Abstract
The procoagulant effect of microparticles (MPs) contributes to hypercoagulability-induced thrombosis. We provide preliminary findings of the MPs-Activated Clotting Time (MPs-ACT) assay to determine the procoagulant activity of MPs. MPs-rich plasma was obtained and recalcified. Changes in plasma viscoelasticity were evaluated and the time to the peak viscoelastic changes was defined as the MPs-ACT. MPs concentration was measured by flow cytometry. Coagulation products produced during plasma clotting were identified by fibrin and fibrinopeptide A. MPs were prepared in vitro and added to standard plasma to simulate pathological samples. In addition, reproducibility and sensitivity were evaluated. We confirmed the linear relationship between MPs-ACT and MP concentrations. Dynamic changes in fibrin production were depicted. We simulated the correlation between MPs-ACT and standard plasma containing MPs prepared in vitro. The reproducibility of high-value and low-value samples was 6.0% and 10.8%, respectively. MPs-ACT sensitively detected hypercoagulable samples from patients with pre-eclampsia, hip fractures, and lung tumors. MPs-ACT largely reflects the procoagulant effect of MPs. MPs-ACT sensitively and rapidly detects hypercoagulability with MPs-rich plasma. It may be promising for the diagnosis of hypercoagulable states induced by MPs.
Keywords: microparticle, hypercoagulability, coagulation function test, tissue factor, phosphatidylserine
Introduction
Hypercoagulable state exists in many diseases, such as bone fracture,1 pre-eclampsia,2 malignancy,3 and even COVID-19.4 The mechanisms underlying hypercoagulability are still confusing due to its complicated disease processes. Recently, the procoagulant activity of microparticles (MPs) in diseases has gradually been discovered, and has been described to play an extremely important role. MPs demonstrate procoagulant activity because they express phosphatidylserine (PS), tissue factor (TF), and other coagulation factors.5 Elevated platelet-derived MPs (PMPs), endothelial cell-derived MPs, and monocyte-derived MPs are observed in almost all thrombotic diseases that occur in the venous and arterial beds.6,7 However, due to the dynamic characteristics of MPs in the progression of various diseases, it is difficult to assess the hypercoagulable state induced by MPs over time.
Recently, we used annexin V to label PS on the MPs and discovered that MPs-rich plasma from patients with brain trauma (elevated circulating MPs) quickly coagulated when calcium buffer was added. But this phenomenon rarely occurs in samples from healthy donors. Subsequently, we observed similar phenomena in samples from patients with bone fracture, pre-eclampsia, and lung cancer, who presented higher plasma MPs levels than healthy individuals. Of note, results of the conventional coagulation testing (activated partial thromboplastin time, aPTT; prothrombin time, PT; thrombin time, TT) are within normal ranges. This suggested that the hypercoagulable state induced by MPs may easily be overlooked by previous detection methods.
To our knowledge, only a few commercially available kits can measure the activity of MPs. The ZYMUPHEN MP-Activity assay or the MP-TF assay from HYPHEN Biomed (France) measure the levels of PS-positive or TF-positive MPs in plasma using chromogenic substrates.11 The Diagnostica Stago (France) Procoagulant phospholipid assay (PPL) assay uses PS-free plasma mixed with test plasma and measures the clotting time to determine the level of procoagulant MPs.12 However, these assays focus more on PS and TF activity, ignoring other factors present in the blood.13 These assays are time-consuming and expensive, and are currently only used in a few high-level studies, with limited clinical application.11,14 Simple and efficient methods are needed in the point-of-care setting to identify patients who experience hypercoagulability caused by MPs.
At present, the main laboratory detection systems used to assess coagulation status are functional indicators reflecting the coagulation system, anticoagulation system, and fibrinolysis system. The detection of the coagulation system is largely based on citrated whole blood and platelet-poor plasma, including mainly PT, aPTT, TT, and viscoelastic hemostatic assays.15,16 The thromboelastometry (TEG), rotational TEG, and Sonoclot analysis are defined by the viscoelastic properties of whole blood samples. Due to the massive blood cells and platelets in whole blood, these tests are more likely to reflect the overall clotting ability of the samples.17 The activity of MPs is easily masked by the complex blood composition in conventional coagulation tests. Furthermore, an exogenous coagulant is added to the reaction during the detection of PT and aPTT, which are mainly used to indicate the lack of clotting factors. Patients with bone fractures are often considered to be at risk of thrombosis, although their conventional coagulation tests are often within the normal reference range reported by others.18 This means that the hypercoagulable state of patients is difficult to detect through traditional laboratory testing.
