Abstract
A total of 24 strains, biochemically identified as members of the Lactobacillus casei group, were identified by PCR with species-specific primers. The same set of strains was typed by randomly amplified polymorphic DNA (RAPD) analysis, ribotyping, and pulsed-field gel electrophoresis (PFGE) in order to compare the discriminatory power of the methods. Species-specific primers for L. rhamnosus and L. casei identified the type strain L. rhamnosus ATCC 7469 and the neotype strain L. casei ATCC 334, respectively, but did not give any signal with the recently revived species L. zeae, which contains the type strain ATCC 15820 and the strain ATCC 393, which was previously classified as L. casei. Our results are in accordance with the suggested new classification of the L. casei group. Altogether, 21 of the 24 strains studied were identified with the species-specific primers. In strain typing, PFGE was the most discriminatory method, revealing 17 genotypes for the 24 strains studied. Ribotyping and RAPD analysis yielded 15 and 12 genotypes, respectively.
Lactobacilli have a worldwide industrial use as starters in the manufacturing of fermented milk products. Moreover, some Lactobacillus strains have probiotic characteristics and are therefore included in fresh fermented products or used in capsular health products, such as freeze-dried powder. The use of some Lactobacillus strains as probiotics is based on studies which show that these species belong to the normal intestinal flora and that the strains have beneficial effects on human and animal health (for reviews, see references 16 and 19). Lactobacillus rhamnosus and L. casei do not belong to the group of primary starters used in the dairy industry, but these species include many important probiotic strains, e.g., L. casei Shirota (26) and L. rhamnosus GG (20). These species are also naturally found in raw milk and in high numbers in cheese after it ripens (8, 15).
Traditionally, the identification of lactobacilli has been based mainly on fermentation of carbohydrates, morphology, and Gram staining, and these methods are still used. However, in recent years, the taxonomy has changed considerably with the increasing knowledge of the genomic structure and phylogenetic relationships between Lactobacillus spp. (14, 24, 30). The identification of some Lactobacillus species by biochemical methods alone is not reliable (6, 14, 22), as evidenced by the L. casei group (21, 32). The L. casei group includes L. casei, L. paracasei, L. rhamnosus, and L. zeae; the rejection of L. paracasei and its inclusion in L. casei has been proposed (7, 9, 10, 17).
Probiotic health products can contain, perhaps due to the lack of good identification methods, Lactobacillus species other than those declared on the product specifications (13, 14, 32). Difficulty in identification has also been reported for clinical isolates (21, 32). The need for rapid and reliable species-specific identification, e.g., by PCR, is obvious. Recently, species-specific oligonucleotide primers for L. paracasei and L. rhamnosus were described (1, 29).
The identification of lactobacilli at the strain level is important for their industrial use. The biotechnology industry needs tools to monitor, e.g., the use of patented strains or to distinguish probiotic strains from natural isolates in the host gastrointestinal tract. As for safety aspects, it is crucial to be able to compare clinical isolates and biotechnological strains and also to monitor the genetic stability of the strains (11, 14). Genotypic methods used for strain typing are typically PCR methods (e.g., randomly amplified polymorphic DNA [RAPD] analysis) or variations of restriction enzyme analysis (e.g., pulsed-field gel electrophoresis [PFGE] and ribotyping) (30). In RAPD analysis (31), short arbitrary sequences are used as primers in PCR, which yields strain-specific amplification product patterns. In PFGE and ribotyping analysis, genomic DNA is digested with restriction enzymes. In PFGE (23), rare-cutting enzymes are used and large genomic fragments are separated, while in ribotyping (25), rRNA genes and/or their spacer regions are used as probes that hybridize with genomic restriction fragments. These basic methodological differences may cause divergences in typing results.
The aims of this study were (i) to compare the identification of L. casei and L. rhamnosus strains by the API 50 CHL test and by species-specific PCR and (ii) to compare PFGE, RAPD analysis, and ribotyping techniques for the discrimination of closely related L. casei and L. rhamnosus strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used throughout the study are listed in Table 1. The strains were maintained at −80°C and subcultured in MRS broth or on MRS agar plates (LabM, Bury, England) anaerobically at 37°C. An API 50 CHL kit and APILAB Plus software using the API 50 CHL version 4.0 database (bioMérieux, Lyon, France) were used to identify strains biochemically.
TABLE 1.
