Abstract
Liver cancer is one of the malignant cancers globally and seriously endangers human health because of its high morbidity and mortality. Plant-derived natural products have been evaluated as potential anticancer drugs due to low side effects and high anti-tumor efficacy. However, plant-derived natural products also have defects of poor solubility and cumbersome extraction process. In recent years, a growing numbers of plant derived natural products have been used in combination therapy of liver cancer with conventional chemotherapeutic agents, which has improved clinical efficacy through multiple mechanisms, including inhibition of tumor growth, induction of apoptosis, suppression of angiogenesis, enhancement of immunity, reversal of multiple drug resistance and reduction of side effects. The therapeutic effects and mechanisms of plant-derived natural products and combination therapy on liver cancer are reviewed to provide references for developing anti-liver-cancer strategies with high efficacy and low side effects.
Keywords: plant-derived natural products, immunotherapy, combination therapy, liver cancer, molecular mechanisms
Introduction
Liver cancer is one of the most common malignant cancers with more than 906,000 new cases and 830,000 deaths annually worldwide (1). Liver cancer has brought significant threats and challenges to human’s physical and mental health as well as social and economic development (2). Currently, the main treatments for liver cancer include surgical resection (3), radiotherapy (4), chemotherapy (5), local ablation therapy (6) and liver transplantation (7). Although traditional therapies are effective for liver cancer at early stage, they have limited efficacy for advanced liver cancer due to severe side effect, drug resistance, multiple recurrences and metastases (8, 9). For the past few years, plant-derived natural products have been evaluated as potential anticancer drugs that preferentially kill tumor cells with low toxic effect on normal cells. A variety of plant-derived natural products with antitumor activities have been identified, such as alkaloids, terpenoids, phenols, flavonoids, which can inhibit tumor-cell invasion and migration (10), induce apoptosis (11), suppress angiogenesis (12) and proliferation (13). Although these plant-derived natural products have many advantages, such as abundant resources, low toxic effect and diversity in targets and molecular mechanisms, they have limited efficacy for clinical application due to low solubility and poor bioavailability (14). Therefore, there is an urgent need to develop new therapy with high efficacy and low side effects to prevent and treat liver cancer.
Numerous studies have shown that combined therapy has great potential value in treating of liver cancer. The combination of chemotherapy with natural compounds is likely to increase the efficacy of drug treatment as well as reduce the adverse responses (15). Some natural products may sensitize to conventional cytotoxic therapy, reinforce the drug effective concentration, reverse drug resistance, intensify the combined effect of both administered therapeutics or exert cytotoxic effects specifically on tumor cells (16). Moreover, natural products combined with immunotherapy, can reduce the development of cancer and enhance the immune function by targeting multiple signaling pathways (17). The desired effect could be to diminish burden on the patient’s organ by replacing part of the dose of a conventional chemotherapeutic with a natural substance with a defined effect. Many natural compounds are well tolerated by the patients and do not cause toxic effects even at high doses. Interaction of conventional chemotherapeutics with natural compounds introduces a new aspect in the research and therapy of cancer. It could be a complementing approach to potentially achieve improvements, while minimizing side effects associated with conventional chemotherapy. In this paper, we review the recent studies on the functions and mechanisms of plant-derived natural products and combination therapy in the treatment of liver cancer.
Search strategy
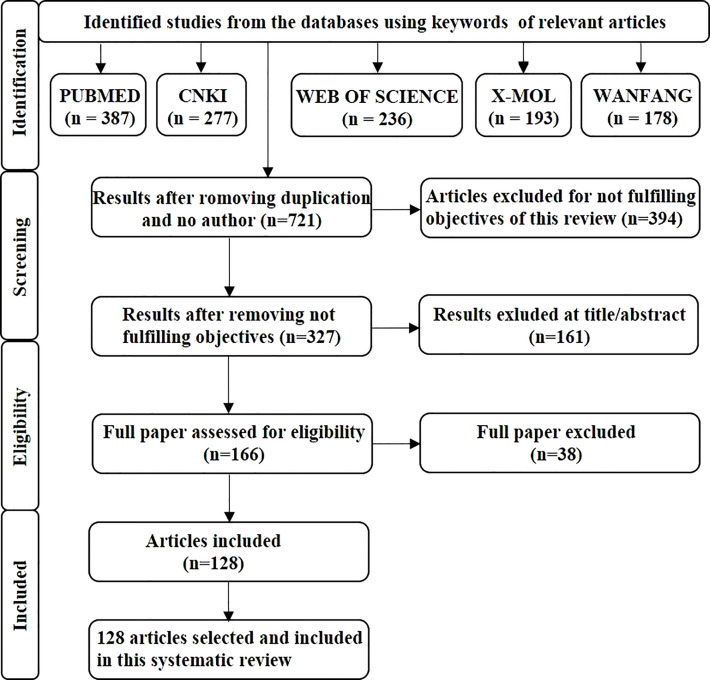
This review followed the PRISMA guidelines. The primary search for article screening used in this review was conducted using PubMed (387), CNKI (277), Web of science (236), X-MOL (193) and the Wanfang database (178), and the medical subject headings (natural product, liver cancer, combination). After completing the initial search, therefore to avoid duplication, we removed 550 articles from the literature, and selected 721 articles. After screening for eligibility of the articles, 128 articles were included in the study. PRISMA flow chart indicates only the identification and screening processes of the anti-liver-cancer activity of plant-derived plant products and combination therapy ( Figure 1 ).
Figure 1.
The flowchart provides an overview of the study search and selection process. A search of the databases PubMed, CNKI, Web of Science, X-MOL, and the Wanfang database was undertaken, and then duplicate articles and any which did not meet the criteria of our study were removed before the relevant articles were selected for further study.
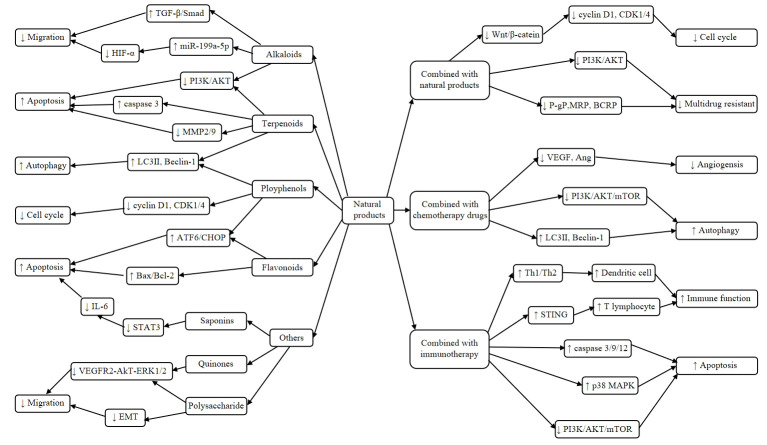
Plant-derived natural products in the treatment of liver cancer
Alkaloids
Alkaloid is a kind of basic organic compounds containing nitrogen in plants. The representative anti-tumor compounds include matrine, berberine, evodiamine, camptothecin, vincristine and caffeine. Matrine exists in dried roots of Sophora flavescent and induces HepG2 cell apoptosis through increasing Bax/Bcl-2 ratio and inhibiting the ERK1/2 signaling pathway (18). Dai et al. reported that matrine inhibits BEL-7402 cell and SMMC-7721 cell invasion and metastasis by upregulating the expression of microRNA-199a-5p (miR-199a-5p) and reduces the levels of hypoxia-inducible factor-1α (HIF-1α) (19). Moreover, matrine also inhibits liver cancer migration and induces apoptosis through increasing the expression of microRNA-345-5p (miR-345-5p) and reduces the levels of circular RNA_0027345 (circ_0027345) and homeobox-containingD3 (HOXD3) (20). In addition, Zhang et al. researched that matrine at non-toxic dose could significantly suppress PLC/PRF/5 and MHCC97L cells migration and invasion. And, it also significantly decreased lung metastasis in orthotopic HCC mouse models (21). Berberine, an isoquinoline alkaloid, has been extracted from the rhizome of Coptis and Phellodendri and considered as anticancer drug in China because of its wide range of pharmacological effects. Berberine induced autophagy of HepG2 and MHCC97-L cells through activating Beclin-1 via enhancing p38 mitogen-activated protein kinase (MAPK) signaling pathway and inhibiting protein kinase B (Akt) activity (22). Hou et al. researched that berberine decreased the expression of CD147, thereby induced apoptosis of HepG2 and SMMC7721 cells in a dose-and time-dependent manner (23). In addition, berberine inhibits transforming growth factor-β/Smad (TGF-β/Smad) signaling pathway and interferes epithelial-mesenchymal transition (EMT), thereby inhibiting migration and invasion of HepG2 cells (24). A study showed that evodiamine induced apoptosis of HepG2 and PLHC-1 cells in a dose-dependent manner by inactivating phosphatidylinositol 3-kinase (PI3K) signaling pathway, downregulating B-cell lymphoma 2 (Bcl-2) expression and upregulating Bcl-2-associated X protein (Bax) (25). Evodiamine can also inhibit the proliferation of hepatocellular carcinoma cells by activating Hippo-Yes-associated protein signaling pathway in vitro and in vivo (26). Camptothecin has been used clinically to treat liver cancer, stomach and colorectal cancer for a long time, which can inhibit Huh7 and H22 cell proliferation, metastasis and angiogenesis by upregulating of reactive oxygen species (ROS) and nuclear factor E2-related factor 2 (Nrf2) expression, reducing the expression of N-cadherin, matrix metalloproteinase 9 (MMP9), Snail and Twist, and increasing E-cadherin (27). Notably, 10-hydroxycamptothecin (0.5 mg/L) inhibited the proliferation of BEL-7402 cells through induction of apoptosis, and showed synergistic effect with human cytokine-induced killer cells (optimal target ratio of 20:1) (28). According to reports, vincristine could decrease the expression of Ki-67, MMP2 and MMP9, and increase the expression of cleaved caspase 3, thereby inhibiting proliferation and reducing metastasis by PI3K/Akt signaling pathway in Hep3B cells (29). Caffeine is an alkaloids compound extracted from tea, cocoa bean and coffee fruit. Caffeine and X-ray irradiation have synergistic effect on the inhibition of cell proliferation and induction of apoptosis, and cell cycle arrest at G2/M phase (30). Cinchonine is a natural compound present in Cinchona bark. It exerts multidrug resistance reversal activity and synergistic apoptotic effect with paclitaxel in uterine sarcoma cells. Jin et al. demonstrated that cinchonine inhibited liver cancer cell proliferation and promoted apoptosis in a dose-dependent manner. It can activate caspase-3 and PARP1 cleavage, increased the expression of GRP78 and PERK, and suppressed HepG2 xenograft tumor growth in mice (31). As a transcription factor, the tumor suppressor p53 can participate in many important biological processes, such as cell cycle arrest and apoptosis. It can activate the expression of antioxidant proteins such as catalase, glutathione peroxidase 1 (GPX 1), manganese superoxide dismutase (MnSOD), which leads to the imbalance of ROS, and finally leads to apoptotic (32). Moreover, Sanguinarine significantly inhibited liver cancer cell proliferation in a p53-dependent manner by inducing cell cycle arrest and ROS-associated apoptosis. Importantly, it dramatically suppressed tumor growth in an HCC xenograft model, with little cytotoxicity (33).
Terpenoids
Terpenoids are hydrocarbons and oxygenic derivatives in natural plants, which contain isoprene structure. It has been found that terpenoids have antitumor activities, such as saikosaponin D, triptolide, ginsenoside, artemisinin, celastrol and norcantharidin. Saikosaponin D is the most important active component of the Chinese medicinal herb Bupleurum, which can selectively up-regulate the protein expression of light chain 3II (LC3II) and Beclin 1, down-regulate the protein expression of P-S6K1, and induce autophagy by negatively regulating the mammalian target of rapamycin complex (mTORC) signaling pathway to reduce liver cancer cell growth (34). Moreover, it can increase radiation-induced apoptosis of liver cells via inhibiting mTOR phosphorylation to provide a possible potential therapeutic method for radio sensitization of liver cancer (35). In addition, Wu et al. discovered that saikosaponin D inhibited HepG2 cell proliferation by induction of apoptosis, improved the survival rate and suppressed the growth of liver tumors (36). Triptolide, a natural diterpenoid compound, is the main active ingredient of Tripterygium wilfordii, which inhibits the proliferation of HepG2 cells through induction of autophagy and apoptosis via up-regulating the expression of microRNA-194 (miR-194), LC3 and Beclin-1, and Bax/Bcl-2 ratio (37). Another study showed that it induced HepG2 cell apoptosis by inhibiting the expression of Ras (38). Gan et al. demonstrated that triptolide significantly suppressed the proliferation of H22 cells with IC50 value of 266.8 ng/mL at 24 h and induced apoptosis in H22 cells through decreasing the expression of cycloxygenase-2 (COX-2) (39). Ginsenoside-Rg3 inhibited cell proliferation and invasion of SMMC-7721 cells through PI3K/AKT pathway, which effectively decreased the levels of phosphorylated (p)-AKT, p-PI3K, MMP2, MMP9 and long non-coding RNA HOX antisense intergenic (lncRNA HOTAIR) (40). Recently, Zhang et al. showed that artemisinin enhanced the effect of anti-programmed cell death-ligand 1 (anti-PD-L1) immunotherapy by blocking the accumulation and function of myeloid-derived suppressor cells (MDSCs) via regulating PI3K/AKT/mTOR and MAPK signaling pathways and polarizing M2-like pro-tumor phenotype to M1-like anti-tumor phenotype (41). Dihydroartemisinine inhibits proliferation and induces apoptosis of HepG2 cells through regulating Bcl-2/Bax/caspase-3 apoptosis signal pathway (42). It can also induce liver cancer ferroptosis by promoting the formation of phosphatidylethanolamine-binding protein 1/15-Lipoxygenases (PEBP1/15-LO) and lipid peroxidation in cell membrane (43). Celastrol is a quinone methyl triterpene monomer compound of T. wilfordii Hook. f. and inhibits HepG2 cell proliferation by activating AMPK signaling pathway to suppress lipid metabolism and proliferation signal (44). Moreover, it can reduce the expression of nuclear factor kappa-B (NF-κB) and tumor necrosis factor alpha (TNF-α) in HepG2 and SMMC-7721 cells in a dose- and time-dependent manner (45). Recent studies have found that p53 can downregulate the expression of asparagine synthetase (ASNS) at the transcriptional level, thereby inhibiting asparagine synthesis and disrupting aspartate homeostasis, which can suppress the growth of lymphoma and colon tumors in vivo and in vitro. And depleting the levels of asparagine in cancer cells could release liver kinase B1 (LKB 1), and activated monophosphate-activated protein kinase (AMPK) -p53 signaling, triggered p21-dependent cell cycle arrest and promoted survival of cancer cells in asparagine-deficient conditions (46). Norcantharidin inhibits Huh7 cell proliferation and induces apoptosis in a time-and dose-dependent manner by activating p53 and p21 proteins and arresting cell cycle at G2/M phase (47). Tan et al. reported that genipin suppressed orthotopic HCC tumour growth through inhibiting IRE1a-mediated infiltration and priming of tumour associated macrophages (TAMs). It could reduce infiltration of inflammatory monocytes into liver and tumour, and inhibited the TAMs migration (48). Natural compound andrographolide (Andro), isolated from medicinal herb Andrographis paniculata, was reported to inhibit hepatoma tumor growth. It can inhibit the hepatoma tumor growth and alters the expression of miRNAs profile and downstream signals (49).
Ployphenols
Polyphenol is a compound with multiple phenolic groups and includes curcumin, resveratrol, 6-gingerol, epigallocatechin gallate, and tannic acid. Curcumin is a spice and food coloring extracted from the rhizome of the perennial herb turmeric in Zingiberaceae. In vitro and in vivo results showed that curcumin downregulated the expression of Bcl2-associated transcription factor (BCLAF1), inhibited the activation of PI3K/AKT/glycogen synthase kinase-3β (GSK-3β) pathway, and triggered mitochondria-mediated apoptosis in liver cancer (50). Sun et al. reported that curcumin inhibited SMMC-7721 cell proliferation by induction of apoptosis via activating C/EBP homologous protein (CHOP) signaling pathway, reducing the expression of Bcl-2 and increasing cleaved caspase-3 and suppressed SMMC-7721 cell growth in xenograft tumor nude mice by activating endoplasmic reticulum (ER) stress pathway (51). Curcumin can induce liver cancer cell death by induction of apoptosis and pyroptosis. In addition, curcumin activates the gasdermin E (GSDME)-related scorch death and mitochondria-dependent apoptosis signaling pathways (52). Resveratrol is a phytoestrogen derived from grapes and berries and has several potential biological effects, including antioxidant, anti-inflammatory, cardioprotective and anticancer properties (53). Resveratrol inhibited the wound healing and invasion of HepG2 and Huh7 cells, increased the expression of E-cadherin, and decreased the expression of vimentin and Twist1. It inhibited metastasis and EMT through up-regulating the expression of microRNA-186-5p (miR-186-5p) (54). Furthermore, resveratrol can decrease the expression of membrane-associated RING-CH protein 1 (MARCH1) and p-Akt, increase the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) dose-dependently, hence inducing apoptosis and inhibiting the proliferation and invasion of HepG2 and Hep3B cells (55). 6-gingerol is an active polyphenolic compound extracted from ginger and can regulate the AKT/extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathway, increase P27/Kip1 and p21/Cip1 expression and inhibit cyclins D1, c-myc and cyclin-dependent kinase 4 (CDK4) protein expression, thereby arresting cell cycle at G2/M phase in Huh7 cells (56). Notably, a study showed that epigallocatechin gallate (EGCG) and EGCG derivative Y6 could significantly inhibit tumor growth and angiogenesis through MAPK/ERK1/2 and PI3K/AKT/HIF-1α/vascular endothelial factor (VEGF) pathways (57). Guo et al. demonstrated that EGCG combined with paclitaxel effectively inhibited the proliferation of HepG2 cells and the tumor growth in bearing cancer nude mice (58). In addition, it combined with adriamycin to exert synergistic anti-liver cancer effect, which induced apoptosis of BEL-7404/ADR cells and arrested cells in S/G2 phase of the cell cycle in a dose-dependent manner (59). Tannic acid is a natural polyphenol compound primarily found in fruits and nuts. Tannic acid can induce apoptosis by DNA disruption via caspase-dependent and caspase-independent mechanisms. It can also induce oxidative stress to promote liver cancer cell death (60). The combination of tannic acid with cisplatin induced HepG2 cell apoptosis through enhancing the activation of ER stress ATF6-CHOP pathway (61). Magnolol, a natural compound extracted from herbal plant Magnolia officinalis, has been recognized as a liver protection and antitumor drugs. It can significantly suppress tumor size and tumor growth rate, decreased the expression of p-ERK, NF-κB p65 (Ser536), MMP-9, VEGF, X-linked inhibitor of apoptosis protein (XIAP), and cyclinD1, and increased the expression of caspase-8 and caspase-9, which in turn induced apoptosis in vivo (62). Rosmarinic acid is a natural polyphenolic compound that exists in many medicinal species of Boraginaceae and Lamiaceae. Rosmarinic acid can regulated immune response and induced apoptosis of H22 cells. It could effectively inhibit the tumor growth through decreasing the expressions of IL-6, IL-10 and signal transducer and activator of transcription 3, up-regulating Bax, caspase-3 and down-regulating Bcl-2 (63).