Therefore, we proposed the hypothesis that the procoagulant activity of MPs could be quantified through the clotting time of MPs-rich plasma. In this study, we verified the role and mechanism of MPs in the coagulation of MPs-rich plasma. We defined the clotting time of this MPs-rich plasma as the MP-induced MPs-ACT (activated clotting time) to distinguish it from other coagulation assays.
Materials and Methods
Sample Preparation and Assay Detection
Blood samples were collected from the median cubital vein using a 3.2% sodium citrate anticoagulant and processed within 2 h. Platelet-free plasma (PFP), namely MPs-rich plasma, was obtained as in previous studies.19 MPs-free plasma was obtained by ultracentrifugation at 100,000g for 1 h. A 200 μL volume of PFP was added to the test cup, followed by 170 μL of 20 mM calcium chloride (CaCl2). Once the sample solution was inserted in the Century Clot analyzer, plasma and CaCl2 were mixed evenly (total volume is 370 μL). The time when the viscoelasticity of the samples changed significantly was defined as the MPs-ACT, which is indicated in “seconds.” The clotting time of the recalcified MPs-rich plasma was measured using the Century Clot analyzer, whose probe can sense changes in plasma viscosity with high-frequency and low-amplitude vibrations. The main principle of the instrument is to measure the time when the viscoelasticity of the sample detected by the probe increases significantly, which is then defined as the coagulation time.
Detection of MPs in MPs-Rich Plasma
The concentration of MPs was determined by flow cytometry (LSR Fortessa, BD, USA) as in a previous study.19 The MPs were first identified by their size (0.1-1.0 μm) using Megamix polystyrene beads (0.5, 0.9, and 3 μm) to gate the MP region by forward and side scatter (Megamix-Plus SSC; Biocytex, Marseille, France). For antibody labeling, PFP (50 μL) was incubated with phycoerythrin (PE)-labeled annexin V (5 μL) (BD Biosciences, USA) to detect PS or PE-labeled TF (5 μL) (BD Biosciences, USA) to detect TF levels according to the manufacturer's instructions, respectively. We used AccuCount Ultra Rainbow Fluorescent Particles (3.8 micron; Spherotech, Lake Forest, IL, USA) to quantify the number of MPs. The following formula was used to calculate the concentration of particles: 10,120*MP (#) /(microbeads*volume) *dilution factor. In addition, MPs were identified by transmission electron microscopy (TEM). PFP was centrifuged at 100,000g for 1 h to enrich the MPs in plasma. The pellets were resuspended in normal saline. The method used for staining MPs was based on a previous study.20 The dried slides were observed using a Hitachi HT-7700 TEM (Japan). MPs were identified based on their diameter and double membrane structure.
Preparation of MPs to Explore the Correlation Between MP Levels and MPs-ACT
PMPs and Brain derived microparticles (BDMPs) are prepared and added to standard plasma to simulate pathological samples. PMPs were prepared as described previously with minor modifications.21 Citrated whole blood samples, obtained from the blood transfusion department, were centrifuged at 150g for 15 min to obtain platelet-rich plasma, then centrifuged at 1500g for 15 min to obtain platelets. After washing with a Tyrode solution, purified platelets were activated with collagen I at 37°C for 1.5 h to produce PMPs. The solution was then centrifuged at 1500g for 15 min to remove platelets and the PMPs were collected after ultracentrifugation at 100,000g for 1 h. The PMPs concentration was determined by flow cytometry. BDMPs obtained from the brains of C57BL/6J mice were generated by freeze-thawing injury.22
Evaluation of the Fibrin Dynamics of the Plasma Clot
We used liquid chromatography–mass spectrometry to detect the dynamics of fibrinopeptide A (FPA) with reaction time during the coagulation process of MPs-ACT from PFP and supernatant samples. After the reaction ended, 0.1 M zinc sulfate dissolved in 50% methanol was immediately added to the samples to stop the coagulation reaction. The samples were then centrifuged and placed in a sample injection vial for analysis.23 Hopping probe ion conductance microscopy (HPICM) was used to study the morphology of fibrin. The scan time was determined by the complexity of the sample and the selected resolution.24 Before adding CaCl2, an image was scanned as a control. Then scanning continued until the plasma coagulated to observe the fibrin production process.
