Abstract
Magnetic resonance imaging (MRI) is a standard imaging modality for diagnosing spinal stenosis, which is a common degenerative disorder in the elderly population. Standardized interpretation of spinal MRI for diagnosing and grading the severity of spinal stenosis is necessary to ensure correct communication with clinicians and to conduct clinical research. In this review, we revisit the Lee grading system for central canal and neural foraminal stenosis of the cervical and lumbar spine, which are based on the pathophysiology and radiologic findings of spinal stenosis.
Keywords: Spinal stenosis, Grading system, Central canal stenosis, Neural foraminal stenosis
INTRODUCTION
Spinal stenosis is a common disease in the elderly population and its incidence has been gradually increasing with an aging society [1]. Magnetic resonance imaging (MRI) is a standard imaging technique for the diagnosis of spinal stenosis, and the demand for spinal MRI is steadily rising every year [2]. Although MRI has been used as the gold standard for diagnosing spinal stenosis, well-established radiologic criteria or parameters to characterize spinal stenosis are currently unavailable. Standardized interpretation of spinal MRI for diagnosing and grading the severity of spinal stenosis is necessary to ensure correct communication with clinicians and to conduct clinical research.
The North American Spine Society has provided guidelines for diagnosis and treatment of degenerative lumbar spinal stenosis [3]. Ironically, the literature does not contain any detailed specifications of the radiologic criteria or parameters to describe the degree of lumbar spinal stenosis. Moreover, researchers have used various radiologic criteria for classifying the severity of lumbar spinal stenosis in patients included in different therapeutic clinical trials [4,5]. Therefore, Lee et al. [6,7,8,9] suggested a practical grading system (the Lee grading system) for central canal and neural foraminal stenosis of the cervical and lumbar spine, which are now widely accepted in clinical practice, included in web-based radiology resources, and cited in many studies. In this review, we revisited the Lee grading system for cervical and lumbar spinal stenosis.
Radiologic Criteria or Parameters for Assessing Spinal Stenosis
Spinal stenosis can be quantitatively or qualitatively assessed. A quantitative parameter is a measurable value, such as the diameter or cross-sectional area. Semiquantitative or qualitative parameters are evaluated via visual assessment.
The word “stenosis” means “narrowing or constriction of the diameter of a bodily passage or orifice.”[10] For blood vessels, any degree of luminal narrowing can cause alterations in fluid dynamics, blood flow, and pressure [11,12]. However, because the spinal cord or cauda equina floats in ample cerebrospinal fluid (CSF) within the dural sac or central canal, a certain degree of central canal narrowing does not significantly compromise the spinal cord or nerve rootlets [13]. However, significant symptoms may occur in cases of close contact with or direct compression of the cauda equina by degenerative spinal structures. The nerve root passing through the neural foramen is surrounded by perineural fat that protects the nerve root from compression due to adjacent osseous, ligamentous, or discal structures, and it functions like the CSF in the dural sac [14,15]. Similar to central canal stenosis, a minor decrease in the cross-sectional area of the neural foramen does not always result in significant radiculopathy symptoms [16]. Considering these pathophysiological characteristics of spinal stenosis, quantitative parameters, such as anterior-posterior diameter or cross-sectional area, seem to have a limited role in the diagnosis of clinically significant central canal stenosis. The consensus meeting of the Lumbar Spine Outcome Study Working Group Zurich identified the criterion, “relationship between the CSF and cauda equina,” as an essential parameter for evaluating lumbar central canal stenosis, which is reflected in the Lee grading system [17].
The Lee grading systems is based on qualitative assessment and was developed to establish criteria that could meet the essential requirements of a grading method, such as reproducibility and reliability, high sensitivity, ease of learning and understanding, ability to account for individual anatomical variations, and correlation with symptoms and outcomes [6,7,8,9].
Lumbar Central Canal Stenosis
Lumbar central stenosis is narrowing of the spinal canal due to degeneration and progressive hypertrophy of the surrounding osseocartilaginous and ligamentous structures. The most common symptom associated with lumbar central stenosis is neurogenic claudication, a clinical condition in which patients experience lower leg pain, cramps, and weakness after walking for a certain distance [18]. Quantitative parameters, such as the anterior-posterior diameter and cross-sectional area of the dural sac, are commonly used radiologic measurements to determine central canal stenosis in patients with neurogenic claudication. These parameters can be helpful in assessing upper lumbar spine level or for modality that cannot visualize the cauda equina within the dural sac such as computed tomography. An anterior-posterior diameter of less than 10 mm or a cross-sectional area of less than 100 mm2 suggests stenosis [19]. Classification based on quantitative measurements has limitations because the number of rootlets decreases at the lower lumbar level as the rootlets exit through the neural foramen sequentially at each level [20]. For instance, at the upper lumbar level, a decrease in the dural sac cross-sectional area of less than 100 mm2 can result in a significant compromise of the cauda equina inside the dural sac. In contrast, at the lower lumbar level, such as at L5/S1, the cauda equina would not be compromised at a cross-sectional area of 100 mm2 because a small number of rootlets remain at that level. Many authors have reported that these quantitative radiological findings alone cannot be the major criteria for predicting clinical severity and outcomes [21,22].