Lactobacillus strains used in the study
| Bacterial strain | Identification by API 50 CHL (identification comment)a | Source |
|---|---|---|
| L. rhamnosus | ||
| GG (ATCC 53103) | L. rhamnosus (doubtful) | Human isolate, Valio Ltd.b |
| VS 1030 | L. rhamnosus (doubtful) | Human isolate, Valio Ltd. |
| VS 1031 | L. rhamnosus (doubtful) | Human isolate, Valio Ltd. |
| VS 1032 | L. rhamnosus (doubtful) | Human isolate, Valio Ltd. |
| VS 1033 | L. rhamnosus (unacceptable) | Human isolate, Valio Ltd. |
| VS 1034 | L. rhamnosus (good) | Human isolate, Valio Ltd. |
| E-78080 | L. rhamnosus (very good) | Isolated from beer, VTTc Culture Collection |
| VS 872 | L. rhamnosus (very good) | Isolated from milk, Valio Ltd. |
| E-97800 | L. rhamnosus (doubtful) | Human isolate, VTT Culture Collection |
| Lactophilus | L. rhamnosus (excellent) | Isolated from Lactophilusd powder |
| VS 495 | L. rhamnosus (good) | Isolated from cheese, Valio Ltd. |
| VS 1017 | L. rhamnosus (doubtful) | Human isolate, Valio Ltd. |
| VS 1018 | L. rhamnosus (unacceptable) | Human isolate, Valio Ltd. |
| VS 1019 | L. rhamnosus (doubtful) | Human isolate, Valio Ltd. |
| VS 1020 | L. rhamnosus (good) | Human isolate, Valio Ltd. |
| VS 1021 | L. rhamnosus (good) | Human isolate, Valio Ltd. |
| VS 1022 | L. rhamnosus (very good) | Human isolate, Valio Ltd. |
| ATCC 7469 | L. rhamnosus (excellent) | American Type Culture Collection |
| ATCC 11443 | L. rhamnosus (very good) | American Type Culture Collection |
| L. casei | ||
| ATCC 393e | L. rhamnosus (good) | American Type Culture Collection |
| ATCC 334 | L. rhamnosus (doubtful) | American Type Culture Collection |
| ATCC 4646 | L. paracasei (very good) | American Type Culture Collection |
| L. paracasei VS 1023 | L. paracasei (excellent) | Human isolate, Valio Ltd. |
| L. zeae ATCC 15820f | L. paracasei (unacceptable) | American Type Culture Collection |
Identification by the API 50 CHL kit and the profile status by APILAB Plus software using the API 50 CHL version 4.0 database. Identification comment given by APILAB Plus software: excellent, the percentage of identification (%ID) ≥ 99.9 and the T index (T) ≥ 0.75; very good, %ID ≥ 99.0 and T ≥ 0.5; good, %ID ≥ 90.0 and T ≥ 0.25; acceptable, %ID ≥ 80.0 and T ≥ 0; doubtful, several tests against identification (e.g., a rare biotype); unacceptable, below threshold value.
Valio Ltd., Helsinki, Finland.
VTT Biotechnology and Food Research, Espoo, Finland.
Manufactured by Laboratoires Lyocentre, Aurillac, France.
L. rhamnosus and L. paracasei species-specific PCR.
Template DNA for the L. rhamnosus species-specific PCR was extracted as described previously (1) or, alternatively, PCR was performed with a fresh single colony grown overnight. The L. rhamnosus species-specific PCR assay described by Alander et al. (1) was used. The sequences of the primer pair (Table 2, RhaI) designed into the 16S rRNA gene were 5′CTTGCATCTTGATTTAATTTTG3′ (forward) and 5′CCGTCAATTCCTTTGAGTTT3′ (reverse). The specificity of the primer pair was defined by the forward primer, and the expected PCR product size was 863 bp. The primers were made with a PCR Mate 391 DNA synthesizer (Perkin-Elmer Applied Biosystems, Foster City, Calif.) according to the manufacturer’s instructions. Taq DNA polymerase and PCR buffer (final concentrations of 10 mM Tris-HCl, 1.5 mM MgCl2, and 50 mM KCl [pH 8.3]) were obtained from Boehringer Mannheim (Mannheim, Germany). The amount of Taq DNA polymerase used was 2.0 U in a total reaction volume of 100 μl. The concentration of each primer was 0.5 μM, and that of each deoxynucleotide (Finnzymes Oy, Espoo, Finland) was 200 μM. The amount of template used was 1 μl of an appropriate dilution of the extracted DNA. A Gene Amp PCR System 9600 apparatus (Perkin-Elmer Applied Biosystems) was used for the PCR cycling. Initial denaturation was carried out at 94°C for 5 min, followed by a touch-down thermocycling program with 30 amplification cycles (annealing for 30 s at 62°C in cycles 1 to 10, 60°C in cycles 11 to 20, and 58°C in cycles 21 to 30; extension for 1 min at 72°C; and denaturation for 40 s at 94°C) and final extension for 10 min at 72°C. Reaction mixtures were subsequently cooled to 4°C. The PCR products were analyzed by agarose gel electrophoresis with 1% agarose in 0.5× Tris-borate-EDTA (10× is 89 mM Tris, 89 mM boric acid, and 25 mM EDTA [pH 8.0]) (TBE) buffer and ethidium bromide staining.
TABLE 2.