Flavonoids
Flavonoids are phenolic phytochemicals and are commonly found in fruits, vegetables and plant-based beverages (e.g., green tea and wine), which have a polyphenolic structure and are usually composed of a 15-carbon skeleton (including two benzene rings) and a heterocyclic nucleus containing an oxygen atom. Many studies have reported that flavonoids have a significant role in tumor treatment, such as kaempferol, silymarin, quercetin, chrysin and diosmetin. Kaempferol is a natural flavonol and widely distributes in many vegetables and fruits. Kaempferol remarkably decreased the expression of miR-21 and increased the expression of PTEN, thereby inhibiting HepG2 cell proliferation and metastasis through inactivating PI3K/AKT/mTOR signaling pathway (64). Moreover, it can promote apoptosis of HepG2 cells in a dose-dependent manner through decreasing the expression of CDK1 and increasing the expression of Bax and JUN (65). Another study showed that kaempferol was able to increase the protein expression of Bax, glucose-regulated protein of 78 kDa (GRP78), GRP94, PERK, cleaved ATF6, IRE1α, CHOP, caspase-3 and caspase-4, thereby inducing HepG2 cell apoptosis by mitochondrial pathway and ER stress pathway (66). Silymarin is a lipid compound extracted from the fruit of Silybum marianum and inhibits MHCC97 cell invasion and metastasis through reducing Akt phosphorylation and integrin β1, VEGF and MMP-9 expression. Moreover, it can restore the cell adhesion ability by upregulating E-cadherin, thereby inhibiting invasion and metastasis of liver cancer cells (67). Silibinin is another bioactive polyphenolic flavonoid isolated from the fruits and seeds of S. marianum (68), which can achieve anti-liver-cancer effects through multiple mechanisms, including induction of apoptosis, suppression of angiogenesis and block of cell cycle (69, 70). Quercetin is a dietary polyphenolic compound with wide distribution in onions, grapes, berries, apples, broccoli and cereals (71), and has the capability to reduce the expression of markers in cancer stem cells and myofibroblasts, as well as the expression of ABCC3 to reduce the acquisition of drug resistance in the early liver cancer stages (72). Quercetin inhibited the growth of HepG2 cells in a dose- and time-dependent manner, with IC50 values of 110.50, 63.73 and 46.38 µmol/L at 24 h, 48 h and 72 h, respectively, and suppressed the growth of subcutaneous xenografts in BALB/c mice (73). Another study showed that quercetin inhibited HepG2 cell proliferation by NF-κB pathway and activator protein-1 (AP-1)/c-Jun N-terminal kinase (JNK) pathway, which increased the expression of p-JNK, p-AKT, p-p38, caspase-3 and decreased the expression of Bcl-xL, p-ERK and NF-κB (74). Chrysin, also known as aspicin, is a natural flavonoid compound extracted from Pterophylla globulina, with extensive pharmacological activities (75). Chrysin significantly inhibited SMMC-7721 cell proliferation and induced apoptosis by activating MAPK signaling pathway (76). Moreover, chrysin could effectively inhibit the progression of tumor and promote the antitumor immunity of mice concomitant with increased CD4+ and CD8+ T cell proportions in tumor tissues of H22 xenograft mice model. Additionally, chrysin reduced the expression of PD-L1 through blocking of STAT3 and NF-κB pathways in vitro and in vivo (77). Diosmetin is an effective component of medicinal plants in southern China. Liu et al. found that diosmetin inhibited liver cancer cell metastasis, invasion and adhesion by regulating the PKCδ/MAPK/MMP pathway (78). It also can significantly downregulate the levels of Bcl-2, CDC2 and cyclinB1, and upregulate the levels of Bax, cleaved-caspase-3, cleaved-caspase-8, cleaved-PARP, Bak, p53 and p21, thereby inhibiting HepG2 cell proliferation and inducing cell apoptosis and arresting cell cycle at G2/M phase (79). Similarly, another study showed that diosmetin reduced the expression of Bcl-2, CDK1 and CCND1 in a dose-dependent manner in vitro and the expression of Ki67 in vivo, thereby inhibiting the proliferation and migration and arresting cell cycle at G1 phase of liver cells (80). Tan et al. observed that oral administration of baicalin completely blocked orthotopic growth of implanted HCC. And it induced repolarisation of TAM and M2-like macrophages, which is associated with elevated autophagy, and transcriptional activation of RelB/p52 pathway (81).
Others
Emodin is an anthraquinone compound extracted from Palmar rhubarb and Amomum amomatum and inhibits liver cancer cell proliferation and metastasis by regulating VEGFR2-AKT-ERK1/2 signaling pathway and miR-34a signaling pathway (82). Aloin is a natural anthracycline compound, which can inhibit MHCC97H cell proliferation, migration and invasion and induce apoptosis through regulating Bcl-2/Bax ratio (83). Tanshinone is a phenoquinone derivative extracted from Salvia miltiorrhiza and inhibits HepG2 and Huh7 cell proliferation by blocking G0/G1 phase cell cycle via decreasing the expression of cyclin D1 and increasing p21, as well as inhibiting p53/damage-regulated autophagy and inducing apoptosis (84). As the most frequently mutated gene in human tumors, p53 can induce apoptosis, cell cycle arrest and cell senescence. And it plays an important role in suppressing proliferation of tumor, and is a major target for developing new drugs to reactivate its tumor suppressing activity for anticancer therapies. Taking p53 mutant Y220C as the core, Joseph et al. studied the structure-guided development of high-affinity small molecules stabilizing p53-Y220C in vitro and the synthetic route developed in the process. They found two new chemical probes with submicromolar binding affinity in vitro, which represents an important step toward the novel and effective Y220C ligand in the clinical evaluation of tumors (85). Cryptotanshinone significantly inhibited the cell viability of HepG2 cells with an IC50 of 93.73 μmol/L and induced ferroptosis by ROS accumulation via reducing glutathione (GSH) level and the expression of cystine/glutamate antiporter system light chain (xCT) and glutathione peroxidase 4 (GPX4) (86).
Natural polysaccharides are rich dietary components with significant roles in tumor prevention and treatment. Astragalus polysaccharides are the active component of the dried root of Radix astragali and can inhibit HepG2 cell proliferation (87), reverse the drug resistance of 5-fluorouracil (88), suppress EMT (89) and induce apoptosis (90). Astragalus polysaccharides can inhibit the growth of HepG2.215 cells by blocking HepG2.215 cells from entering the G2/M phase and inducing apoptosis (91). Poria cocos polysaccharides induced HepG2 cell apoptosis by inhibiting the expression of NOD-like receptor thermal protein domain associated protein 3 (NLRP3)/caspase-1/gasdermin D (GSDMD) in the classical pyroptosis pathway (92). Another study showed that Lycium barbarum polysaccharides inhibited SMMC-7721 cell proliferation and metastasis and induced apoptosis by reducing the expression of MMP-2, MMP-9 and VEGF (93). Saponins can significantly inhibit tumor cell proliferation. Astragaloside IV, also known as astragalus saponins, was able to upregulate the expression of caspase-3 and downregulate the expression of interleukin (IL)-6 by inhibiting the STAT3 signaling pathway, thereby inducing the apoptosis of liver cancer cells (94). Astragaloside IV also inhibits HepG2 cell proliferation and induces apoptosis by regulating oxidative stress and NF-κB signaling pathway (95). In addition, astragalus saponins promoted the secretion of IL-2 to enhance the proliferation and transformation of lymphocytes, thereby improving the immune function of rats and inhibiting liver cancer cells growth (96).
Compounds
Zhu et al. investigated the influence of Huangqi Sijunzi decoction on 80 patients undergoing primary liver cancer operation. They found that Huangqi Sijunzi decoction can promote postoperative recovery of the liver function and immunologic function of the patients undergoing primary liver cancer operation, alleviate clinical symptoms and facilitate postoperative recovery of the patients (97). Yiqi Huoxue Fuzheng Jiedu detoxification is a compound with many kinds of traditional Chinese medicine, Jiang et al. explored the clinical effect of supplementing qi and activating blood circulation to assist the treatment of patients with advanced liver cancer. It is an effective method in adjuvant treatment of patients with advanced liver cancer, and it may be helpful to improve its clinical symptoms and liver function (98). Moreover, Zheng et al. researched that in the treatment of severe cancer pain due to primary liver cancer, external application of compound Gleditsiae Spina ointment has a marked analgesic effect, and can reduce the dose of opioid painkillers and the number of times of breakthrough pain and improve patients’ quality of life (99). Jing et al. observed the clinical effect of the therapy of Xingqi Sanjie Huayu prescription combined with reduced glutathione on 80 patients who had undergone surgery for primary liver cancer. It can effectively improve the clinical symptoms, reduce the levels of serum tumor markers, enhance the immune function and liver function, and reduce the incidence of adverse reactions (100). To investigate the clinical effect of Thymalfasin injection combined with Kanglaite injection and Chinese medicine Zhenggan prescription in the prevention of postoperative recurrence of primary liver cancer, chen et al. selected sixty patients with primary liver cancer. The triple therapy of Thymalfasin injection combined with Kanglaite injection and Chinese medicine Zhenggan prescription can significantly improve patients’ clinical syndrome and quality of life, promote the improvement of liver function, regulate immune function, reduce AFP level, has the advantages of low recurrence rate, has significant curative effect, and is worthy of further promotion and application (101). Furthermore, Zhao et al. founded that the therapy of Fuzheng Huayu Jiedu prescription can effectively improve the clinical effect on advanced liver cancer with less adverse reactions and high safety, and its mechanism may be related to its remarkable reduction of levels of inflammatory factors, inhibition of inflammatory responses, improvement of immune function and enhancement of resistance (102). Hedyotis diffusa injection is a Chinese patent medicine, which has the effect of clearing heat and detoxifying, benefiting dampness and swelling. Hedyotis diffusa injection combined with transarterial chemoembolization in the treatment of the patients with primary liver cancer can improve the disease control rate and improve the levels of T cell subsets. Moreover, it is superior to single transarterial chemoembolization (103).
Supplementary Table 1 summarized the effects and mechanisms of plant-derived natural products on liver cancer. Although many plant-derived natural products are effective, they have limited efficacy for clinical application. Conventional mono-therapeutic methods target rapidly proliferating cells and destroy their growth, which it does not differentiate between healthy and cancerous cells. Chemotherapy can be toxic to the patient with multiple side effects and risks, such as nausea and vomiting and tumor metastasis (104). And it can also strongly reduce their immune system by affecting peripheral blood lymphocyte subsets and increasing susceptibility to host diseases (105). Although combination therapy can be toxic if one of the technologies is chemotherapeutic, the toxicity is remarkably less because a lower therapeutic dosage of each individual drug is demanded (106). It may be able to prevent the toxic effects on normal cells while concurrently producing cytotoxic effects on cancer cells. Further, monotherapy treatment is more develop drug resistance because continuous treatment with a single compound induces cancer cells to create alternative salvage approach (107). The most common mechanism of multidrug resistance is the elimination of drugs from the cell by ATP-dependent efflux pumps, thereby reducing intracellular drug concentrations and giving rise to resistance. As an example, cells in adenocarcinoma, when treated with adriamycin, upregulate the expression of ATP-binding cassette (ABC) transporter family, P-glycoprotein, to eliminate the drug, leading to a state of drug resistance (108). However, combination therapy also produced a more effective treatment response which target different pathways, and therefore this treatment modality reverses the drug resistance (109). Generally, conventional chemotherapy only targets non-stem cell tumor cells, resulting in an increasing proportion of stem cells in tumors after chemotherapy. And it is also a key mechanism for induction of drug resistance, regulation of metastasis and recurrence of malignant (110). However, combination therapy (for example quercetin in combination with chemotherapy drug doxorubicin) could reduce drug resistance and attenuated the likelihood of relapse (111). Therefore, combination therapy of plant-derived natural products with chemotherapeutics or immunotherapy is drawing researcher’s attention on liver cancer to induce apoptosis and inhibit cell proliferation and angiogenesis. It has the potential to develop therapeutic or adjuvant agents for human liver cancers.
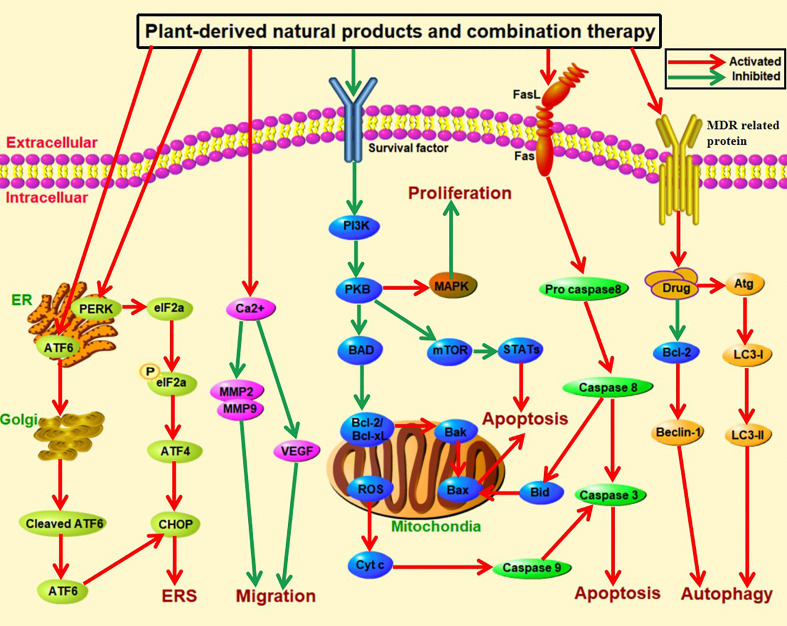
Anti-liver cancer mechanism by combination of plant-derived natural product ingredients
Mutations and epigenetic changes allow cancer cells to grow and replicate uncontrollably and invade normal tissues. This uncontrolled growth and proliferation arise due to loss of function mutations in tumour organelles such as lysosome (112), mitochondria (113), as well as endoplasmic reticulum (114). The limited efficiency and serious side effects associated with the use of conventional anticancer therapies encouraged scientists to focus on the discovery and development of new anticancer agents derived from natural products. For much of history, people have used plants and plant extracts to treat their ailments. Although the established natural plants as monotherapy of cancer had many advantages, they had limited efficacy for clinical application. If they are used in combination with other natural products, their anti-cancer effect is more effective. Secondary metabolites from plant sources like flavonoids, alkaloids, terpenoids, saponins, and others had been reported as important sources for potent anticancer agents. It was demonstrated that combination treatment acts by reducing the concentration of the compound required for drug efficacy through enhancing the effect on the same target or mechanism of action. The anticancer effect of these natural products in combination with others is mediated by different mechanisms, including inhibiting proliferation, blocking cell cycle and reversing multidrug resistance.
Inhibit liver cancer cells proliferation
Proliferation and invasion of liver cancer cells were inhibited through decreasing the expression of the tumor suppressor gene deleted in liver cancer 1 (DLC1), translationally controlled tumor protein (TCTP) and Cdc42 (115). Resveratrol in combination with dihydroartemisinine acts by regulating DLC1/TCTP protein expression and inhibiting migration-related signaling pathways in HepG2 and MDA-AB231 cells through inducing Cdc42 regulation of JNK/NF-κB and N-WASP signaling pathways (116). PI3K-AKT signaling pathway is closely related to the proliferation of liver cancer cells (117). The combination of natural products acts by inducing anti-tumor effect through inhibiting the invasion and proliferation of tumor cells. Sodium arsenite and astrageoside IV inhibit HepG2 cell proliferation, block cell cycle, promote apoptosis, and suppress tumor growth. It can reduce the expression of PI3K, AKT, mTOR through PI3K/AKT/mTOR signaling effect, and the synergistic effect is more significant (118). Apoptosis an active and ordered multistep cell death process that controlled by genes, and involves two major pathways, including intrinsic and extrinsic apoptosis pathways in human cancer cells. The intrinsic pathway of apoptosis is mediated mainly by mitochondria within the cell, and this pathway is regulated by pro-apoptosis Bcl-2 family proteins (119). Natural products principally induced liver cancer cells apoptosis and inhibited proliferation through caspase cascade reaction and NF-κB signaling pathway.
Wen et al. developed citrus net skin black tea (CRPBT) by combining citrus reticulate peel and black tea. CRPBT can significantly down-regulate the phosphorylation of PI3K and AKT in liver cancer cells, up-regulate the Bax/Bcl-2 ratio, and inhibit the expression of MMP2/3, N-cadherin and Vimetin proteins. It can inhibit invasion, proliferation and induce apoptosis of liver cancer cells (120). A combination of curcuma zedoary and kelp could inhibit liver cancer cell proliferation and metastasis by inhibiting endogenous H2S production and down-regulating the transcription activator 3 (STAT3)/BCL-2 and VEGF pathway in vitro and in vivo (121). Bufalin (BFL) and cinobufagin (CBF) have a synergistic effect. Metabolic pathways, including methionine metabolism, energy metabolism, lipid metabolism, and amino acid metabolism, were modulated and subsequently led to apoptosis and cell cycle arrest of HepG2 cells. Zhang et al. researched that the cotreatment group can significantly decrease mitochondrial membrane potential (MMP) and oxygen consumption rate (OCR), which associated with maximal respiration (122). Triptolide and sodium cantharidinate exert a synergistic toxic effect on hepatoma cell line 7721, which is related to increasing capase-3 activity and suppression of NF-κB (123). Quercetin and rosmarinic acid had a more significant synergistic effect on inhibiting HepG2 cells proliferation and migration in a dose-dependent manner by up-regulating the expression of E-cadherin and down-regulating N-cadherin gene expression (124). Manikandan et al. showed that curcumin in combination with catechin which is polyphenolic compound have a synergistic effect on inhibiting the proliferation and inducing cell apoptosis. It treated HepG2 cells showed small morphological changes which significantly destruction of monolayer, and change the DNA fragmentation (125). Wang et al. evaluated the effects of co-treatment of vincristine and berberine on hepatic carcinoma cell lines. They find that combinational usage of these two drugs can significantly induce cell growth inhibition and apoptosis even under a concentration of vincristine barely showing cytotoxicity in the same cells when used alone (126). The combination of arsenic trioxide and tanshinone have synergistic effect, attenuation and antihepatocarcinoma effect. Tanshinone can reduce the blood routine changes and liver and kidney function damage caused by arsenic trioxide (127). Cryptotanshinone induced liver cancer cells apoptosis and enhanced the effect of Arsenic trioxide by downregulating phosphorylated STAT3, Bcl-2 in vitro and in vivo (128).
Block liver cancer cells cycle
The cell cycle system plays a critical role in regulating activities such as cancer cell proliferation and apoptosis. The pathogenesis of neoplasms because of lost control in cycle progression is common. Uncontrol cell division is an essential factor in the development of cancer. The cell cycle consists of G1, the S-phase, G2, and the M (mitotic) phase (129). Cells are preparing for DNA synthesis during G1 and they perform surveillance to establish the integrity of newly synthesized DNA during G2 before initiating mitosis. Chromosomal DNA is replicated during the S-phase. If this phase arrest, the cells will undergo variation or abnormal division, which will then terminate the proliferative (130). In the G2 phase, after the nucleic acid is amplified, the other cellular components are partitioned between two daughter cells during the M-phase. If the stagnation occurs, the normal growth and gathering of the cells is hindered, which inhibit proliferation (131). Cyclins and their cognate cyclin-dependent protein kinases (CDKs) are necessary components required for traversing the cell division cycle, with controlling balance of all stages in cell cycle (132). CDK2/4/6, Cyclin D1, and Cyclin E are the major regulatory proteins in the G1 phase; and the reduction of Cyclin B1 and CDK1/2 activity is a key marker of cycle arrest in the G2/M phase (133, 134).