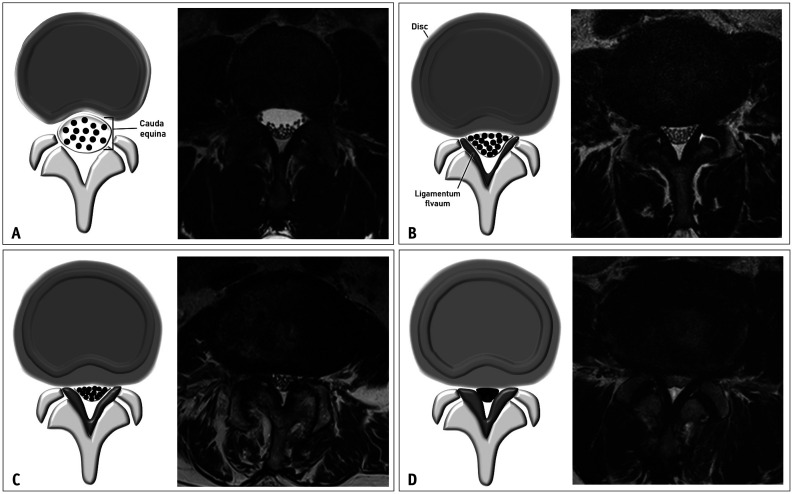
The Lee grading system for lumbar central canal stenosis is described in Table 1 and Figure 1. Stenosis can be graded as mild, moderate, or severe, depending on the degree of CSF space obliteration and cauda equina clumping. Axial T2-weighted images were examined for grading purpose. In “mild” central canal stenosis, each nerve rootlet remains separated inside the dural sac without clumping. In “moderate” central canal stenosis (grade 2), there is some clumping of the nerve rootlets inside the dural sac. If the cauda equina appears as a single bundle due to marked clumping, without any separation of the nerve rootlets, that would indicate “severe” central canal stenosis (grade 3). Determining grades 0 and 1 can sometimes be confusing. In such cases, sagittal T2-weighted images may be helpful. Obliteration of the ventral CSF space on consecutive sagittal images can be used to distinguish “mild” stenosis (grade 1) from “no” stenosis (grade 0).
Table 1. Grading Criteria for Lumbar Central Canal Stenosis.
| Grade | Severity | Description |
|---|---|---|
| Grade 0 | Normal | No lumbar central canal stenosis. The anterior CSF space is not obliterated. |
| Grade 1 | Mild | The anterior CSF space is mildly obliterated, but all the cauda equina can be clearly separated from each other. |
| Grade 2 | Moderate | The anterior CSF space is moderately obliterated and some of the cauda equina is aggregated, making it impossible to visually separate them. |
| Grade 3 | Severe | The anterior CSF space is obliterated severely as to show marked compression of the dural sac, and none of the cauda equina can be visually separated from each other, appearing as single bundle. |
CSF = cerebrospinal fluid
Fig. 1. The Lee grading system for lumbar central canal stenosis. A. Schematic drawing of normal central canal and the corresponding T2-weighted axial magnetic resonance image at the L3/4 disc level show no obliteration of the anterior cerebrospinal fluid space. B. Schematic drawing of mild central canal stenosis and the corresponding T2-weighted axial magnetic resonance image at the L4/5 disc level show mild obliteration of the anterior cerebrospinal fluid space and all cauda equina clearly separated from each other. C. Schematic drawing of moderate central canal stenosis and the corresponding T2-weighted axial magnetic resonance image at the L4/5 disc level shows moderate obliteration of the anterior cerebrospinal fluid space and some cauda equina aggregation. D. Schematic drawing of severe central canal stenosis and the corresponding T2-weighted axial magnetic resonance image at the L4/5 disc level show severe obliteration of the anterior cerebrospinal fluid space, marked compression of the dural sac, and the entire cauda equina appearing as single bundle.
Considering the underlying morphological and anatomical variations in individuals, this rapid visual assessment may contribute to the strength of this grading system. In fact, this grading system showed excellent to perfect intraobserver reliability, excellent to perfect interobserver agreement, and was significantly correlated with clinical manifestations [6,23,24].
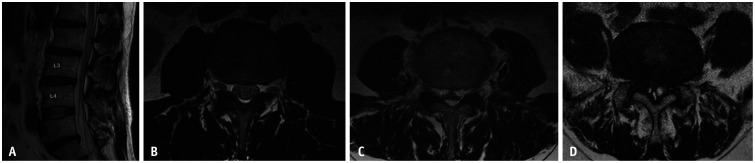
Although it would not be difficult to follow this grading system, readers may encounter confusing cases. The first case concerns flow-related artifacts in the ventral CSF space. When there is a flow-related artifact, the CSF space shows heterogeneous hypointensity or inhomogeneous signal intensity on axial T2-weighted images, which might appear as crowding of the cauda equina [25]. This finding might lead to an overestimation of central canal stenosis (Fig. 2A, B). The other cases are those with ample hyperintensity within the bony central canal (Fig. 2C, D). Although the dural sac is severely collapsed in both cases due to prominent epidural fat or synovial cysts, there is sufficient hyperintensity area ventral or dorsal to the dural sac, which can be misjudged as visible CSF. These cases should not be overlooked and should be considered severe central canal stenosis because the cauda equina appears as a single bundle due to severe compression of the dural sac.