Bacterial species detected by PCR with species-specific primer pairs
| Bacterial strain | Result with primer pair
|
||
|---|---|---|---|
| RhaIa | RhaIIb | Casc | |
| L. rhamnosus | |||
| GG | + | + | − |
| VS 1030 | + | + | − |
| VS 1031 | + | + | − |
| VS 1032 | + | + | − |
| VS 1033 | − | − | − |
| VS 1034 | + | + | − |
| E-78080 | + | + | − |
| VS 872 | + | + | − |
| E-97800 | + | + | − |
| Lactophilus | + | + | − |
| VS 495 | + | + | − |
| VS 1017 | + | + | − |
| VS 1018 | + | + | − |
| VS 1019 | + | + | − |
| VS 1020 | + | + | − |
| VS 1021 | + | + | − |
| VS 1022 | + | + | − |
| ATCC 7469 | + | + | − |
| ATCC 11443 | + | + | − |
| L. casei | |||
| ATCC 393d | − | − | − |
| ATCC 334 | − | − | + |
| ATCC 4646 | − | − | + |
| VS 1023 | − | − | + |
| L. zeae ATCC 15820 | − | − | − |
Other sets of species-specific primers, designed into the 16S-23S ribosomal DNA (rDNA) spacer region, were used to identify L. rhamnosus (Table 2, RhaII) and L. paracasei (Table 2, Cas) as described previously (29). Primer 5′CAGACTGAAAGTCTGACGG3′ was used with primers 5′GCGATGCGAATTTCTATTATT3′ and 5′GCGATGCGAATTTCTTTTTC3′ to amplify L. rhamnosus and L. paracasei species-specific sequences, respectively. PCR amplification was performed with a DyNAzyme DNA polymerase kit (Finnzymes Oy) according to the instructions of the manufacturer. The PCR buffer contained 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl, and 0.1% Triton X-100 (pH 8.8). The primers were used at 1 μM and deoxynucleotides were used at 200 μM. Initial denaturation was at 94°C for 2 min, and the thermocycling program was 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. With both the L. rhamnosus and L. paracasei primers, two PCR products of 350 and 185 bp were amplified.
RAPD genotyping.
Template DNA for RAPD analysis was extracted from lactobacilli according to a modification of the method of Bollet et al. (4). Briefly, bacterial cells from a plate of a single-colony subculture of lactobacilli on MRS agar were harvested and transferred to Eppendorf tubes containing 100 μl of Tris-EDTA (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Tubes were vortexed well, 50 μl of 10% sodium dodecyl sulfate was added, and after vortexing, the tubes were incubated for 30 min at 65°C. The bacterial suspension was centrifuged (2,200 × g for 5 min), the supernatant was discarded, and the Eppendorf tubes containing the cells were heated in a microwave oven for 5 min at a power of 650 W. The pellets were dissolved in 500 μl of TE buffer, and a 1:100 dilution of cell lysate in water was used as a template in RAPD analysis. RAPD analysis was performed in a 50-μl reaction volume consisting of 200 μM deoxynucleoside triphosphate (Finnzymes Oy) a 0.4 μM concentration of random sequence primer 5′AGTCAGCCAC3′, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 4 mM MgCl2, 2.5 U of Taq DNA polymerase (Boehringer Mannheim), and 5 μl of template. The temperature profile in the Gene Amp PCR System 9600 thermocycler was 35 cycles as follows: 94°C for 1 min, 32°C for 2 min, and 72°C for 2 min. The initial denaturation was performed at 94°C for 5 min, and the final extension was done at 72°C for 5 min. Amplification products were analyzed electrophoretically in 1% (wt/vol) agarose gels containing ethidium bromide (0.5 μg/ml) and visualized under UV light. RAPD profiles of the strains were visually compared, and every clearly distinguishable profile was considered one RAPD genotype (A1, etc.)
Ribotyping.
Ribotyping was performed by the automated ribotyping device RiboPrinter microbial characterization system (Qualicon, Wilmington, Del.). Standard reagents were used in all steps of the analysis. The method involves the release of DNA from cells, EcoRI digestion of chromosomal DNA, and the separation of the resulting fragments by agarose gel electrophoresis, followed by Southern hybridization probing with the rrnB rRNA operon from Escherichia coli (5) as a chemiluminescent probe. Images were acquired with a charge-coupled-device camera and processed by RiboPrinter analysis software that normalizes fragment pattern data for band intensity and relative band position compared to the molecular weight marker. Similar fingerprint patterns (similarity of >0.95) were automatically clustered into ribogroups (R1, etc.). All strains were ribotyped at least twice to ensure the reproducibility of the fingerprint patterns.
PFGE.
