevalence of dental anomalies in patients with moderate–severe atopic dermatitis which may warrant further investigation due to its clinical implications.
KEY WORDS: Atopic dermatitis, dental caries, genetics, tooth abnormalities
Introduction
Atopic dermatitis (AD) is a common inflammatory skin condition.[1] A 2017 cross-sectional survey in Singapore showed an overall AD prevalence of 13.1%, with a higher prevalence in children (20.6%) than in adults (11.1%). Patients with AD experience a lower quality of life than people without AD.[2] The high prevalence and impact that AD has on patients are of considerable clinical interest.
The pathophysiology of AD is complex and likely involves a combination of genetic and exogenous factors. A variety of genes are implicated in AD. Twin–twin studies revealed loss-of-function mutations in the filaggrin gene that resulted in epidermal barrier dysfunction and the entry of allergens and microbes into the skin, which conferred the highest risk of the development of AD.
Immune dysregulation with predominant activation of type 2 immune responses in the skin leads to further epidermal barrier dysfunction. Defective barrier function and immune dysregulation are central to the pathogenesis of AD, and each factor perpetuates the other factor.
The GUSTO birth cohort further expounded on the primary barrier defect in AD and demonstrated a higher prevalence/incidence of early childhood caries (ECC) in pediatric AD compared to children without AD.[3] Ectodermal developmental defects regulated by genetic mutations (e.g. MBL2, TLR2, DLX3 and filaggrin) may be the common pathogenic pathway between ECC and AD. Therefore, the structural defect hypothesis was proposed.
However, few studies investigated other structural dental anomalies in patients with AD. An observational study described anatomical dental abnormalities in 14.4% (13/90) of pediatric AD patients, but there was no differentiation of AD severity.[4] This result suggests that the hypothesized developmental structural defect in patients with AD may extend beyond ECC and include other dental anomalies.
Genetic impact on the severity of AD: Patients homozygous for filaggrin loss-of-function mutations have more severe disease than heterozygotes or patients without these mutations. Therefore, we hypothesized that patients with moderate–severe AD were more likely to have a greater burden of AD-specific genetic mutations and/or structural defect (s) that manifest as dental caries/dental anomalies.
Materials and Methods
We identified 15 patients with moderate–severe AD in a tertiary dermatology clinic. AD was diagnosed using the Hanifin and Rajka criteria, and severity was assessed using the Scoring Atopic Dermatitis (SCORAD). All included patients had a SCORAD greater than 25. A pediatric dentist examined these 15 patients for any specific anomalous dental findings. Table 1 shows the demographics of patients studied.
Table 1.
Demographic of patients and dental anomalies
| Patient | Age of onset | Family history of atopy | Age | Dental caries/fillings | Microdontia | Hypoplasia | Agenesis of wisdom teeth | Hypodontia | Others | Max SCORAD |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | − | 19 | 2 | NIL | NIL | #28 | Delayed development of #18 | 57 | |
| 2 | Childhood | + | 19 | 7 | NIL | NIL | NIL | NIL | 51.5 | |
| 3 | 8 | + | 15 | 9 | NIL | NIL | NIL | NIL | 44 | |
| 4 | Infantile | + | 15 | 2 | NIL | NIL | NIL | Retention of #53 (baby teeth) | 33.4 | |
| 5 | 5 | + | 14 | 11 | NIL | NIL | NIL | #13/23 | #53/63 retention | 29.6 |
| 6 | 8 | + | 25 | 3 | #12 | #11/21/22/24/25/33 | #28/38/48 | Bruxism, deep palatal pit of #22 | 30 | |
| 7 | 5 | + | 7 | 3 | NIL | #11/21/26 (mesio-buccal and palatal surfaces) | NIL | Molar–incisor hypomineralization | 26.5 | |
| 8 | Infantile | + | 7 | 2 | NIL | #26 | NIL | Bruxism | 32.5 | |
| 9 | 2 | + | 25 | 0 | NIL | #13/15/16/17/23/26 | NIL | Bruxism, crossbite, rotated #15/25 | 33 | |
| 10 | Childhood | + | 18 | 2 | NIL | #11/12/13/14/15/16/21/24/26/31/32/33/34/36/43 | #18/28/38/48 | Bruxofacets of 13/23 | 42.5 | |
| 11 | Infantile | + | 16 | 7 | NIL | NIL | NIL | Bruxism with bruxofacets on all canines #13/23/33/43 | 44.5 | |
| 12 | 9 | + | 17 | 0 | #12/22 | NIL | #18/28/38/48 | #37 | #47 rotated; bruxofacets (all mild except moderate on #33); peg lateral with flat surface without palatal pits #12/22; pronounced palatal fossa with prominent marginal ridges of #11/21, | 48.8 |
| 13 | 3 | − | 11 | 3 | NIL | #43 | #18/28 | Potential missing tooth germs of #18/28 | 50.5 | |
| 14 | 15 | − | 22 | 2 | NIL | NIL | NIL | Bruxofacets (all moderate except mild on #33) | 36.2 | |
| 15 | Infantile | − | 24 | 0 | NIL | NIL | NIL | NIL | 30.5 |
Results
Hypodontia, defined as the absence of teeth (except for 3rd molars), was noted in 13% (2/15). This finding is significantly greater than the tooth-specific prevalence rate (P < 0.05) reported by a quality meta-analysis with a combined sample size of 48,274 derived from 10 studies.[5] Figure 1 shows the dental anomalies of a 14-year-old male with moderate AD and the congenital absence of two maxillary permanent canine teeth. The positions are occupied by two unexfoliated baby teeth/canines.