Fig. 2. Confusing cases in grading lumbar central canal stenosis. Sagittal T2-weighted image (A) shows no obliteration of the ventral cerebrospinal fluid space at the L3/4 level, indicating that there is no central canal stenosis. However, in the corresponding axial T2-weighted image (B), the ventral cerebrospinal fluid space shows heterogeneous hypointensity, which appears as clumping of the cauda equina. Axial T2-weighted image of a 67-year-old male with epidural lipomatosis (C) showing severe collapse of the dural sac. In addition, on an axial T2-weighted image of a 72-year-old male with synovial cysts (D), the cauda equina appear as a single bundle. Severe dural sac collapse by epidural lipomatosis or synovial cysts (C, D) should be determined as severe central canal stenosis.
A qualitative grading system proposed by Schizas et al. [26] focused on the CSF/rootlet ratio and effacement of the dorsal epidural fat, sharing the basic concept of the Lee grading system [23]. The Schizas et al. [26] grading system additionally described the inhomogeneous distribution of the cauda equina within the dural sac in cases with no or minor central canal stenoses.
Lumbar Neural Foraminal Stenosis
Lumbar neural foraminal stenosis occurs when the nerve root at the neural foramen is compromised because of a combination of degenerative changes in the lumbar spine, including decreased height of the intervertebral disc, osteoarthritis of the facet joint, associated cephalad subluxation of the superior articular process, buckling of the ligamentum flava, or protrusion of the annulus fibrosus [27]. Similarly, in lumbar central canal stenosis, a minor decrease in the cross-sectional area of the neural foramen does not represent neural foraminal stenosis. The Lumbar Spine Outcome Study Working Group Zurich considered the presence of perineural intraforaminal fat a reliable finding in neural foraminal stenosis [17].
A few radiologic criteria or parameters have been reported for the evaluation of lumbar neural foraminal stenosis. The grading system proposed by Wildermuth et al. [28] focuses only on the degree of epidural fat obliteration. Another classification proposed by Kunogi and Hasue [29] defines stenosis according to the direction of obliteration of perineural fat. However, a major difference from the Lee grading system criteria is that neither of these systems considers the morphological change or deformity of the nerve root as a factor in determining severity, which is a major strength of the Lee criteria for diagnosing lumbar neural foraminal stenosis. The Lee grading system showed good reproducibility and perfect interobserver agreement, and was significantly correlated with clinical manifestations [7,30,31].
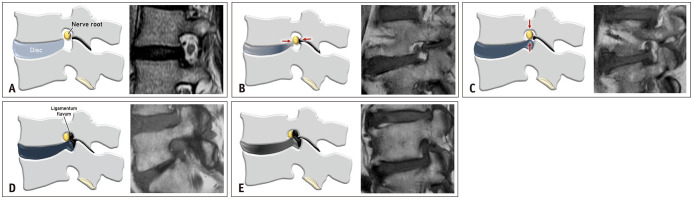
The Lee grading system for lumbar neural foraminal stenosis is presented in Table 2 and Figure 3. This system graded lumbar neural foraminal stenosis in terms of perineural fat obliteration and morphological changes in the nerve root. To diagnose lumbar neural foraminal stenosis, perineural fat in the neural foramen should be obliterated in at least one direction by the surrounding structures during the degenerative process. To clearly define neural foraminal stenosis, we suggest that at least one direction (i.e., one side and its opposite side) of the perineural fat should be obliterated. If perineural fat obliteration is observed only on a single side of the nerve and if the opposite side is preserved, the condition is not defined as neural foraminal stenosis. “Mild” lumbar neural foraminal stenosis (grade 1) is defined as perineural fat obliteration occurring in one opposing direction, such as the anterior-posterior (transverse) or superior-inferior (vertical) direction, without any morphological changes in the nerve root. “Moderate” lumbar neural foraminal stenosis (grade 2) is defined when perineural fat obliteration occurs in two or more directions but without morphological changes in the nerve root. “Severe” lumbar neural foraminal stenosis (grade 3) is defined when compression/collapse or morphological changes of the nerve root are observed. In some cases, especially those with spondylolytic spondylolisthesis, the nerve root can severely collapse in only one direction, and perineural fat can be visible. These cases are defined as having “severe” lumbar neural foraminal stenosis because of the presence of morphological changes in the compressed nerve root (Fig. 4).