Zhang Di demonstrated that chrysin in combination with diosmetin or triptolide acted by the induction of apoptosis through inhibiting HepG2 cells migration as well as arresting the cell cycle in G1 and G2 phases in vitro. Moreover, it had a stronger inhibitory effect on H22 solid tumors than single drug application (135). Xiao et al. revealed that a combination of biochanin A with BRAF inhibitor SB590885 synergistically inhibiting proliferation by disrupting of the ERK MAPK and the PI3K/AKT pathways in vitro, and promoting cell cycle arrest and apoptosis (136). Quercetin in combination with maleic anhydride derivatives acts by inducing apoptosis of liver cancer cells through arresting cells in S-phase by oxidative stress response, causing DNA damageactivating the intrinsic apoptosis pathway (137). Curcumin (20 µmol/L) combined with vincristine (1 pg/L) inhibits proliferation and clone formation of HepG2 cells, reduces mitochondrial membrane potential and MDR1 and LRP protein expression, increases P21 expression, thereby blocking G2/M phase cell cycle and inducing apoptosis (138). Curcumin (5 pg/L) and glycyrrhetinic acid (10 pg/L) obviously inhibited HepG2 cells proliferation in a concentration- and time-dependent manner, arrested the G2 phase, and the synergistic effect is more remarkably (139). The combination of Curcumin (40 µmol/L) and artemisinin (80 µmol/L) could effectively inhibit HepG2 cells proliferation and arrest cell cycle and promote apoptosis. In addition, it can inhibit telomerase activity in liver cancer cells (140). Tanshinone IIA block subG1 phase cell cycle, while resveratrol arrest S phase and G2/M phase of HepG2 cells. The combination of them induce cell apoptosis and arrest subG1 cell cycle. Moreover, it exerted synergistic cytotoxicity and robustly induces apoptosis, and DNA fragmentation (141). Berberine (15 µmol/L) combined with tarpinine derivative HMQ1611 (15 µmol/L) inhibits cyclin D1, cyclin E, CDK1 expression and arrests the cell cycle at G1 phase through PI3K/AKT/mTOR signaling pathways to induce apoptosis of HepG2 cells. Furthermore, the combination therapy had strong suppressive effect on proliferation by inhibiting the phosphorylation of LDL receptor-associated protein, which was related to Wnt/β-catenin signaling pathway (142). Liu et al. showed that different concentrations of norcantraridin (12.5-200 µmol/L) and evodiamine (2.5-40 µmol/L) inhibited HepG2 cells growth and arrested G2/M phase cell cycle and induced apoptosis. Moreover, it has obvious synergistic inhibitory effect on anti-proliferation and pro-apoptosis through increasing the expression of Bax and decreasing the expression of Bcl-2 (143). Triptolide (0.01-5 pg/L), glycyrrhetinic acid (1-10 pg/L), rhein (1-10 pg/L) and paclitaxel (1-20 pg/L) inhibit HepG2 cell proliferation in a dose-dependent manner through blocking G2/M phase cell cycle. In addition, glycyrrhetinic acid, paclitaxel and rhein can enhance the inhibitory effect of low concentration of tripterine on HepG2 cell proliferation (144).
Reverse liver cancer cells multidrug resistance
Liver cancer cells will be exposed to a certain chemotherapy drug for a long time under chemotherapy. And it can develop multidrug resistance (MDR) to this drug and cross-resistance to other chemotherapy drugs with different functional structures, leading to chemotherapy failure (145). This mechanism of multidrug resistance arises due to activity of the superfamily members of adenosine triphosphate binding cassette (ABC) such as P-glycoprotein (P-gP), multidrug resistance associated protein (MRP) and breast tumor resistance protein (BCRP) (146). Plant-derived natural products act by enhancing the tumor killing effect of chemotherapeutic drugs and inducing their anti-tumor activity through reversing the multidrug resistance of liver cancer cells.
Sun et al. demonstrated that curcumin reversed the multi-drug resistance caused by cucurbitacin B through inhibiting the expression of P-gP, thereby inducing liver cancer cell apoptosis (147). Shen et al. researched that coumarin derivatives, as an inhibitor of microtubule affinity-regulating kinase 4 (MARK4), induce apoptosis of liver cancer cell by increasing microtubule dynamics and the sensitivity of paclitaxel (148). The curcumin antiproliferative effect was confirmed by Nan et al., who reported marked induction apoptosis of liver cancer cells, reversed the drug resistance of paclitaxel through inhibiting NF-κB pathway stimulated Lin28B expression (149). Xu et al. prepared folate-PEG-mesoporous silica nanoparticles loaded with paclitaxel, which had a good targeting effect on liver cancer cells and enhanced the anti-tumor effect of paclitaxel (150). A combination of esculetin with paclitaxel acted by induce apoptosis of liver cancer cells through up-regulating Bax/Bcl, inducing the activation of caspase-8 and caspase-3, as well as ERK signal pathway (151). Jiang et al. identified the antitumor effects of resveratrol on HepG2 cell, in addition to up-regulate p53, Bax and other proteins, and reverse drug resistance of paclitaxel (152). The combination of emodin (10 mg/L) and curcumin (10 mg/L) enhances sensitivity of BEL-7402 hepatoma cells and reduces the dosage of emodin and induces apoptosis (153). Curcumin (5-20 µmol/L) and paclitaxel (0.05-0.2 µmol/L) have synergistic anti-proliferation and pro-apoptosis effects on Hep3B cells. It can decrease paclitaxel-induced NF-κB activation, mediate Lin28 level, thereby enhancing sensitivity of liver cancer cells to paclitaxel (154). Matrine also reverses resistance of K562/ADM cells to doxorubicin and vincristine by arresting cell cycle and inducing autophagy (155). Table 1 summarizes their effects and mechanisms on liver cancer.
Table 1.
Effect and mechanism by combination of plant-derived natural product ingredients on liver cancer.
| Combination Therapy | Concentration | Cell Line/Model | Mechanism | Reference |
|---|---|---|---|---|
| Dihydroartemisinine/ Resveratrol |
dihydroartemisinine: 25 μmol/L resveratrol: 50 μmol/L |
HepG2 MDA-AB-231 |
Regulated DLC1/TCTP protein expression and induced Cdc42 regulation of JNK/NF-κB and N-WASP signaling pathways | (116) |
| Sodium arsenite/ Astrageoside IV |
sodium arsenite: 0.5 μg/mL astrageoside IV: 0.8 μg/mL |
HepG2 | Reduced the expression of PI3K, AKT, mTOR through PI3K/AKT/mTOR signaling effect | (118) |
| Citrus reticulate peel/ Black tea |
CRPBT: 0-1 mg/mL | HepG2 Bel 7402 |
Down-regulated the phosphorylation of PI3K and AKT, up-regulated the Bax/Bcl-2 ratio, and inhibited the expression of MMP2/3, N-cadherin and Vimetin proteins | (120) |
| Curcuma zedoary/ Kelp |
curcuma zedoary: 4000 mg/kg kelp: 4000 mg/kg |
H22 | Inhibited endogenous H2S production and down-regulated the pSTAT3/BCL-2 and VEGF pathway in vitro and in vivo | (121) |
| Bufalin/ Cinobufagin |
bufalin: 8 nmol/L cinobufagin: 50 nmol/L |
HepG2 | Decreased mitochondrial membrane potential (MMP) and oxygen consumption rate (OCR), which associated with maximal respiration | (122) |
| Triptolide/ Sodium cantharidinate |
triptolide: 9-36 μg/mL sodium cantharidinate: 12-50 μg/mL |
SMMC7721 | Increased capase-3 activity and suppression of NF- κB | (123) |
| Quercetin/ rosmarinic acid |
quercetin: 12.5-100 μmol/L rosmarinic acid:12.5-100μmol/L |
HepG2 | Up-regulated the expression of E-cadherin and down-regulated N-cadherin gene expression | (124) |
| Curcumin/ Catechin |
curcumin: 50 μmol/L catechin: 25 μmol/L |
HepG2 | Inhibited the proliferation and induce cell apoptosis | (125) |
| Vincristine/ Berberine |
vincristine: 0-4 nmol/L berberine: 0-40 nmol/L |
HepG2 SMMC7721 |
Regulated the signals related to mitochondrial function, apoptotic pathway and endoplasmic reticulum stress | (126) |
| Arsenic trioxide/ Tanshinone |
arsenic trioxide: 2.5 mg/kg tanshinone: 500 mg/kg |
Bel-7404 nude mice | Reduced the blood routine changes and liver and kidney function damage caused by As2O3 | (127) |
| Cryptotanshinone/ Arsenic trioxide |
cryptotanshinone: 10,20 μmol/L arsenic trioxide: 1, 2 μmol/L |
Bel-7404 | Induced liver cancer cells apoptosis and enhanced the effect of Arsenic trioxide by downregulating phosphorylated STAT3, Bcl-2 in vitro and in vivo | (128) |
| Chrysin/ Diosmetin/ Triptolide |
chrysin: 1.25-20 μmol/L diosmetin: 3.125-50 μmol/L triptolide: 0.78-12.5 nmol/L |
HepG2 H22 |
Induced apoptosis and inhibited cell migration as well as arrested the cell cycle in G1 and G2 phases | (135) |
| Biochanin A/ SB590885 |
biochanin A: 75 μmol/L SB590885: 12 μmol/L |
Bel-7402 SK-Hep-1 |
Inhibited proliferation by disrupting of the ERK MAPK and the PI3K/AKT pathways in vitro, and promoted cell cycle arrest and apoptosis | (136) |
| Quercetin/ Maleic anhydride derivatives |
quercetin: 50 mmol/L maleic anhydride derivatives: 0.01 mmol/L |
HuH7 HepG2 |
Induced apoptosisand arresting S-phase cell cycle by oxidative stress response, causing DNA damageactivating the intrinsic apoptosis pathway | (137) |
| Curcumin/ Vincristine |
curcumin: 20 µmol/L vincristine: 1 pg/L |
HepG2 | Reduced mitochondrial membrane potential and MDR1 and LRP protein expression, increased P21 expression | (138) |
| Curcumin/ Glycyrrhetinic acid |
curcumin: 5 pg/L glycyrrhetinic acid: 10 pg/L |
HepG2 | Inhibited the proliferation, promoted apoptosis and arrested the G2 phase in a concentration- and time-dependent manner | (139) |
| Curcumin/ Artemisinin |
curcumin: 40 µmol/L artemisinin: 80 µmol/L | HepG2 | Inhibited proliferation and telomerase activity and arrested cell cycle and promoted apoptosis | (140) |
| Tanshinone IIA/ Resveratrol |
tanshinone IIA: 5 µg/mL resveratrol: 5 µg/mL |
HepG2 | Induced cell apoptosis and arrested subG1 cell cycle and DNA fragmentation | (141) |
| Berberine/ HMQ1611 |
berberine: 15 µmol/L HMQ1611: 15 µmol/L |
HepG2 Bel-7402 SMMC-7721 |
Inhibited the phosphorylation of LDL receptor-associated protein, which is related to Wnt/β-catenin signaling pathway | (142) |
| Norcantraridin/ Evodiamine |
norcantraridin: 12.5-200 µmol/L evodiamine: 2.5-40 µmol/L |
HepG2 | Increased the expression of Bax and decreased the expression of Bcl-2 | (143) |
| Triptolide/ Glycyrrhetinic acid/ Rhein/ Paclitaxel |
triptolide: 0.01-5 pg/L glycyrrhetinic acid: 1-10 pg/L rhein: 1-10 pg/L Paclitaxel: 1-20 pg/L |
HepG2 | Inhibited proliferation through block G2/M phase cell cycle | (144) |
| cucurbitacin B/ Curcumin |
cucurbitacin B: 143.2 μmol/L curcumin: 108.6 μmol/L |
BEL7402/5-Fu | Reversed the multi-drug resistance caused by terpenoid cysurin B through inhibiting the expression of P-gP, thereby inducing liver cancer cell apoptosis | (147) |
| Coumarinderivatives/ Paclitaxel |
coumarinderivatives: 2.5 μmol/L paclitaxel: 2.5 μmol/L |
HepG2 SMMC-7721 |
Induced apoptosis by increasing microtubule dynamics and the sensitivity of paclitaxel | (148) |
| Curcumin/ Paclitaxel |
curcumin: 5-20 μmol/L paclitaxel: 1 μmol/L |
Hep3B HepG2 |
Induced apoptosis, and reversed the drug resistance of paclitaxel through inhibiting NF-κB pathway stimulated Lin28B expression | (149) |
| Folate/ Paclitaxel |
FA-PEG-MSNs-PTX: 100 μg/mL |
SMMC-7721 | Had a good targeting effect on SMMC-7721 cells and enhanced the anti-tumor effect of paclitaxel | (150) |
| Esculetin/ Paclitaxe |
esculetin: 50-100 μmol/L paclitaxe: 0.5 μmol/L |
HepG2 | Induced apoptosis through up-regulating Bax/Bcl, inducing the activation of caspase-8 and caspase-3, as well as ERK signal pathway | (151) |
| Resveratrol/ Paclitaxel |
resveratrol: 10 μg/mL paclitaxel: 5, 10 μg/mL |
HepG2 | Up-regulated P53, Bax and other proteins, and reversed drug resistance of paclitaxel | (152) |
| Emodin/ Curcumin |
emodin: 10 mg/L curcumin: 10 mg/L |
BEL-7402 | Enhanced sensitivity and reduce the dosage of emodin and induce apoptosis | (153) |
| Curcumin/ Paclitaxel |
curcumin: 5-20 µmol/L paclitaxel: 0.05-0.2 µmol/L |
Hep3B | Decreased paclitaxel-induced NF-κB activation, mediated Lin28 level, thereby enhancing sensitivity of liver cancer cells to paclitaxel | (154) |
The synergistic effect of plant-derived natural products in combination with chemotherapy
Clinical chemotherapy drugs mainly include cisplatin which is platinum compounds, and doxorubicin which is antibiotics, and hormones. Cisplatin and adriamycin mainly affect cell division and induce apoptosis by interfering with DNA replication (156). However, chemotherapeutic drugs significantly reduce the immune function of the body, and induce multiple organ damage (157, 158). Radiation and chemotherapeutic agents as the backbone for cancer are not very effective and toxic not only to tumor cells but also to normal cells, and are not affordable for most. Therefore, plant-derived natural products in combination with chemotherapeutic drugs are generally free of deleterious side effects and usually inexpensive, which have played a significant role in the development of anticancer drugs over the years. It remains to mention that extraction from natural plants is also contributed to the synergistic effects with other anticancer drugs such as cisplatin, doxorubicin, and hormones. Plant-derived natural products in combination with chemotherapeutic agents act as an anticancer agent by inhibition of tumor cell angiogenesis, invasion and migration, induction of autophagy.
Suppress liver cancer cells angiogenesis
Liver cancer is a kind of tumor rich in blood vessels. And blood vessels providing essential nutrients and oxygen for the occurrence and development of liver cancer cells, and are closely related to the growth and metastasis of liver cancer cells (159). Vascular endothelial growth factor (VEGF) is the most important type of angiogenesis regulatory factor, which promotes the division and proliferation of vascular endothelial cells through paracrine and promoting formation of blood vessels (160). Angiopoietin (Ang)/Tie2 pathway regulates vascular and lymphatic development, vascular permeability, angiogenesis remodeling, and tumor vascularization (161, 162). Tumor cells act by up-regulating VEGF through inducing miR-21 targeting AKT and ERK signaling pathways, thereby promoting tumor angiogenesis (163). The mTOR pathway can also promote tumor angiogenesis through inducing the transformation of tumor-associated macrophages (TAM) into M2-type. Therefore, it can reduce proliferation and metastasis of liver cancer cells through preventing the formation of new blood vessels (164, 165).
Ao et al. found that the main component of kanglite injections is the extract from coix seed in traditional Chinese medicine, this active compound in combination with thalidomide can significantly inhibit human liver cancer blood vessels through inhibiting VEGF and B-FGF, thereby inducing angiogenesis. At the same time, it can regulate cellular immunity of the body, which has synergistic effect with chemotherapy (166). Compared with cisplatin group, the alcohol extracts of Liuwei rehmannium (mainly composed of polysaccharide, paeonol, flavonoids, trace elements, etc.) in combination with cisplatin, can significantly induce apoptosis of BEL-7402 cells, and inhibit angiogenesis by down-regulating the expression of VEGF and ANG-2 proteins, up regulating the expression of TSP and TIMP-2 (167). Lin et al. reported that compared to model group, a combination of astragalus polysaccharide with cisplatin and adriamycin could significantly reduce the expressions of Ki-67, HIF-1α and VEGF, and had a significant synergistic and toxic effect on inhibition of tumor growth (168). Zang et al. confirmed that radix astragali or curcuma in combination with cisplatin acted by inhibiting angiogenesis and metastasis of liver cancer through down-regulating the expression of CD147 and iNOS (169). Luo et al. showed that a combination of cisplatin with astragaloside IV or curcumin which is flavonoid compound have a significant inhibition the formation of new blood vessels in liver cancer by down-regulating the expression of HIF-1α and VEGF (170). Chen et al. researched that combining epigallocatechin gallate (EGCG) derivative Y6 with dunorubicin which is anthracycline antitumor drug, acts by inhibiting of angiogenesis and toxic effects in vivo through inhibiting of MAPK/ERK and PI3K/AKT signaling pathways to down-regulate the expression of HIF-1α, Carbonyl Reductase1 (CBR1) and VEGF (171). Zhao et al. developed doxorubicin (DOX) and curcumin (Cur) co-delivery lipid nanoparticles (DOX/Cur-NPs) and examined its inhibitory effect on diethylnitrosamine (DEN)-induced liver cancer in mice. The mRNA levels of MDR1, Bcl-2 and HIF-1α, and protein levels of P-gP, Bcl-2 and HIF-1α were decreased in DOX/Cur-NPs than those in DOX-NPs, indicating that Cur might reverse multidrug resistance (MDR) through these pathways (172).
Inhibit invasion and migration of liver cancer cells
A critical event during tumorigenesis is the transformation from a static primary tumor to an aggressive and invasive oner. During the processes, tumor cells show an increased capacity to migrate, and multitudinous intracellular molecules participate in migration. Metastasis is significantly pathological characterized by malignant tumors, while the invasion and migration of liver cancer cells lead to poor prognosis of liver cancer patients, prone to recurrence and short median survival (173). The invasion and migration of tumor cells is a complex process controlled by multiple factors (174). It mainly included EMT, inhibition of matrix metalloproteinases (MMPs) hydrolysis and cell adhesion to basement membrane and extracellular matrix (175). And it is affected by the regulation of tumor metastasis genes and tumor metastasis suppressor genes (176). Combining natural products with chemotherapy drugs can prevent and cure cancer metastasis safely and effectively.
Shan et al. confirmed that mannose and cisplatin had a synergistic effect, which inhibited proliferation and migration of HepG2 cells, furthermore, it can change morphology with separation, shedding and aggregation (177). A combination of emodin with cisplatin can up-regulate the expression of E-cadherin, acts by inhibiting proliferation, invasion and migration of HepG2 cells through inhibiting EMT (178). Yang et al. showed that kaempferol and doxorubicin synergistically inhibited the proliferation, migration and invasion of liver cancer cells by inhibiting PI3K/mTOR/MMP protein pathway in a dose-and time-dependent manner (179). The sinosinine thiocyanate extracted from the herbaceous plants of The Cross family, could target the multidrug-resistant related proteins ABCB1 and ABCG2, enhance the sensitivity of HepG2 cells to gemcitabine, and inhibit the invasion and migration (180). Wang et al. conducted an investigation about the potential anti-tumor mechanism of alkaloid caffeine in combination with 5-fluorouracil. It can exhibit a synergistic anti-liver cancer effect by increasing the expression of cleaved PARP, p-JNK, p-p38, and decreasing the expression of Bcl-2, Bcl-xL, CDK2/4/6, p-ERK, p38, via inducing generation of ROS and influencing MAPK signaling pathway, thereby inhibiting the proliferation and metastasis and inducing apoptosis of SMMC-7721 and Hep3B cells (181). Data from a study in vitro revealed that combined metformin (10 mmol/L) and curcumin (5 and 10 µmol/L) could induce apoptosis and inhibit metastasis in HepG2 and PLC/PRF/5 cells. The anticancer effects could be attributed to inhibition of VEGF, MMP2/9, and VEGFR-2 protein expression (182).