Figure 1.
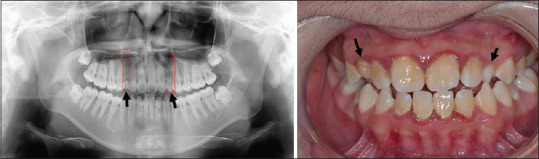
Dental anomalies of a 14-year-old male patient with moderate AD. (a) Missing two maxillary canines (highlighted by two red dotted boxes) in the panoramic radiograph; (b) retention of 2 maxillary primary canines (marked by black arrows) shown in the intraoral photograph
Microdontia, defined as teeth that are physically smaller in size than usual, was identified in 13% (2/15) of this cohort, which was more prevalent than a high-risk orthodontic patient pool (P = 0.013).[6] Figure 2 shows the dental anomalies of a 17-year-old male with severe AD. He presented with hypodontia (missing lower left 2nd molars and all the 3rd molars) and microdontia, which manifested as bilateral 'peg laterals'-undersized tapered lateral incisors, unusual barrel-shaped central incisors and extremely rare prominent palatal fossa of central incisors.
Figure 2.
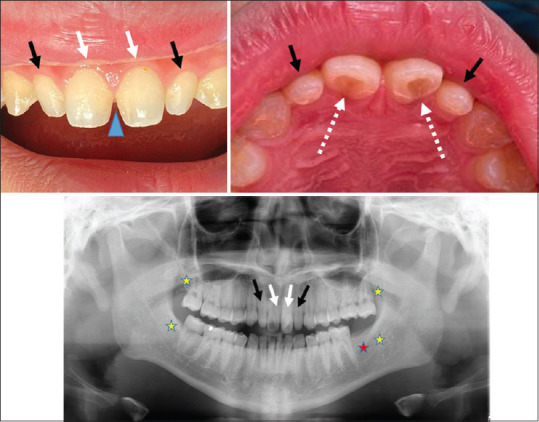
Dental anomalies of a 17-year-old male patient with severe AD. (a) Bilateral 'peg laterals'-undersized tapered lateral incisors (marked with black arrows) together with an unusual barrel-shaped central incisors (marked with white arrows) which cause the visual diastema (indicated with a blue triangle; (b) prominent shovel-shaped palatal fossa of central incisors (pointed by dotted white arrows) fenced by protuberant marginal ridges; (c) a panoramic radiograph showing the missing of lower left 2nd molar (marked with a red asterisk) besides all 4 wisdom teeth-3rd molars (yellow asterisks) and undersized maxillary incisors described in figures (a) and (b).
Dental caries was found in 80% (12/15) of this cohort, and enamel hypoplasia was found in 33% (5/15). Agenesis of 3rd molars was observed in 33% (5/15), which showed greater prevalence than other studies. However, the difference did not reach statistical significance due to the wide range of previously reported prevalence rates.
We postulated that patients with a significant caries burden or dental anomalies were more likely to have earlier onset AD or a strong family history of AD. However, there was no significant correlation. Similarly, we did not find a correlation between maximal SCORAD during review and the presence of dental caries or anomalies.