The preparation of genomic DNA in situ in agarose blocks was performed by a slight modification of the method of Tanskanen et al. (27). Lactobacillus strains were grown to an A600 of 0.6 in MRS broth containing 1% glycine to facilitate lysis. Chloramphenicol (100 μg/ml) was added, and incubation was continued for 1 to 2 h. Cells were harvested from 1.5 ml of culture, washed with 10 mM Tris–20 mM NaCl–50 mM EDTA (pH 7.2), and suspended in 300 μl of the same buffer. The suspension was heated in 50°C, and 300 μl of 2% agarose in 0.5× TBE buffer at the same temperature was added before solidifying the suspension in molds. The agarose blocks were incubated overnight at 37°C in lysis buffer, 6 mM Tris–1 M NaCl–100 mM EDTA–1% sarcosyl–0.2% deoxycholate (pH 7.6), containing 2.5 mg of lysozyme (Sigma, St. Louis, Mo.) per ml and 20 U of mutanolysin (Sigma) per ml. Proteinase K (1 mg/ml) treatment was performed in 100 mM EDTA–1% sarcosyl–0.2% deoxycholate buffer (pH 8.0) for 18 h at 50°C. The agarose blocks were washed four times for 1 h per wash with 20 mM Tris–50 mM EDTA (pH 8.0), the two first washes containing 1 mM phenylmethylsulfonyl fluoride (Sigma). Before restriction enzyme digestion, the agarose blocks were washed twice for 1 h per wash with TE buffer and then balanced for 1 h in an appropriate restriction enzyme buffer. Restriction enzyme digestions with NotI and SfiI were performed overnight at 37°C. Electrophoresis was carried out with a CHEF DR II apparatus (Bio-Rad, Hercules, Calif.) in 1% PFGE certified agarose (Bio-Rad) with 0.5× TBE buffer. The pulse time was 1 to 15 s, the current was 5 V/cm, the temperature was 14°C, and the running time was 22 h. The agarose gel was stained with ethidium bromide (0.5 μg/ml) and visualized under UV light. The PFGE profiles of the strains were visually compared, and every clearly distinguishable profile was considered one NotI or SfiI genotype. The final classification of PFGE genotypes (P1, etc.) combines the separate results obtained with these two restriction enzymes.
RESULTS
Identification of bacterial species.
Biochemical identification of species was performed with an API 50 CHL kit. The identification results given by APILAB Plus software with the API 50 CHL version 4.0 database are shown in Table 1. For 13 strains, identification levels from good to excellent were obtained, and identification levels of 11 strains were considered doubtful or unacceptable due to atypical fermentation reactions.
The ribosomal intergenic regions are reported to be more variable between species than are the 16S or 23S RNA genes (2). Therefore, two sets of L. rhamnosus species-specific oligonucleotide primers were used to identify bacterial strains; the first pair of primers was designed into 16S rDNA (1) and the second into the 16S-23S rDNA spacer region (29). Both L. rhamnosus primer pairs gave PCR products of expected sizes with all strains except L. zeae ATCC 15820, L. rhamnosus VS 1033, L. paracasei VS 1023, and L. casei ATCC 393, ATCC 334, and ATCC 4646 (Table 2). The L. paracasei species-specific primers produced PCR products of expected sizes with L. paracasei VS 1023 and L. casei ATCC 334 and ATCC 4646. All three of these strains were classified as L. casei since the rejection of L. paracasei has been proposed (9, 10, 17); further, only the name L. casei is used. The L. zeae type strain, ATCC 15820, and L. casei ATCC 393, which was recently reclassified as L. zeae (10, 17), were not identified by either L. rhamnosus- or L. casei-specific primers. L. rhamnosus VS 1033 gave an API 50 CHL profile (Table 1) and was earlier identified as belonging to the L. casei group by 16S rRNA sequencing (unpublished results). It did not, however, give positive results with either of the L. rhamnosus or L. casei primers. This very likely indicates that this strain also belongs to L. zeae. PCR identifications of bacterial strains with the L. rhamnosus and L. casei species-specific oligonucleotide primers are in Table 2.
RAPD analysis.
Twelve RAPD genotypes (A1 to A12) were detected among the 24 Lactobacillus strains. Genotypes A1 (Fig. 1, lanes 1 to 6), A2 (lanes 7 to 12), A3 (lanes 13 and 14), and A5 (lanes 15 and 18) were represented by six, six, two, and two strains, respectively, whereas the remaining eight strains each had a unique RAPD genotype (Fig. 1 and Table 3). All L. rhamnosus strains (Fig. 1, lanes 1 to 18) except for VS 1033 (Fig. 1, lane 20) produced a strong 1-kb amplification product that was either missing or weak in the L. zeae (Fig. 1, lanes 21 and 22) and L. casei (Fig. 1, lanes 19, 23, and 24) strains.
FIG. 1.
RAPD patterns and genotypes (in parentheses) of the strains. Lanes: 1 to 18, L. rhamnosus GG (A1), VS 1031 (A1), VS 1032 (A1), VS 1034 (A1), VS 1017 (A1), VS 1018 (A1), ATCC 7469 (A2), ATCC 11443 (A2), E-78080 (A2), VS 872 (A2), VS 495 (A2), VS 1022 (A2), VS 1020 (A3), VS 1021 (A3), E-97800 (A4), VS 1030 (A5), Lactophilus (A6), and VS 1019 (A5), respectively; 19, L. casei VS 1023 (A7); 20, L. rhamnosus VS 1033 (A8); 21, L. zeae ATCC 15820 (A9); 22, L. casei ATCC 393 (A10); 23, L. casei ATCC 334 (A11); 24, L. casei ATCC 4646 (A12); 25, molecular weight marker (in kilobase pairs).
TABLE 3.