Induce liver cancer cells autophagy
Autophagy is a genetically controlled and evolutionarily conserved form of cell death, which is also related to occurrence and development of tumor, thereby has becoming a new tumor therapy method. Several cellular classical signaling pathways of natural plants target intracellular autophagy including Beclin-1, phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin pathway (PI3K/AKT/mTOR) and p53, have been known to control cell proliferation and apoptosis (183, 184). Beclin-1 and LC3 are two key markers of autophagy. Beclin-1 can enhance autophagy and induce apoptosis of tumor cells (185). p53 can induce autophagy by activating cathepsin D (CTSD), and further enhance the p53-mediated tumor suppression effect (186). In addition, p53 can activate the expression of transglutaminase 2 (TGM 2) to promote autophagy and tumor suppression functions, and TGM 2 contributes to the development of p53-induced autophagy program and the function of tumor suppressor (187). Moreover, autophagy is also regulated by p53 in the elimination of aberrant mitochondria. In radiation-resistant cancer cells, p53 induces mitophagy by increasing the levels of BCL 2 interacting protein 3 (BNIP 3) to remove abnormal mitochondria to maintain mitochondrial oxidative phosphorylation (OXPHOS) and inhibit the glycolytic pathway (188). Importantly, PI3K-AkT-mTOR signaling pathway is a well-characterized anti-apoptotic cascade in human cancers, since its activation leads to cell proliferation and cell survival. The inducing cell survival of this pathway was mediated by activating anti-apoptotic factors and inhibiting pro-apoptotic factors. AKT can induce anti-apoptotic effect through phosphorylating target proteins by a variety of pathways, such as prevent the apoptosis cascade through inhibiting caspase-9 activity (189). Natural products act by inducing autophagy and apoptosis of cells through down-regulating the expression of mTOR, AKT, PI3K and other proteins (190).
Hu et al. suggested that a combination of matrine with cisplatin promoted apoptosis through activating the caspase apoptosis pathway and inhibiting expression of survivin-related caspase-9 protein (191). A combination of tannic acid with cisplatin acts by inducing apoptosis through activating caspase-3 signaling cascade (192). Xu et al. revealed that saikosaponin D in combination with doxorubibin significantly induced autophagy and apoptosis of HepG2 cells. It suppressed cell proliferation and invasion through up-regulating of ROS, Oatp1b1 as well as TGF-β1 (193). Wang et al. showed that ginsenosides in combination with anti-cancer drugs regorafenib acted by inhibiting growth of HepG2 cells through regulating the expression of Survivin and Caspase-3 genes (194). Ginkgol C17:1 inhibited cisplatin-induced autophagy via AMP-activated protein kinase/ULK1signaling and increased cisplatin-induced apoptosis in HepG2 cells via the PI3K/AKT/mTOR signaling pathway (195). Astragaloside IV effectively protected against cisplatin-induced injury by inducing autophagy and limiting the expression of NLRP3 (196). Aloin in combination with metformin synergistically inhibited HepG2 and Bel-7402 cell proliferation and invasion and induced apoptosis and autophagy through activating PI3K/AKT/mTOR pathway in vitro. In addition, it has a synergistic effect by inhibiting growth and promoting apoptosis and autophagy in HepG2 xenograft mice model (197). Sinapic acid combined with cisplatin inhibits proliferation and migration of HepG2 and SMMC-7221 cells. At the same time, this combination therapy induces autophagy by upregulating the protein expressions of LC3II, Beclin-1 and Atg5, and downregulating the expression of p62 (198). The combination of 6-shogaol and 5-FU inhibit AKT/mTOR/MRP1 signaling pathway and induce liver cancer cell apoptosis by decreasing the expression of AKT, mTOR, MRP1 and cyclin-related proteins (199).
With the development of nanotechnology, nano-drug delivery system can improve drug solubility improve drug stability, increase drug targeting, reduce drug toxicity and side effects. Ceria Nanoparticles can kill cancer cells through induce cancer cells to produce ROS, reduce the expression level of various antioxidant enzymes, cause mitochondrial damage and apoptosis (200). Xu et al. synthesized a composite material DCQ which dextran-coated cerium oxide nanoparticlesloaded quercetin, and discovered that the composite material was more toxic to HepG2 cells, and had no obvious toxic effect on normal cells, human umbilical vein endothelial cells (HUVEC). The composite material can induce the production of ROS, inhibit the fusion of autophagosomes and lysosomes in HepG2 cells and cause the accumulation of autophagosomes, block autophagy and promote apoptosis of HepG2 cells (201). Dong et al. synthesized a nano-layered double hydroxide (NLDH) co-loaded fluorouracil (Fu) and curcumin (Cur) mixed dosage form (LDH-Fu-Cur, LFC). Compared with pure fluorouracil, fluorouracil plus curcumin, and LDH-Fu, LDH-Fu-Cur more efficiently inhibited the growth and promoted the apoptosis of 7721, LM3 and Hep G2 cell lines. It also could significantly down-regulate the Bcl-2 gene expression of 7721 cells and induce cell apoptosis by activating caspase 3 and caspase 9 (202). Moreover, han explored a unique amphiphilic PCL-AuNC/Fe (OH)3-PAA Janus nanoparticle (JNP) to simultaneously preserve the hydrophilic drug (doxorubicin) and hydrophobic drug (docetaxel) in their distinct domains. It realized the optional sequential drug release by a single inorganic JNP for the first time, and the simultaneous release of the two drugs can improve efficacy and reduce toxic side effects. Furthermore, the mice treated with dual drug loaded PCL-AuNC/Fe (OH)3-PAA JNPs under near infrared (NIR) laser irradiation showed better tumor inhibition than solo drug, cocktail and dual drug treated groups, induced aopotosis and blocked the cell cycle of HepG2, and inhibited the growth of tumor in vivo, indicated the effectivity and significance of combined cancer therapy (203). To improve the utilization of curcumin (CUR) and 5-fluorouracil (5-FU) chemotherapeutic drugs in the treatment of liver tumors, increase the accumulation of drugs in tumor sites and reduce the side effects of drugs on the systemic system, Ni Wenfeng constructed a novel targeting nanocarrier to transport the drug to the liver tumor site. In their study, an amphiphilic triblock PEG-PLGA-PEG copolymer was coupled with two targeting ligands (BIO and LAC) to prepare dual-targeted nanoparticles (BLPPNPs), which encapsulated curcumin (CUR) and 5-FU. And the mechanism of synergistic anti-hepatocarcinogenesis of the two drugs may be that CUR down-regulates the expression of DPYP protein by up-regulating the expression of p53 protein, increasing the cytotoxicity of 5-FU and enhancing the anti-tumor effect. At the same time, CUR may decrease the expression of Bcl-2 protein, increase the release of cyt c, and promote the apoptosis of hepatoma cells (204). Zhang successfully prepared a kind of multifunctional nanoparticles: Janus-magnetic mesoporous silica nanoparticles (Fe3O4-MSNs), with the surface modified by hyaluronic acid (HA) and nano fluorescent probe quantum dots (QD), and chemotherapy drug doxorubicin and berberine (ber), Fe3O4-MSN/Dox+Ber@HA-QD. The in vitro experiments showed that the nanoparticles could selectively enter HepG2 cells through the CD44 receptor mediated endocytosis pathway and release dox and rer rapidly, which had better cytotoxic effects at low concentrations. The in vivo results also suggested that the nanoparticles have a significantly lower recurrence of the hepatocellular carcinoma induced by dox and significantly reduced the toxic and side effect of doxorubicin while having a better antitumor effect than other groups. In addition, the nanoparticles can also achieve MRI and fluorescence imaging of the tumor site, providing the possibility of drug delivery and therapeutic effects (205). Table 2 summarizes their effects and mechanisms on liver cancer.
Table 2.
Effect and mechanism of plant-derived natural products combination Chemotherapy drugs.
| Combination Therapy | Concentration | Cell Line/Model | Mechanism | Reference |
|---|---|---|---|---|
| Kanglite injection/ Thalidomide |
kanglite injections: 200 mL/d thalidomide: 100 mg/d |
76 patients of primary liver cancer | Inhibited blood vessels through inhibiting VEGF and B-FGF induced angiogenesis, and regulate cellular immunity of the body | (166) |
| Liuwei Dihuang/ Cisplatin |
liuwei dihuang: 100 mg/mL cisplatin: 1 mg/mL |
BEL-7402 | Downregulated the expressions of VEGF and Ang-2, upregulated the expression of TSP and TIMP-2 | (167) |
| Astragalus polysaccharide/ Cisplatin/ Doxorubicin |
astragalus polysaccharide: 50-200 mg/kg cisplatin: 2 mg/kg doxorubicin: 6 mg/kg |
HepG2 tumor-bearing mice | Reduced the expressions of Ki-67, HIF-1α and VEGF | (168) |
| Radix astragali/ Curcuma/ Cisplatin |
radix astragali: 3-12 g/kg/d curcuma: 3-12 g/kg/d cisplatin: 2 mg/kg |
HepG2 tumor-bearing mice | Down-regulated the expression of CD147 and In-OS | (169) |
| Astragaloside IV/ Curcumin/ Cisplatin |
astragaloside IV: 12 g/kg/d curcumin: 10 g/kg cisplatin: 2 mg/kg |
HepG2 tumor-bearing mice | Inhibited the formation of new blood vessels in liver cancer by down-regulating the expression of HIF-1α and VEGF | (170) |
| Epigallocatechin gallate/Derivative Y6/ Daunrrubicin |
EGCG: 40 mg/kg Y6: 27.5-110 mg/kg daunrrubicin:2 mg/kg |
HepG2 tumor-bearing mice | Inhibited MAPK/ERK and PI3K/AKT signaling pathways and down-regulated the expression of HIF-1 α, CBR1 and VEGF | (171) |
| Curcumin/ Doxorubicin |
DOX/Cur-NPs: 2 mg/kg | Liver cancer mice | Decreased the mRNA levels of MDR1, Bcl-2 and HIF-1α, and protein levels of P-gP, Bcl-2 and HIF-1α | (172) |
| Mannose/ Cisplatin |
mannose: 25 mmol/L cisplatin: 10 µmol/L |
HepG2 | Inhibited proliferation and migration and changed morphology with separation, shedding and aggregation | (177) |
| Emodin/ Cisplatin |
emodin: 6.25-50 µg/mL cisplatin: 2.5 µg/mL |
HepG2 | Up-regulated the expression of e-cadherin, and inhibited EMT | (178) |
| Kaempferol/ Doxorubicin |
kaempferol: 0-40 mmol/L doxorubicin: 300-1200 nmol/L |
Huh-7, Huh-1, HepG2 HepG2.2.15 SK-Hep-1 PLC/PRF/5 HLE, HLF, Hep3B |
Inhibited PI3K/mTOR/MMP protein pathway in a dose-and time-dependent manner153 | (179) |
| Sinosinine thiocyanate/ Gemcitabine |
sinosinine thiocyanate: 50 µmol/L gemcitabine: 0-20 µmol/L |
HepG2 | Inhibited the expression of multidrug-resistant related proteins ABCB1 and ABCG2,155 enhance the sensitivity to gemcitabine | (180) |
| Caffeine/ 5-fluorouracil |
caffeine: 0.5-1 mmol/L 5-fluorouracil: 0-50 µmol/L |
SMMC-7221 HepG3B |
Increased the e156xpression of cleaved PARP, p-JNK, p-p38, and decreased the expression of Bcl-2, Bcl-xL, CDK2/4/6, p-ERK, p38, via inducing generation of ROS and influencing MAPK signaling pathway, | (181) |
| Curcumin/ Metformin |
curcumin: 5-10 µmol/L metformin: 10 mmol/L |
HepG2 PLC/PRF/5 |
Inhibted the expression of VEGF, MMP2/9, and VEGFR-2 | (182) |
| Matrine/ Cisplatin |
matrine: 100 mg/kg cisplatin: 2 mg/kg |
HepG2 nude mice | Activated the caspase apoptosis pathway and inhibiting expression of Survivin-related caspase-9 protein | (188) |
| Tannic acid/ Cisplatin |
tannic acid: 90-540 µmol/L cisplatin: 0.6-3.6 µg/L |
HepG2 | Induced apoptosis through activating caspase-3 signaling cascade | (192) |
| Saikosaponin D/ Doxorubibin |
saikosaponin D: 2-16 µg/mL doxorubibin: 0.5-16 µg/mL |
H22 mice model | Suppressed cell proliferation and invasion through up-regulating of ROS, Oatp1b1 as well as TGF-β1 | (193) |
| Ginsenoside/ Regorafenib |
ginsenoside: 10 mg/L regorafenib: 1 mg/L |
HepG2 | Regulated the expression of Survivin and Caspase-3 genes | (194) |
| Ginkgol C17:1/ Cisplatin |
ginkgol C17:1: 0-80 µg/mL cisplatin: 2 µg/mL |
HepG2 | Induced AMP-activated protein kinase/ULK1signaling and the phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin pathway | (195) |
| Astragaloside IV/ Cisplatin |
astragaloside IV:40-80 mg/kg cisplatin:15 mg/kg |
Liver cancer SD rats | Induced autophagy and limiting the expression of NLRP3 | (196) |
| Aloin/ Metformin |
aloin: 50 μmol/L metformin: 400 μmol/L |
HepG2 Bel-7402 |
Activated PI3K/AKT/mTOR pathway | (197) |
| Sinapic acid/ Cisplatin |
sinapic acid: 0-2,000 μmol/L cisplatin: 5 μmol/L |
HepG2 SMMC-7721 |
Upregulated the protein expressions of LC3II, Beclin-1 and Atg5, and downregulated the expression of P62 | (198) |
| 6-shogaol/ 5-fluorouracil |
6-Shogaol: 10 μmol/L 5-fluorouracil: 10 μmol/L |
HepG2 Li-7 |
Inhibited AKT/mTOR/MRP1 signaling pathway and decreased the expression of AKT, mTOR, MRP1 and cyclin-related proteins | (199) |
| Quercetin/ Ceria nanoparticles |
DCQ: 33-165 μmol/L | HepG2 | Induced the production of ROS, inhibited the fusion of autophagosomes and lysosomes in HepG2 cells and caused the accumulation of autophagosomes, blocked autophagy and promoted apoptosis of HepG2 cells | (201) |
| Curcumin/ Fluorouracil |
LFC: 15-35 µg/mL | LM3 Hep G2 7721 |
Down-regulated the Bcl-2 gene expression of 7721 cells and induced cell apoptosis by activating caspase 3 and caspase 9 | (202) |
| Docetaxel/ Doxorubicin |
PCL-AuNC/Fe(OH)3-PAA JNPs: 1.56-100 µg/mL | HepG2/H22 BALB/c tumor-bearing mice | Induced apoptosis and blocked the cell cycle of HepG2, and inhibited the growth of tumor in vivo | (203) |
| Curcumin/ 5-fluorouracil |
combined feeding ratio: 1:2.5 (5-FU: CUR) | HepG2 HL7702 HepG2 tumor-bearing nude mice |
Down-regulated the expression of DPYP protein by up-regulating the expression of p53 protein, increasing the cytotoxicity of 5-FU and enhancing the anti-tumor effect, decreased the expression of Bcl-2 protein, increased the release of cyt c, and promoted the apoptosis of hepatoma cells | (204) |
| Berberine/ Doxorubicin |
Fe3O4-MSN/Dox+Ber@HA-QD: 4 mg/kg | HepG2 H22 ICR tumor-bearing mice |
Reduced the toxic and side effect of doxorubicin while having a better antitumor effect than other groups | (205) |
Anti-liver cancer mechanism of plant-derived natural products in combination with immunotherapy
The immune system of the body can recognize and eliminate foreign invading antigens, mutated cells and senescent cells of the body, and maintain the stability of the internal environment. However, tumor cells have the ability to escape immune surveillance, immune identification, and eradication, and immune system dysfunction lead to the development of malignant cells with clinical manifestationsb (206). According to the immune escape mechanism of tumor cells, who established a variety of immunotherapies of cancer (207). This kind of breakthrough therapy is defined that regulates immunological response through activating the organism’s immune defense system or action of biological compounds to suppress and prevent tumor growth. At present, the commonly immunotherapy methods include immune checkpoint blockade therapy (208), antibody therapy (209), tumor vaccine therapy (210). Cancer immunotherapy has become an irreversible trend herald in the field of cancer therapy and is regarded as the fourth type of anti-tumor treatment after surgery, radiotherapy, and chemotherapy due to the obvious efficacy and low side effects. Although immunotherapy has made significant progress in the treatment of liver cancer, it needed to be further improved the clinical efficacy, find more specific immune targets, while avoid unnecessary targeting and off-target toxicity (211). Therefore, a combination of natural products and tumor immunotherapy can learn from each other and coordinate with each other to improve the effective rate of treatment.
Regulate immune function
It has been known that immune function of body is closely related to the occurrence and development of tumors. As host immune function is low or suppressed, the incidence of tumor will be increased. And in the progressive growth of tumor, the immune function will also be inhibited by the tumor. These two factors are mutual causation and the fluctuation directly affects the occurrence and development of tumor. Tumor microenvironment is an extremely complex system that involves several kinds of multifunctional immunizing cells and molecules, as well as tumor cells. It is a dynamic system composed of cancer cells, cytokines, extracellular matrix and immune cell subsets (212). Formation of Individual tumor is likely to develop because the tumor-associated microenvironment promotes tumor cell growth and protects tumor cells from elimination of systemic effector cells (213). Natural products in combination with immunotherapy act by inducing antitumor effects through restoring immune recognition and immune elimination by regulating the immune function.
Hu et al. revealed that Lycium barbarum polysaccharide in combination with cytokine CXCL10 acts by improving immune function against liver cancer. It also promotes secretion of Th1 cytokines and restores balance of Thl/Th2, improving number and function of dendritic cells (DC) and intervening immune suppression state of tumor-bearing bodies (214). Chen et al. demonstrated that a combination of anti-PD-L1 antibody and high concentration of vitamin C acts by promoting the expression of PD-L1 in vivo and in vitro and enhance the efficacy of anti- PD-L1 antibody. It can promote liver tumor blood vessels normalization and eventually increase T cell infiltration through activating cyclic GMP-AMP synthase (cGAS) and promoting the secretion of its product cGAMP, and then activating the STING pathway of vascular endothelial cells (215). Song et al. proved that a combination of 10-hydroxycamptotenin and DC hepa-6 fusion vaccine acts by inducing improving immune function and significantly inducing CTL cytotoxicity, and resisted against the rechallenge of Hepal-6 cells (216). Yang et al. reported that Chinese herb medicine combine with targeted therapy of TCM immune for patients with end-stage liver cancer, after treatment, improved symptoms of abdominal distension and jaundice, nutritional status and quality, alleviated the pain, and prolonged overall survival time (217). Yu et al. suggested that sorafenib combined with Poria Lingsini decoction can significantly improve immune function and the survival outcome of patients with advanced primary liver cancer (218).
Induce liver cancer cell apoptosis
Endoplasmic reticulum (ER) stress results from the accumulation of unfolded or misfolded proteins in the ER, is involved in regulating apoptotic process in tumor cells (219). It will trigger endogenous and exogenous apoptotic signals as ER dysfunction persists in eukaryotic cells, in addition, the heavy and continuing endoplasmic reticulum stress can lead to cell dysfunction and even cell death. When endoplasmic reticulum stress (ERS) occurs in cells, the expression of anti-apoptotic molecule BiP (immunoglobulin binding protein) and pro-apoptotic molecules, such as CHOP and caspase-12, is regulated to determine whether the cells will undergo apoptosis or survive to adapt to the environment (220). Plant-derived natural products in combination with immunotherapy can induce apoptosis of tumor cells.
