Abstract
Introduction:
The differential diagnosis of pleural effusion is difficult, and studies have reported on the potential role of adenosine deaminase (ADA) in the differential diagnosis of undiagnosed pleural effusion. This retrospective study aimed to investigate the diagnostic role of ADA in pleural effusion.
Methods:
266 patients with pleural effusion from three centers were enrolled. The concentrations of ADA and lactate dehydrogenase (LDH) were measured in pleural fluids and serum samples of the patients. The diagnostic performance of ADA-based measurement for tuberculous pleural effusion (TPE), malignant pleural effusion (MPE), and parapneumonic effusion (PPE) was examined by receiver operating characteristic (ROC) curve analysis.
Results:
An area under the ROC curve (AUC) value of 0.909 was obtained using the pleural ADA values as the indicator for TPE identification (sensitivity: 87.50%, specificity: 87.82%). The ratio of serum LDH to pleural ADA (cancer ratio) provided the predictive capacity with an AUC of 0.879 for MPE diagnosis (sensitivity: 95.04%, specificity: 67.06%). At a cut-off value of 14.29, the pleural ADA/LDH ratio showed a sensitivity and specificity of 81.13% and 83.67%, respectively, and a high AUC value of 0.888 for the differential diagnosis of PPE from TPE.
Conclusion:
ADA-based measurement is helpful for the differential diagnosis of pleural effusion. Further studies should be performed to validate these results.
Keywords: Adenosine deaminase, diagnosis, malignant pleural effusion, parapneumonic effusion, pleural effusion, tuberculous pleural effusion
Introduction
Pleural effusion is a commonly clinic manifestation associated with more than 50 recognized diseases and disorders. In the clinic, malignant pleural effusion (MPE), tuberculous pleural effusion (TPE), and parapneumonic effusion (PPE) are the most likely causes of exudative effusions.1 Approximately one-third of patients with tuberculosis develop extra-pulmonary tuberculosis, while a quarter of them have TPE.2 However, traditional microbiology and molecular biology methods show poor performance when pleural fluids are used to diagnose TPE, especially in an acute setting.3–5 MPE is distinct from TPE and PPE in that it has worse prognosis and a median survival time of only 3–12 months.6 When pleural biopsy is used, cytological examination has a low rate of MPE detection because of the poor preservation of tumor cells and small sample volume.7,8 Thoracoscopic biopsy is an efficient diagnostic method for both TPE and MPE, but its invasiveness limits clinical application.9–11 Furthermore, the performances of tumor biomarkers such as vascular endothelial growth factor, carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, CA 15-3, CA 19-9, and CYFRA 21-1 (a fragment of cytokeratin 19) in the diagnosis of MPE are also limited.12–14 Therefore, it is important to identify invasive and effective methods to differentiate subtypes of exudative effusions.
Adenosine deaminase (ADA), an enzyme secreted by mononuclear cells, lymphocytes, neutrophils, and red blood cells (RBCs),15 plays an important role in purine nucleoside metabolism15,16 and is related to intracellular infection such as tuberculosis. An increased total pleural fluid ADA level usually helps to discriminate TPE from PPE.17,18 Although a meta-analysis revealed that ADA can diagnose TPE with a high sensitivity and specificity of 92% and 90%, respectively,17 a similar or even higher level of total pleural fluid ADA has occasionally been reported in PPE, especially in patients with pyothorax,15,19,20 which may be due to the high pleural level of ADA in the case of empyema.21,22 Thus, it remains a challenge to distinguish TPE from PPE on the basis of elevated pleural fluid ADA. In addition, a decreased level of ADA was reported in patients with MPE,15 but it remains unclear if pleural fluid ADA alone can help to diagnose MPE.
Lactate dehydrogenase (LDH) is a ubiquitous enzyme present in a variety of tissues including liver, kidney, myocardium, skeletal muscles, and RBCs.23 Verma et al.24 reported that an increased serum LDH to pleural ADA ratio (cancer ratio, CR) could diagnose MPE with a sensitivity and specificity of 98% and 94%, respectively. Another study found that the LDH/ADA ratio in the pleural fluid was highly predictive of differentiating TPE from PPE at a cut-off level of 16.20.25 Although several studies have been performed to validate whether ADA-based measurements can be used as novel markers for differential diagnosis of pleural effusion, these results have been inconsistent. Therefore, we conducted a retrospective study across multiple centers to evaluate the diagnostic performance of ADA-based measurements in pleural effusion as parameters to distinguish between the different subtypes of pleural effusion.
Patients and methods
Study design and subjects
This was a retrospective, multi-center study conducted across West China Hospital (Cohort 1, between June 2020 and January 2021), Chengdu Fifth People’s Hospital (Cohort 2, between June 2020 and December 2020), and Hospital of Chengdu University of Traditional Chinese Medicine (Cohort 3, between January 2018 and December 2020). The study participants were patients aged >18 years with accumulation of pleural fluid through chest ultrasonic examinations. Those who had inconclusive final diagnosis, incomplete data, a coexisting systemic disease, immunodeficiency, autoimmune disease, or hemothorax were excluded from this study. This study was designed according to the requirements of the Chinese Guidelines for Good Clinical Practice and was approved by the Ethics Committee of West China Hospital. The need for written informed consent was waived given the retrospective nature of the study.