Abilities of RAPD analysis, ribotyping, and PFGE to differentiate L. rhamnosus and L. casei strains
| Bacterial strain | Genotype by:
|
||
|---|---|---|---|
| RAPD analysis | RiboPrint | PFGEa | |
| L. rhamnosus | |||
| GG | A1 | R1 | P1 |
| VS 1032 | A1 | R1 | P1 |
| VS 1034 | A1 | R1 | P1 |
| VS 1018 | A1 | R1 | P1 |
| VS 1031 | A1 | R1 | P2 |
| VS 1017 | A1 | R1 | P3 |
| ATCC 7469 | A2 | R2 | P4 |
| ATCC 11443 | A2 | R2 | P4 |
| E-78080 | A2 | R2 | P4 |
| VS 872 | A2 | R3 | P5 |
| VS 1022 | A2 | R3 | P5 |
| VS 495 | A2 | R4 | P6 |
| VS 1020 | A3 | R5 | P7 |
| VS 1021 | A3 | R6 | P7 |
| E-97800 | A4 | R7 | P8 |
| VS 1019 | A5 | R7 | P9 |
| VS 1030 | A5 | R8 | P10 |
| Lactophilus | A6 | R9 | P11 |
| VS 1033 | A8 | R10 | P13 |
| L. casei | |||
| VS 1023 | A7 | R11 | P12 |
| ATCC 393b | A10 | R12 | P14 |
| ATCC 334 | A11 | R13 | P15 |
| ATCC 4646 | A12 | R14 | P16 |
| L. zeae ATCC 15820 | A9 | R15 | P17 |
Ribotyping.
Ribotyping with the EcoRI restriction enzyme produced 15 distinct fingerprint patterns for the 24 strains studied (Fig. 2 and Table 3). The triple band located between 4.8 and 6.2 kb seemed to be a feature typical of the L. rhamnosus fingerprint patterns; 16 of the 18 L. rhamnosus strains (identified by species-specific PCR) gave this type of fingerprint (Fig. 2, R1 to R4, R6, R7, and R9). L. casei VS 1023 (R11), ATCC 334 (R13), and ATCC 4646 (R14) (identified by species-specific PCR) shared bands of approximately 4.2 and 6.5 kb; in addition, strains VS 1023 (R11) and ATCC 334 (R13) shared bands of approximately 5 and 7 kb. The band pattern of L. rhamnosus VS 1030 (R8) resembled those of strains of both L. rhamnosus and L. casei. L. zeae ATCC 15820 (R15) and L. casei ATCC 393 (R12), which was proposed to belong to L. zeae (10, 17), had bands of approximately 1, 3.5, and 7 kb and a double band between 4.5 and 5.5 kb in common. VS 1033 (R10), which we suggest belongs to L. zeae according to the results of species-specific PCR, shared the bands of approximately 1 and 3.5 kb and the larger band of the double band between 4.5 and 5.5 kb with the L. zeae strains. The fingerprint of L. rhamnosus VS 1020 (R5) did not show similarity to any other fingerprints. Strains belonging to the same species were found to also share bands of >10 kb (Fig. 2). These bands are not listed individually because it was difficult to estimate the sizes of the bands with the coarse scale.
FIG. 2.
RiboPrint patterns of L. rhamnosus, L. casei, and L. zeae strains. The patterns are composites of several individual patterns. Ribotypes: R1, L. rhamnosus GG, VS 1032, VS 1034, VS 1018, VS 1031, and VS 1017; R2, L. rhamnosus ATCC 7469, ATCC 11443, and E-78080; R3, L. rhamnosus VS 872 and VS 1022; R4, L. rhamnosus VS 495; R5, L. rhamnosus VS 1020; R6, L. rhamnosus VS 1021; R7, L. rhamnosus E-97800 and VS 1019; R8, L. rhamnosus VS 1030; R9, L. rhamnosus Lactophilus; R10, L. rhamnosus VS 1033; R11, L. casei VS 1023; R12, L. casei ATCC 393; R13, L. casei ATCC 334; R14, L. casei ATCC 4646; R15, L. zeae ATCC 15820.
PFGE.
L. rhamnosus genomic DNA digested with SfiI and NotI yielded fragments of approximately 23 to 250 and 4 to 250 kb, respectively (Fig. 3 and 4). SfiI revealed 16 (S1 to S16) and NotI revealed 15 (N1 to N15) distinct genotypes. Combining the results (Table 3), 17 distinct genotypes (P1 to P17) were found in the 24 Lactobacillus strains studied. Thirteen unique genotypes were found, and genotypes P1, P4, P5, and P8 were represented by four, three, two, and two strains, respectively (Table 3). All L. rhamnosus and L. zeae strains produced a typical double band (approximately 250 kb) and, possibly, additional bands with restriction enzyme SfiI (Fig. 3a and b). NotI cut L. rhamnosus genomic DNA more often, and similar kinds of typical bands were not distinguishable (Fig. 4a and b). With the L. casei strains, a typical restriction pattern was not produced by either enzyme (Fig. 3c and 4c).