Table 2. Grading Criteria for Lumbar Neural Foraminal Stenosis.
| Grade | Severity | Description |
|---|---|---|
| Grade 0 | Normal | There is absence of foraminal stenosis. |
| Grade 1 | Mild | Perineural fat surrounding the nerve root is obliterated in two opposing directions (vertical or transverse), contacting the superior and inferior portions or anterior and posterior portions of the nerve root. There is no visible morphological change of the nerve root. |
| Grade 2 | Moderate | Perineural fat surrounding the nerve root is obliterated in four directions (both vertical and transverse) without morphological change. |
| Grade 3 | Severe | There is collapse or morphological change of the nerve root. |
Fig. 3. The Lee grading system for lumbar neural foraminal stenosis. A. Schematic drawing of normal neural foramen and the corresponding T1-weighted sagittal magnetic resonance image shows normal nerve root without compression. B. Schematic drawing of mild neural foraminal stenosis and the corresponding T1-weighted sagittal magnetic resonance image show obliteration of the perineural fat in transverse direction (red arrows). There is narrowing of the foraminal width due to disc bulging and ligamentum flavum hypertrophy. There is no visible morphological change of the nerve root. C. Schematic drawing of mild neural foraminal stenosis and the corresponding T1-weighted sagittal magnetic resonance image show obliteration of the perineural fat in vertical direction (red arrows). There is narrowing of the foraminal height due to disc bulging at the foraminal zone. There is no visible of morphological change of the nerve root. D. Schematic drawing of moderate neural foraminal stenosis and the corresponding T1-weighted sagittal magnetic resonance image show obliteration of the perineural fat in both vertical and transverse directions, without morphological change of the nerve root. There is narrowing of the foraminal width and height due to thickened ligamentum flavum, facet arthropathy, and disc bulging at the foraminal zone. E. Schematic drawing of severe neural foraminal stenosis and the corresponding T1-weighted sagittal magnetic resonance image show complete obliteration of the perineural fat and morphological change of the nerve root caused by severe ligamentum flavum hypertrophy, facet arthropathy, and disc bulging at the foraminal zone.
Fig. 4. A 63-year-old male with spondylolytic spondylolisthesis suffering from chronic lower back pain and bilateral lower extremity radiculopathy. T1-weighted sagittal magnetic resonance image shows severe L5/S1 neural foraminal stenosis. Although there is visible perineural fat around L5 nerve root and nerve root is collapsed in only one direction, it should be determined as severe neural foraminal stenosis because of morphological changes in the compressed nerve root.
Cervical Central Canal Stenosis
The narrowing of the cervical spinal canal is caused by degenerative changes in several elements of the spinal architecture. As intervertebral disc degeneration progresses, the disc loses its ability to bear axial loads. These axial loads are transferred to the uncovertebral and facet joints, leading to joint space narrowing, subchondral sclerosis, and osteophyte formation at the joints [32]. These changes subsequently narrow the cervical spinal canal along with neural foramina, resulting in cervical spondylotic myelopathy or cervical spondylotic radiculopathy. Common symptoms of cervical spondylotic myelopathy are gait disturbances and hand clumsiness. These symptoms are caused by chronic mechanical compression resulting in Wallerian degeneration of the posterior columns and posterolateral tracts cephalad to the site of compression, together with the loss of anterior horn cells and corticospinal tract degeneration at and caudal to the site of compression. The histologic characteristics, cystic cavitation, gliosis of the central gray matter, and demyelination of the white matter tracts indicate chronic compression and correspond to a signal alteration of the spinal cord on T2-weighted images in advanced stage central canal stenosis [33].
Various attempts have been made to determine the degree of cervical central canal stenosis. Similar to lumbar stenosis, several quantitative measurements are available for cervical spinal stenosis. The diameter of the cervical spinal canal, distance between the posterior vertebral line and spinolaminar line, and spinal canal-to-vertebral body ratio on lateral radiographs have been proposed as criteria for assessing cervical myelopathy or spinal canal stenosis [34,35]. The cross-sectional area and dural sac diameter were measured on MRI. These measurements are a better assessment of spinal canal stenosis than that of the lumbar segment, but it still cannot represent a compromise of the spinal cord. Muhle et al. [36] proposed a qualitative classification system based on the degree of obliteration of the subarachnoid space and cord compression. However, according to our experience over the years, there are several limitations to their system: 1) the description of the degree of stenosis, such as partial or complete, was ambiguous or impractical and 2) the system did not account for signal alteration of the spinal cord, which is a hallmark of compressive myelopathy, the most severe stage of central canal stenosis.
The Lee grading system for cervical central canal stenosis takes into consideration the following obvious findings: CSF obliteration, spinal cord deformity, and intramedullary signal change, which reflect the mechanism and natural prognosis of spinal canal stenosis. Based on these findings, and with the aim of providing a clearer description, we proposed a grading system for cervical central canal stenosis. The Lee grading system for cervical central canal stenosis showed excellent intraobserver reliability and excellent to perfect interobserver agreement, and was significantly correlated with clinical manifestations [8,37].