Reproducibility Evaluation of the Assay
Samples from healthy donors and patients were measured repeatedly on the same analyzer 10 times within 2 h. The mean, standard deviation (SD), and coefficient of variation (CV) were calculated to evaluate the repeatability of the experiment.
Sensitivity Evaluation of the Assay
Blood samples from healthy donors and patients with pre-eclampsia, hip fracture, and lung cancer were collected and tested according to the above method to verify the sensitivity of MPs-ACT in identifying the hypercoagulable states caused by MPs.
Statistical Analysis
SPSS statistical software (version 23.0, IBM) was used for all statistical analyses in the present study. The results are presented as the mean ± SD and were analyzed using a t-test to compare two groups. The CV was calculated as the SD/mean*100%. The normal distribution of the data was confirmed by the Shapiro-Wilk test. We used G*Power (version 3.1.9.7) for power analysis and to determine the minimum sample size. P < .05 was considered statistically significant.
Results
An Introduction to the Century Clot Analyzer
Physical image and schematic diagram of the Century Clot analyzer (Century Yikang Medical Technology Development Co., Ltd, Tianjin, China) are shown in Figure 1A and B. This device consists of an Electronic Signal Converter, Probe, and Test Cup. Machine quality control includes two parts: the first step is air quality control, to determine whether the test platform is in a stable state and probe is physically disturbed. The second step is to test with standard viscosity oil to make sure that the resistance value detected by the probe is within the set range.
Figure 1.
(A) and (B) The physical image and schematic diagram of the Century Clot analyzer. (C) and (D) Schematic diagram of MPs gating and representative bivariate flow cytometry plots (forward and side scatter). (E) MPs concentration decreased after ultracentrifugation and re-elevated in suspension (n=8). (F) The MPs-ACT were compared among PFP, supernatant, and suspension groups (n=8). (G) Diagram of MPs-ACT from three representative samples results (from left to right are suspension, PFP and supernatant). (H) TEM image of sediment MPs (bar =1000 nm). Data are shown as mean ± SD. **P<.01. Abbreviations: MPs, microparticles; MPs-ACT, MPs-Activated Clotting Time; PFP, platelet-free plasma; SD, standard deviation; TEM, transmission electron microscopy.
MPs Concentration Determines MPs-ACT
Flow cytometric characterization was performed after calibration with fluorescent beads (0.5, 0.9, and 3.0 µm), and the MPs gate was set above the 0.9 µm bead cloud (Figure 1C). A forward scatter versus side scatter (FSC vs SSC) dot plot for MPs concentrate is shown as an example (Figure 1D). The total concentration of MPs in the supernatant (249 ± 42/μL) of PFP decreased significantly after ultracentrifugation, which was significantly lower than that of PFP (1449 ± 310/μL; P < .0001) (Figure 1E). The concentration of resuspended sediment (1817 ± 311/μL) was significantly higher than that of supernatant (P < .0001) and the PFP (P < .0001). Consequently, there was a statistically significant difference in the MPs-ACT of PFP (300 ± 38 s) and the supernatant (593 ± 118 s; P = .0003) (Figure 1F). In MPs-enriched plasma, the MPs-ACT was significantly shortened (207 ± 36 s; P = .0023) compared with the PFP group. MPs-ACT results from three representative samples (from left to right in suspension solutions, PFP, and supernatant, respectively, as shown in Figure 1G). The sediment was analyzed by TEM, to confirm the vesicle-like membrane structures having a diameter of 100-1000 nm (Figure 1H).
PS and TF Influenced MPs-ACT
PS and TF are expressed on the surface of many cell-derived MPs and are involved in the hypercoagulable state. We quantified the concentration of PS-positive and TF-positive MPs by flow cytometry and verified its relationship to MPs-ACT. MPs-ACT and PS-positive MPs levels were negatively correlated (R2 = 0.4012, P = .0084) (Figure 2A). Similarly, MPs-ACT and TF-positive MPs were negatively correlated (R2 = 0.3348, P = .0189) (Figure 2B).
Figure 2.