Jin et al. prepared paclitaxel-loaded nanoparticles decorated with anti-CD133 antibody, which could effectively target liver cancer stem cells and induce apoptosis of liver cancer cells (221). Wang et al. demonstrated that paclitaxel in combination with anti-human stathmin1 antibody inhibits proliferation and induces apoptosis of liver cancer cells (222). Lin et al. found that the combination of taxol and immunosuppressant cyclosporin A increases the expression of caspase-9 and caspase-3, induces apoptosis of liver cancer cells through PI3K-mTOR pathway, and effectively reverses drug resistance of paclitaxel (223). Huang et al. confirmed that a combination of gambogic acid and proteasome inhibitors induces liver cancer cell apoptosis and inhibits growth through inducing cytotoxicity and enhancing proteasome inhibition and ER stress (224). A combination of n-glycosylation inhibitor tunicamycin and camptothecin acts by inducing resistance through up-regulating GRP78 and blocking G1 phase cell cycle (225). Nicotinamide and STAT3 inhibitor significantly induced apoptosis and inhibited proliferation by reducing phosphorylation of STAT3 Y705 in HepG2 cells, down-regulating the expression of SNAIL1, VEGFA and ZEB1, EMT-related gene and glucose metabolism. Moreover, the synergistic effect is more significant (226).
Photodynamic therapy (PDT), with its unique advantages of minimally invasive, good selectivity, high safety and unique efficacy, has been used as a new therapeutic method for liver tumor treatment. As a natural product, hypocrellin B (HB) can be used as a photosensitive agent for PDT treatment of many diseases, especially for tumor treatment. In order to improve poor water solubility, fast metabolism in vivo and no specific tissue distribution of HB, zhang collected liver cell membrane (CCCM) and modified tumor-targeting ligand (transferrin, TF) into homologous liver cancer cell membrane (TF-CCCM), and prepared ethylene nanoparticles (HB NPs) by double emulsion method. Then TF-CCCM-HB NPs was prepared by extrusion method by mixing the two in a certain proportion. TF-CCCM-HB NPs has strong targeting ability and phagocytosis in homologous liver cells, and suppress the proliferation of tumor cells in a dose-dependent manner. Moreover, it has a synergistic effect with PDT, which can promote the production of ROS and reduce mitochondrial membrane potential. At the same time, TF-CCCM-HB NPs combined with PDT can inhibit the growth of tumor in mice model with liver cancer, prolong the survival time of tumor-bearing mice, and inhibit tumor metastasis and recurrence, which has good biosafety (227). Bai prepared a form drug/gene co-loaded nanoparticles (sCDP/DOX/miR-122), which a nanosystem based on β-cyclodextrin-cored star polymer (sCDP) and co-deliver antitumor drug doxorubicin (DOX) and miRNA-122. s CDP/DOX/miR-122 nanoparticles can remarkably inhibit HepG2 cell proliferation, promote cell apoptosis and increase the expression of p53 and cleaved caspase 3; it also can downregulate the expression of Bcl-w and CCNG1 leading to irreversible cell apoptosis, decrease the expression of MDR1, MRP and P-gp, and improve the sensitivity to DOX of HepG2 cells, thus realizing the synergistic antitumor effect in vitro. sCDP/DOX/miR-122 nanoparticles can slow down the weight loss of nude mice bearing tumor and significantly inhibit tumor growing, thus realizing a synergistic anti-tumor effect in vivo (228). Yuan synthesis iron oxide nanoparticles loaded polydopamine (PDA) and doxorubicin (Dox). Rats were treated with hepatic artery via interventional methods, and it was verified that Fe2O3-PDA-Dox nanoparticles can simultaneously have the ecceft of Transcatheter arterial chemoembolization (TACE) and photothermal ablation (PTA). It also confirmed that combretastatin A-4 phosphate disodium (CA4P) can increase the uptake of Fe2O3-PDA-Dox nanoparticles by rat hepatocellular carcinoma, which can enhance the combined treatment of transcatheter arterial chemoembolization and photothermal ablation without additional increase in liver and kidney toxicity (229).
Reduce liver cancer cells metastasis
Plant-derived natural products in combination with immunotherapy act by inhibiting tumor cell proliferation and metastasis through inhibiting angiogenesis and arresting cell cycle. Lai Chunhui prepared nanoparticles which mannose (M)/CpG oligodeoxynucleotide (CpG-ODN)-conjugated liposomes lipo loaded with tumor-associated antigens. It specifically induced the activation and maturation of DCs in vivo and the activated DCs stimulated effector cells to kill tumor cells in mice, thereby achieving the effect of anti-tumor immunotherapy (230). Zhong et al. revealed that tubacin, an inhibitor of histone deacetylase 6 (HDAC6), in combination with docetaxel, could arrest the cell cycle, inhibit metastasis and proliferation, as well as induce apoptosis of liver cancer cells (231). Shen et al. found that evocorine in combination with autophagy inhibitor chloroquine (CQ) acts by inhibiting angiogenesis of HepG2 cells through inducing autophagy. It can decrease the expression of VEGFA and inhibit invasion (232). Lai et al. demonstrated that evodiamine or paclitaxel in combination with CDK1 inhibitors acts by inducing the apoptosis of hepatocarcinoma cells through arresting G2/M phase cell cycle. Evodiamine in combination with R03306 have a synergistic effect on upregulating the expression of cyclin E and decreasing the expression of cyclin B1. Moreover, paclitaxel combined with R03306 can decrease the expression of Bcl-2 and cyclin B1, increase the expression of Bax, thereby inducing apoptosis in HepG2 cells (233). Compound kushen injection significantly enhanced the anticancer activity of sorafenib at a subclinical dose without obvious side effects, and prevented the postsurgical recurrence and rechallenged tumor growth. It activated proinflammatory responses and relieved immunosuppression of tumor-associated macrophages in the liver cancer cell microenvironment by triggering tumor necrosis factor receptor superfamily member 1 (TNFR1)-mediated NF-κB and p38 MAPK signaling cascades, which subsequently resulted in apoptosis of liver cancer cells (234). Table 3 summarizes their effects and mechanisms on liver cancer.
Table 3.
Effect and mechanism of plant-derived natural products in combination with immunotherapy.
| Combination Therapy | Concentration | Cell Line/Model | Mechanism | Reference |
|---|---|---|---|---|
| Lycium barbarum polysaccharide/ CXCL10 |
lycium barbarum polysaccharide: 100 mg/kg CXCL10: 15 µg/kg |
H22 mice model | Improved immune function and promoted secretion of Th1 cytokines and restore balance of Thl/Th2, improved number and function of DC cells and intervened immune suppression state of tumor-bearing bodies | (214) |
| Vitamin C/ Anti-PD-L1 antibody |
vitamin C: 4 g/kg Anti-PD-L1 antibody: 75 µg/3day |
Hepa1-6 cancer mice | Activated cyclic GMP-AMP synthase (cGAS) and promoted the secretion of its product cGAMP, and then activated the STING pathway of vascular endothelial cells | (215) |
| 10-hydroxycamptothecinc/DC hepa1-6fusion vaccine | 10-hydroxycamptothecinc: 50-100 µg/mL | Hepa1-6 cancer mice | Induced improving immune function and inducing significantly CTL cytotoxicity | (216) |
| Chinese herb medicine/ Camrelizumab/ Lenvatinib |
camrelizumab: 200 mg/3weeks lenvatinib: 8 mg/day |
Advanced liver cancer patient | Improved symptoms of abdominal distension and jaundice, nutritional status and quality, alleviated the pain, and prolonged overall survival time | (217) |
| Fuling sini decoction/Sorafenib | sorafenib: 800 mg/day | Liver cancer patient | Improved the survival outcome of patients with advanced primary liver cancer, improve immune function | (218) |
| Paclitaxel/ Anti-CD133 antibody |
nanoparticles: 7.51-56.92 ng/mL |
Huh-7 HepG2 |
Targeted liver cancer stem cells and induced apoptosis | (221) |
| Paclitaxel/ Anti-human stathmin1antibody |
paclitaxel: 0.1-1.6 µg/mL anti-human stathmin l antibody: 10-160 µg/mL |
HepG2 | Inhibited proliferation and induced apoptosis | (222) |
| Taxol/ Cyclosporin A |
taxol: 0.1 µmol/L cyclosporin A: 0-10 µmol/L |
Hep3B HepG2 HA22T VGH Hepa 1-6 |
Increased the expression of caspase-9 and caspase-3, induced apoptosis of liver cancer cells through PI3K-mTOR pathway, and effectively reversed drug resistance of paclitaxel | (223) |
| Gambogic acid/ Bortezomib |
gambogic acid: 0.4-0.6 µmol/L bortezomib: 40-60 nmol/L |
HepG2 H22 |
Induced cytotoxicity and enhanced proteasome inhibition and ER stress | (224) |
| Camptothecin/ Etoposide/ Tunicamycin |
camptothecin:3 μmol/L etoposide: 5 μmol/L tunicamycin: 1 μg/mL |
Hep3B | Induced resistance through up-regulating GRP78 and block G1 phase cell cycle | (225) |
| Nicotinamide/ STAT3 inhibitor |
nicotinamide: 5 mmol/L S3I-201:100 µmol/L stattic: 1.5 µmol/L |
HepG2 | Reduced phosphorylation of STAT3 Y705, down-regulated the expression of SNAIL1, VEGFA and ZEB1, and decreased epithelial-mesenchymal transition and glucose metabolism | (226) |
| Hypocrellin B/ Transferrin/ Membrane |
TF-CCCM-HB-NPs: 0-100 µg/mL | HepG2 LO2 HepG2 tumor-bearing mice model |
Promoted the production of ROS and reduced MMP, inhibited the growth of tumor in mice model with liver cancer, prolonged the survival time of tumor-bearing mice | (227) |
| Doxorubicin/ miRNA-122 |
sCDP/DOX/miR-122: 2.1 µg/mL | Hep G2 Liver nude mice bearing tumor |
Increased the expression of p53 and cleaved caspase-3, downregulated the expression of Bcl-w and CCNG1 leading to irreversible cell apoptosis, decreased the expression of MDR1, MRP and P-gp, and improved the sensitivity to DOX of HepG2 cells | (228) |
| Doxorubicin/ Combretastatin A-4 phosphate disodium |
Fe2O3-PDA-Dox: 0.4 mg/mL CA4P: 0.1 mg/mL |
Liver tumor rats model | Enhanced the combined treatment of transcatheter arterial chemoembolization and photothermal ablation without additional increase in liver and kidney toxicity | (229) |
| Mannose/ Tumor-assoclated antigens |
M/CpG-ODN-H22-Lipo: 100 µL/mice |
H22 bearing mice model | Induced the activation and maturation of DCs in vivo and the activated DCs stimulated effector cells to kill tumor cells in mice | (230) |
| Docetaxel/ Tubacin |
docetaxel: 0-32 µmol/L tubacin: 10 µmol/L |
SNU449 SNU387 |
Arrested the cell cycle, inhibit metastasis and proliferation, as well as induce apoptosis of liver cancer cells | (231) |
| Evodiamine/ Chloroquine |
evodiamine: 10 μmol/L chloroquine: 25 μmol/L |
HepG2 | Inhibited angiogenesis through inducing autophagy, and decreased the expression of VEGFA and inhibited invasion | (232) |
| Evodiamine/ Paclitaxel/ CDK1 inhibitors |
evodiamine:1-4 μmol/L paclitaxel: 0.2 μmol/L R0306: 2 μmol/L |
HepG2 | Decreased the expression of Bcl-2 and cyclin B1, increased the expression of Bax and cyclin E | (233) |
| Compound kushen injection/ Sorafenib |
compound kushen injection: 0.43-1.32 mg/mL sorafenib: 10, 30 mg/kg |
Hepa1-6 tumor nude mice | Activated proinflammatory responses and relieved immunosuppression of tumor-associated macrophages in the liver cancer cell microenvironment by triggering tumor necrosis factor receptor superfamily member 1 (TNFR1)-mediated NF-κB and p38 MAPK signaling cascades | (234) |
Conclusion
The high mortality and morbidity of liver cancer remain a primary challenge for scientific research. Although traditional therapies, such as surgical resection, radiotherapy and chemotherapy, are effective for liver cancer at early stage, the five-year survival rate of liver cancer patients is meager and often ends in failure. In recent years, plant derived natural products and their secondary metabolites possess characteristics of abundant products, low toxicity and side effects, diverse biologic activities, and high content of active ingredients. It has been considered as the most promising candidates for oncology therapies. However, due to poor water solubility, difficult extraction, and easy to develop drug resistance, a single treatment may not be able to achieve satisfactory efficacy. Therapies that combine natural products with chemotherapy drugs or immunotherapy have been developed to target a variety of cancer pathways. The dose requirement of each agent in combination therapy can be reduced, which reduces the side-effects compared to monotherapy. Targeting various pathways via multiple drug combinations can control disease preferably and decrease the chance of cancer cells becoming increasingly malignant and incurable. It can also target the heterogeneity of the tumor by two or more pathways to toxicity of cancer cells and disruption of their homeostasis (235). This review mainly focused on the mechanisms of plant-derived natural products and combination therapy against liver cancer. We have listed the mainly compounds and combination therapy and individually summarized their antitumour effects and mechanisms ( Figure 2 ). Like many other cancers, liver cancer arises as a result of accumulation of multiple genetic mutations that cause abnormal cellular proliferation. Our study reviewed natural products and different combination therapy that have antitumor effects on liver cancer, which showed possible benefits in treating patients with liver cancer through mechanisms, such as activation of continuing endoplasmic reticulum stress, suppression of migration, inhibition of cell proliferation, induction of apoptosis and autophagy ( Figure 3 ). Therefore, it could provide effectively alternative or adjuvant treatment strategies for cancer patients.
Figure 2.
Mechanisms of natural products and combination therapy against liver cancer. Alkaloids could inhibits migration through up-regulating TGF-β/Smad and miR-199a-5p, and down-regulating HIF-α expression. Terpeniods promotes apoptosis by inhibiting the PI3K/AKT pathway and the expression of caspase3. It also induces autophagy through up-regulating the expression of LC3II and Beclin-1. Ployphenols inhibits Cyclin D1 and CDK1/4 expression, which in turn block the cell cycle. It also induces apoptosis by activating ATF6/CHOP signaling pathway. Flavonoids induces apoptosis through activation of the ATF6/CHOP pathway and upregulation the ratio of Bax/Bcl-2. Moreover, other natural products inhibit migration through down-regulating VEGFR2-AKT-ERK1/2 signaling pathway and suppressing EMT. Combination of Natural product ingredients inhibits Cyclin D1 and CDK1/4 expression through downregulation of the Wnt/β-catenin pathway, which in turn inhibits cell proliferation. It also reverses multidrug resistant by decreasing the expression of P-gP, MRP and BCRP. Natural product in combination with chemotherapy suppress angiogensis through down-regulating VEGF and Ang expression. It also induces autophagy by upregulating expression of LC3ii and Beclin-1. Natural product in combination with immunotherapy act by inducing antitumor effects through restoring immune recognition and immune elimination by regulating the immune function. It also induces apoptosis by inhibiting the PI3K/AKT and p38MAPK pathway, which in turn induces apoptosis.
Figure 3.
Overview of signaling pathways against liver cancer. Plant-derived natural products and different combination therapy can achieve practical anti-liver-cancer effects through multiple mechanisms, including activation of continuing endoplasmic reticulum stress, suppression of migration, inhibition of cell proliferation, induction of apoptosis and autophagy. For example, it could promote apoptosis through the heavy and continuing endoplasmic reticulum stress; and it coula promote the expression of ATF6, PERK, eIF2α, p-eIF2α, ATF4, CHOP. It also could suppress migration through increasing the concentration of intracellular calciumions, reduce transcriptional activities of MMP-2 and MMP9 and VEGF to inhibit metastasis. Besides, it could increase the expression of Bcl-x; downregulating the expression of Bcl-xL and Bcl-2; suppressing the PI3K/AKT/mTOR signaling pathway and caspase cascade reaction. In the next place, combination therapy could reduce cancer cell proliferation by binding PI3K and subsequently suppressing PKB function and regulating MAPK signaling pathway. It also coula induce apoptosis through activation of STAT3 via inhibiting the phosphorylation of mTOR. Moreover, it could promote aopotosis through caspase cascade reactor and. In addition, autophagy is also related to occurrence and development of tumor, natural plants target intracellular autophagy including Beclin-1, PI3K/AKT/mTOR, have been known to control cell proliferation and apoptosis.
However, it is important to note that the study also revealed some disadvantages of combination therapy for cancer. Multiple drug combinations can synergistically increase efficacy, but may also produce unnecessary side effects (236). The target molecular mechanisms of most natural products have not been fully elucidated, especially those related to combinations. Researchers need to further systematically and comprehensively study the immunity or molecular pathology of liver cancer to clarify the relevant mechanisms. Meanwhile, there is still a considerable long way in developing the antitumor strategy of combining natural products with other methods.
Author contributions
YW: Conceptualization, Data curation, Formal analysis, Investigation, Writing-original draft, Visualization. JL: Writing-review & editing, Supervision. LX: Writing-review & editing, Supervision. All authors contributed to the article and approved the submitted version.