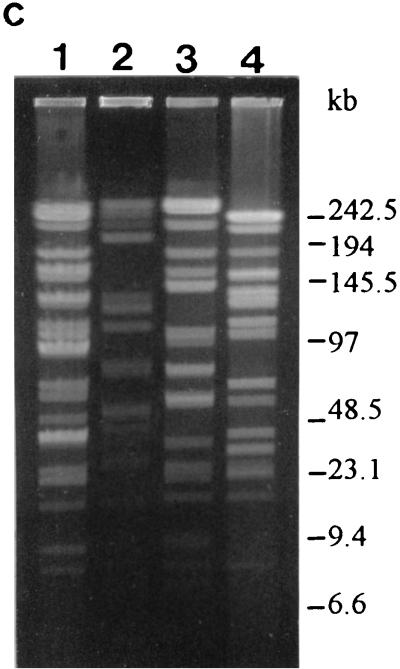
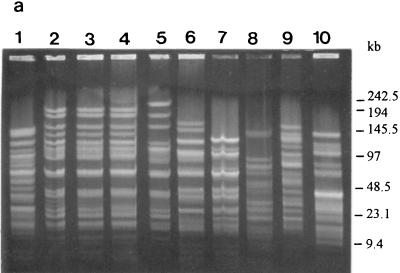
FIG. 3.
PFGE profiles and genotypes (in parentheses) of the strains as determined with restriction enzyme SfiI. (a) Lanes: 1 to 9, L. rhamnosus GG (S1), ATCC 7469 (S2), ATCC 11443 (S2), E-78080 (S2), VS 872 (S2), E-97800 (S3), VS 1030 (S4), VS 1033 (S5), and VS 1021 (S6), respectively; 10, L. zeae ATCC 15820 (S7). (b) Lanes: 1 to 10, L. rhamnosus VS 1031 (S8), VS 1032 (S1), VS 1034 (S1), Lactophilus (S9), VS 495 (S10), VS 1017 (S11), VS 1018 (S1), VS 1019 (S12), VS 1020 (S6), and VS 1022 (S2), respectively. (c) Lanes: 1 to 4, L. casei VS 1023 (S13), ATCC 393 (S14), ATCC 334 (S15), and ATCC 4646 (S16), respectively.
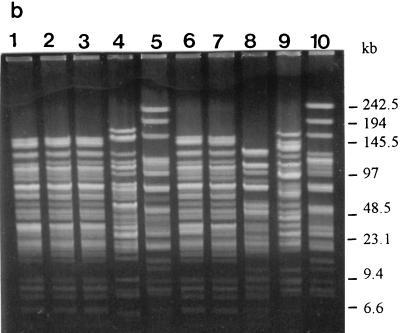
FIG. 4.
PFGE profiles and genotypes (in parentheses) of the strains as determined with restriction enzyme NotI. (a) Lanes: 1 to 9, L. rhamnosus GG (N1), ATCC 7469 (N2), ATCC 11443 (N2), E-78080 (N2), VS 872 (N3), E-97800 (N4), VS 1030 (N5), VS 1033 (N6), and VS 1021 (N7), respectively; 10, L. zeae ATCC 15820 (N8). (b) Lanes: 1 to 10, L. rhamnosus VS 1031 (N1), VS 1032 (N1), VS 1034 (N1), Lactophilus (N9), VS 495 (N10), VS 1017 (N11), VS 1018 (N1), VS 1019 (N5), VS 1020 (N7), and VS 1022 (N3), respectively. (c) Lanes: 1 to 4, L. casei VS 1023 (N12), ATCC 393 (N13), ATCC 334 (N14), and ATCC 4646 (N15), respectively.
L. rhamnosus GG (Fig. 3a, lane 1, and 4a, lane 1), VS 1032 (Fig. 3b, lane 2, and 4b, lane 2), VS 1034 (Fig. 3b, lane 3, and 4b, lane 3), and VS 1018 (Fig. 3b, lane 7, and 4b, lane 7) had identical PFGE profiles with both enzymes and could not be distinguished from each other (Table 3, genotype P1). The SfiI-produced profiles of L. rhamnosus VS 1017 (Fig. 3b, lane 6) and VS 1031 (Fig. 3b, lane 1) differed from those of the previous group by one and two extra bands, respectively. Another group with identical PFGE profiles (Table 3, genotype P4) with both enzymes consisted of L. rhamnosus ATCC 7469 (Fig. 3a, lane 2, and 4a, lane 2), ATCC 11443 (Fig. 3a, lane 3, and 4a, lane 3), and E-78080 (Fig. 3a, lane 4, and 4a, lane 4). The third group with identical PFGE patterns (Table 3, genotype P5) contained L. rhamnosus VS 872 (Fig. 3a, lane 5, and 4a, lane 5) and VS 1022 (Fig. 3b, lane 10, and 4b, lane 10), and the last group (Table 3, genotype P7) contained strains L. rhamnosus VS 1021 (Fig. 3a, lane 9, and 4a, lane 9) and VS 1020 (Fig. 3b, lane 9, and 4b, lane 9). All the other PFGE profiles of the L. rhamnosus strains were unique. The L. casei and L. zeae strains all had unique profiles.
DISCUSSION
Polyphasic taxonomy, which integrates phenotypic, genotypic, and phylogenetic information, has changed the classification of lactobacilli in recent years (for a review, see reference 30). Reliable identifications of some species are not obtained by traditional biochemical methods alone; genotypic methods are needed as well. This may cause problems for routine laboratories performing analyses if reliable and easy genetic methods, e.g., species-specific PCR, are not available.