(A) The MPs-ACT is negatively correlated with PS-positive MPs (R2=0.4012, P=.0084, n=16). (B) MPs-ACT is negatively correlated with TF-positive MPs (R2=0.3348, P=.0189, n=16). (C) The negative correlation between MPs-ACT and MPs is simulated by BDMPs (n=4). (D) The negative correlation between MPs-ACT and MPs is simulated by PMPs (n=4). Data are shown as mean ± SD. Abbreviations: MPs, microparticles; MPs-ACT, MPs-Activated Clotting Time; PS, phosphatidylserine; SD, standard deviation; TF, tissue factor; PMPs, Platelet derived microparticles.
Preparation of BDMPs and PMPs to Verify the Correlation Between MPs and MPs-ACT
PMPs and BDMPs have been shown to exert procoagulant activity. We added different concentrations of PMPs or BDMPs separately to plasma to simulate the effect of MPs on MPs-ACT. MPs-ACT showed a good negative correlation with increasing MPs concentration decreasing from 500s to 100 s (Figure 2C and D). This time interval covers the clotting time of most patients and healthy individuals.
Comparison of MPs-ACT and the Conventional Coagulation Assay
Samples from hip fractured patients were collected and centrifuged. We tested the PT, aPTT, TT, and fibrinogen (FIB), international normalized ratio (INR) of PFP, and supernatant after removing most MPs by ultracentrifugation, to reveal changes in clotting factors. There was no significant difference in PT (10.9 ± 0.4 s; 10.9 ± 0.5 s; P = 1.000) or TT (21.9 ± 0.4 s; 21.5 ± 0.7 s; P = .140) (Figure 3A), INR (1.0 ± 0.04; 1.0 ± 0.05; P = .9489) (Figure 3B) or FIB (2.7 ± 0.2 g/L; 2.9 ± 0.4 g/L; P = .091) (Figure 3C) between the PFP and the supernatant. Differences in the aPTT of the PFP and the supernatant were significant (28.8 ± 1.1 s; 31.3 ± 2.1 s; P = .004). But the test results of both groups were within the normal reference range.
Figure 3.
(A) and (B) Detect PFP and supernatant by conventional coagulation function assay (n=6). (C) Fibrinogen concentration did not change before and after ultracentrifugation (n=6). (D) Dynamic production of FPA during MPs-ACT reaction (n=5). (E) Representative molecular ion peak for FPA. (F)-(H) The production of fibrin during the MPs-ACT reaction scanned by HPICM. Data are shown as mean ± SD. ns, P>.05, **P<.01. Abbreviations: HPICM, Hopping probe ion conductance microscopy; FPA, fibrinopeptide A; MPs, microparticles; MPs-ACT, MPs-Activated Clotting Time; ns, non significant; PFP, platelet-free plasma; SD, standard deviation.
Fibrin Production During MPs-ACT
FPA is released when FIB is transformed into fibrin by thrombin. It is a reliable indicator of coagulation activity and fibrin formation and is positively correlated with fibrin production. FPA in the PFP samples can be detected after 2 min, whereas in the supernatant it can be detected after about 6 min. The FPA generation speed is significantly slower, and the total amount is reduced after removing MPs from the PFP (Figure 3D). The MPs-ACT of the PFP samples was approximately 600 s and that of the supernatant group was 900 s. Both MPs-ACT cover approximately the point of time when FPA changes the most. The molecular ion peak of FPA was identified by mass spectrometry (Figure 3E). To synchronize with the FPA result, we also tested fibrin production by HPICM. HPICM scans fibrin in the shape of Chinese character “Hui,” starting from the lower left corner of the picture. Scans in the absence of CaCl2 were used as a negative control (Figure 3F). When the first 7 min scan was completed, a small amount of fibrin was found in the center of the image (Figure 3G). When scanning for 20 min, significant fibrin was produced (Figure 3H). The scan results confirmed that fibrin was produced during the MPs-ACT.