Diagnostic criteria and data extraction
Based on previous study,19 this study only included patients with exudates and focused on patients with MPE, TPE, and PPE. Further, the diagnostic criterion for MPE was based on the presence of malignant cells in pleural effusion or pleural biopsy specimens.7 The inclusion criteria for patients with TPE were chronic granulomas in the pleural tissue, clinical response to anti-tuberculosis treatment, or acid-fast bacteria found in pleural fluid or sputum. PPE was identified as exudative effusions associated with bacterial pneumonia, lung abscesses, or bronchiectasis, absence of Mycobacterium tuberculosis (MTB) in the pleural fluid, pathological manifestations of inflammatory pleuritis, pleural fibrosis and plaques, or chronic empyema without evidence of MTB and good response to antibiotic therapy.26 Demographic and baseline characteristics including age, sex, and color of the pleural fluid were collected from all study participants.
Sample collection and quantification of ADA and LDH levels
A volume of 3 ml of venous blood was collected and centrifuged at 4000 r/min for 10 min to separate serum. The pleural fluid samples were collected via thoracentesis and centrifuged at 3000 g for 10 min. The levels of pleural ADA, LDH, protein, glucose and serum LDH, albumin were measured on the automated chemistry analyzer in clinical laboratory. The operation was performed in strict accordance with the SOP (standard operating procedure). All experiments were performed in accordance with the manufacturers’ protocols.
Statistical analysis
After a normality test, the data were summarized as the mean and standard deviation or median and interquartile range. When comparing two groups, an independent samples t-test was used to evaluate normally distributed data and the Mann–Whitney test was used to examine non-normally distributed data. Receiver operating characteristic (ROC) curves were used to identify the diagnostic ability of these markers. Univariate logistic regression analysis was performed to assess risk factors responsible for the presence of TPE, MPE, or PPE and variables having a P < 0.1 were included for multivariate analysis to calculate the odds ratios (OR) and corresponding 95% confidence interval (CI). All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Figures were drawn using GraphPad Prism 8.0.1 (GraphPad Software Inc., La Jolla, CA, USA). Significance for statistical analyses was set at P < 0.05.
Results
Baseline characteristics of patients
Data for a total of 266 patients suffering from pleural effusion were collected and reviewed. There were 102, 109, and 55 patients enrolled in Cohorts 1, 2, and 3, respectively. Of the patients with benign pleural effusion (BPE), 58 and 62 had TPE and PPE, respectively. The mean ages of the MPE and BPE groups were 69.00(60.00−76.00) and 64.00(46.25−75.75) years, respectively (P < 0.05, Supplementary Table 1), and patients with PPE were older than patients with TPE (P < 0.05, Supplementary Table 1). In Cohorts 1 and 3, patients with MPE were older than those with BPE, and the PPE group was also more aged than the TPE group (Supplementary Table 1). Among all patients and patients in Cohort 2, there were more smokers in patients with MPE than those with BPE (P < 0.05, Supplementary Table 1). Among all patients with MPE, 132 patients had lung cancer, 8 patients had hematological malignancies, 5 patients had metastatic cancer, and 1 patient was diagnosed with pleural mesothelioma (Supplementary Table 2).
Pleural levels of ADA and LDH and serum LDH levels
The clinical and laboratory findings of patients with MPE and BPE are summarized in Table 1. Compared with the BPE group, the MPE group had a significantly higher serum LDH ((217.50(179.50−299.00) versus 188.00 (158.00−232.00) U/L, P = 0.006)) and a lower pleural fluid ADA level ((7.40(5.38−11.00) versus 19.60(8.20−42.15) U/L, P = 0.003)) in all subjects. Therefore, the CR values significantly increased in MPE patients ((29.84(18.47−50.50)) than in BPE patients ((7.65(4.09−18.09), P < 0.0001) in all subjects. Only in Cohort 2, a significant increase of serum LDH level was detected in MPE patients (P < 0.05). All three cohorts showed significantly lower pleural fluid ADA levels and higher CR values in the MPE groups than the BPE groups (all P < 0.05).
Table 1.
Pleural fluid parameters of patients with MPE, TPE, or PPE in three cohorts.