The Lee grading system for cervical central canal stenosis is shown in Table 3 and Figure 5. The diagnosis of cervical central canal stenosis can be made based on a quick visual assessment of sagittal T2-weighted images. If more than 50% obliteration of the anterior or posterior subarachnoid space is observed, cervical central canal stenosis is considered present in that segment. Specifically, in “mild” central canal stenosis (grade 1), narrowing of the central canal induces > 50% obliteration of the anterior and/or posterior subarachnoid space around the spinal cord without any spinal cord deformity or signal change. If a deformity or compression of the spinal cord is observed without any medullary signal change on T2-weighted images, it is defined as “moderate” central canal stenosis (grade 2). If a signal change in the spinal cord is observed on T2-weighted images, it is defined as “severe” central canal stenosis (grade 3).
Table 3. Grading Criteria for Cervical Central Canal Stenosis.
| Grade | Severity | Description |
|---|---|---|
| Grade 0 | Normal | There is absence of central canal stenosis. |
| Grade 1 | Mild | The subarachnoid space is near completely obliterated, including obliteration of the arbitrary subarachnoid space exceeding 50%, without signs of cord deformity. |
| Grade 2 | Moderate | The spinal cord is compressed and deformed but without cord signal change. |
| Grade 3 | Severe | There is signal change of the spinal cord near the compressed level on T2-weighted images. |
Fig. 5. The Lee grading system for cervical central canal stenosis. A. Schematic drawing of normal cervical central canal and the corresponding T2-weighted sagittal magnetic resonance image show cervical spine without central canal compromise. There is no obliteration of cerebrospinal fluid space around the spinal cord. Deformity or signal alteration of the spinal cord is not observed. B. Schematic drawing of mild cervical central canal stenosis and the corresponding T2-weighted sagittal magnetic resonance image show mild central canal stenosis with over 50% obliteration of cerebrospinal fluid space at the C5/6 disc level, without deformity or signal alteration of the spinal cord. C. Schematic drawing of moderate cervical central canal stenosis and the corresponding T2-weighted sagittal magnetic resonance image show moderate central canal stenosis. Ventral aspect of the spinal cord is compressed and deformed. There is no signal change of the spinal cord. D. Schematic drawing of severe cervical central canal stenosis and the corresponding T2-weighted sagittal magnetic resonance image show severe central canal stenosis. Spinal canal is significantly narrow at the C5/6 disc level with severe deformity of the spinal cord. There is focal medullary hyperintensity at the compressed segment.
As mentioned above, this system emphasizes the direct effect on the spinal cord; therefore, several factors should be considered when determining the grade. When the spinal cord is compressed by a bulging disc or thickened ligament, it should be graded as moderate stenosis (grade 2) and not mild stenosis (grade 1), even though the CSF cleft is still preserved between the spinal cord and the compressing structures. Likewise, if a signal change is observed in the spinal cord on T2-weighted images at a level, it should be graded as severe stenosis, regardless of the spinal cord deformity or CSF cleft obliteration. Such cases of severe central canal stenosis (grade 3) are occasionally encountered in dynamic compression setting (Fig. 6) [38].
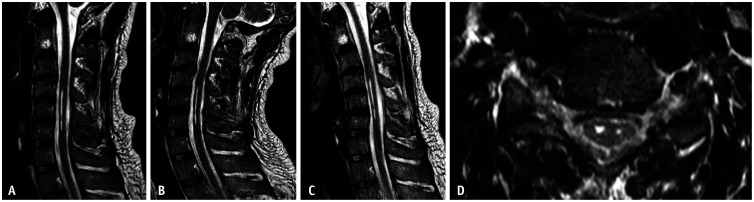
Fig. 6. A 34-year-old male presented with a 2-year history of progressive weakness and paresthesia of the right hand. T2-weighted sagittal magnetic resonance images in neutral position (A), extension (B), and flexion (C) show segmental linear medullary hyperintensity at C4 to C6 level. There is no significant narrowing of the cerebrospinal fluid space nor deformity of the spinal cord. T2-weighted axial magnetic resonance image (D) reveals mild atrophic and cystic change in the spinal cord gray matter at the corresponding level. These findings indicate long-standing compromise of the spinal cord by dynamic compression and should be determined as severe degree cervical central canal stenosis.
Cervical Neural Foraminal Stenosis
Cervical neural foraminal stenosis is a mechanical distortion of the cervical nerve roots due to neural foraminal narrowing caused by degenerative structural changes such as hypertrophied facet or uncovertebral joints, spurring of the vertebral body, lateral disc herniation, or a combination of these factors [39]. These structural changes cause nerve root compression and subsequent radiculopathy. The symptoms of cervical spondylotic radiculopathy include neck pain, radiating pain, and paresthesia in the arms.
Several attempts have been made to establish a method to grade cervical neural foraminal stenosis. These systems are mostly based on axial CT or MRI [40]. Park et al. [41] suggested a grading system based on oblique sagittal T2-weighted images. In this system, the basic rule of grading is similar to that for lumbar neural foraminal stenosis on sagittal T1-weighted images. A basic assumption in this system is that the oblique sagittal image shows the true foraminal view. However, the orientation angle of the cervical neural foramen varies from C2-3 to C7-T1 [42]. Therefore, obtaining images in a plane perfectly perpendicular to all levels of the cervical neural foramina course is impossible.
