Take-home points
• Proton density fat fraction measurements enable unconfounded quantification of hepatic fat accumulation.
• Currently, magnetic resonance elastography is the most established magnetic resonance imaging (MRI) technique for the assessment of hepatic fibrosis.
• Abbreviated MRI protocols rely on acquisition of only a limited number of sequences tailored to a specific disease.
INTRODUCTION
Magnetic resonance imaging (MRI) has emerged as the imaging modality of choice for evaluating patients with focal or diffuse liver disease. Its unique advantages compared with computed tomography include the lack of ionizing radiation exposure and an inherently high soft tissue signal. Therefore, additional intravenous contrast is not mandatory. However, with the advancement of hepatobiliary contrast agents (gadoxetate disodium: Eovist or Primovist, Bayer; gadobenate dimeglumine: MultiHance, Bracco Imaging), the assessment of hepatocellular function has become feasible [1].
In recent years, MRI has developed from being considered as a diagnostic tool for the assessment of morphological disorders to an advanced technique for multiparametric imaging [2], enabling evaluation and prediction of disease course [3] and therapeutic success [4]. Development is ongoing with a vast number of emerging techniques for morphological and quantitative imaging, many of which are primarily used in research settings. Discussion of all of these is beyond the scope of this article. Instead, this manuscript focuses on new techniques that are already applied in routine clinical practice or are likely to be more broadly applied in the near future. MRI techniques are addressed from a clinical perspective, specifically for the assessment of hepatic steatosis, fibrosis, and focal liver lesions (FLLs).
Hepatic Steatosis
Clinical Relevance
Hepatic steatosis refers to a pathologically elevated fat content in hepatocytes. It is graded histologically depending on the percentage of hepatocytes presenting intracellular lipid-containing vacuoles [5]. Non-alcoholic fatty liver disease (NAFLD) is the most frequent cause of hepatic steatosis, affecting more than 25% of the adult population and with an increasing prevalence worldwide [6]. Approximately 20% of patients with NAFLD progress to non-alcoholic steatohepatitis (NASH), which is characterized by additional inflammation with or without fibrosis [7]. Liver biopsy is the reference standard for diagnosing fat accumulation and differentiating NAFLD from NASH. However, biopsy is invasive and prone to sampling bias, particularly in cases of heterogeneous disease distribution. There is a great need for non-invasive biomarkers for the detection and quantification of fat accumulation, especially in the early stages.
Emerging MRI Techniques for Steatosis Assessment
Current MRI techniques exploit the differences in the resonance frequencies of water and fat protons. Until recently, magnetic resonance spectroscopy (MRS) has been considered a non-invasive reference standard for the quantification of hepatic fat accumulation. In MRS, the proton signals of water and fat are acquired in a single breath hold and then depicted as a high-resolution spectrum. Currently, point-resolved spectroscopy (PRESS) and stimulated-echo acquisition mode (STEAM) are the most common techniques in practice. Although PRESS is characterized by a higher signal-to-noise ratio (SNR), STEAM is often preferred because it is less affected by J-coupling [8]. Despite rapid MRS techniques (such as, high-speed T2-corrected multi-echo single voxel spectroscopy), MRS is limited to a voxel basis and, therefore, prone to sampling bias. Multivoxel MRS increases the imaging time proportionally, but can be used to cover larger volumes.
More recently, proton density frat fraction (PDFF) has been introduced and has already superseded MRS. PDFF refers to the fraction of mobile protons in the liver that are attributable to fat. It is an uncorrected confounded measure of steatosis that is influenced by biological, physical, and technical factors. Therefore, imaging techniques need to address all confounding factors (T1 bias, T2 relaxation, T2* decay, the spectral complexity of fat, J-coupling, noise bias, and eddy currents). PDFF is an unconfounded measure for the quantification of fat accumulation, independent of magnetic field strength, scanner platform, and technical parameters [9]. To calculate PDFF, multi-echo gradient echo (GRE) sequences with chemical shift-encoding (CSE) are used, enabling coverage of the whole liver in a single breath-hold. PDFF measurements correlate closely with histology [10] and are expressed in percentage (0%–100%) (Fig. 1). Different strategies for post-processing and calculation exist, with the exact strategy for CSE depending on the vendor. Magnitude-based techniques are simpler to implement but suffer from a low SNR and coverage of a limited PDFF range (0%–50%). Complex-based CSE techniques enable measurement of the full PDFF range (0%–100%). However, they are more sensitive to phase errors and may suffer from occasional water-fat swaps. Lastly, hybrid-based methods combine the advantages of both approaches [9].
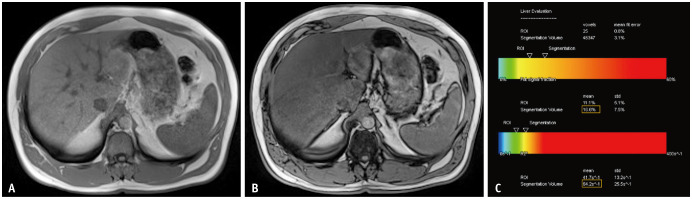
Fig. 1. A 51-year-old male patient referred for magnetic resonance imaging (MRI) with clinical suspicion of iron storage disorder. On the in-phase image (A), a slightly increased signal intensity of the liver can be observed compared to that of the spleen. Opposed-phase imaging (B) reveals a distinct signal drop in the liver parenchyma. Quantification using chemical-shift encoded MRI (C) demonstrates a proton density fat fraction of approximately 17%, consistent with mild steatosis, but with no significant iron deposition (R2* below 70 s-1). ROI = region of interest, std = standard deviation.