| Cohort 1 | Cohort 2 | Cohort 3 | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPE (n = 55) | TPE (n = 29) | PPE (n = 18) | MPE (n = 48) | TPE (n = 22) | PPE (n = 39) | MPE (n = 43) | TPE (n = 7) | PPE (n = 5) | MPE (n = 146) | TPE (n = 58) | PPE (n = 62) | |
| Color | ||||||||||||
| Yellow | 37 | 20 | 13 | 38 | 18 | 34 | 25 | 6 | 3 | 100 | 44 | 50 |
| Red | 12 | 5 | 4 | 8 | 2 | 3 | 16 | 1 | 1 | 36 | 8 | 8 |
| Yellow-red | 5 | 4 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 9 | 6 | 3 |
| Purulent/chylous | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Cytology | ||||||||||||
| Monocytes (%) | 82.00 (44.00–90.00) | 72.00 (29.00–90.50) | 83.00 (56.00–90.00) | 81.00 (66.80–92.00) | 94.00 (85.00–97.00) | 86.00 (51.50–93.50)** | 65.50 ± 16.95 | 81.85 ± 10.11 | 65.00 (12.50–73.00) | 78.00 (57.00–90.00) | 89.00 (66.50–94.50) | 83.00 (51.50–90.75) |
| Coenocyte (%) | 10.00 (5.00–29.00) | 17.00 (7.50–49.00) | 17.00 (4.00–44.00) | 19.00 (8.00–33.20) | 6.00 (3.00–15.00) | 14.00 (6.50–48.50)** | 33.06 ± 17.14 | 18.15 ± 10.11 | 33.00 (26.00–82.50) | 16.00 (7.00–37.60) | 10.00 (5.00–26.65) | 17.00 (7.00–48.50) |
| Glucose (mmol/L) | 6.60 (5.98−7.52)* | 6.02 (2.65−7.72) | 5.42 (1.05−7.88) | 6.36 (5.16−7.77) | 6.00 (5.12−7.27) | 6.83 (5.34−7.86)** | 6.96 ± 3.36 | 4.69 ± 1.33 | 7.37 ± 3.29** | 6.56 (5.47 −7.72) | 6.00 (4.24−7.27) | 6.30 (3.61−7.84)** |
| Protein (g/L) | 38.31 ± 11.18 | 42.04 ± 23.38 | 36.51 ± 10.46 | 41.20 (32.29−48.50) | 47.60 (38.00−52.00) | 39.10 (34.60−51.70)** | 42.11 ± 11.59 | 46.30 ± 3.58 | 16.38 ± 10.78 | 40.28 ± 11.31 | 43.86 ± 16.95 | 34.93 ± 12.95** |
| Chlorine (mmol/L) | 105.00 (101.00–107.00) | 104.00 (101.00–107.50) | 104.00 (101.00–106.00) | 106.60 (104.40–109.20) | 105.90 (101.70–107.70) | 108.70 (101.05–112.30) | 107.00 ± 8.29 | 108.53 ± 3.11 | 106.16 ± 11.96 | 106.10 (102.40–109.40) | 105.90 (101.00–107.70) | 105.50 (101.11–112.30) |
| Pleural LDH (U/L) | 272.00 (185.50−379.50) | 205.00 (149.50−506.50) | 255.00 (135.75−1250.50) | 301.00 (166.50−515.50)* | 317.00 (217.00−528.00) | 194.00 (148.50−453.50)** | 402.71 ± 344.51 | 335.50 ± 95.19 | 1164.00 ± 1913.08 | 288.50 (173.00−457.75)* | 281.00 (185.00−496.00) | 194.00 (142.00−746.00)** |
| ADA (U/L) | 8.55 (5.98−11.20)* | 28.00 (17.10−48.50) | 12.05 (8.85−34.00)** | 7.00 (5.00−11.00)* | 42.00 (35.00−47.00) | 13.00 (8.00−15.00)** | 9.37 ± 5.04* | 46.81 ± 14.62 | 2.40 ± 0.78** | 7.40 (5.38−11.00)* | 35.00 (21.00−47.00) | 13.00 (8.00−18.80)** |
| Cancer ratio | 26.79 (16.10−42.05)* | 9.09 (3.72−14.64) | 18.98 (4.68−25.88) | 32.40 (20.64−64.17)* | 4.93 (3.62−6.16) | 17.54 (10.70−24.14)** | 38.46 ± 31.11* | 4.24 ± 1.48 | 6.05 ± 6.44 | 29.84 (18.47−50.50)* | 4.96 (3.62−11.46) | 18.17 (10.47−24.29)** |
| Pleural LDH/ ADA ratio | – | 8.85 (6.01−19.04) | 23.83 (16.31−39.81)** | – | 8.43 (6.89−11.55) | 19.50 (12.53−28.71)** | – | 8.81 ± 3.74 | 21.72 ± 13.37 | – | 8.78 (6.51−13.25) | 20.00 (15.05−35.64)** |
ADA, adenosine deaminase; Cancer ratio, serum LDH/ pleural ADA; MPE, malignant pleural effusion; LDH, lactate dehydrogenase; PPE, parapneumonic pleural effusion; TPE, tuberculous pleural effusion.
Data are presented as the mean ± standard deviation or as the median (interquartile range). Statistical significance was set at P < 0.05.
P < 0.05 versus patients with benign pleural effusion.
P < 0.05 versus patients with TPE.
Patients with TPE had a significantly elevated level of pleural ADA ((35.00(21.00−47.00) versus 13.00(8.00−18.80) U/L, P < 0.0001, Table 1)) and pleural LDH (281.00(185.00−496.00) versus 194.00(142.00−746.00) U/L, P = 0.005, Table 1) than those with PPE in all subjects. Moreover, the pleural fluid LDH/ADA ratio in the TPE group was significantly lower than in the PPE group in all subjects ((8.78(6.51−13.25) versus 20.00(15.05−35.64), P < 0.0001, Table 1)). Meanwhile, Elevated levels of pleural ADA were observed in patients with TPE than in those with PPE in all three cohorts (all P < 0.05, Table 1). Only in Cohort 2, the pleural LDH level was lower in PPE group ((194.00(148.50−453.50)) than in the TPE group (317.00(217.00−528.00), P < 0.0001, Table 1). In Cohorts 1 and 2, the pleural fluid LDH/ADA ratio significantly decreased in the TPE group (all P < 0.0001, Table 1).