The Lee grading system for cervical neural foraminal stenosis suggests the use of axial T2-weighted images to avoid overestimation of stenosis. Nevertheless, it might still be difficult to determine the degree of neural foraminal narrowing because the original widths of the neural foramen and nerve root are difficult to define. As spondylotic changes progress, the neural foramen becomes structurally narrowed, and the root is already compressed within the foramen. Therefore, we developed an internal reference, that is, the width of the extraforaminal nerve root, to assess neural foraminal narrowing. The extraforaminal nerve root, which is located between the superior articular process and vertebral artery, is often unaffected by neural foraminal narrowing because cervical neural foraminal stenosis is commonly induced by uncovertebral osteophytes. Based on our clinical experience, we used the width of the extraforaminal nerve root, including the ipsilateral or contralateral side, as a reference to define and grade the neural foraminal stenosis. This grading system showed fair to good reproducibility (intraclass correlation coefficient ranged from 0.68 to 0.73) and perfect interobserver agreement [9]. Moreover, a clinical validity study of this grading system concluded that this system demonstrated a high correlation with clinical manifestations and could be useful in clinical settings [43,44].
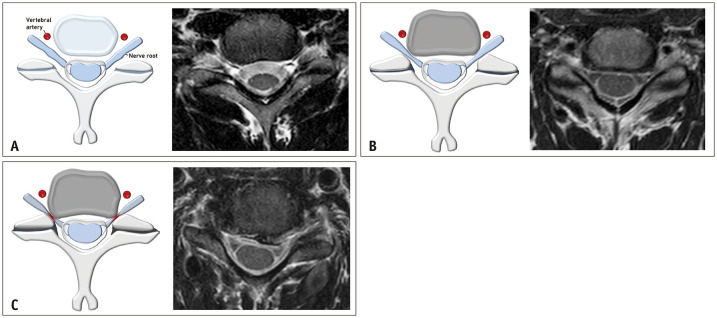
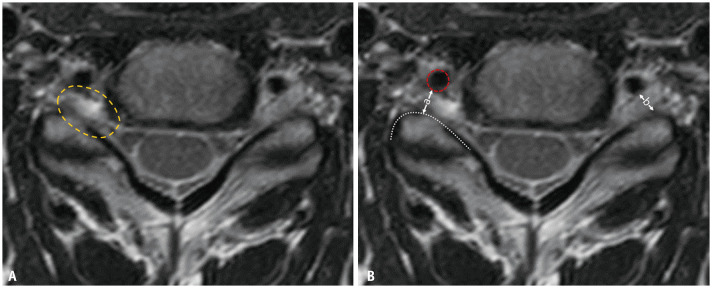
The Lee grading system for cervical neural foraminal stenosis is shown in Table 4 and Figure 7. The narrowest width of the neural foramen is narrower than that of the extraforaminal nerve root, which indicates the presence of cervical neural foraminal stenosis. If the width of the neural foramen is less than half the width of the extraforaminal nerve root, it indicates “severe” neural foraminal stenosis (grade 2). If the width of the neural foramen is more than half, but narrower than the width of the extraforaminal nerve root, it indicates “non-severe” neural foraminal stenosis (grade 1). Occasionally, the extraforaminal nerve root is not clearly visible. In such cases, the width of the contralateral extraforaminal nerve root or the distance between the posterior margin of the vertebral artery and anterior margin of the superior articular process can be used as an alternative (Fig. 8).
Table 4. Grading Criteria for Cervical Neural Foraminal Stenosis.
| Grade | Severity | Description |
|---|---|---|
| Grade 0 | Normal | The narrowest width of the neural foramen is greater than the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process. |
| Grade 1 | Non-severe | The narrowest width of the neural foramen is 51%–100% of the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process. |
| Grade 2 | Severe | The width of the neural foramen is the same as or less than 50% of the width of the extraforaminal nerve roots. |
Fig. 7. The Lee grading system for cervical neural foraminal stenosis. A. Schematic drawing of normal cervical neural foramen and the corresponding T2-weighted axial magnetic resonance image show normal bilateral C5/6 neural foramina without nerve root compromise. B. Schematic drawing of non-severe neural foraminal stenosis and the corresponding T2-weighted axial magnetic resonance image show non-severe neural foraminal stenosis of the left C5/6 neural foramen. The narrowest width of the left side neural foramen is less than (but more than 50% of) the extraforaminal nerve root width. C. Schematic drawing of severe neural foraminal stenosis and the corresponding T2-weighted axial magnetic resonance image show severe neural foraminal stenosis of the right C5/6 neural foramen. The narrowest width of the right side neural foramen is less than 50% of the extraforaminal nerve root width.
Fig. 8. Method for grading cases without a visible extraforaminal nerve root. A. The extraforaminal nerve root on the right side is not visible on the T2-weighted axial magnetic resonance image (yellow dotted-circle). B. The distance (a) between the posterior margin of the vertebral artery (red dotted-circle) and the anterior margin of the superior articular process (white dotted-line) or the width (b) of the contralateral extraforaminal nerve root can be used as reference.