Confounder-corrected CSE-MRI further enables the simultaneous assessment of iron deposition. The presence of iron results in tissue signal loss in T2 and T2*-weighted images, which is proportional to the iron content. Based on R2* measurements, fat-corrected R2*maps are a by-product of multi-echo CSE acquisitions used to create R2*-corrected PDFF maps [11].
There is growing evidence for the use of PDFF, which is an increasingly accepted technique, especially for the evaluation of NAFLD. In a meta-analysis comparing PDFF measurements with transient elastography, MRI offered pooled sensitivities and specificities of 0.71–0.91 and 0.88–0.93, respectively, for the staging of hepatic steatosis [12]. Studies have shown that PDFF can be used to monitor and predict therapeutic effects in patients with NAFLD [4,13]. Other potential applications for PDFF measurements include surveillance of patients undergoing bariatric surgery [14] and preoperative evaluation of living liver donor candidates [15].
Future Expectations
Concomitant fat and/or iron deposition is often present in patients with chronic liver disease (CLD), and quantification is clinically useful for diagnosis and follow-up. PDFF measurements with simultaneous evaluation of iron content provide a great opportunity for the implementation of abbreviated MRI protocols specifically tailored to these clinical indications. Automated assessment of PDFF and T2* maps based on deep learning algorithms may facilitate analysis; until then, a consensus on a standardized approach for placement of regions of interest would be desirable. Free-breathing techniques, including navigator-based image acquisition or non-cartesian sampling strategies, are the current areas of research.
Hepatic Fibrosis
Clinical Relevance
Hepatic fibrosis results from progressive liver injury in all causes of CLDs. It is defined as an excessive accumulation of extracellular matrix proteins and is commonly staged from F0-F4 in the radiology literature [16]. CLD is a major health problem, with significant morbidity and mortality worldwide. In the USA, CLD and cirrhosis are the 10th leading causes of death [17]. Liver biopsy is the reference standard for diagnosis and staging of hepatic fibrosis. Early detection and treatment of the cause are critical because early stage fibrosis is potentially reversible, and liver transplantation is the only curative treatment for decompensated cirrhosis [16].
Emerging MRI Techniques for Fibrosis Assessment
Magnetic resonance elastography (MRE) is currently the leading MRI technique for staging hepatic fibrosis, with stiffness measurements directly related to fibrosis stage [18]. A passive driver that generates mechanical waves is positioned over the patient’s liver. Mechanical vibrations are produced by an active driver outside the MRI room and transported through a flexible tube to the passive driver. A phase-contrast pulse sequence with motion-encoding gradients is used to evaluate wave propagation. Post-processing enables the visualization of wave images and quantitative maps of liver stiffness, known as elastograms [16]. Higher liver stiffness causes faster wave propagation and is directly associated with a higher fibrosis stage (Fig. 2).
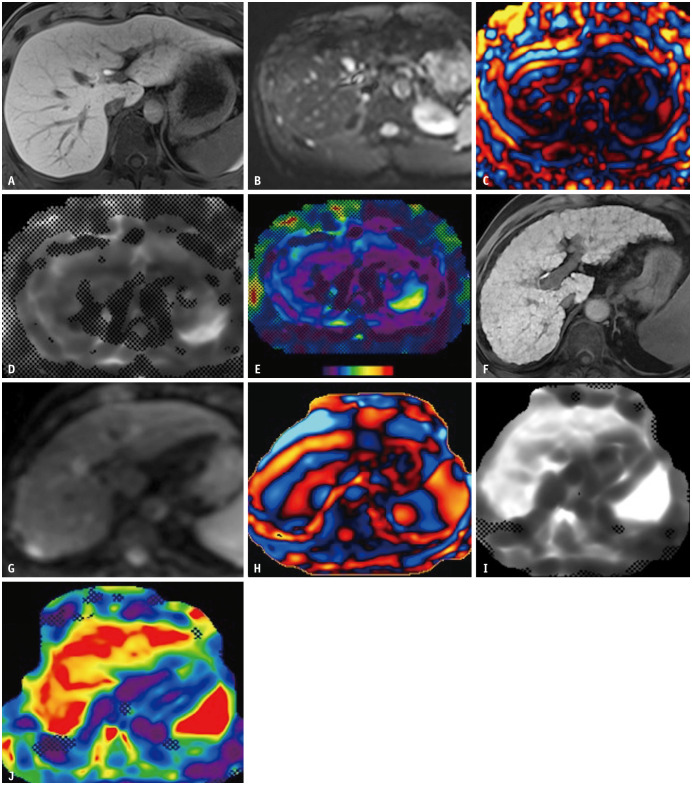
Fig. 2. Spin-echo magnetic resonance elastography (MRE) at 3T in a 29-year-old male living liver donor candidate (A-E) and gradient echo MRE at 3T in a 63-year-old cirrhotic male patient (F-J). Hepatobiliary phase images (A, F) demonstrate homogeneous contrast uptake. Magnitude (B, G) and wave (C, H) image data are converted into gray-scale (D, I) and color elastograms (E, J). Compared to the healthy donor, faster wave speed is depicted on wave images of the cirrhotic patient (H), consistent with higher liver stiffness on gray-scale and color elastograms (I, J).