Diagnostic accuracy of pleural ADA for TPE
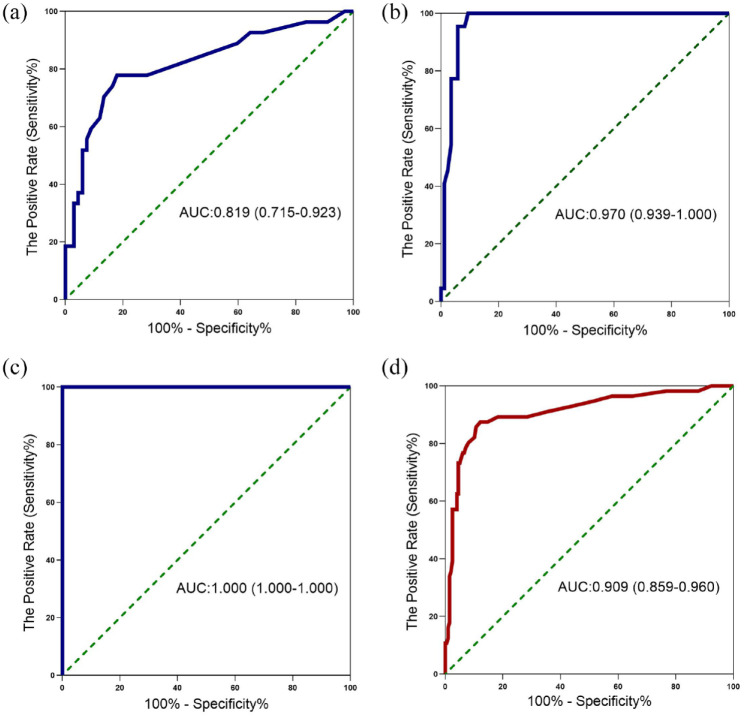
The ability of pleural fluid ADA level to diagnose TPE from other causes of pleural effusion was explored by ROC curve analysis in Figure 1 and Table 2. In Cohort 2, the pleural fluid ADA level provided an AUC of 0.970, along with a sensitivity of 100.00%, a specificity of 90.59% and a PLR value of 10.63 at a cut-off value of 18.50 U/L to differentiate patients with TPE (Table 2, Figure 1(b)). At a cut-off value of 16.50 U/L, pleural fluid ADA level had an AUC value of 0.909, along with a sensitivity value of 87.50%, a specificity value of 87.82%, a PLR value of 7.18 and a NLR value of 0.14 in all subjects (Table 2, Figure 1(d)). It was found that pleural protein (OR = 1.029, 95% CI: 1.005–1.053) and pleural ADA (OR = 1.115, 95% CI: 1.083–1.148) were significantly associated with the diagnosis of patients with TPE (all P < 0.05) in univariate analysis. Further multivariate analysis revealed that only pleural ADA (OR = 1.124, 95% CI: 1.089–1.160) was independently correlated with the diagnosis of patients with TPE (Supplementary Table 3).
Figure 1.
Receiver operating characteristic curve for pleural ADA to diagnose patients with TPE. Receiver operating characteristic curves showing the performances of the pleural ADA level for diagnosing TPE in cohort 1 (a), cohort 2 (b), cohort 3 (c), and all subjects (d). ADA, adenosine deaminase; TPE, tuberculous pleural effusion.
Table 2.
The cut-off values, sensitivity, specificity, and AUC of the pleural ADA distinguishing TPE, cancer ratio detecting MPE, and pleural LDH/ADA ratio differentiating PPE from TPE.
| Cohort 1 | Cohort 2 | Cohort 3 | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pleural ADA | Cancer ratio | Pleural LDH/ADA | Pleural ADA | Cancer ratio | Pleural LDH/ADA | Pleural ADA | Cancer ratio | Pleural LDH/ADA | Pleural ADA | Cancer ratio | Pleural LDH/ADA | |