Funding Statement
The work was financially supported by the National Natural Science Foundation of China, China (NSFC) (Grant No: 31860258, and 32060229).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1116532/full#supplementary-material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Su TS, Lu HZ, Cheng T, Zhou Y, Huang Y, Gao YC, et al. Long-term survival analysis in combined transarterial embolization and stereotactic body radiation therapy versus stereotactic body radiation monotherapy for unresectable hepatocellular carcinoma >5 cm. BMC Cancer (2016) 16(1):834. doi: 10.1186/s12885-016-2894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom Radiol (NY) (2018) 43(1):13–25. doi: 10.1007/s00261-017-1209-1 [DOI] [PubMed] [Google Scholar]
- 4. Zaheer J, Kim H, Lee YJ, Kim JS, Lim SM. Combination radioimmunotherapy strategies for solid tumors. Int J Mol Sci (2019) 20(22):5579. doi: 10.3390/ijms20225579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hindson J. Combined TACE and sorafenib for HCC treatment. Nat Rev Gastroenterol Hepatol (2020) 17(2):66. doi: 10.1038/s41575-020-0265-0 [DOI] [PubMed] [Google Scholar]
- 6. Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency ablation and microwave ablation in liver tumors: An update. Oncologist (2019) 24(10):e990–e1005. doi: 10.1634/theoncologist.2018-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, et al. Liver transplantation outcomes in a U.S. multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond Milan criteria. Hepatology (2020) 72(6):2014–28. doi: 10.1002/hep.31210 [DOI] [PubMed] [Google Scholar]
- 8. Xia F, Wu LL, Lau WY, Huan HB, Wen XD, Ma KS, et al. Adjuvant sorafenib after heptectomy for Barcelona clinic liver cancer-stage c hepatocellular carcinoma patients. World J Gastroenterol (2016) 22(23):5384–92. doi: 10.3748/wjg.v22.i23.5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol Res (2015) 45(10):E1–E11. doi: 10.1111/hepr.12459 [DOI] [PubMed] [Google Scholar]
- 10. Yang MD, Sun Y, Zhou WJ, Xie XZ, Zhou QM, Lu YY, et al. Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer MDA-MB-231 cell models. In Vivo In Vitro Molecules (2021) 26(8):2204. doi: 10.3390/molecules26082204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin M, Wang Y, Ren Q, Guo Y. Formononetin and metformin act synergistically to inhibit growth of MCF-7 breast cancer cells in vitro . BioMed Pharmacother (2019) 109:2084–9. doi: 10.1016/j.biopha.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 12. Piao XM, Gao F, Zhu JX, Wang LJ, Zhao X, Li X, et al. Cucurbitacin b inhibits tumor angiogenesis by triggering the mitochondrial signaling pathway in endothelial cells. Int J Mol Med (2018) 42(2):1018–25. doi: 10.3892/ijmm.2018.3647 [DOI] [PubMed] [Google Scholar]
- 13. Wei T, Xiaojun X, Peilong C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. BioMed Pharmacother (2020) 121:109139. doi: 10.1016/j.biopha.2019.109139 [DOI] [PubMed] [Google Scholar]
- 14. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget (2017) 8(23):38022–43. doi: 10.18632/oncotarget.16723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pons-Fuster López E, Gómez García F, López Jornet P. Combination of 5-florouracil and polyphenol EGCG exerts suppressive effects on oral cancer cells exposed to radiation. Arch Oral Biol (2019) 101:8–12. doi: 10.1016/j.archoralbio.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 16. Panji M, Behmard V, Zare Z, Malekpour M, Nejadbiglari H, Yavari S, et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene (2021) 787:145638. doi: 10.1016/j.gene.2021.145638 [DOI] [PubMed] [Google Scholar]
- 17. Kamat AM, Tharakan ST, Sung B, Aggarwal BB. Curcumin potentiates the antitumor effects of bacillus calmette-guerin against bladder cancer through the downregulation of NF-kappaB and upregulation of TRAIL receptors. Cancer Res (2009) 69(23):8958–66. doi: 10.1158/0008-5472.CAN-09-2045 [DOI] [PubMed] [Google Scholar]
- 18. He YL, Yang W, Wang P, Li B, Zheng Y, Lu LX, et al. Experimental study on matrine promoting hepatoma cells apoptosis through ERK1/2 signaling pathway. Chin J Gastroenterol Hepatol (2022) 31(09):1045–9. [Google Scholar]
- 19. Dai M, Chen N, Li J, Tan L, Li X, Wen J, et al. In vitro and in vivo anti-metastatic effect of the alkaliod matrine from sophora flavecens on hepatocellular carcinoma and its mechanisms. Phytomedicine (2021) 87:153580. doi: 10.1016/j.phymed.2021.153580 [DOI] [PubMed] [Google Scholar]
- 20. Lin S, Zhuang J, Zhu L, Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int (2020) 20:246. doi: 10.1186/s12935-020-01293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Gao Y, Han H, Zou C, Feng Y, Zhang H. Matrine suppresses lung metastasis of human hepatocellular carcinoma by directly targeting matrix metalloproteinase-9. Biochem Biophys Res Commun (2019) 515(1):57–63. doi: 10.1016/j.bbrc.2019.04.063 [DOI] [PubMed] [Google Scholar]
- 22. Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J Cell Biochem (2010) 111(6):1426–36. doi: 10.1002/jcb.22869 [DOI] [PubMed] [Google Scholar]
- 23. Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing X, et al. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci (2011) 102(7):1287–92. doi: 10.1111/j.1349-7006.2011.01933.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CM, Zhang GZ, Liu PP, Huo YF, Li TL. Berberine inhibits TGF-β1-induced epithelial-mesenchymal transition in human liver cancer HepG2 cells via TGF-β/Smad pathway. Chin Pharmacol Bull (2020) 36(02):261–7. [Google Scholar]
- 25. Jia J, Kang X, Liu Y, Zhang J. Inhibition of human liver cancer cell growth by evodiamine involves apoptosis and deactivation of PI3K/AKT pathway. Appl Biol Chem (2020) 63(1):1–8. doi: 10.1186/s13765-020-00551-9 [DOI] [Google Scholar]
- 26. Zhao S, Xu K, Jiang R, Li DY, Guo XX, Zhou P, et al. Evodiamine inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells via the hippo-Yes-Associated protein signaling pathway. Life Sci (2020) 251:117424. doi: 10.1016/j.lfs.2020.117424 [DOI] [PubMed] [Google Scholar]
- 27. Liu Q, Zhao S, Meng F, Wang H, Sun L, Li G, et al. Nrf2 down-regulation by camptothecin favors inhibiting invasion, metastasis and angiogenesis in hepatocellular carcinoma. Front Oncol (2021) 11:661157. doi: 10.3389/fonc.2021.661157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma QQ. The inhibitory effect of hydroxycamptothecin combined with human cytokine-induced killer cells on the proliferation of hepatoma cells. Jilin Med J (2020) 41(04):773–6. [Google Scholar]
- 29. Diao YH, Liu L, Sha JP, Xue M. Vincristine regulates PI3K/Akt signaling pathway to inhibit the proliferation, migration, invasion of hepatocellular carcinoma cell line Hep3B and promote apoptosis. Chin J Int Tradit Wes Med Liver Dis (2019) 31(08):726–30. [Google Scholar]
- 30. Yu L, Wang ZC, Wang TJ. Inhibitory effect of caffeine combined with ionizing radiation on proliferation of hepatocellular carcinoma stem cells silenced by chk-1 and its apoptosis-induced effect. J Jilin Univ (Med Edition) (2018) 44(01):52–7. doi: 10.13481/j.1671-587x.20180110 [DOI] [Google Scholar]
- 31. Jin ZL, Yan W, Qu M, Ge CZ, Chen X, Zhang SF. Cinchonine activates endoplasmic reticulum stress-induced apoptosis in human liver cancer cells. Exp Ther Med (2018) 15(6):5046–50. doi: 10.3892/etm.2018.6005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res (2004) 64(7):2350–6. doi: 10.1158/0008-5472.can-2287-2 [DOI] [PubMed] [Google Scholar]
- 33. Zhang B, Wang X, Deng J, Zheng H, Liu W, Chen S, et al. p53-dependent upregulation of miR-16-2 by sanguinarine induces cell cycle arrest and apoptosis in hepatocellular carcinoma. Cancer Lett (2019) 459:50–8. doi: 10.1016/j.canlet.2019.05.042 [DOI] [PubMed] [Google Scholar]
- 34. Wang ZM, Wang M, Xiao HZ. Effect of mechanism of saikosaponin d on autophagy by regulating mTORC signaling pathway in human hepatocellular carcinoma cells. Chin Pharmaceut J (2018) 53(19):1652–7. [Google Scholar]
- 35. Wang B, Min W, Lin S, Song L, Yang P, Ma Q, et al. Saikosaponin-d increases radiation-induced apoptosis of hepatoma cells by promoting autophagy via inhibiting mTOR phosphorylation. Int J Med Sci (2021) 18(6):1465–73. doi: 10.7150/ijms.53024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu QX, Li HC, Qiao ZQ, Jia TY. Inhibitory of saikosaponin d on cell proliferation of HepG2 cells and tumor growth if liver cancer. Chin J Immunol (2018) 34(11):1664–8. [Google Scholar]
- 37. Li CY, Yang H. Triptolide induces apoptosis and autophagy in HepG2 cells by regulating the expression of miR-194. Jilin Med J (2022) 43(01):7–11. [Google Scholar]
- 38. Zhang N, Liu T, Chen D, Zeng CJ, Zeng J. Influence and the mechanisms of triptolide on growth of hepatocellular carcinoma cells. Contemp Med (2020) 26(24):103–5. [Google Scholar]
- 39. Gan CL, Zou YL, Huang XW, Xu JH. Effects of triptolide on proliferation, apoptosis and cycloxygenase-2 expression in hepatic cancer cell line H22. J Fujian Med Univ (2017) 51(03):146–149+165. [Google Scholar]
- 40. Pu Z, Ge F, Wang Y, Jiang Z, Zhu S, Qin S, et al. Ginsenoside-Rg3 inhibits the proliferation and invasion of hepatoma carcinoma cells via regulating long non-coding RNA HOX antisense intergenic. Bioengineered (2021) 12(1):2398–409. doi: 10.1080/21655979.2021.1932211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang M, Wang L, Liu W, Wang T, De Sanctis F, Zhu L, et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors. J Immunol Res (2022) 2022:2253436. doi: 10.1155/2022/2253436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng XY, Li RC, Rao Q, Gao QH, Duan YJ, Zhao G. Dihydroartemisinin inhibits proliferation and induces apoptosis of hepatocellular carcinoma HepG2 cells and its mechanism. Lishizhen Med Materia Med Res (2022) 33(09):2139–42. [Google Scholar]
- 43. Su Y, Zhao D, Jin C, Li Z, Sun S, Xia S, et al. Dihydroartemisinin induces ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid Med Cell Longev (2021) 2021:3456725. doi: 10.1155/2021/3456725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun Y, Song YY, Zhang C, Lu YZ, Zheng GH, Tian XX, et al. Effect and mechanism of celastrol on the proliferation of hepatocellular carcinoma HepG2 cells by activating AMPK signaling pathway. China Pharmacist (2021) 24(11):1961–1966+1982. doi: 10.19962/j.cnki.issn1008-049X.2021.11.001 [DOI] [Google Scholar]
- 45. Sheng L, Li X, Rao H, Zheng GH, Wang GH, Hu JJ. Celastrol delays hepatocellular carcinoma via downregulating NF-κB signaling pathway. Modernizat Tradit Chin Med Materia Medica-World Sci Technol (2021) 23(07):2372–9. [Google Scholar]
- 46. Deng L, Yao P, Li L, Ji F, Zhao S, Xu C, et al. p53-mediated control of aspartate-asparagine homeostasis dictates LKB1 activity and modulates cell survival. Nat Commun (2020) 11(1):1755. doi: 10.1038/s41467-020-15573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dong X, Gao H. Mechanisms of norcantharidin induced apoptosis and cycle arrest in human hepatocellular carcinoma Huh7 cells. Global Tradit Chin Med (2021) 14(08):1366–70. [Google Scholar]
- 48. Tan HY, Wang N, Tsao SW, Che CM, Yuen MF, Feng Y. IRE1α inhibition by natural compound genipin on tumour associated macrophages reduces growth of hepatocellular carcinoma. Oncotarget (2016) 7(28):43792–804. doi: 10.18632/oncotarget.9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu B, Sheng Y, Zhang J, Zheng Z, Ji L. The altered microRNA profile in andrographolide-induced inhibition of hepatoma tumor growth. Gene (2016) 588(2):124–33. doi: 10.1016/j.gene.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 50. Bai C, Zhao J, Su J, Chen J, Cui X, Sun M, et al. Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling. Life Sci (2022) 306(1):120804. doi: 10.1016/j.lfs.2022.120804 [DOI] [PubMed] [Google Scholar]
- 51. Sun J, Xu W, Lu RZ, Zhu XR, Xu XM, Jiang YQ, et al. Inhibitory effect of curcumin on human carcinoma SMMC-7721 cells xenografted in nude mice and its mechanism. J Jiangsu Univ (Med Sci) (2022) 32(03):219–225+230. doi: 10.13312/j.issn.1671-7783.y210066 [DOI] [Google Scholar]
- 52. Tian S, Liao L, Zhou Q, Huang X, Zheng P, Guo Y, et al. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol Lett (2021) 21(4):286. doi: 10.3892/ol.2021.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, et al. The role of resveratrol in cancer therapy. Int J Mol Sci (2017) 18(12):2589. doi: 10.3390/ijms18122589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Song F, Zhang Y, Pan Z, Zhang Q, Lu X, Huang P. Resveratrol inhibits the migration, invasion and epithelial-mesenchymal transition in liver cancer cells through up- miR-186-5p expression. Zhejiang Da Xue Xue Bao Yi Xue Ban (2021) 50(5):582–90. doi: 10.3724/zdxbyxb-2021-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dai H, Li M, Yang W, Sun X, Wang P, Wang X, et al. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging (Albany NY) (2020) 12(12):11717–31. doi: 10.18632/aging.103338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El-Melegy MG, Eltaher HM, Gaballah A, El-Kamel AH. Enhanced oral permeability of trans-resveratrol using nanocochleates for boosting anticancer efficacy; in-vitro and ex-vivo appraisal. Eur J Pharm Biopharm (2021) 168:166–83. doi: 10.1016/j.ejpb.2021.08.020 [DOI] [PubMed] [Google Scholar]
- 57. Liao ZH, Zhu HQ, Chen YY, Chen RL, Fu LX, Li L, et al. The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/ HIF-1α/VEGF dependent pathways. J Ethnopharmacol (2020) 259:112852. doi: 10.1016/j.jep.2020.112852 [DOI] [PubMed] [Google Scholar]
- 58. Guo HL, Bai Y. Inhibitory effect of paclitaxel combined with epigallo catechin galate on hepatocellular carcinoma cell multiplication and tumor growth in bearing cancer nude mice. J Xinxiang Med Univ (2015) 32(03):216–9. [Google Scholar]
- 59. Ou BB, Lin XZ, Liang G, Sun YW. The-epigallocatechin gallate and adriamycin apoptosis of BEL-7404/ADR in vivo . Lishizhen Med Materia Med Res (2012) 23(08):1854–5. [Google Scholar]
- 60. Mhlanga P, Perumal PO, Somboro AM, Amoako DG, Khumalo HM, Khan RB. Mechanistic insights into oxidative stress and apoptosis mediated by tannic acid in human liver hepatocellular carcinoma cells. Int J Mol Sci (2019) 20(24):6145. doi: 10.3390/ijms20246145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geng NN, Wu MS, Zheng X, Yang L, Wang HY, Li XY. Tannic acid enhances cisplatin-induced apoptosis of hepatocellular carcinoma HepG2 cells by activating ATF6-CHOP pathway. J Pract Med (2018) 34(01):12–5. [Google Scholar]
- 62. Tsai JJ, Chen JH, Chen CH, Chung JG, Hsu FT. Apoptosis induction and ERK/NF-κB inactivation are associated with magnolol-inhibited tumor progression in hepatocellular carcinoma in vivo . Environ Toxicol (2020) 35(2):167–75. doi: 10.1002/tox.22853 [DOI] [PubMed] [Google Scholar]
- 63. Cao W, Mo K, Wei S, Lan X, Zhang W, Jiang W. Effects of rosmarinic acid on immunoregulatory activity and hepatocellular carcinoma cell apoptosis in H22 tumor-bearing mice. Korean J Physiol Pharmacol (2019) 23(6):501–8. doi: 10.4196/kjpp.2019.23.6.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu G, Liu X, Li H, Yan Y, Hong X, Lin Z. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int J Immunopathol Pharmacol (2018) 32:2058738418814341. doi: 10.1177/2058738418814341 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Zhang Q. Study on the effect of kaempferol on the proliferation and apoptosis of human hepatoma cells HepG2 and its mechanism. Hebei Med University; (2022). [Google Scholar]
- 66. Guo HQ, Liu YL, Ren F. Zahng J.Research on the effect of kaempferol on HepG2 apoptosis and its mechanism. Beijing Med J (2021) 43(09):899–904. doi: 10.15932/j.0253-9713.2021.09.015 [DOI] [Google Scholar]
- 67. Zhang J, Ma H, Yang L. Silymarin inhibits invasion and migration of hepatoma cell line MHCC97. Basic Clin Med (2019) 39(12):1741–5. doi: 10.16352/j.issn.1001-6325.2019.12.019 [DOI] [Google Scholar]
- 68. Shi WY, Li HQ, Wang DQ, Yang YJ, Pan KJ, Xu J. Study on extraction and purification of silhistbinin in tistle. China Food Addit (2022) 33(10):99–105. doi: 10.19804/j.issn1006-2513.2022.10.013 [DOI] [Google Scholar]
- 69. Liu ZG, Li XL, Weng LD, Liu Q, Zhu HX, Huang ZG. Research progress in pharmacological effects of silymarin. J Liaoning Univ Tradit Chin Med (2012) 14(10):91–3. doi: 10.13194/j.jlunivtcm.2012.10.93.liuzhg.066 [DOI] [Google Scholar]
- 70. Zhao YF, Wan J, Dai Y. Silibinin inhibits the proliferation and migration of HepG2 hepatic cancer cells. Chin J Histochem Cytochem (2017) 26(02):142–6. doi: 10.16705/j.cnki.1004-1850.2017.02.008 [DOI] [Google Scholar]
- 71. Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin modulates NF-kappa b and AP-1/JNK pathways to induce cell death in human hepatoma cells. Nutr Cancer (2010) 62(3):390–401. doi: 10.1080/01635580903441196 [DOI] [PubMed] [Google Scholar]
- 72. Reyes-Avendaño I, Reyes-Jiménez E, González-García K, Pérez-Figueroa DC, Baltiérrez-Hoyos R, Tapia-Pastrana G, et al. Quercetin regulates key components of the cellular microenvironment during early hepatocarcinogenesis. Antioxidants (Basel) (2022) 11(2):358. doi: 10.3390/antiox11020358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou M, Liao XM, Wang S, Gong ZP, Zhang RH. In vivo and in vitro anticancer activity of quercetin against human liver cancer HepG2 cells. Anhui Med Pharmaceut J (2019) 23(11):2136–41. [Google Scholar]
- 74. Liao CY, Lee CC, Tsai CC, Hsueh CW, Wang CC, Chen IH, et al. Novel investigations of flavonoids as chemopreventive agents for hepatocellular carcinoma. BioMed Res Int (2015) 2015:840542. doi: 10.1155/2015/840542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu SH, Luo ZY, Zhang JS. Study on the antitumor effect of chrysin and its mechanism. J Hunan Normal University(Med Sci) (2006) 2006(04):78–81. [Google Scholar]
- 76. Wei XT, Peng WR, Jiang Q, Li Q, Feng ZY, Qi ZL, et al. Chrysin promotes SMMC-7721 cells apoptosis by regulating MAPKs signaling pathway. J South Med Univ (2018) 38(10):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rong W, Wan N, Zheng X, Shi G, Jiang C, Pan K, et al. Chrysin inhibits hepatocellular carcinoma progression through suppressing programmed death ligand 1 expression. Phytomedicine (2022) 95:153867. doi: 10.1016/j.phymed.2021.153867 [DOI] [PubMed] [Google Scholar]