Although MRE is the most established MRI technique for the assessment of hepatic fibrosis, the need for additional hardware is a drawback for the expansion of clinical implementation. Reliability has been reported to be lower in patients with iron overload, massive ascites, or high body mass index [16,19]. The recently implemented MRE using spin-echo echo-planar imaging (SE-EPI) may overcome some of these limitations. Acquisition of MRE is traditionally performed with GRE sequences, which tend to have a relatively long echo time (TE) and are therefore sensitive to iron overload. Compared with GRE sequences, SE or SE-EPI sequences have shorter TEs and are inherently insensitive to T2* effects due to a 180° refocusing pulse, as shown in a study by Zhan et al. [20] for MRE at 3T. Studies have shown that 2D or 3D MRE using SE-EPI has lower technical failure rates and a larger confidence area while maintaining a comparable or higher diagnostic performance than conventional 2D GRE-MRE [20,21,22]. Notably, the cut-off values for staging hepatic fibrosis are not significantly different between these two sequences [20,23].
Recently, 3D MRE has been introduced for the evaluation of hepatic fibrosis. In 2D MRE, elastograms are reconstructed based on waves propagating in the slice plane, whereas waves propagating oblique to the splice plane are discarded. In a 3D MRE, wave motions are encoded in three planes (x, y, and z), providing more accurate information on the properties of materials in theory. Specifically, in a 3D MRE, the storage and loss moduli can be calculated independently, as compared to the magnitude of the complex shear modulus (that is, liver stiffness value) of a 2D MRE. For 3D MRE, more data are required; SE-EPI sequences are usually used to acquire multiple slices in a limited timeframe. Recent studies have shown that the liver damping ratio in 3D MRE is further associated with inflammation in patients without or early stage fibrosis [24]. Notably, the cut-off values for the staging of fibrosis between 3D and conventional 2D MRE may be different [25,26].
A more advanced technical approach is the multi-frequency MRE. Currently, the staging of hepatic fibrosis is usually performed at a single frequency (60 Hz). Even though single-frequency MRE has been applied successfully for the staging of hepatic fibrosis, its accuracy, especially for the detection of early stage fibrosis, is relatively low [27]. This can be improved by adopting the concept of viscoelasticity. For multi-frequency MRE, tissue oscillations are acquired either separately or simultaneously at multiple frequencies (25–62.5 Hz) [28,29]. The storage and loss moduli are then calculated using different rheological models, for example, Voit, Maxwell, or spring-pot models [29]. Recent studies have shown that multi-frequency MRE may improve the performance of MRE in the diagnosis of hepatic fibrosis and in differentiating fibrosis from inflammation [28,30].
With the use of MRE, high areas under the receiver operating characteristic or area under receiver operating characteristics have been determined for the classification of fibrosis as ≥ 1, ≥ 2, ≥ 3, or 4 [18,31,32,33]. Compared with ultrasound elastography, MRE has a higher diagnostic performance for staging liver fibrosis, especially in patients with ascites [19,34]. However, it is important to note that the threshold for liver stiffness on MRE needs to be further validated depending on the etiology of CLD and scan parameters, including applied sequences, dimensions, and frequencies [16].
MRI evaluation of hepatic fibrosis using hepatobiliary contrast agents is also feasible. Hepatocellular contrast uptake in the hepatobiliary phase is quantified as a surrogate biomarker for the estimation of liver fibrosis. Different techniques have been developed for quantifying hepatocellular contrast uptake. Signal intensity-based indices such as relative liver enhancement and liver-to-spleen index are easy to implement because there is no need for specific sequences or post-processing [35]. Studies have shown a good correlation of signal intensity (SI)-based indices with liver function tests and established clinical scores [3,36]. However, it must be noted that SI measurements are only relative values and are dependent on technical parameters. In addition, the nonlinear relationship between gadolinium (Gd) concentration and SI is a potential caveat.
T1 mapping, also known as relaxometry, overcomes some of the limitations of direct SI measurements. Contrast-induced T1 relaxation time changes are directly correlated with Gd concentration and are therefore more reliable than SI measurements [37]. For hepatic fibrosis evaluation, the variable flip-angle method with B1 inhomogeneity correction, modified look-locker, and look-locker inversion recovery sequences have been proposed [38,39,40]. In addition to T1 relaxation time, the contrast uptake ratio or contrast uptake rate can be calculated using a dedicated software [41,42] (Fig. 3). Encouragingly, T1 relaxation time and calculated contrast uptake ratio have been shown to correlate with liver function and clinical outcome [40,43]. Although T1 relaxometry has theoretical advantages over direct SI measurements, the requirement for dedicated sequences and software limits its application in research settings. Moreover, confounding factors, such as iron, fat, and off-resonance effects, may affect T1 values. For example, if fat is not suppressed correctly when performing T1 mapping using the modified look-locker technique, T1 values may be overestimated owing to the fat. Recent studies have demonstrated the feasibility of obtaining confounding factor-corrected T1 values [44,45,46].