| Cut off values | 14.50 | 12.61 | 12.68 | 18.50 | 13.56 | 14.53 | 22.50 | 10.92 | 11.34 | 16.50 | 12.50 | 14.29 |
| Sensitivity (%) (95% CI) | 77.78 (59.24–89.39) | 93.48 (82.50–97.76) | 100.00 (80.64–100.0) | 100.00 (85.13–100.00) | 95.12 (83.86– 99.13) | 79.41 (63.20–89.65) | 100 (64.57–100.0) | 97.06 (85.08–99.85) | 100 (30.06–98.72) | 87.50 (76.37–93.81) | 95.04 (89.60–97.71) | 81.13 (68.00–90.60) |
| Specificity (%) (95% CI) | 82.09 (71.25–89.45) | 60.00 (44.60– 73.65) | 62.50 (42.71–78.84) | 90.59 (82.51–95.15) | 70.27 (54.22–82.51) | 100.00 (84.54–100.00) | 100 (92.13–100.0) | 100 (67.56–100.0) | 75 (43.85–100.0) | 87.82 (82.51–91.67) | 67.06 (56.52–76.12) | 83.67 (70.30–92.70) |
| PLR (95%CI) | 4.34 (2.50–7.50) | 2.37 (1.60–3.50) | 2.67 (1.60–4.50) | 10.63 (5.50–20.50) | 3.20 (1.9–5.3) | NaN | NaN | NaN | 4.00 (0.70–21.80) | 7.18 (4.90–10.60) | 2.89 (2.10–3.90) | 4.97 (2.60–9.50) |
| NLR (95%CI) | 0.27 (0.10–0.60) | 0.11 (0.04–0.30) | NaN | NaN | 0.069 (0.02–0.3) | 0.21 (0.10–0.40) | NaN | 0.029 (0.004–0.20) | NaN | 0.14 (0.07–0.30) | 0.074 (0.03–0.20) | 0.23 (0.10–0.40) |
| AUC (95% CI) |
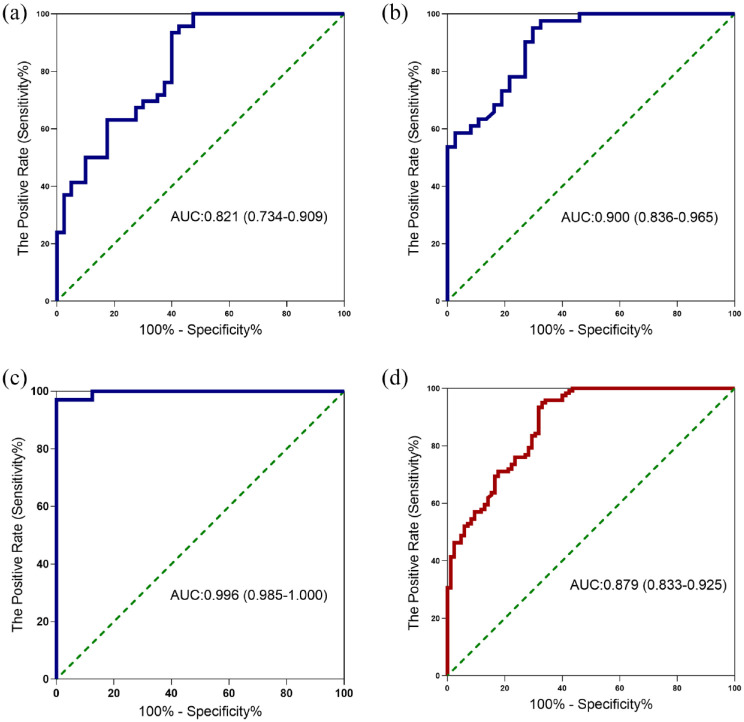
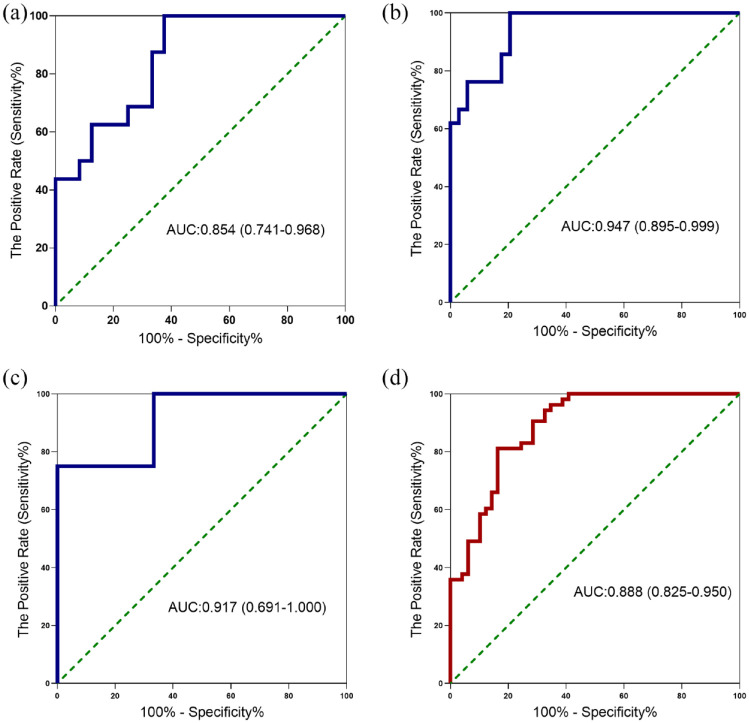
0.819 (0.715–0.923) | 0.821 (0.734–0.909) | 0.854 (0.741–0.968) | 0.970 (0.939–1.000) | 0.900 (0.836–0.965) | 0.947 (0.895–0.999) | 1.000 (1.000–1.000) | 0.996 (0.985–1.000) | 0.917 (0.691–1.000) | 0.909 (0.859–0.960) | 0.879 (0.833–0.925) | 0.888 (0.825–0.950) |
Statistical significance was set at P < 0.05.
ADA, adenosine deaminase; AUC, area under the curve; Cancer ratio, serum LDH / pleural ADA; CI, confidence interval; LDH, lactate dehydrogenase; MPE, malignant pleural effusion; NaN, the conclusion could not be performed because the values entered include one or more instances of zero; NLR, negative likelihood ration; PLR, positive likelihood ration; PPE, parapneumonic pleural effusion; TPE, tuberculous pleural effusion.