We tested two pairs of recently published L. rhamnosus specific primers, one pair complementary to 16S rDNA and the other complementary to the spacer between 16S and 23S rDNA. Similar results were obtained with the primer pairs, and their specificity to the studied strains was good. No PCR signal was obtained with either L. rhamnosus- or L. casei-specific primers for L. zeae ATCC 15820 or L. casei ATCC 393, which was recently reclassified as L. zeae. Neotype strain L. casei ATCC 334 and the L. rhamnosus type strain, ATCC 7469, were correctly identified with their species-specific primers. Primers specific for L. zeae are needed for the complete identification of this bacterial group. All the strains studied were identified as belonging to the L. casei group, i.e., to L. casei, L. rhamnosus, or L. zeae, by the API 50 CHL test. However, the exact identifications of these closely related species were not reliable. Identifications of 11 strains were doubtful or unacceptable, and one strain, L. casei ATCC 393 (reclassified as L. zeae), was misidentified as L. rhamnosus with a good identification level.
At the species level, RAPD analysis yielded typical amplification products of 1 kb from all L. rhamnosus strains except for VS 1033, whose identification by the API 50 CHL test was unacceptable; we suggest that VS 1033 belongs to L. zeae, according to the results of species-specific PCR. The band representing the 1-kb amplification product was missing or weak with the L. casei and L. zeae strains. Ribotyping revealed a triple band (between 4.8 and 6.2 kb) which seems to be typical for most L. rhamnosus strains. In PFGE, all L. rhamnosus and L. zeae strains yielded a typical double band (over 250 kb) when cut with SfiI, while no typical bands were distinguished by NotI. Typical bands in the fingerprints are very helpful but, of course, are not adequate alone for the identification of L. rhamnosus.
For strain typing, PFGE was the most discriminating method; it revealed 17 genotypes of the 24 strains studied, while 15 and 12 genotypes were distinguished by ribotyping and RAPD analysis, respectively. PFGE was performed with two enzymes, SfiI and NotI, which increased its discrimination capability. However, even if the results obtained with SfiI (which revealed 16 genotypes) or NotI (15 genotypes) are considered separately, PFGE remains the most discriminating or at least as discriminating as ribotyping. All non-L. rhamnosus strains (according to species-specific PCR) were distinguished from the L. rhamnosus strains by all three methods. The 18 L. rhamnosus strains were typed into 11 (10 genotypes by SfiI and 9 by NotI), 9, and 6 genotypes by PFGE, ribotyping, and RAPD analysis, respectively. Table 3 shows that some L. rhamnosus strains were typed as belonging to the same genotype group by all three methods, which can be considered a very reliable identification. Based on our experience, PFGE analysis alone, performed with two or three appropriate enzymes, can be used for reliable strain typing. In several Lactobacillus studies, PFGE has been shown to be the most powerful method for strain typing (3, 12, 18), and it is also used in epidemiological studies (28). However, it is a laborious and expensive method; therefore, only a limited number of samples can be analyzed. Screening new primers in RAPD analysis and using other restriction enzymes in ribotyping could possibly increase their specificity for strain typing. Ribotyping can be done automatically (RiboPrinter) and is therefore easily applied, but the equipment is rather expensive. RAPD analysis is a rapid and cheap method, but careful optimization is needed to ensure the repeatability of the results.
To conclude, species-specific PCR, due to rapid and easy performance, is a very useful method for identifying species of the L. casei group. RAPD analysis, ribotyping, and PFGE are all primarily typing methods, but they do have the potential to also give species-specific information. Highly standardized and automated ribotyping could be suitable in forming large databases, giving rise to the possibility of using a typing method for identification purposes. Principal identification is still based on microbiological and biochemical methods, but for thorough analysis, conventional identification methods should be combined with genotypic methods.
ACKNOWLEDGMENTS
This work was partly supported by EU grant no. FAIR-CT96-1028.
Tuula Vähäsöyrinki is acknowledged for valuable technical help in PFGE experiments.