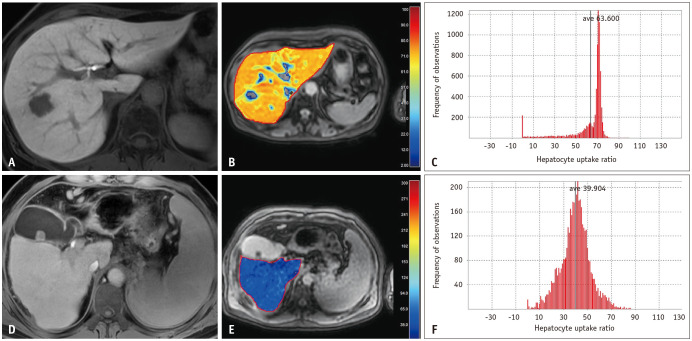
Fig. 3. Evaluation of contrast uptake for fibrosis assessment. A 53-year-old female patient with chronic hepatitis B (A-C). Hepatobiliary phase imaging (A) and hepatocyte uptake ratio (HUR) map (B) using Look-Locker sequence depict homogeneous and high contrast uptake (HUR: 63.6). In comparison, hepatobiliary phase imaging (D) and HUR map (E) in a 65-year-old male patient with Child B cirrhosis demonstrate decreased contrast uptake (HUR: 39.9). Histogram analyses (C, F) reveal comparatively more heterogeneous contrast uptake in the patient with advanced cirrhosis (F). ave = average.
Future Expectations
MRE is likely to be more widely implemented in the near future, along with verification of etiology-based cut-off values. The possibility of differentiating between fibrosis and inflammation, as well as an increase in the diagnostic accuracy for early stage fibrosis are promising expectations. T1 mapping may improve in precision by corrections in confounding factors and liver MR fingerprinting may enable water-specific T1 acquisition [47,48]. The integration of automatic liver segmentation and automated reporting of liver stiffness or T1 values will ease the radiology workflow [49]. The same is true for the implementation of deep- or machine-learning algorithms for the evaluation of fibrosis and liver function derived from routine MRI sequences [50,51]. These techniques are expected to play an important role in the context of abbreviated MRI in combination with PDFF measurements for the evaluation of diffuse liver diseases, including NAFLD and NASH [26,52]. Finally, improving the cost-effectiveness of these applications by adopting low-field point-of-care MRI [53] will help to further popularize the implementation of MRI for the evaluation of diffuse liver disease.
FLLs
Clinical Relevance
In healthy livers, most incidentally detected FLLs in patients without known malignancies are benign. Most malignant FLLs are metastases, especially from the gastrointestinal tract, pancreas, breast, and lungs. In the cirrhotic liver, however, approximately 75% of FLLs are consistent with hepatocellular carcinoma (HCC) [54]. Therefore, differential diagnosis is dependent on patient history, age, and clinical examination. Imaging is a mainstay not only for diagnosis but also for the determination of the number and localization of lesions. In this context, MRI has evolved into the imaging modality of choice for evaluating patients with suspected or known FLLs. A conventional full MRI exam, however, is time consuming, which is in contrast to the rising clinical demand and overall limited scanner availability.
Emerging MRI Techniques for Assessment of FLLs
Recently, abbreviated MRI protocols have been introduced. This approach is characterized by the acquisition of only a limited number of selected sequences tailored to a specific disease. With acquisition times of approximately 10 min or less, abbreviated MRI aims to reduce exam complexity while maintaining a high diagnostic sensitivity. Therefore, it is of utmost importance that each sequence has a consistent and acceptable image quality. Rapid imaging techniques may facilitate adherence to this timeframe. For example, compressed sensing-based rapid imaging has been successfully adopted for T1-weighted imaging in full MRI protocols [55]. Deep-learning-based T2- or diffusion-weighted imaging may shorten acquisition times while simultaneously improving image quality and lesion detection [56,57,58]. The implementation of these techniques may further reduce the acquisition times without compromising the image quality, which is critical for abbreviated MRI.
Three general abbreviated MRI approaches have been developed for FLLs, as follows, and are currently being discussed in the literature: non-contrast abbreviated MRI, dynamic abbreviated MRI, and hepatobiliary abbreviated MRI (Fig. 4). Each protocol has specific advantages and disadvantages [59,60]. The clinical potential of abbreviated MRI protocols is promising, for example, for the evaluation and follow-up of diffuse liver disease [61] and the detection and follow-up of metastatic liver disease [62,63]. However, most evidence is available in the context of HCC screening and surveillance. A meta-analysis showed that abbreviated MRI had higher sensitivity than ultrasound (82% vs. 53%) for HCC screening [64]. Similarly, for HCC surveillance, abbreviated MRI had high sensitivity, especially for very early tumor detection, with pooled sensitivities and specificities of 85% and 94%, respectively, for any tumor stage [65].