CR for the diagnosis of MPE
ROC curves were created to analyze the diagnostic performance of CR as a marker for MPE from BPE in Figure 2 and Table 2. In Cohort 2, the CR provided an AUC of 0.900, along with a sensitivity of 95.12%, a specificity of 70.27%, a PLR of 3.20 and a NLR of 0.069 at a cut-off value of 13.56 to differentiate patients with MPE (Table 2, Figure 2(b)). In Figure 2(d), CR had an AUC of 0.879 at a cut-off value of 12.50, along with a sensitivity value of 95.04%, a specificity value of 67.06%, a PLR of 2.89 and a NLR of 0.074 in all subjects (Table 2, Figure 2(d)). Univariate analysis found that age (>65 years, OR = 0.490, 95% CI: 0.300–0.800), smoking status (smoker, OR = 0.597, 95% CI: 0.358–0.995), serum CA199(OR = 0.986, 95% CI: 0.974–0.998), serum CA153(OR = 0.974, 95% CI: 0.956–0.993), serum NSE (OR = 0.967, 95% CI: 0.937–0.997), serum CYFRA211 (OR = 0.959, 95% CI: 0.924–0.995) and cancer ratio (OR = 0.871,95% CI: 0.936–0.908) were associated with the diagnosis of patients with MPE (all P < 0.05). However, it was found that only CR (OR = 0.888, 95% CI: 0.826–0.954) was independently associated with diagnosis of patients with MPE in multivariate analysis (Supplementary Table 4).
Figure 2.
Receiver operating characteristic curve for cancer ratio to distinguish patients with MPE from BPE. Receiver operating characteristic curves showing the performances of the cancer ratio as the indicator for MPE identification in cohort 1 (a), cohort 2 (b), cohort 3 (c), and all subjects (d). BPE, benign pleural effusion; MPE, malignant pleural effusion.
Pleural fluid LDH/ADA ratio for differentiating PPE from TPE
Next, we evaluated the diagnostic performances of pleural fluid LDH/ADA ratios in identifying PPE from TPE in Figure 3 and Table 2. In Cohort 2, with an AUC of 0.947, the pleural fluid LDH/ADA ratio diagnosed PPE with a sensitivity of 79.41%, a specificity of 100.00% and a NLR of 0.21 at a cut-off value of 14.53 (Table 2, Figure 3(b)). In all subjects, the AUC for distinguishing PPE from TPE was 0.888 at a cut-off value of 14.29, with a sensitivity of 81.13%, a specificity of 83.67%, a PLR of 4.97 and a NLR of 0.23 (Table 2, Figure 3(d)). It was revealed that pleural protein (OR = 0.950, 95% CI: 0.920–0.982) and pleural LDH/ADA ratio (OR = 1.238, 95% CI: 1.133–1.354) were associated with the diagnosis of patients with PPE from TPE (all P < 0.05) in univariate analysis. Further multivariate analysis revealed that pleural protein (OR = 0.934, 95% CI: 0.886–0.985) and pleural LDH/ADA ratio (OR = 1.205, 95% CI: 1.102–1.319) was independently associated with diagnosis of patients with PPE from TPE (Supplementary Table 5).
Figure 3.
Receiver operating characteristic curve for pleural fluid LDH/ADA ratio to distinguish patients with PPE from TPE. Receiver operating characteristic curves showing the performances of the pleural fluid LDH/ADA ratio as the indicator for PPE differentiation from TPE in cohort 1 (a), cohort 2 (b), cohort 3 (c), and all subjects (d). ADA, adenosine deaminase; LDH, lactate dehydrogenase; PPE, parapneumonic effusion; TPE, tuberculous pleural effusion.
Discussion
It is necessary to identify noninvasive, quick, and effective biomarkers to differentiate multiple types of pleural fluid. For patients with undiagnosed pleural effusion, the conventional blood and pleural biochemical tests regarding pleural fluid ADA and LDH levels are usually performed without any additional costs. We conducted a retrospective study in multiple centers and aimed to explore the diagnostic performance of pleural ADA-based measurements in the differential diagnosis of pleural effusion. Overall, our results showed that the pleural fluid ADA can be an effective marker for the diagnosis of TPE with an AUC of 0.909. Moreover, CR is a useful biomarker in predicting MPE with an AUC of 0.879, and the pleural fluid LDH/ADA ratio is also meaningful to distinguish PPE and TPE, providing an AUC of 0.888, suggesting that pleural ADA plays a critical role in the diagnosis of TPE, MPE, and PPE.
Some investigations during the past decade have addressed the potential role of pleural ADA level in the differential diagnosis of TPE with high sensitivity and specificity.17,27 However, inconsistent data were reported by Zaric et al.28 who reported a poor specificity as low as 70.4% for the ADA level in diagnosing TPE, although its sensitivity of 89.2% was considered acceptable. Therefore, these results suggested that the use of ADA level in pleural fluid for differentiating TPE from other types of pleural effusion in clinical practice can be challenging. In the present study, an elevated pleural fluid ADA level was evident in patients with TPE, with an AUC of 0.909, specificity of 87.50%, and sensitivity of 87.82% in diagnosing TPE. The diagnostic sensitivity of ADA was moderate, in that it was not sufficiently low to exclude non-TPE when the pleural ADA level was lower than the cut-off values; therefore, it should be interpreted together with clinical findings and routine laboratory tests in clinical practice. Univariate and multivariate analyses also demonstrated a strong association between pleural ADA levels and different types of pleural fluid.