Discussion
Despite our study's small sample size with resultant potential type II error (false negatives) due to insufficient statistical power, the association of AD and microdontia/hypodontia identified suggest a link between moderate–severe AD and microdontia/hypodontia and support the developmental structural defect hypothesis. Because tooth germs and the epidermis are derived from the ectoderm, the dysregulated ectodermal differentiation that results in an epidermal barrier defect may also result in defective dental development.
Microdontia and hypodontia may cause malocclusion of teeth, which adversely impacts articulation, phonation, dental function and aesthetics. Hypodontia is multifactorial in origin, and hypotheses on evolution, environmental factors and/or genetic factors, such as mutations in MSX1, PAX9, AXIN2 and EDA, were described.[7]
Genome-wide association studies investigating candidate genes that contribute to AD showed no single gene overlap with candidate genes associated with microdontia/hypodontia.[8] However, genodermatoses, such as ectodermal dysplasia (EDA gene mutation) with cutaneous and dental anomalies, may reinforce common genetic pathways that lead to abnormalities in the skin and dentition. We postulated that the association between moderate–severe AD and microdontia/hypodontia was due to mutations in unidentified genes, which may be elucidated in similar genome-wide studies performed in patients with microdontia/hypodontia. Further work into this area is required to delineate the role of other potential candidate genes.
Recent studies demonstrated the role of Sonic Hedgehog (Shh) intercellular signaling proteins and their effects on immune regulation in inflammatory diseases, including AD.[9] Notably, Shh signaling also plays a role in tooth morphogenesis and development.[10] Beyond genetic factors, downstream signaling proteins may be a separate mechanistic pathway to explore.
Conclusion
We present the novel finding of an increased incidence of dental anomalies, specifically microdontia and hypodontia, in moderate–severe AD patients. This finding supports the ectodermal/developmental structural defect hypothesis, which may link dental caries and dental anomalies to AD. The clinical implications for the early prevention and management of both diseases warrant further investigation.
This study was approved by the institution ethics review board (National Healthcare Group DSRB Reference 2018/00498) and all participants provided informed consent to participate in this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–60. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 2.Cheok S, Yee F, Song Ma JY, Leow R, Ho MSL, Yew YW, et al. Prevalence and descriptive epidemiology of atopic dermatitis and its impact on quality of life in Singapore. Br J Dermatol. 2018;178:276–7. doi: 10.1111/bjd.15587. [DOI] [PubMed] [Google Scholar]
- 3.Kalhan TA, Loo EXL, Kalhan AC, Kramer MS, Karunakaran B, Un Lam C, et al. Atopic dermatitis and early childhood caries: Results of the GUSTO study. J Allergy Clin Immunol. 2017;139:2000–3. doi: 10.1016/j.jaci.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Perugia C, Saraceno R, Ventura A, Lorè B, Chiaramonte C, Docimo R, et al. Atopic dermatitis and dental manifestations. G Ital Dermatol Venereol. 2017;152:122–5. doi: 10.23736/S0392-0488.16.05224-X. [DOI] [PubMed] [Google Scholar]
- 5.Polder BJ, Van't Hof MA, Van der Linden FPGM, Kuijpers-Jagtman AM. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32:217–26. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 6.Fekonja A. Prevalence of dental developmental anomalies of permanent teeth in children and their influence on esthetics. J Esthet Restor Dent. 2017;29:276–83. doi: 10.1111/jerd.12302. [DOI] [PubMed] [Google Scholar]
- 7.Cobourne MT. Familial human hypodontia—is it all in the genes? Br Dent J. 2007;203:203–8. doi: 10.1038/bdj.2007.732. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shobaili HA, Ahmed AA, Alnomair N, Alobead ZA, Rasheed Z. Molecular genetic of atopic dermatitis: An update. Int J Health Sci (Qassim) 2016;10:96–120. [PMC free article] [PubMed] [Google Scholar]
- 9.Papaioannou E, Yánez DC, Ross S, Lau CI, Solanki A, Chawda MM, et al. Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation. J Clin Invest. 2019;129:3153–70. doi: 10.1172/JCI125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoya A, Shalehin N, Takebe H, Shimo T, Irie K. Sonic Hedgehog signaling and tooth development. Int J Mol Sci. 2020;21:E1587. doi: 10.3390/ijms21051587. [DOI] [PMC free article] [PubMed] [Google Scholar]