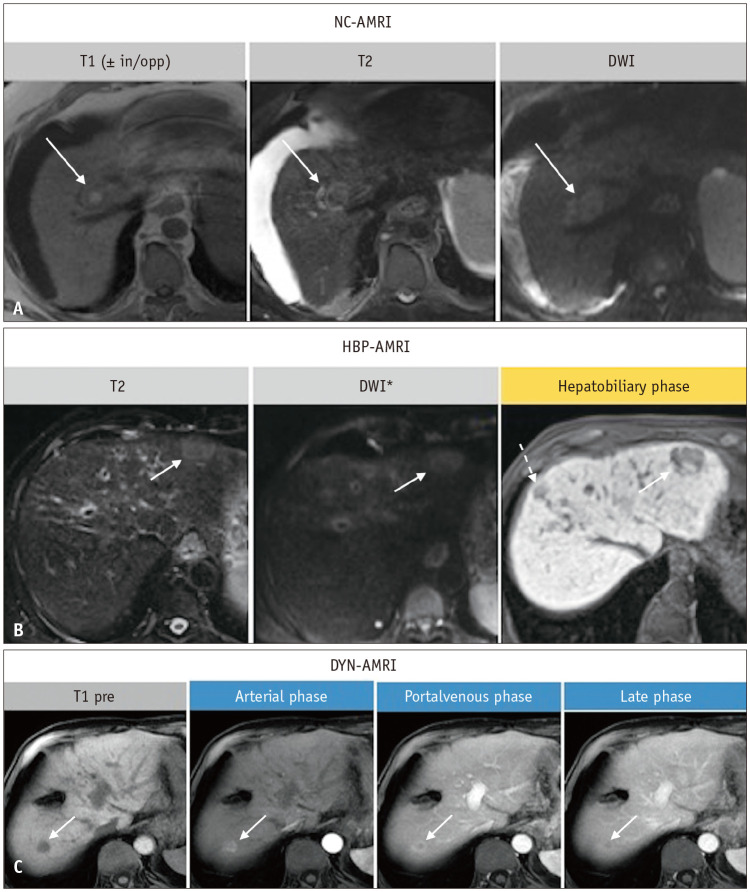
Fig. 4. Abbreviated magnetic resonance imaging (AMRI) in three different patients with hepatocellular carcinoma. Non-contrast (NC)-AMRI (A), hepatobiliary (HBP)-AMRI (B) and dynamic (DYN)-AMRI (C) in three different patients with hepatocellular carcinoma (arrows in A-C). In the hepatobiliary phase an additional lesion can be appreciated (dashed arrow in B). *There is no consensus on whether diffusion-weighted imaging (DWI) should be a part of this protocol or not. in/opp = in and opposed phase imaging, pre = pre-contrast.
Future Expectations
Abbreviated MRI protocols are likely to become firmly established in clinical routine in the near future. Implementation of deep-learning algorithms may improve the diagnostic confidence and accuracy for the detection of FLLs [66]. However, despite apparent advantages, some open issues for the use of abbreviated MRI still need to be addressed: the optimal target population, which patients benefit the most, how findings should be reported and followed-up, and the appropriate cost of abbreviated MRI. In addition, there is a lack of large prospective studies directly comparing abbreviated MRI with full MRI or other imaging modalities; until then, the best abbreviated MRI protocol for each setting needs to be defined.
CONCLUSION
MRI has advanced from a mere diagnostic tool for the assessment of morphological disorders to a non-invasive quantitative biomarker for predicting prognosis and therapeutic success. Multiparametric imaging enables the comprehensive assessment of diffuse liver disease. PDFF is an emerging technique for the quantification of hepatic steatosis. Similarly, MRE) and the use of hepatocyte-specific contrast agents are promising applications for the quantification of hepatic fibrosis. Abbreviated MRI protocols tailored to a specific disease (such as focal lesions and diffuse liver disease) are likely to become firmly established and will help fulfill the increasing demand for imaging.
Footnotes
Conflicts of Interest: Kristina I. Ringe received honoraria from Bayer Healthcare in the past 12 months. Jeong Hee Yoon received honoraria from Bayer, Philips Healthcare and Accuzen in the past 12 months.