Effect of Sample Volume on MPs-ACT
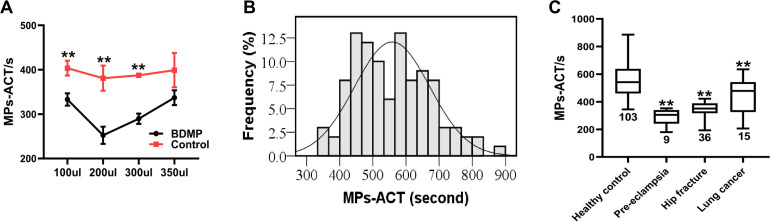
To explore how the sample volume influenced MPs-ACT, we kept the total reaction volume and the final concentration of CaCl2 unchanged and varied the amount of sample added. Patient samples were replaced with standard plasma solutions spiked with BDMPs, and healthy donor samples were simulated with standard plasma with the addition of normal saline. We explored 4 different volumes of plasma and found that there was a significant difference between the BDMPs and the control groups, when the plasma volume is 100 μL (333 ± 14 s vs 404 ± 17 s; P = .005), 200 μL (252 ± 20 s vs 381 ± 28 s; P = .003), and 300 μL (290 ± 12 s vs 387 ± 5 s; P = .000). However, there was no significant difference between the two groups (337 ± 17 s vs 366 ± 96 s; P = .637) (Figure 4A) when the sample volume was 350 μL. Thus, when the sample volume was 200 μL, the difference between the BDMPs and the control groups was the most significant, and defined the maximum detection sensitivity.
Figure 4.
(A) Effect of sample volume on MPs-ACT (n=4). (B) Normal distribution graph of MPs-ACT from healthy donors’ samples (n=103, P=.071). (C) The MPs-ACT of preeclampsia, hip fracture, and lung cancer significantly shortened compared to healthy donors. Data are shown as mean ± SD. **P<.01. Abbreviations: MPs-ACT, microparticles-Activated Clotting Time; SD, standard deviation.
Reproducibility of the MPs-ACT Assay
We measured high-value (healthy donors) and low-value (patients) samples 10 times to confirm the stability of the results as evaluated by the CV. The CV of the high-value and low-value samples were, respectively, 6.0% and 10.8% (Table 1). As preliminary data for new detection method, a CV of <15% is currently acceptable.
Table 1.
Reproducibility Assay of MPs-ACT.
| Repeated times | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | SD | CV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low value | 304 | 294 | 310 | 279 | 300 | 338 | 295 | 334 | 316 | 297 | 307 | 18 | 6.0% |
| High value | 544 | 501 | 650 | 626 | 466 | 521 | 589 | 512 | 528 | 603 | 554 | 60 | 10.8% |
High-value and low-value samples are repeated 10 times to evaluate the reproducibility.
Abbreviations: CV, coefficient of variation; MPs-ACT, microparticles-Activated Clotting Time; SD, standard deviation.
Sensitivity of MPs-ACT Assay
Whole blood treated with 3.2% sodium citrate obtained from healthy donors and patients was used to test the sensitivity of the MPs-ACT assay. Samples were analyzed within 2 h of collection time. We initially tested samples from healthy donors (n = 103) to verify the feasibility of the test method. Our results showed that the results of the MPs-ACT in healthy subjects was consistent with a normal distribution (Shapiro-Wilk test, P = .071) (Figure 4B). The normal reference range of the MPs-ACT assay in healthy people was 335s-781s, according to the formula of the normal distribution (average ± 1.96 SD). The sensitivity of the MPs-ACT assay was verified in patient samples. Patients with pre-eclampsia, hip fracture, and lung cancer are widely considered to have a high incidence of a hypercoagulable state. We used the MPs-ACT assay to evaluate the clotting time of PFP samples and the results showed that MPs-ACT of patients with pre-eclampsia (n = 9, 290 ± 58 s; P < .0001), hip fractures (n = 36, 349 ± 49 s; P < .0001), and lung cancer (n = 15, 447 ± 140 s; P = .0004) were significantly reduced compared to healthy control subjects (558 ± 114 s; Figure 4C).
Discussion
The role of MPs in the hypercoagulable state has been confirmed by some studies.6,7 Moreover, MPs may play a central role in physiological hemostasis. Unlike other coagulopathies, pathological hypercoagulation caused by MPs has unique characteristics and requires targeted treatment.25 However, we lack a specific assay that can high-effectively and rapidly identify coagulopathy caused by MPs.26 In this study, the hypercoagulable state caused by MPs was determined by detecting changes in the viscoelasticity of the MPs-rich plasma (namely PFP). We provide reliable findings associating the MPs-ACT assay with the activity of MPs.