- 78. Liu J, Wen X, Liu B, Zhang Q, Zhang J, Miao H, et al. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep (2016) 13(3):2401–8. doi: 10.3892/mmr.2016.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang Y, Lin MZ, Huang YW, Chen M. Promoting effect of diosmetin on the cell cycle arrest and cell apoptosis in HepG2 cell and its mechanism. Hainan Med J (2016) 27(3):354–7. [Google Scholar]
- 80. Pan L, Feng F, Wu J, Li L, Xu H, Yang L, et al. Diosmetin inhibits cell growth and proliferation by regulating the cell cycle and lipid metabolism pathway in hepatocellular carcinoma. Food Funct (2021) 12(23):12036–46. doi: 10.1039/d1fo02111g [DOI] [PubMed] [Google Scholar]
- 81. Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis (2015) 6(10):e1942. doi: 10.1038/cddis.2015.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bai J, Wu J, Tang R, Sun C, Ji J, Yin Z, et al. Emodin, a natural anthraquinone, suppresses liver cancer in vitro and in vivo by regulating VEGFR2 and miR-34a. Invest New Drugs (2020) 38(2):229–45. doi: 10.1007/s10637-019-00777-5 [DOI] [PubMed] [Google Scholar]
- 83. Du YC, Zheng TX, Shi H, Qian BL, Chen H, Zhang H, et al. Effect of aloin on proliferation, migration and invasion of hepatocellular carcinoma MHCC97H cells. Chin J Clin Pharmacol (2020) 36(12):1669–71. doi: 10.13699/j.cnki.1001-6821.2020.12.018 [DOI] [Google Scholar]
- 84. Liu X, Liu J. Tanshinone I induces cell apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and by suppressing p53/DRAM-mediated autophagy in human hepatocellular carcinoma. Artif Cells Nanomed Biotechnol (2020) 48(1):488–97. doi: 10.1080/21691401.2019.1709862 [DOI] [PubMed] [Google Scholar]
- 85. Stephenson Clarke JR, Douglas LR, Duriez PJ, Balourdas DI, Joerger AC, Khadiullina R, et al. Discovery of nanomolar-affinity pharmacological chaperones stabilizing the oncogenic p53 mutant Y220C. ACS Pharmacol Transl Sci (2022) 5(11):1169–80. doi: 10.1021/acsptsci.2c00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu JL, Tong L, Luo Y, Gao YJ. Cryptotanshinone may induce ferroptosis of human liver cancer HepG2 cells[J]. Zhongguo yi xue ke xue yuan xue bao. (2021) 43(3):366–70. Acta Academiae Medicinae Sinicae. [DOI] [PubMed] [Google Scholar]
- 87. Liu L, Yuan CJ, Guo JJ, Yu T, Lv XW, Liu JP, et al. Inhibitory effect of astragalus polysaccharide on the proliferation of HepG2 cells and its potential mechanism. Pract Prevent Med (2018) 25(4):385–7. [Google Scholar]
- 88. Mei J, Wang XM, Xie L, Jin XW, Li YL. Astragalus polysaccharide inhibits proliferation of Bel-7402/5-FU drug-resistant cells and its effect on drug-resistance genes. Chin J Integrat Tradit West Med Liver Dis (2020) 30(04):326–9. [Google Scholar]
- 89. Li X, Li P, Chang Y, Xu Q, Wu Z, Ma Q, et al. The SDF-1/CXCR4 axis induces epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Cell Biochem (2014) 392(1-2):77–84. doi: 10.1007/s11010-014-2020-8 [DOI] [PubMed] [Google Scholar]
- 90. Lu J, Zhu PF, Liu YM, Zeng QL, Yu ZJ. Astragalus polysaccharides downregulated apoptosis in HepG2 cell through wnt/β-catenin signaling pathway. Chin Tradit Herbal Drugs (2018) 49(21):5155–60. [Google Scholar]
- 91. Chan LJ, Yang SW, Yan YD, Wang WJ, Li R, Chen J. Et.al. study on the inhibitory effect of astragalus polysaccharides on HepG2.215 cells and its mechanism. Infect Dis Informat (2022) 35(02):130–4. [Google Scholar]
- 92. Yang Y, Song N, Guo JF, Yu N, Zhao Z, Gao H, et al. Inhibitory effect and its mechanism of pachymaran on HepG2 hepatoma cells by regulating NLRP3/pyrogen pathway. Chin Arch Tradit Chin Med (2022) 40(09):171–175+282-283. doi: 10.13193/j.issn.1673-7717.2022.09.038 [DOI] [Google Scholar]
- 93. Zhang DQ, Xin GJ. Lycium barbarum polysaccharide inhibits VEGF expression, migration and invasion iof SMMC-7721 hepatocellular carcinoma cells. Chin J Histochem Cytochem (2019) 28(01):26–31. doi: 10.16705/j.cnki.1004-1850.2019.01.005 [DOI] [Google Scholar]
- 94. Yan CL, Luo YL, An FY, Liu XS, Liu YQ, Wang YF, et al. Inhibitory effects of total astragalosides combined with cisplatin on STAT3-mediated inflammatory signaling pathway in H22 tumor-bearing mice. Chin J Biochem Molr Biol (2020) 36(11):1359–66. doi: 10.13865/j.cnki.cjbmb.2020.09.1200 [DOI] [Google Scholar]
- 95. An XC, Zhu RX, Lin SM, Shi L. Mechanism of astragaloside IV promoting proliferation and apoptosis of hepatoma cells by inhibiting ROSNF-κB signaling pathway. Modern Digest Intervent (2019) 24(12):1399–403. [Google Scholar]
- 96. An FY, Liu XS, Yan CL, Luo YL, Liu YQ, Shi XF, et al. Inhibitory effect of total astragalus saponins on tumor and its mechanism in H22 bearing mice. Chin J Public Health (2017) 33(4):596–8. [Google Scholar]
- 97. Zhu HY, Shen HJ, Wang Y. Analysis on influence of huangqi sijunzi decoction on postoperative recovery and immunologic function of patients undergoing primary liver cancer operation. Liaoning J Tradit Chin Med (2022), 1–9. [Google Scholar]
- 98. Jiang CM, Ding SZ, Zhang ZF. Effect of supplementing qi and activating blood circulation to help the patients with primary hepatocellular carcinoma to assist the treatment and improve the effect of digestive tract symptoms. Pract J Cancer (2018) 33(09):1528–31. [Google Scholar]
- 99. Zheng WC, Li H, Gong WJ. Clinical effect of external application of compound gleditsiae spina ointment in treatment of severe cancer pain due to primary liver cancer: An analysis of 28 cases. Hunan J Tradit Chin Med (2022) 38(10):8–11. doi: 10.16808/j.cnki.issn1003-7705.2022.10.003 [DOI] [Google Scholar]
- 100. Jing T. Clinical study on xingqi sanjie huayu prescription combined with reduced glutathione for patients after surgery for primary liver cancer. New Chin Med (2022) 54(17):187–91. doi: 10.13457/j.cnki.jncm.2022.17.040 [DOI] [Google Scholar]
- 101. Chen WL, Fan XX, Liang F, Lv JZ, Zhang YC, Ran Y, et al. Clinical observation on triple therapy in the prevention and treatment of recurrence of primary liver cancer after hepatectomy. Guangming J Chin Med (2022) 37(16):2949–2951+2994. [Google Scholar]
- 102. Zhao Y, Yu JJ, Li H. Clinical study on fuzheng huayu jiedu prescription for advanced liver cancer. New Chin Med (2022) 54(15):153–7. doi: 10.13457/j.cnki.jncm.2022.15.032 [DOI] [Google Scholar]
- 103. Yang HJ, Jing XH. Effects of hedyotis diffusa injection combined with transarterial chemoembolization in treatment of patients with primary liver cancer. Med J Chin People's Health (2022) 34(13):107–9. [Google Scholar]
- 104. Tokumoto H, Akita S, Nakamura R, Yamamoto N, Kubota Y, Mitsukawa N. Investigation of the association between breast cancer-related lymphedema and the side effects of taxane-based chemotherapy using indocyanine green lymphography. Lymphat Res Biol (2022) 20(6):612–7. doi: 10.1089/lrb.2021.0065 [DOI] [PubMed] [Google Scholar]
- 105. Liu K, AiKeM WFR, Wang RZ. Changes of peripheral blood lymphocyte subsets in patients with nasopharyngeal carcinoma before and after radiotherapy. J Xinjiang Med Univ (2015) 38(01):6–10+16. [Google Scholar]
- 106. Mokhtari RB, Kumar S, Islam SS, Yazdanpanah M, Adeli K, Cutz E, et al. Combination of carbonic anhydrase inhibitor, acetazolamide, and sulforaphane, reduces the viability and growth of bronchial carcinoid cell lines. BMC Cancer (2013) 13:378. doi: 10.1186/1471-2407-13-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Elmeliegy M, Vourvahis M, Guo C, Wang DD. Effect of p-glycoprotein (P-gP) inducers on exposure of p-gP substrates: Review of clinical drug-drug interaction studies. Clin Pharmacokinet (2020) 59(6):699–714. doi: 10.1007/s40262-020-00867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sun L, Chen W, Qu L, Wu J, Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and pglycoprotein expression. Mol Med Rep (2013) 8(6):1883–7. doi: 10.3892/mmr.2013.1742 [DOI] [PubMed] [Google Scholar]
- 109. Chen XR, Zhou SH, Wang RC, Xu L. Effect and mechanism of β-elemene on SKOV3/DDP resistant ovarian cancer cells. Pharmaceut Clin Res (2021) 29(02):81–5. [Google Scholar]
- 110. Carnero A, Garcia-Mayea Y, Mir C, Lorente J, Rubio IT, LLeonart ME. The cancer stem-cell signaling network and resistance to therapy. Cancer Treat Rev (2016) 49:25–36. doi: 10.1016/j.ctrv.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 111. Wang XY, Zhao Q, Wang B, Yuan S, Li K. Mechanism of quercetin reversing drug resistance of human breast cancer MCF-7 cells to doxorubicin. Modern Prevent Med (2018) 45(10):1844–1849+1859. [Google Scholar]
- 112. Li L. Antcancer mechanism investigations of natural product derivatives targeting for subcellular organelles. Shandong University; (2018). [Google Scholar]
- 113. Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion (2013) 13(3):199–208. doi: 10.1016/j.mito.2012.07.112 [DOI] [PubMed] [Google Scholar]
- 114. Ebrahimi N, Saremi J, Ghanaatian M, Yazdani E, Adelian S, Samsami S, et al. The role of endoplasmic reticulum stress in the regulation of long noncoding RNAs in cancer. J Cell Physiol (2022) 237(10):3752–67. doi: 10.1002/jcp.30846 [DOI] [PubMed] [Google Scholar]
- 115. Chai RF. Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT/DLC1 in hepatocellular carcinoma cells. Shandong Normal University; (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gao J, Ma F, Wang X, Li G. Combination of dihydroartemisinin and resveratrol effectively inhibits cancer cell migration via regulation of the DLC1/TCTP/Cdc42 pathway. Food Funct (2020) 11(11):9573–84. doi: 10.1039/d0fo00996b [DOI] [PubMed] [Google Scholar]
- 117. Feng Y, Zu LL, Zhang L. MicroRNA-26b inhibits the tumor growth of human liver cancer through the PI3K/Akt and NF-κB/MMP-9/VEGF pathways. Oncol Rep (2018) 39(5):2288–96. doi: 10.3892/or.2018.6289 [DOI] [PubMed] [Google Scholar]
- 118. Guo Z, Xia Y, Hao G, Gao ZZ, Li R, Yang Y. In vitro analysis on inhibitory effect of sodium arsenite combined with astragaloside IV on HepG2 liver cancer cells. Alexandria Engin J (2021) 60(6):5749–64. doi: 10.1016/j.aej.2021.03.043 [DOI] [Google Scholar]
- 119. Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ (2018) 25(1):37–45. doi: 10.1038/cdd.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wen S, Sun L, An R, Zhang W, Xiang L, Li Q, et al. A combination of citrus reticulata peel and black tea inhibits migration and invasion of liver cancer via PI3K/AKT and MMPs signaling pathway. Mol Biol Rep (2020) 47(1):507–19. doi: 10.1007/s11033-019-05157-z [DOI] [PubMed] [Google Scholar]
- 121. Han H, Wang L, Liu Y, Shi X, Zhang X, Li M, et al. Combination of curcuma zedoary and kelp inhibits growth and metastasis of liver cancer in vivo and in vitro via reducing endogenous H2S levels. Food Funct (2019) 10(1):224–34. doi: 10.1039/c8fo01594e [DOI] [PubMed] [Google Scholar]
- 122. Zhang J, Hong Y, Jiang L, Yi X, Chen Y, Liu L, et al. Global metabolomic and lipidomic analysis reveal the synergistic effect of bufalin in combination with cinobufagin against HepG2 cells. J Proteome Res (2020) 19(2):873–83. doi: 10.1021/acs.jproteome.9b00681 [DOI] [PubMed] [Google Scholar]
- 123. Zhou Y, Wang M, Pan X, Dong Z, Han L, Ju Y, et al. Combination of triptolide with sodium cantharidinate synergistically enhances apoptosis on hepatoma cell line 7721. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2016) 41(9):911–7. doi: 10.11817/j.issn.1672-7347.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 124. Liu QD, Bai TY, Wang YF, Yao Y. Effect of combined use of quercetin and rosmarinic acid on proliferation and apoptosis of HepG2 cells in vitro . Anhui Med Pharmaceut J (2020) 24(09):1705–7. [Google Scholar]
- 125. Manikandan R, Beulaja M, Arulvasu C, Sellamuthu S, Dinesh D, Prabhu D, et al. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microsc Res Tech (2012) 75(2):112–6. doi: 10.1002/jemt.21032 [DOI] [PubMed] [Google Scholar]
- 126. Wang L, Wei D, Han X, Zhang W, Fan C, Zhang J, et al. The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J Cell Biochem (2014) 115(4):721–30. doi: 10.1002/jcb.24715 [DOI] [PubMed] [Google Scholar]
- 127. Liao GH, Zhang GS, Lou ZH, Cheng RB, Zhang GJ. Study on the effeciency and attenuating mechanism of arsenic trioxide combined with tanshinone against liver cancer. J Zhejiang Chin Med Univ (2020) 44(01):15–23. doi: 10.16466/j.issn1005-5509.2020.01.003 [DOI] [Google Scholar]
- 128. Shen L, Zhang G, Lou Z, Xu G, Zhang G. Cryptotanshinone enhances the effect of arsenic trioxide in treating liver cancer cell by inducing apoptosis through downregulating phosphorylated- STAT3 in vitro and in vivo . BMC Complement Altern Med (2017) 17(1):106. doi: 10.1186/s12906-016-1548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Roskoski R., Jr. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol Res (2019) 139:471–88. doi: 10.1016/j.phrs.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 130. Cao YJ, Zhou YJ, He XZ, Zhou CX, Cui L, Zhuang QF, et al. Overexpression of β-arrestin2 induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. Eur Rev Med Pharmacol Sci (2017) 21(8):1729–37. [PubMed] [Google Scholar]
- 131. Ye K, Wei Q, Gong Z, Huang Y, Liu H, Li Y, et al. Effect of norcantharidin on the proliferation, apoptosis, and cell cycle of human mesangial cells. Ren Fail (2017) 39(1):458–64. doi: 10.1080/0886022X.2017.1308257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Roskoski R., Jr. Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol Res (2016) 107:249–75. doi: 10.1016/j.phrs.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 133. Song D, Liang H, Qu B, Li Y, Liu J, Chen C, et al. Moxidectin inhibits glioma cell viability by inducing G0/G1 cell cycle arrest and apoptosis. Oncol Rep (2018) 40(3):1348–58. doi: 10.3892/or.2018.6561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lohberger B, Leithner A, Stuendl N, Kaltenegger H, Kullich W, Steinecker-Frohnwieser B. Diacerein retards cell growth of chondrosarcoma cells at the G2/M cell cycle checkpoint via cyclin B1/CDK1 and CDK2 downregulation. BMC Cancer (2015) 15:891. doi: 10.1186/s12885-015-1915-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang D. Study on anti-hepatocellular carcinoma activity of chrysin combined with diosmetin or triptolide in vivo and in vitro. Wuhan Polytechnic University; (2020). [Google Scholar]
- 136. Xiao Y, Gong Q, Wang W, Liu F, Kong Q, Pan F, et al. The combination of biochanin a and SB590885 potentiates the inhibition of tumour progression in hepatocellular carcinoma. Cancer Cell Int (2020) 20:371. doi: 10.1186/s12935-020-01463-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Carrasco-Torres G, Baltiérrez-Hoyos R, Andrade-Jorge E, Villa-Treviño S, Trujillo-Ferrara JG, Vásquez-Garzón VR. Cytotoxicity, oxidative stress, cell cycle arrest, and mitochondrial apoptosis after combined treatment of hepatocarcinoma cells with maleic anhydride derivatives and quercetin. Oxid Med Cell Longev (2017) 2017:2734976. doi: 10.1155/2017/2734976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang W, Wang L, Xu PY, Xiao HY. Synergistic tumor suppression by curcumin and low-concentration vincristine in hepatoma cell lines. Carcinog Teratog Mutag (2012) 24(4):270–4. [Google Scholar]
- 139. Chang MX, Wu MM, Li HM. Inhibition on proliferation of hepatoma HepG2 cell treated with curcumin combined with glycyrrhetinic acid. Drug Evaluat Res (2017) 40(01):42–7. [Google Scholar]
- 140. Li GZ. Effects of combination of artesunate with curcumin of proliferation apoptosis and telomerase activity of human hepatoma cell line HepG2. Guangxi Med University; (2013). [Google Scholar]
- 141. Chang TW, Lin CY, Tzeng YJ, Lur HS. Synergistic combinations of tanshinone IIA and trans-resveratrol toward cisplatin-comparable cytotoxicity in HepG2 human hepatocellular carcinoma cells. Anticancer Res (2014) 34(10):5473–80. [PubMed] [Google Scholar]
- 142. Dai B, Ma Y, Yang T, Fan M, Yu R, Su Q, et al. Synergistic effect of berberine and HMQ1611 impairs cell proliferation and migration by regulating wnt signaling pathway in hepatocellular carcinoma. Phytother Res (2019) 33(3):745–55. doi: 10.1002/ptr.6267 [DOI] [PubMed] [Google Scholar]
- 143. Liu YM, Zhang JQ, You ZM, Liao H. Inhibitory effect of norcantharidin combined with evodiamine on the growth of human hepatic carcinoma cell line HepG2 in vitro . Chin J Cell Mol Immunol (2014) 8):824–8. doi: 10.13423/j.cnki.cjcmi.006975 [DOI] [PubMed] [Google Scholar]
- 144. Zhang X, Ouyang HZ, Xu W, Wang XY. Effect of celastrol combined medication on the inhibition of HepG2 cell proliferation. J China Pharmaceut Univ (2020) 51(02):185–92. [Google Scholar]
- 145. Ling S, Li J, Shan Q, Dai H, Lu D, Wen X, et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol (2017) 11(6):682–95. doi: 10.1002/1878-0261.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. He S, Liu F, Xie Z, Zu X, Xu W, Jiang Y. P-Glycoprotein/MDR1 regulates pokemon gene transcription through p53 expression in human breast cancer cells. Int J Mol Sci (2010) 11(9):3309–051. doi: 10.3390/ijms11093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sun Y, Zhang J, Zhou J, Huang Z, Hu H, Qiao M, et al. Synergistic effect of cucurbitacin b in combination with curcumin via enhancing apoptosis induction and reversing multidrug resistance in human hepatoma cells. Eur J Pharmacol (2015) 768:28–40. doi: 10.1016/j.ejphar.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 148. Shen X, Liu X, Wan S, Fan X, He H, Wei R, et al. Discovery of coumarin as microtubule affinity-regulating kinase 4 inhibitor that sensitize hepatocellular carcinoma to paclitaxel. Front Chem (2019) 7:366. doi: 10.3389/fchem.2019.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tian N, Shangguan W, Zhou Z, Yao Y, Fan C, Cai L. Lin28b is involved in curcumin-reversed paclitaxel chemoresistance and associated with poor prognosis in hepatocellular carcinoma. J Cancer (2019) 10(24):6074–87. doi: 10.7150/jca.33421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Xu X, Wu C, Bai A, Liu X, Lv H, Liu Y. Folate-functionalized mesoporous silica nanoparticles as a liver tumor-targeted drug delivery system to improve the antitumor effect of paclitaxel. J Nanomater (2017) 2017:1–13. doi: 10.1155/2017/2069685 [DOI] [Google Scholar]
- 151. Kuo HC, Lee HJ, Hu CC, Shun HI, Tseng TH. Enhancement of esculetin on taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol Appl Pharmacol (2006) 210(1-2):55–62. doi: 10.1016/j.taap.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 152. Jiang Q, Yang M, Qu Z, Zhou J, Zhang Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement Altern Med (2017) 17(1):477. doi: 10.1186/s12906-017-1956-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lei X, Li J, Chen KL. Inhibitory effect of emodin and curcumine on hepatoma BEL-7402 cell. Chin J Hosp Pharm (2009) 29(12):971–3. [Google Scholar]