- Conceptualization: all authors.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Funding Statement: None
References
- 1.Tan CH, Chou SC, Inmutto N, Ma K, Sheng R, Shi Y, et al. Gadoxetate-enhanced MRI as a diagnostic tool in the management of hepatocellular carcinoma: report from a 2020 Asia-Pacific multidisciplinary expert meeting. Korean J Radiol. 2022;23:697–719. doi: 10.3348/kjr.2021.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomaides-Brears HB, Lepe R, Banerjee R, Duncker C. Multiparametric MR mapping in clinical decision-making for diffuse liver disease. Abdom Radiol (NY) 2020;45:3507–3522. doi: 10.1007/s00261-020-02684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze J, Lenzen H, Hinrichs JB, Ringe B, Manns MP, Wacker F, et al. An imaging biomarker for assessing hepatic function in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2019;17:192–199.e3. doi: 10.1016/j.cgh.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Chen HC, Lafata KJ, Bashir MR. Week 4 liver fat reduction on MRI as an early predictor of treatment response in participants with nonalcoholic steatohepatitis. Radiology. 2021;300:361–368. doi: 10.1148/radiol.2021204325. [DOI] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145–152. doi: 10.1002/jmri.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshizawa E, Yamada A. MRI-derived proton density fat fraction. J Med Ultrason (2001) 2021;48:497–506. doi: 10.1007/s10396-021-01135-w. [DOI] [PubMed] [Google Scholar]
- 10.McDonald N, Eddowes PJ, Hodson J, Semple SIK, Davies NP, Kelly CJ, et al. Multiparametric magnetic resonance imaging for quantitation of liver disease: a two-centre cross-sectional observational study. Sci Rep. 2018;8:9189. doi: 10.1038/s41598-018-27560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: state of the art. Radiology. 2021;301:250–262. doi: 10.1148/radiol.2021204288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Q, Cen L, Lai J, Zhang Z, Pan J, Zhao F, et al. A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease. Eur J Clin Invest. 2021;51:e13446. doi: 10.1111/eci.13446. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pooler BD, Wiens CN, McMillan A, Artz NS, Schlein A, Covarrubias Y, et al. Monitoring fatty liver disease with MRI following bariatric surgery: a prospective, dual-center study. Radiology. 2019;290:682–690. doi: 10.1148/radiol.2018181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satkunasingham J, Nik HH, Fischer S, Menezes R, Selzner N, Cattral M, et al. Can negligible hepatic steatosis determined by magnetic resonance imaging-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transpl. 2018;24:470–477. doi: 10.1002/lt.24965. [DOI] [PubMed] [Google Scholar]
- 16.Petitclerc L, Gilbert G, Nguyen BN, Tang A. Liver fibrosis quantification by magnetic resonance imaging. Top Magn Reson Imaging. 2017;26:229–241. doi: 10.1097/RMR.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451.e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Zhan C, Kannengiesser S, Chandarana H, Fenchel M, Ream J, Shanbhogue KP. MR elastography of liver at 3 tesla: comparison of gradient-recalled echo (GRE) and spin-echo (SE) echo-planar imaging (EPI) sequences and agreement across stiffness measurements. Abdom Radiol (NY) 2019;44:1825–1833. doi: 10.1007/s00261-019-01932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DW, Kim SY, Yoon HM, Kim KW, Byun JH. Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: a systematic review and meta-analysis. J Magn Reson Imaging. 2020;51:1086–1102. doi: 10.1002/jmri.26918. [DOI] [PubMed] [Google Scholar]
- 22.Kim SW, Lee JM, Park S, Joo I, Yoon JH, Chang W, et al. Diagnostic performance of spin-echo echo-planar imaging magnetic resonance elastography in 3T system for noninvasive assessment of hepatic fibrosis. Korean J Radiol. 2022;23:180–188. doi: 10.3348/kjr.2021.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JW, Lee ES, Park HJ, Park SB, Cho YY, Kannengiesser S, et al. Comparison of spin-echo echo-planar imaging magnetic resonance elastography with gradient-recalled echo magnetic resonance elastography and their correlation with transient elastography. Diagn Interv Radiol. 2022;28:294–300. doi: 10.5152/dir.2022.201014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Qi YF, Lan GY, Wu Q, Ma B, Zhang XY, et al. Three-dimensional MR elastography depicts liver inflammation, fibrosis, and portal hypertension in chronic hepatitis B or C. Radiology. 2021;301:154–162. doi: 10.1148/radiol.2021202804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morisaka H, Motosugi U, Glaser KJ, Ichikawa S, Ehman RL, Sano K, et al. Comparison of diagnostic accuracies of two- and three-dimensional MR elastography of the liver. J Magn Reson Imaging. 2017;45:1163–1170. doi: 10.1002/jmri.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaapman JJ, Tushuizen ME, Coenraad MJ, Lamb HJ. Multiparametric MRI in patients with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2021;53:1623–1631. doi: 10.1002/jmri.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huwart L, Peeters F, Sinkus R, Annet L, Salameh N, ter Beek LC, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed. 2006;19:173–179. doi: 10.1002/nbm.1030. [DOI] [PubMed] [Google Scholar]