Although a higher pleural ADA level in pleural fluid strongly suggests TPE, PPE may also lead to a relatively high pleural ADA level (~40 U/L),15,29 and an extremely high ADA level is associated with a greater risk of empyema or lymphoma.23 Conversely, pleural fluid LDH level might be a specific diagnostic indicator for empyema,29 suggesting that pleural ADA or LDH level alone is limited in its ability to differentiate between clinical TPE and PPE. Thus, the two parameters were combined as a predictor of PPE, and the pleural fluid LDH/ADA ratio provided a sensitivity of 81.13% and a specificity of 83.67% yielded an AUC of 0.888. The reason why performance of pleural LDH/ADA ratio was inferior to that of previous studies might be due to lack of consideration of pleural fluid cellular predominance. However, the cut-off value of pleural fluid ADA for TPE diagnosis was controversial, further studies are required to validate these findings.
CR has been proposed as a predictor of MPE, and this ratio was found to be significantly higher in patients with MPE than in those with TPE or PPE.24 The results of our study are consistent with previous research,30 reporting an AUC of 0.879, along with high sensitivity of 95.04% and a relatively low specificity of 67.06%, suggesting that CR can be a sensitive biomarker of MPE. However, the specificity (67.06%) is lower than that reported in previous studies,24,31 because less patients with lung cancer (90.41%) were enrolled in our study (95% and 97.6%).24,31 The different subtypes of MPE should be considered in the clinical interpretation of CR results, given that LDH levels may be influenced by different types of tumors. Obviously, the relatively low specificity limited the diagnostic value of CR. The high sensitivity indicates that clinicians should exercise caution when patients have high CR value and conduct further examinations such as repeated cytologic test and invasive procedures such as medical thoracoscopy and pleural biopsy if needed. Although the optimal cut-off value of CR has not yet been determined, a prespecified threshold value is needed because a data-driven threshold can overstate indicator tests.32
Among the three cohorts, the performance for ADA-based measurements of Cohort 3 was better than those of Cohorts 1 and 2. The main reason may be that there were only seven patients with TPE and five with PPE in Cohort 3, much less than the number of patients with TPE or PPE in Cohorts 1 and 2. It has been reported that the sample size can influence the diagnostic accuracy of the ROC curve.33 Therefore, the pooled analysis of all three cohorts was representative when assessing the diagnostic performance of ADA-based measurements in differentiating MPF/TPE.
This study has some limitations. First, we conducted these analyses in a retrospective manner. The data obtained from the hospital’s medical records were limited and we were unable to further analyze the differences between the PPE subgroups. Second, the patients suffering from pleural effusions enrolled in our study were not representative of other diseases that could lead to pleural effusion, such as connective tissue diseases,34 which may also have an increased pleural fluid ADA or LDH level.
Conclusion
Overall, our study has provided evidence that pleural fluid ADA-based measurements are effective biomarkers to discriminate multiple types of pleural effusion. Consequently, it may assist in the early diagnosis and treatment for patients with pleural effusion.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666231155747 for Adenosine deaminase-based measurement in the differential diagnosis of pleural effusion: a multicenter retrospective study by Lijuan Gao, Wujun Wang, Ying Zhang, Xueru Hu, Jing An, Yang Li, Mei Chen and Yongchun Shen in Therapeutic Advances in Respiratory Disease
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Lijuan Gao  https://orcid.org/0000-0002-2848-7629
https://orcid.org/0000-0002-2848-7629
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lijuan Gao, Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, Chengdu, China.
Wujun Wang, Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China.
Ying Zhang, Department of Respiratory and Critical Care Medicine, Army Medical Center of PLA, Chongqing, China.
Xueru Hu, Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, Chengdu, China.
Jing An, Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, Chengdu, China.
Yang Li, Department of Respiratory and Critical Care Medicine, Chengdu Fifth People’s Hospital, Chengdu, China.
Mei Chen, Department of Respiratory and Critical Care Medicine, Chengdu Fifth People’s Hospital, Chengdu 611130, China; School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu 611130, China.
Yongchun Shen, Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, Chengdu 610041, China.
Declarations
Ethics approval and consent to participate: This is a retrospective study. This study was designed according to the requirements of the Chinese Guidelines for Good Clinical Practice and was approved by the Ethics Committee of West China Hospital. The need for written informed consent was waived given the retrospective nature of the study.
Consent for publication: All data used to support the findings of the current study are available from the corresponding authors upon reasonable request.
Author contributions: Lijuan Gao: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Wujun Wang: Data curation; Investigation; Resources.
Ying Zhang: Data curation; Investigation; Resources.
Xueru Hu: Investigation; Resources.
Jing An: Investigation; Resources.
Yang Li: Investigation; Resources.
Mei Chen: Project administration; Investigation; Resources; Writing – review & editing.