CONCLUSION
This review highlights the structured qualitative MRI grading system, the Lee grading system, for lumbar central canal stenosis, lumbar neural foraminal stenosis, cervical central canal stenosis, and cervical neural foraminal stenosis. Beyond detecting the presence of central canal or neural foraminal stenosis on MRI, accurate grading of stenosis severity is necessary to establish management plans for patients with spinal stenosis. These grading systems may promote effective communication between clinicians and fellow radiologists, and provide practical guidance for deciding treatments for patients with spinal stenosis.
Footnotes
Conflicts of Interest: Joon Woo Lee who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. Other author has declared no conflicts of interest.
- Conceptualization: all athors.
- Methodology: all athors.
- Resources: all athors.
- Supervision: all athors.
- Visualization: Jiwoon Seo.
- Writing—original draft: all athors.
- Writing—review & editing: all athors.
Funding Statement: None
References
- 1.Jensen RK, Jensen TS, Koes B, Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J. 2020;29:2143–2163. doi: 10.1007/s00586-020-06339-1. [DOI] [PubMed] [Google Scholar]
- 2.Statistics of annual lumbar magnetic resonance imaging in south korea 2010-2021. Healthcare Bigdata Hub.com Web site. [Accessed April 11, 2022]. http://opendata.hira.or.kr/op/opc/olapDiagBhvInfo.do .
- 3.Andreisek G, Hodler J, Steurer J. Uncertainties in the diagnosis of lumbar spinal stenosis. Radiology. 2011;261:681–684. doi: 10.1148/radiol.11111086. [DOI] [PubMed] [Google Scholar]
- 4.Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine (Phila Pa 1976) 2010;35:803–811. doi: 10.1097/BRS.0b013e3181bc9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update) Spine J. 2013;13:734–743. doi: 10.1016/j.spinee.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Lee GY, Lee JW, Choi HS, Oh KJ, Kang HS. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40:1033–1039. doi: 10.1007/s00256-011-1102-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Lee JW, Yeom JS, Kim KJ, Kim HJ, Chung SK, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194:1095–1098. doi: 10.2214/AJR.09.2772. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol. 2011;197:W134–W140. doi: 10.2214/AJR.10.5560. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Lee JW, Chai JW, Yoo HJ, Kang Y, Seo J, et al. A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol. 2015;16:1294–1302. doi: 10.3348/kjr.2015.16.6.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Merriam-Webster Dictionary [Internet] Springfield (MA): Merriam-Webster Inc.; 2022. Stenosis. https://www.merriam-webster.com/dictionary/stenosis . [Google Scholar]
- 11.Mates RE, Gupta RL, Bell AC, Klocke FJ. Fluid dynamics of coronary artery stenosis. Circ Res. 1978;42:152–162. doi: 10.1161/01.res.42.1.152. [DOI] [PubMed] [Google Scholar]
- 12.Roy M, Sikarwar BS, Bhandwal M, Ranjan P. Modelling of blood flow in stenosed arteries. Procedia Computer Science. 2017;115:821–830. [Google Scholar]
- 13.Telano LN, Baker S. Physiology, cerebral spinal fluid [Internet] Treasure Island (FL): StatPearls Publishing; 2022. [Last updated Jul 4, 2022]. https://www.ncbi.nlm.nih.gov/books/NBK519007 . [PubMed] [Google Scholar]
- 14.Hasue M, Kikuchi S, Sakuyama Y, Ito T. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine (Phila Pa 1976) 1983;8:50–58. doi: 10.1097/00007632-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Jenis LG, An HS. Spine update: Lumbar foraminal stenosis. Spine (Phila Pa 1976) 2000;25:389–394. doi: 10.1097/00007632-200002010-00022. [DOI] [PubMed] [Google Scholar]
- 16.Choi YK. Lumbar foraminal neuropathy: an update on non-surgical management. Korean J Pain. 2019;32:147–159. doi: 10.3344/kjp.2019.32.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreisek G, Deyo RA, Jarvik JG, Porchet F, Winklhofer SF, Steurer J, et al. Consensus conference on core radiological parameters to describe lumbar stenosis - an initiative for structured reporting. Eur Radiol. 2014;24:3224–3232. doi: 10.1007/s00330-014-3346-z. [DOI] [PubMed] [Google Scholar]
- 18.Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253–265. doi: 10.1016/j.berh.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steurer J, Roner S, Gnannt R, Hodler J LumbSten Research Collaboration. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Wall EJ, Kerber CW, Abitbol JJ, Garfin SR. The anatomy of the cauda equina on CT scans and MRI. J Bone Joint Surg Br. 1991;73:381–384. doi: 10.1302/0301-620X.73B3.1670432. [DOI] [PubMed] [Google Scholar]
- 21.Lohman CM, Tallroth K, Kettunen JA, Lindgren KA. Comparison of radiologic signs and clinical symptoms of spinal stenosis. Spine (Phila Pa 1976) 2006;31:1834–1840. doi: 10.1097/01.brs.0000227370.65573.ac. [DOI] [PubMed] [Google Scholar]