- 28.Asbach P, Klatt D, Schlosser B, Biermer M, Muche M, Rieger A, et al. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology. 2010;257:80–86. doi: 10.1148/radiol.10092489. [DOI] [PubMed] [Google Scholar]
- 29.Klatt D, Hamhaber U, Asbach P, Braun J, Sack I. Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: a study of brain and liver viscoelasticity. Phys Med Biol. 2007;52:7281–7294. doi: 10.1088/0031-9155/52/24/006. [DOI] [PubMed] [Google Scholar]
- 30.Yin M, Glaser KJ, Manduca A, Mounajjed T, Malhi H, Simonetto DA, et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology. 2017;284:694–705. doi: 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: a meta-analysis. Hepatology. 2012;56:239–247. doi: 10.1002/hep.25610. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–1440. doi: 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Parthasarathy S, Goyal P, McCarthy RJ, Larson AC, Miller FH. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom Imaging. 2015;40:818–834. doi: 10.1007/s00261-014-0137-6. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772–782. doi: 10.1148/radiol.14132000. [DOI] [PubMed] [Google Scholar]
- 35.Poetter-Lang S, Bastati N, Messner A, Kristic A, Herold A, Hodge JC, et al. Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom Radiol (NY) 2020;45:3532–3544. doi: 10.1007/s00261-020-02779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastati N, Beer L, Ba-Ssalamah A, Poetter-Lang S, Ambros R, Kristic A, et al. Gadoxetic acid-enhanced MRI-derived functional liver imaging score (FLIS) and spleen diameter predict outcomes in ACLD. J Hepatol. 2022;77:1005–1013. doi: 10.1016/j.jhep.2022.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Bae KE, Kim SY, Lee SS, Kim KW, Won HJ, Shin YM, et al. Assessment of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig Dis. 2012;30:617–622. doi: 10.1159/000343092. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Lee JM, Paek M, Han JK, Choi BI. Quantitative assessment of hepatic function: modified look-locker inversion recovery (MOLLI) sequence for T1 mapping on Gd-EOB-DTPA-enhanced liver MR imaging. Eur Radiol. 2016;26:1775–1782. doi: 10.1007/s00330-015-3994-7. [DOI] [PubMed] [Google Scholar]
- 39.Yoon JH, Lee JM, Kim E, Okuaki T, Han JK. Quantitative liver function analysis: volumetric T1 mapping with fast multisection B1 inhomogeneity correction in hepatocyte-specific contrast-enhanced liver MR imaging. Radiology. 2017;282:408–417. doi: 10.1148/radiol.2016152800. [DOI] [PubMed] [Google Scholar]
- 40.Kim JE, Kim HO, Bae K, Choi DS, Nickel D. T1 mapping for liver function evaluation in gadoxetic acid-enhanced MR imaging: comparison of look-locker inversion recovery and B1 inhomogeneity-corrected variable flip angle method. Eur Radiol. 2019;29:3584–3594. doi: 10.1007/s00330-018-5947-4. [DOI] [PubMed] [Google Scholar]
- 41.Hinrichs H, Hinrichs JB, Gutberlet M, Lenzen H, Raatschen HJ, Wacker F, et al. Functional gadoxetate disodium-enhanced MRI in patients with primary sclerosing cholangitis (PSC) Eur Radiol. 2016;26:1116–1124. doi: 10.1007/s00330-015-3913-y. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JH, Lee JM, Kang HJ, Ahn SJ, Yang H, Kim E, et al. Quantitative assessment of liver function by using gadoxetic acid-enhanced MRI: hepatocyte uptake ratio. Radiology. 2019;290:125–133. doi: 10.1148/radiol.2018180753. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JH, Choi JI, Jeong YY, Schenk A, Chen L, Laue H, et al. Pre-treatment estimation of future remnant liver function using gadoxetic acid MRI in patients with HCC. J Hepatol. 2016;65:1155–1162. doi: 10.1016/j.jhep.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Mozes FE, Tunnicliffe EM, Moolla A, Marjot T, Levick CK, Pavlides M, et al. Mapping tissue water T1 in the liver using the MOLLI T1 method in the presence of fat, iron and B0 inhomogeneity. NMR Biomed. 2019;32:e4030. doi: 10.1002/nbm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tunnicliffe EM, Banerjee R, Pavlides M, Neubauer S, Robson MD. A model for hepatic fibrosis: the competing effects of cell loss and iron on shortened modified Look-Locker inversion recovery T1 (shMOLLI-T1) in the liver. J Magn Reson Imaging. 2017;45:450–462. doi: 10.1002/jmri.25392. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Cao T, Han F, Xie Y, Zhong X, Ma S, et al. Free-breathing multitasking multi-echo MRI for whole-liver water-specific T1, proton density fat fraction, and R2* quantification. Magn Reson Med. 2022;87:120–137. doi: 10.1002/mrm.28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita S, Sano K, Cruz G, Fukumura Y, Kawasaki H, Fukunaga I, et al. MR fingerprinting for liver tissue characterization: a histopathologic correlation study. Radiology. 2023;306:150–159. doi: 10.1148/radiol.220736. [DOI] [PubMed] [Google Scholar]
- 48.Jaubert O, Arrieta C, Cruz G, Bustin A, Schneider T, Georgiopoulos G, et al. Multi-parametric liver tissue characterization using MR fingerprinting: simultaneous T1, T2, T2*, and fat fraction mapping. Magn Reson Med. 2020;84:2625–2635. doi: 10.1002/mrm.28311. [DOI] [PubMed] [Google Scholar]