- 154. Zhou M, Li Z, Han Z, Tian N. Paclitaxel-sensitization enhanced by curcumin involves down-regulation of nuclear factor-κB and Lin28 in Hep3B cells. J Recept Signal Transduct Res (2015) 35(6):618–25. doi: 10.3109/10799893.2015.1041644 [DOI] [PubMed] [Google Scholar]
- 155. Li Z, Wang N, Yue T, Liu L. Matrine reverses the drug resistance of K562/ADM cells to ADM and VCR via promoting autophagy. Transl Cancer Res (2020) 9(2):786–94. doi: 10.21037/tcr.2019.12.11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156. Wang YX, Liu XJ. Research progress of cisplatin target and resistance mechanism. China Med Biotechnol (2020) 15(03):316–9. [Google Scholar]
- 157. Kim IH, Kwon MJ, Jung JH, Nam TJ. Protein extracted from porphyra yezoensis prevents cisplatin-induced nephrotoxicity by downregulating the MAPK and NF-κB pathways. Int J Mol Med (2018) 41(1):511–20. doi: 10.3892/ijmm.2017.3214 [DOI] [PubMed] [Google Scholar]
- 158. Xue DF, Pan ST, Huang G, Qiu JX. ROS enhances the cytotoxicity of cisplatin by inducing apoptosis and autophagy in tongue squamous cell carcinoma cells. Int J Biochem Cell Biol (2020) 122:105732. doi: 10.1016/j.biocel.2020.105732 [DOI] [PubMed] [Google Scholar]
- 159. Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) (2008) 291(6):721–34. doi: 10.1002/ar.20668 [DOI] [PubMed] [Google Scholar]
- 160. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev (1992) 13(1):18–32. doi: 10.1210/edrv-13-1-18 [DOI] [PubMed] [Google Scholar]
- 161. Dalton AC, Shlamkovitch T, Papo N, Barton WA. Correction: Constitutive association of Tie1 and Tie2 with endothelial integrins is functionally modulated by angiopoietin-1 and fibronectin. PLoS One (2017) 12(5):e0179059. doi: 10.1371/journal.pone.0179059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol (2009) 10(3):165–77. doi: 10.1038/nrm2639 [DOI] [PubMed] [Google Scholar]
- 163. Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One (2011) 6(4):e19139. doi: 10.1371/journal.pone.0019139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fayette J, Soria JC, Armand JP. Use of angiogenesis inhibitors in tumour treatment. Eur J Cancer (2005) 41(8):1109–16. doi: 10.1016/j.ejca.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 165. Zhuang PY, Shen J, Zhu XD, Lu L, Wang L, Tang ZY, et al. Prognostic roles of cross-talk between peritumoral hepatocytes and stromal cells in hepatocellular carcinoma involving peritumoral VEGF-c, VEGFR-1 and VEGFR-3. PLoS One (2013) 8(5):e64598. doi: 10.1371/journal.pone.0064598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ao M, Xiao X, Ao YZ. Observation on effect and adverse reactions of thalidomide combined with kanglaite injection in treating primary liver cancer. Doctor (2017) 3(7):9–10+24. doi: 10.19604/j.cnki.dys.2017.07.005 [DOI] [Google Scholar]
- 167. Zhou MN, Liu J, Liu R, Shu P, Zou ZZ, Shi D. Effects of alcohol extract from luwei dihuang combined with cisplatin on apoptosis and tumor angiogenesis factors of hepatocellular carcinoma BEL-7402 cells. Pharmacol Clin Chin Materia Med (2020) 36(03):61–6. doi: 10.13412/j.cnki.zyyl.2020.03.005 [DOI] [Google Scholar]
- 168. Lin XY, Song GC. Effects of astragalus polysaccharide injection on the expression of ki-67,HIF-1α and VEGF in HepG2 mice during chemotherapy. J North Pharm (2020) 17(08):77–8. [Google Scholar]
- 169. Zang WH, Hu JL, Tang DC, Yin G, Zhi L, Ding SC. Effects on expression of CD147, iNOS in nude mice of human hepatocarcinoma transplantation model by compatibility of radix astragali and curcuma and combination with cisplatin. Lishizhen Med Materia Med Res (2020) 31(04):785–8. [Google Scholar]
- 170. Luo S, Yin G, Tang DC. Effect of astragaloside IV and curcumin on angiogenesis of human hepatocellular carcinoma xenografts in nude mice. Chin J Cancer Prevent Treat (2016) 23(S1):12–3. doi: 10.16073/j.cnki.cjcpt.2016.s1.006 [DOI] [Google Scholar]
- 171. Chen YY. Effect of EGCG derivative Y6 on anti-angiogenesis and synergistic attenuated daunorubicin anti-hepatocarcinoma and its mechanism in vivo. Guangxi Med University; (2018). [Google Scholar]
- 172. Zhao X, Chen Q, Li Y, Tang H, Liu W, Yang X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm (2015) 93:27–36. doi: 10.1016/j.ejpb.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 173. Yan Y, Zhang HY, Pan JH, Wang DY, Zhou XS, Yuan YF, et al. Effect of dopamine receptor 4 on proliferation and metastasis of hepatocellular carcinoma cells and its prognostic significance. J Sun Yat-sen Univ (Med Sci) (2020) 41(05):669–80. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2020.0088 [DOI] [Google Scholar]
- 174. Neelakantan D, Zhou H, Oliphant MUJ, Zhang X, Simon LM, Henke DM, et al. EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat Commun (2017) 8:15773. doi: 10.1038/ncomms15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bae MJ, Karadeniz F, Oh JH, Yu GH, Jang MS, Nam KH, et al. MMP-inhibitory effects of flavonoid glycosides from edible medicinal halophyte limonium tetragonum. Evid Based Complement Alternat Med (2017) 2017:6750274. doi: 10.1155/2017/6750274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sun B, Lin Y, Wang X, Lan F, Yu Y, Huang Q. Single nucleotide polymorphism of the enhancer of zeste homolog 2 gene rs2072408 is associated with lymph node metastasis and depth of primary tumor invasion in gastric cancer. Clin Lab (2016) 62(11):2099–105. doi: 10.7754/Clin.Lab.2016.160302 [DOI] [PubMed] [Google Scholar]
- 177. Shan TT. The inhibitory effect of mannose on human hepatocellular carcinoma cell line HepG2. Hunan University Chin Med; (2020). [Google Scholar]
- 178. Wang HL. Experimental study on the effects of emodin and cisplatin on the invasion and migration og HepG2 cells. Changchun Univ Chin Med (2020). [Google Scholar]
- 179. Yang G, Xing J, Aikemu B, Sun J, Zheng M. Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncol Rep (2021) 45(4):32. doi: 10.3892/or.2021.7983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sun YM, Zeng ZR, Wang JY, Peng MB, Lei S, Yang YS, et al. Effects of sinapine thiocyanate in promoting the sensitivity of HepG2 cells to gemcitabine. Chin Tradit Patent Med (2021) 43(4):901–7. [Google Scholar]
- 181. Wang ZL. Mechanism research on caffeine synergistically promotes the anti-tumor effects of 5-fluorouracil in hepatocellular carcinoma. Guangzhou Med University; (2020). [Google Scholar]
- 182. Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao ZQ, Yu LG, et al. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo . Mol Carcinog (2018) 57(1):44–56. doi: 10.1002/mc.22718 [DOI] [PubMed] [Google Scholar]
- 183. Wu LH. Correlation analysis of hepatitis b virus X gene mutation with autophagy-related proteins (Beclin-1, LC3B and P62) on hepatocellular carcinoma. Guangxi Med University; (2019). [Google Scholar]
- 184. Wang F, Mao Y, You Q, Hua D, Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int J Immunopathol Pharmacol (2015) 28(3):362–73. doi: 10.1177/0394632015598849 [DOI] [PubMed] [Google Scholar]
- 185. Fernández ÁF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature (2018) 558(7708):136–40. doi: 10.1038/s41586-018-0162-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zheng W, Chen Q, Wang C, Yao D, Zhu L, Pan Y, et al. Inhibition of cathepsin d (CTSD) enhances radiosensitivity of glioblastoma cells by attenuating autophagy. Mol Carcinog (2020) 59(6):651–60. doi: 10.1002/mc.23194 [DOI] [PubMed] [Google Scholar]
- 187. Yeo SY, Itahana Y, Guo AK, Han R, Iwamoto K, Nguyen HT, et al. Transglutaminase 2 contributes to a TP53-induced autophagy program to prevent oncogenic transformation. ELife (2016) 5:e07101. doi: 10.7554/eLife.07101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chang HW, Kim MR, Lee HJ, Lee HM, Kim GC, Lee YS, et al. p53/BNIP3-dependent mitophagy limits glycolytic shift in radioresistant cancer. Oncogene (2019) 38(19):3729–42. doi: 10.1038/s41388-019-0697-6 [DOI] [PubMed] [Google Scholar]
- 189. Liu Z, Shi A, Song D, Han B, Zhang Z, Ma L, et al. Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction. Am J Cancer Res (2017) 7(3):574–83. [PMC free article] [PubMed] [Google Scholar]
- 190. Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev (2014) 40(7):862–71. doi: 10.1016/j.ctrv.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 191. Hu G, Cao C, Deng Z, Li J, Zhou X, Huang Z, et al. Effects of matrine in combination with cisplatin on liver cancer. Oncol Lett (2021) 21(1):66. doi: 10.3892/ol.2020.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Geng N, Zheng X, Wu M, Yang L, Li X, Chen J. Tannic acid synergistically enhances the anticancer efficacy of cisplatin on liver cancer cells through mitochondria-mediated apoptosis. Oncol Rep (2019) 42(5):2108–16. doi: 10.3892/or.2019.7281 [DOI] [PubMed] [Google Scholar]
- 193. Xu YW, Geng SN, Wang MQ, Du GJ. Effect of saposide d on hepatocellular carcinoma in mice treated with doxorubicin. Chin Tradit Herbal Drugs (2021) 52(03):778–88. [Google Scholar]
- 194. Wang B, Wang F, Ding A, Zhao H, Bu X. Regorafenib and ginsenoside combination therapy: Inhibition of HepG2 cell growth through modulating survivin and caspase-3 gene expression. Clin Transl Oncol (2020) 22(9):1491–8. doi: 10.1007/s12094-019-02283-9 [DOI] [PubMed] [Google Scholar]
- 195. Liu J, Li Y, Yang X, Dong Y, Wu J, Chen M. Effects of ginkgol C17:1 on cisplatin-induced autophagy and apoptosis in HepG2 cells. Oncol Lett (2018) 15(1):1021–9. doi: 10.3892/ol.2017.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Qu X, Gao H, Tao L, Zhang Y, Zhai J, Sun J, et al. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J Toxicol Sci (2019) 44(3):167–75. doi: 10.2131/jts.44.167 [DOI] [PubMed] [Google Scholar]
- 197. Sun R, Zhai R, Ma C, Miao W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med (2020) 9(3):1141–51. doi: 10.1002/cam4.2723 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 198. Zhao J, Li H, Li W, Wang Z, Dong Z, Lan H, et al. Effects of sinapic acid combined with cisplatin on the apoptosis and autophagy of the hepatoma cells HepG2 and SMMC-7721. Evid Based Complement Alternat Med (2021) 2021:6095963. doi: 10.1155/2021/6095963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhang Y, Qu Y, Chen YZ. Influence of 6-shogaol potentiated on 5-fluorouracil treatment of liver cancer by promoting apoptosis and cell cycle arrest by regulating AKT/mTOR/MRP1 signalling. Chin J Nat Med (2022) 20(5):352–63. doi: 10.1016/S1875-5364(22)60174-2 [DOI] [PubMed] [Google Scholar]
- 200. Kalashnikova I, Mazar J, Neal CJ, Rosado AL, Das S, Westmoreland TJ, et al. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis via modulation of bcl-2/Bax in human neuroblastoma. Nanoscale (2017) 9(29):10375–87. doi: 10.1039/c7nr02770b [DOI] [PubMed] [Google Scholar]
- 201. Xu WD, Yang L, Chen C, Zhou W. Quercetin-loaded ceria nanoparticles induce autophagy blockage in hepatoma cells. Chin J Biochem Molr Bio (2022) 38(11):1538–46. doi: 10.13865/j.cnki.cjbmb.2022.08.1107 [DOI] [Google Scholar]
- 202. Dong W, Liu FC, Ni JS, Li PP, Guo XG, Liu H. Co-Delivery of fluorouracil and curcumin by nano-layered double hydroxide for inhibition of hepatocellular carcinoma cells in vitro . China J Modern Med (2016) 26(09):16–22. [Google Scholar]
- 203. Han QH. Synthesis and application of dual drug Delivery/Controlled release nanotheranostic agent in liver cancer. Jilin University; (2019). [Google Scholar]
- 204. Ni WF. Dual-targeted nanoparticles transporting curcumin and 5-fluorouracil for the treatment of hepatocarcinoma. Tianjin Med University; (2019). doi: 10.27366/d.cnki.gtyku.2019.000567 [DOI] [Google Scholar]
- 205. Zhang F. Study on the treatment of hepatocellular carcinoma and recurrence by magnetic mesoporous nanoparticles with doxorubicin and berberine. Jilin University; (2018). [Google Scholar]
- 206. Xu J, Fang Y, Xu RZ, Li Y. Effect of Chinese medicine on immune regulation mechanism of lung cancer. Acta Chin Med (2021) 36(06):1210–6. doi: 10.16368/j.issn.1674-8999.2021.06.258 [DOI] [Google Scholar]
- 207. Xing XY, Wang XC, He W. Advances in research on tumor immunotherapy and its drug development. J China Pharmaceut Univ (2021) 52(01):10–9. [Google Scholar]
- 208. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med (2017) 9(410):eaal4291. doi: 10.1126/scitranslmed.aal4291 [DOI] [PubMed] [Google Scholar]
- 210. Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology (2016) 5(5):e1129483. doi: 10.1080/2162402X.2015.1129483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, et al. FDA Approval summary: Atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncologist (2017) 22(6):743–9. doi: 10.1634/theoncologist.2017-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci U.S.A. (2017) 114(29):E5900–9. doi: 10.1073/pnas.1706559114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Du NW, Bai RL, Cui JW. Mechanism of tumor immune escape and treatment strategy. Chin J Cancer Biother (2019) 26(04):454–62. [Google Scholar]
- 214. Hu N. Immunological mechanisms of the antitumor effects of lycium barbarum polysaccharide alone or combined with CXCL10. Guangzhou University Chin Med; (2015). [Google Scholar]
- 215. Chen CA. Study on effect and mechanism of high-dose vitamin c combined with immune checkpoint inhibitor in treating liver cancer. PLA Naval Med University; (2020). doi: 10.26998/d.cnki.gjuyu.2020.000166 [DOI] [Google Scholar]
- 216. Song WG, Xun QU, Li YL, Xu YP, Wu C, Qin QL. Anti-tumor effects of 10-hydroxycamptothecinc-treated DC-Hepa1-6 fusion vaccines. Chin J Hepatol (2004) 12(6):344–6. [PubMed] [Google Scholar]
- 217. Yang N, Xu JL, Lu LB, Sheng GG. Chinese Medicine combined with immunosuppressive agents and targeted drugs in the treatment of advanced liver cancer with multiple lung metastases: A case report. Chin J Integrat Tradit West Med Liver Dis (2022) 32(07):658–60. [Google Scholar]
- 218. Yu JF, Li ZP, Zhou XL, Li C, Liu Y, Zhang Y, et al. Effect of fuling sini decoction combined with sorafenib on advanced primary liver cancer. Acta Chin Med Pharmacol (2021) 49(06):76–80. doi: 10.19664/j.cnki.1002-2392.210143 [DOI] [Google Scholar]
- 219. Wang Y, Guo SH, Shang XJ, Yu LS, Zhu JW, Zhao A, et al. Triptolide induces sertoli cell apoptosis in mice via ROS/JNK-dependent activation of the mitochondrial pathway and inhibition of Nrf2-mediated antioxidant response. Acta Pharmacol Sin (2018) 39(2):311–27. doi: 10.1038/aps.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ariyasu D, Yoshida H, Hasegawa Y. Endoplasmic reticulum (ER) stress and endocrine disorders. Int J Mol Sci (2017) 18(2):382. doi: 10.3390/ijms18020382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Jin C, Wang SQ, Bai L. Preparation of paclitaxel nanoparticles targeting hepatocellular carcinoma stem cells and its effect on hepatocellular carcinoma huh-7 and HepG2 cells. Cancer Res Clin (2021) 33(02):99–103. [Google Scholar]
- 222. Wang S, Liu X, Yuan S, Chen WJ, Wang SM, Xu N. Effects of monoclonal antibodies against human stathmin 1 combined paclitaxel on proliferation of human hepatocellular carcinoma cell lines. Chin German J Clin Oncol (2009) 8(10):603–6. doi: 10.1007/s10330-009-0138-z [DOI] [Google Scholar]
- 223. Lin HL, Lui WY, Liu TY, Chi CW. Reversal of taxol resistance in hepatoma by cyclosporin a: involvement of the PI-3 kinase-AKT 1 pathway. Br J Cancer (2003) 88(6):973–80. doi: 10.1038/sj.bjc.6600788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Liu N, Huang H, Xu L, Hua X, Li X, Liu S, et al. The combination of proteasome inhibitors bortezomib and gambogic acid triggers synergistic cytotoxicity in vitro but not in vivo . Toxicol Lett (2014) 224(3):333–40. doi: 10.1016/j.toxlet.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 225. Hsu JL, Chiang PC, Guh JH. Tunicamycin induces resistance to camptothecin and etoposide in human hepatocellular carcinoma cells: Role of cell-cycle arrest and GRP78. Naunyn Schmiedebergs Arch Pharmacol (2009) 380(5):373–82. doi: 10.1007/s00210-009-0453-5 [DOI] [PubMed] [Google Scholar]
- 226. Zhan WJ, Liang MJ, Liu Y, Huang YF, Wang WX. Effects and mechanism of STAT3 inhibitor and nicotinamide combination on the inhibiting proliferation of hepatocarcinoma HepG2 cells. Tianjin Med J (2022) 50(07):686–92. [Google Scholar]
- 227. Zhang ZQ. Targeted inhibition of hepatocellular carcinoma by membrane loaded natural product hypocrellin b nanovesicles and its mechanism. Nanjing University Chin Med; (2021). doi: 10.27253/d.cnki.gnjzu.2021.000076 [DOI] [Google Scholar]
- 228. Bai Y. Study on the co-delivery of doxorubicin and miR-122 with nanosystem for hepatocellular carcinoma therapy. Tianjin Med University; (2017). [Google Scholar]
- 229. Yuan HJ. Experimental study of interventional photothermal ablation combinaed with Fe2O3-PDA-Dox nanocomposites and combretastatin a-4 phosphate disodium for hepatocellular carcinoma in the rats. Chinese general hospital&medical school PLA; (2019). doi: 10.27637/d.cnki.gjyjc.2019.000262 [DOI] [Google Scholar]
- 230. Lai CH. Investigation of the anti-tumor effects of mannose/CPG oligodeoxynucleotide-conjugated liposome containing tumor-assoclated antigens via specific activation of dendritic cell. Guangxi Med University; (2014). [Google Scholar]
- 231. Zhong XR. Study on the mechanism of HDAC6 inhibitor tubacin enhancing the sensitivity of docetaxel against liver cancer. Jilin University; (2020). [Google Scholar]
- 232. Shen XX, Shi L, Yao LY, Niu YZ, Liang T. Effect of evodiamine combined with chloroquine on hepatocarcinoma. J Nanjing Univ Tradit Chin Med (2021) 37(03):400–3. doi: 10.14148/j.issn.1672-0482.2021.0400 [DOI] [Google Scholar]
- 233. Lai XC. Study on the mechanism of evodiamine joint CDK1 inhibitor on hepatoma cell lines. Guangzhou University Chin Med; (2012). [Google Scholar]
- 234. Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer (2020) 8(1):e000317. doi: 10.1136/jitc-2019-000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discovery Today (2012) 17(17-18):1044–52. doi: 10.1016/j.drudis.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 236. Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA (2004) 292(4):470–84. doi: 10.1001/jama.292.4.470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.