Yongchun Shen: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the 1•3•5 Project for Disciplines of Excellence at West China Hospital of Sichuan University [2019HXFH042] and by the Sichuan Key Research and Development Program [2020YFS0147]. These funding agencies were not involved in designing the study, collecting or analyzing the data, writing the manuscript, or making decisions related to publication.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346: 1971–1977. [DOI] [PubMed] [Google Scholar]
- 2. Pang Y, An J, Shu W, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis 2019; 25: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bielsa S, Palma R, Pardina M, et al. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis 2013; 17: 85–89. [DOI] [PubMed] [Google Scholar]
- 4. Choi BY, Yoon MJ, Shin K, et al. Characteristics of pleural effusions in systemic lupus erythematosus: differential diagnosis of lupus pleuritis. Lupus 2015; 24: 321–326. [DOI] [PubMed] [Google Scholar]
- 5. Li S, Lin L, Zhang F, et al. A retrospective study on Xpert MTB/RIF for detection of tuberculosis in a teaching hospital in China. BMC Infect Dis 2020; 20: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014; 69: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018; 198: 839–849. [DOI] [PubMed] [Google Scholar]
- 8. Casado-Rey P, Vázquez-Iglesias L, Botana-Rial M, et al. A rapid calprotectin test for the diagnosis of pleural effusion. PLoS ONE 2021; 16: e0252714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrera Lara S, Fernández-Fabrellas E, Juan Samper G, et al. Predicting malignant and paramalignant pleural effusions by combining clinical, radiological and pleural fluid analytical parameters. Lung 2017; 195: 653–660. [DOI] [PubMed] [Google Scholar]
- 10. Botana-Rial M, Vazquez-Iglesias L, Casado-Rey P, et al. Validation of calprotectin as a novel biomarker for the diagnosis of pleural effusion: a multicentre trial. Sci Rep 2020; 10: 5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez-Otero N, Blanco-Prieto S, Paez de la Cadena M, et al. Calprotectin: a novel biomarker for the diagnosis of pleural effusion. Br J Cancer 2012; 107: 1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen AH, Miller EJ, Wichman CS, et al. Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res 2015; 166: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Liu YL, Shi HZ. Diagnostic accuracy of combinations of tumor markers for malignant pleural effusion: an updated meta-analysis. Respiration 2017; 94: 62–69. [DOI] [PubMed] [Google Scholar]
- 14. Shen YC, Liu MQ, Wan C, et al. Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: a meta-analysis. Exp Ther Med 2012; 3: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med 2010; 21: 419–423. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: a systematic review and meta-analysis. PLoS ONE 2019; 14: e0213728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008; 102: 744–754. [DOI] [PubMed] [Google Scholar]
- 18. Krenke R, Korczyński P. Use of pleural fluid levels of adenosine deaminase and interferon gamma in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med 2010; 16: 367–375. [DOI] [PubMed] [Google Scholar]
- 19. van Keimpema AR, Slaats EH, Wagenaar JP. Adenosine deaminase activity, not diagnostic for tuberculous pleurisy. Eur J Respir Dis 1987; 71: 15–18. [PubMed] [Google Scholar]
- 20. Manuel Porcel J, Vives M, Esquerda A, et al. Usefulness of the British Thoracic Society and the American College of Chest Physicians guidelines in predicting pleural drainage of non-purulent parapneumonic effusions. Respir Med 2006; 100: 933–937. [DOI] [PubMed] [Google Scholar]
- 21. Valdés L, San José E, Alvarez D, et al. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 1996; 9: 747–751. [DOI] [PubMed] [Google Scholar]
- 22. Pérez-Rodriguez E, Jiménez Castro D. The use of adenosine deaminase and adenosine deaminase isoenzymes in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med 2000; 6: 259–266. [DOI] [PubMed] [Google Scholar]
- 23. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001; 292: 504–507. [DOI] [PubMed] [Google Scholar]
- 24. Verma A, Abisheganaden J, Light RW. Identifying malignant pleural effusion by a cancer ratio (serum LDH: pleural fluid ADA ratio). Lung 2016; 194: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Liu J, Xie X, et al. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm Med 2017; 17: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(Suppl. 2): ii41–ii53. [DOI] [PubMed] [Google Scholar]
- 27. Greco S, Girardi E, Masciangelo R, et al. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis 2003; 7: 777–786. [PubMed] [Google Scholar]
- 28. Zarić B, Kuruc V, Milovancˇev A, et al. Differential diagnosis of tuberculous and malignant pleural effusions: what is the role of adenosine deaminase? Lung 2008; 186: 233–240. [DOI] [PubMed] [Google Scholar]
- 29. Lee J, Lee SY, Lim JK, et al. Radiologic and laboratory differences in patients with tuberculous and parapneumonic pleural effusions showing non-lymphocytic predominance and high adenosine deaminase levels. Infection 2015; 43: 65–71. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Li X, Liu J, et al. Diagnostic accuracy of the cancer ratio for the prediction of malignant pleural effusion: evidence from a validation study and meta-analysis. Ann Med 2021; 53: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verma A, Dagaonkar RS, Marshall D, et al. Differentiating malignant from tubercular pleural effusion by Cancer Ratio Plus (cancer ratio: pleural lymphocyte count). Can Respir J 2016; 2016: 7348239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leeflang MM, Moons KG, Reitsma JB, et al. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem 2008; 54: 729–737. [DOI] [PubMed] [Google Scholar]
- 33. Obuchowski NA, Bullen JA. Receiver operating characteristic (ROC) curves: review of methods with applications in diagnostic medicine. Phys Med Biol 2018; 63: 07TR01. [DOI] [PubMed] [Google Scholar]
- 34. Ip H, Sivakumar P, McDermott EA, et al. Multidisciplinary approach to connective tissue disease (CTD) related pleural effusions: a four-year retrospective evaluation. BMC Pulm Med 2019; 19: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666231155747 for Adenosine deaminase-based measurement in the differential diagnosis of pleural effusion: a multicenter retrospective study by Lijuan Gao, Wujun Wang, Ying Zhang, Xueru Hu, Jing An, Yang Li, Mei Chen and Yongchun Shen in Therapeutic Advances in Respiratory Disease