- 22.Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008;17:679–685. doi: 10.1007/s00586-008-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko YJ, Lee E, Lee JW, Park CY, Cho J, Kang Y, et al. Clinical validity of two different grading systems for lumbar central canal stenosis: Schizas and Lee classification systems. PLoS One. 2020;15:e0233633. doi: 10.1371/journal.pone.0233633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HJ, Kim SS, Lee YJ, Lee SY, Park NH, Choi YJ, et al. Clinical correlation of a new practical MRI method for assessing central lumbar spinal stenosis. Br J Radiol. 2013;86:20120180. doi: 10.1259/bjr.20120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisanti C, Carlin C, Banks KP, Wang D. Normal MRI appearance and motion-related phenomena of CSF. AJR Am J Roentgenol. 2007;188:716–725. doi: 10.2214/AJR.05.0003. [DOI] [PubMed] [Google Scholar]
- 26.Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919–1924. doi: 10.1097/BRS.0b013e3181d359bd. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa T, An HS, Haughton VM, Nowicki BH. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am. 1995;77:32–38. [PubMed] [Google Scholar]
- 28.Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, et al. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology. 1998;207:391–398. doi: 10.1148/radiology.207.2.9577486. [DOI] [PubMed] [Google Scholar]
- 29.Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine (Phila Pa 1976) 1991;16:1312–1320. doi: 10.1097/00007632-199111000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Park HJ, Kim SS, Lee SY, Park NH, Rho MH, Hong HP, et al. Clinical correlation of a new MR imaging method for assessing lumbar foraminal stenosis. AJNR Am J Neuroradiol. 2012;33:818–822. doi: 10.3174/ajnr.A2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong TS, Ahn Y, Lee SG, Kim WK, Son S, Kwon JH. Correlation between MRI grading system and surgical findings for lumbar foraminal stenosis. J Korean Neurosurg Soc. 2017;60:465–470. doi: 10.3340/jkns.2016.1010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara LA. The biomechanics of cervical spondylosis. Adv Orthop. 2012;2012:493605. doi: 10.1155/2012/493605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi SH, Kang CN. Degenerative cervical myelopathy: pathophysiology and current treatment strategies. Asian Spine J. 2020;14:710–720. doi: 10.31616/asj.2020.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards WC, LaRocca H. The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine (Phila Pa 1976) 1983;8:20–27. doi: 10.1097/00007632-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164:771–775. doi: 10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 36.Muhle C, Metzner J, Weinert D, Falliner A, Brinkmann G, Mehdorn MH, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. ANJR Am J Neuroradiol. 1998;19:1763–1771. [PMC free article] [PubMed] [Google Scholar]
- 37.Park HJ, Kim SS, Chung EC, Lee SY, Park NH, Rho MH, et al. Clinical correlation of a new practical MRI method for assessing cervical spinal canal compression. AJR Am J Roentgenol. 2012;199:W197–W201. doi: 10.2214/AJR.11.7599. [DOI] [PubMed] [Google Scholar]
- 38.Joaquim AF, Baum GR, Tan LA, Riew KD. Dynamic cord compression causing cervical myelopathy. Neurospine. 2019;16:448–453. doi: 10.14245/ns.1938020.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbed KM, Coumans JV. Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery. 2007;60(1 Suppl 1):S28–S34. doi: 10.1227/01.NEU.0000249223.51871.C2. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett RJ, Hill CR, Gardiner E. A comparison of T2 and gadolinium enhanced MRI with CT myelography in cervical radiculopathy. Br J Radiol. 1998;71:11–19. doi: 10.1259/bjr.71.841.9534693. [DOI] [PubMed] [Google Scholar]
- 41.Park HJ, Kim SS, Han CH, Lee SY, Chung EC, Kim MS, et al. The clinical correlation of a new practical MRI method for grading cervical neural foraminal stenosis based on oblique sagittal images. AJR Am J Roentgenol. 2014;203:412–417. doi: 10.2214/AJR.13.11647. [DOI] [PubMed] [Google Scholar]
- 42.Simpson AK, Sabino J, Whang P, Emerson JW, Grauer JN. The assessment of cervical foramina with oblique radiographs: the effect of film angle on foraminal area. J Spinal Disord Tech. 2009;22:21–25. doi: 10.1097/BSD.0b013e3181639b62. [DOI] [PubMed] [Google Scholar]
- 43.Lee JE, Park HJ, Lee SY, Lee YT, Kim YB, Lee KH, et al. Interreader reliability and clinical validity of a magnetic resonance imaging grading system for cervical foraminal stenosis. J Comput Assist Tomogr. 2017;41:926–930. doi: 10.1097/RCT.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 44.Lee KH, Park HJ, Lee SY, Chung EC, Rho MH, Shin H, et al. Comparison of two MR grading systems for correlation between grade of cervical neural foraminal stenosis and clinical manifestations. Br J Radiol. 2016;89:20150971. doi: 10.1259/bjr.20150971. [DOI] [PMC free article] [PubMed] [Google Scholar]