REFERENCES
- 1.Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol. 1999;65:351–354. doi: 10.1128/aem.65.1.351-354.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Björkroth J, Ridell J, Korkeala H. Characterization of Lactobacillus sake strains associating with production of ropy slime by randomly amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) patterns. Int J Food Microbiol. 1996;31:59–68. doi: 10.1016/0168-1605(96)00964-6. [DOI] [PubMed] [Google Scholar]
- 4.Bollet C, Gevaudan M J, de Lamballerie X, Zandotti C, de Micco P. A simple method for the isolation of chromosomal DNA from gram positive or acid fast bacteria. Nucleic Acids Res. 1991;19:1955. doi: 10.1093/nar/19.8.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius J, Ullrich A, Raker M A, Gray A, Dull T J, Gutell R R, Noller H F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981;6:112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- 6.Charteris W P, Kelly P M, Morelli L, Collins J K. Selective detection, enumeration and identification of potentially probiotic Lactobacillus and Bifidobacterium species in mixed bacterial populations. Int J Food Microbiol. 1997;35:1–27. doi: 10.1016/s0168-1605(96)01222-6. [DOI] [PubMed] [Google Scholar]
- 7.Collins M D, Phillips B A, Zanoni P. Deoxyribonucleic acid homology studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int J Syst Bacteriol. 1989;39:105–108. [Google Scholar]
- 8.Crow V L, Coolbear T, Gopal P K, Martley F G, McKay L L, Riepe H. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int Dairy J. 1995;5:855–875. [Google Scholar]
- 9.Dellaglio F, Dicks L M T, du Toit M, Torriani S. Designation of ATCC 334 in place of ATCC 393 (NCDO 161) as the neotype strain of Lactobacillus casei subsp. casei and rejection of the name Lactobacillus paracasei (Collins et al., 1989) Int J Syst Bacteriol. 1991;41:340–342. [Google Scholar]
- 10.Dicks L M T, du Plessis E M, Dellaglio F, Lauer E. Reclassification of Lactobacillus casei subsp. casei ATCC 393 and Lactobacillus rhamnosus ATCC 15820 as Lactobacillus zeae nom. rev., designation of ATCC 334 as the neotype of L. casei subsp. casei, and rejection of the name Lactobacillus paracasei. Int J Syst Bacteriol. 1996;46:337–340. doi: 10.1099/00207713-46-1-337. [DOI] [PubMed] [Google Scholar]
- 11.Donohue D C, Salminen S. Safety of probiotic bacteria. Asia Pac J Clin Nutr. 1996;5:25–28. [PubMed] [Google Scholar]
- 12.Ferrero M, Cesena C, Morelli L, Scolari G, Vescovo M. Molecular characterization of Lactobacillus casei strains. FEMS Microbiol Lett. 1996;140:215–219. [Google Scholar]
- 13.Holzapfel W H, Haberer P, Snel J, Schillinger U, Huis in’t Veld J H J. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 14.Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol. 1998;41:103–125. doi: 10.1016/s0168-1605(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg A-M, Christiansson A, Rukke E-O, Eklund T, Molin G. Bacterial flora of Norwegian and Swedish semi-hard cheese after ripening, with special reference to Lactobacillus. Neth Milk Dairy J. 1996;50:563–572. [Google Scholar]
- 16.Marteau P, Rambaud J-C. Potential of using lactic acid bacteria for therapy and immunomodulation in man. FEMS Microbiol Rev. 1993;12:207–220. doi: 10.1111/j.1574-6976.1993.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Yamazaki K, Ishiyama T, Katsumata M, Kobayashi K, Kawai Y, Inoue N, Shinano H. Comparative sequence analyses of the genes coding for 16S rRNA of Lactobacillus casei-related taxa. Int J Syst Bacteriol. 1997;47:54–57. doi: 10.1099/00207713-47-1-54. [DOI] [PubMed] [Google Scholar]
- 18.Roussel Y, Colmin C, Simonet J M, Decaris B. Strain characterization, genome size and plasmid content in the Lactobacillus acidophilus group (Hansen and Mocquot) J Appl Bacteriol. 1993;74:549–556. [PubMed] [Google Scholar]
- 19.Salminen S, Isolauri E, Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Leeuwenhoek. 1996;70:347–358. doi: 10.1007/BF00395941. [DOI] [PubMed] [Google Scholar]
- 20.Saxelin M. Lactobacillus GG—a human probiotic strain with thorough clinical documentation. Food Rev Int. 1997;13:293–313. [Google Scholar]
- 21.Saxelin M, Rautelin H, Salminen S, Mäkelä P H. Safety of commercial products with viable Lactobacillus strains. Infect Dis Clin Pract. 1996;5:331–335. [Google Scholar]
- 22.Schleifer K-H, Ehrmann M, Beimfohr C, Brockmann E, Ludwig W, Amann R. Application of molecular methods for the classification and identification of lactic acid bacteria. Int Dairy J. 1995;5:1081–1094. [Google Scholar]
- 23.Schwartz D C, Cantor C R. Separation of yeast chromosome sized DNAs by pulsed-field gradient gel electrophoresis. Cell. 1984;37:67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 24.Stiles M E, Holzapfel W H. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997;36:1–29. doi: 10.1016/s0168-1605(96)01233-0. [DOI] [PubMed] [Google Scholar]
- 25.Stull T L, LiPuma J J, Edlind T D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988;157:280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- 26.Sugita T, Togawa M. Efficacy of Lactobacillus preparation Biolactis powder in children with rotavirus enteritis. Jpn J Pediatr. 1994;47:2755–2762. [Google Scholar]
- 27.Tanskanen E I, Tulloch D L, Hillier A J, Davidson B E. Pulsed-field gel electrophoresis of SmaI digests of lactococcal genomic DNA, a novel method of strain identification. Appl Environ Microbiol. 1990;56:3105–3111. doi: 10.1128/aem.56.10.3105-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilsala-Timisjärvi A, Alatossava T. Development of oligonucleotide primers from 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int J Food Microbiol. 1997;35:49–56. doi: 10.1016/s0168-1605(97)88066-x. [DOI] [PubMed] [Google Scholar]
- 30.Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong W, Millsap K, Bialkowska-Hobrzanska H, Reid G. Differentiation of Lactobacillus species by molecular typing. Appl Environ Microbiol. 1998;64:2418–2423. doi: 10.1128/aem.64.7.2418-2423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]