- 49.Dzyubak B, Li J, Chen J, Mara KC, Therneau TM, Venkatesh SK, et al. Automated analysis of multiparametric magnetic resonance imaging/magnetic resonance elastography exams for prediction of nonalcoholic steatohepatitis. J Magn Reson Imaging. 2021;54:122–131. doi: 10.1002/jmri.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nowak S, Mesropyan N, Faron A, Block W, Reuter M, Attenberger UI, et al. Detection of liver cirrhosis in standard T2-weighted MRI using deep transfer learning. Eur Radiol. 2021;31:8807–8815. doi: 10.1007/s00330-021-07858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HJ, Yoon JS, Lee SS, Suk HI, Park B, Sung YS, et al. Deep learning-based assessment of functional liver capacity using gadoxetic acid-enhanced hepatobiliary phase MRI. Korean J Radiol. 2022;23:720–731. doi: 10.3348/kjr.2021.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsaqal S, Hockings P, Ahlström H, Gummesson A, Hedström A, Hulthe J, et al. The combination of MR elastography and proton density fat fraction improves diagnosis of nonalcoholic steatohepatitis. J Magn Reson Imaging. 2022;56:368–379. doi: 10.1002/jmri.28040. [DOI] [PubMed] [Google Scholar]
- 53.Barahman M, Grunvald E, Prado PJ, Bussandri A, Henderson WC, Wolfson T, et al. Point-of-care magnetic resonance technology to measure liver fat: phantom and first-in-human pilot study. Magn Reson Med. 2022;88:1794–1805. doi: 10.1002/mrm.29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seitz K, Greis C, Schuler A, Bernatik T, Blank W, Dietrich CF, et al. Frequency of tumor entities among liver tumors of unclear etiology initially detected by sonography in the noncirrhotic or cirrhotic livers of 1349 patients. Results of the DEGUM multicenter study. Ultraschall Med. 2011;32:598–603. doi: 10.1055/s-0031-1281858. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Yoon JH, Bae JS, Park S, Han S, Lee JM. Multiarterial phase acquisition in gadoxetic acid-enhanced liver MRI for the detection of hypervascular hepatocellular carcinoma in high-risk patients: comparison of compressed sensing versus view sharing techniques. Invest Radiol. 2023;58:139–147. doi: 10.1097/RLI.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 56.Ginocchio LA, Smereka PN, Tong A, Prabhu V, Nickel D, Arberet S, et al. Accelerated T2-weighted MRI of the liver at 3 T using a single-shot technique with deep learning-based image reconstruction: impact on the image quality and lesion detection. Abdom Radiol (NY) 2023;48:282–290. doi: 10.1007/s00261-022-03687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han S, Lee JM, Kim SW, Park S, Nickel MD, Yoon JH. Evaluation of HASTE T2 weighted image with reduced echo time for detecting focal liver lesions in patients at risk of developing hepatocellular carcinoma. Eur J Radiol. 2022;157:110588. doi: 10.1016/j.ejrad.2022.110588. [DOI] [PubMed] [Google Scholar]
- 58.Bae SH, Hwang J, Hong SS, Lee EJ, Jeong J, Benkert T, et al. Clinical feasibility of accelerated diffusion weighted imaging of the abdomen with deep learning reconstruction: comparison with conventional diffusion weighted imaging. Eur J Radiol. 2022;154:110428. doi: 10.1016/j.ejrad.2022.110428. [DOI] [PubMed] [Google Scholar]
- 59.Brunsing RL, Fowler KJ, Yokoo T, Cunha GM, Sirlin CB, Marks RM. Alternative approach of hepatocellular carcinoma surveillance: abbreviated MRI. Hepatoma Res. 2020;6:59. doi: 10.20517/2394-5079.2020.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park HJ, Seo N, Kim SY. Current landscape and future perspectives of abbreviated MRI for hepatocellular carcinoma surveillance. Korean J Radiol. 2022;23:598–614. doi: 10.3348/kjr.2021.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunha GM, Villela-Nogueira CA, Bergman A, Lobo Lopes FPP. Abbreviated mpMRI protocol for diffuse liver disease: a practical approach for evaluation and follow-up of NAFLD. Abdom Radiol (NY) 2018;43:2340–2350. doi: 10.1007/s00261-018-1504-5. [DOI] [PubMed] [Google Scholar]
- 62.Ghorra C, Pommier R, Piveteau A, Rubbia-Brandt L, Vilgrain V, Terraz S, et al. The diagnostic performance of a simulated “short” gadoxetic acid-enhanced MRI protocol is similar to that of a conventional protocol for the detection of colorectal liver metastases. Eur Radiol. 2021;31:2451–2460. doi: 10.1007/s00330-020-07344-0. [DOI] [PubMed] [Google Scholar]
- 63.Hayoz R, Vietti-Violi N, Duran R, Knebel JF, Ledoux JB, Dromain C. The combination of hepatobiliary phase with Gd-EOB-DTPA and DWI is highly accurate for the detection and characterization of liver metastases from neuroendocrine tumor. Eur Radiol. 2020;30:6593–6602. doi: 10.1007/s00330-020-06930-6. [DOI] [PubMed] [Google Scholar]
- 64.Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol. 2021;75:108–119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, et al. Magnetic resonance imaging for surveillance of hepatocellular carcinoma: a systematic review and meta-analysis. Diagnostics (Basel) 2021;11:1665. doi: 10.3390/diagnostics11091665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bousabarah K, Letzen B, Tefera J, Savic L, Schobert I, Schlachter T, et al. Automated detection and delineation of hepatocellular carcinoma on multiphasic contrast-enhanced MRI using deep learning. Abdom Radiol (NY) 2021;46:216–225. doi: 10.1007/s00261-020-02604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]