First, we eliminated any interference by blood cells and platelets, and prepared samples consisting of only suspended MPs, coagulation factors, and fibrinolytic factors in MPs-rich plasma. Therefore, the plasma clotting time was highly dependent on the amount of procoagulant present. The main hurdle in performing MPs-based clotting tests is contamination with residual platelets. The centrifugal force used in sample preparation is sufficient to remove most platelets. We found that MPs-ACT was reversed when MPs were removed after ultracentrifugation. This confirmed the role of MPs in MPs-ACT. Indeed, a previous study also reported that removing MPs from plasma prolongs the clotting time.27 The supernatant aPTT was prolonged, indicating that MPs affected the intrinsic coagulation pathway. The fact that MPs activate the intrinsic pathway has been reported previously.5,28 MPs also influence intrinsic pathways through direct kallikrein activation of Clotting factor FIX, which complicates the coagulation mechanism of MPs.29 However, significantly prolonged aPTT is still within the normal range, indicating that it is not sufficiently sensitive to MP-induced hypercoagulation. We speculate that a possible reason is that an exogenous coagulant is added during the aPTT assay process to reduce its sensitivity. This explains the confusion whereby patients with hip fractures or pre-eclampsia present almost no obvious abnormalities in aPTT and PT detected during a hospital stay. However, the TT is not affected by ultracentrifugation, which is consistent with the results of the FIB assays and suggests that FIB levels do not decrease.
The PS and TF are bound to the surface of most MPs and are the most studied markers.29 We also explored the association between MPs-ACT and PS and TF. Our results show that the MPs-ACT assay findings were negatively correlated with PS-positive or TF-positive MPs. This implied that the procoagulant effect of MPs was produced by the different coagulation factors carried by the MPs. However, the reduced MPs did not extend the PT in Figure 3A. These two observations seem to be contradictory. This might mean that TT is not sensitive enough to detect the abnormal extrinsic coagulation pathway activated by MP. Previous studied have shown that the procoagulant effect caused by MPs originating from different cells is quite different.30–32 Due to the diversity of MPs, we did not study the effects of different MP types on the results of the MPs-ACT assay. However, the coagulation time of PFP largely depends on the number of procoagulant substances in a cell-free plasma and PFP, mainly the concentration of MPs. As there is currently no evidence that MPs can bypass the intrinsic and extrinsic coagulation pathways. As long as the MPs are in PFP, their procoagulant effect on plasma will be detected.
Second, in the simulation experiment, we observed that MPs-ACT decreased as BDMP concentrations increased. BDMPs induce coagulopathy after brain trauma and have higher procoagulant activity than other MPs.22 Our study demonstrated that the MPs-ACT assay time shortened as the concentration of BDMPs increased. The negative correlation between MPs-ACT and exogenous coagulants was verified in vitro. However, it may not be possible to fully simulate the coagulation effect of complex MPs in the body due to its artifacts resulting from the sample preparation of MPs. Translocated expression of coagulation factors can be found in the central nervous system.33–35 We speculate that MPs derived from brain tissue may also carry these paternal clotting factors and involved in the clotting process, thus allowing MPs to promote coagulation beyond PS and TF, especially for BDMPs. Both prothrombin and its active form thrombin, have also been detected locally in the brain and exert physiological and pathological functions in the central nervous system.36 This may complicate coagulation disorders subsequent to brain trauma due to the clotting factor expressed in BDMPs.
In addition to procoagulant factors, there are anticoagulant factors present in plasma. Anticoagulant proteins naturally occur in human plasma, which can block the procoagulant effect of MPs, such as mfge8, del-1, and annexin V.37–39 They influence the coagulation effect by binding the PS on the surface of the MPs. The MPs-ACT assay reflects the balance between procoagulant and anticoagulant activity, which means there is an increase in pathological MPs or a relative shortage of anticoagulant proteins when the MPs-ACT of PFP is shortened. The increase in pathological MPs is a common factor.
We determined that different amounts of plasma showed different sensitivity in the MPs-ACT assay. We added known concentrations of BDMPs to standard plasma to simulate patient sample and found that the difference between the BDMPs group and control groups was the most significant when a 200 μL sample was added. Thus, we identified the optimal sample volume required for this detection method, to achieve the best sensitivity. MPs-ACT assay detected a hypercoagulable state using a sample volume of 100 μL. This may be the result of the dilution of coagulation factors when hemodilution increases.40 Although the mechanism behind this observation still needs to be further clarified as MPs are also diluted in this preparation. MPs-ACT also detected a hypocoagulable trend using sample volumes of 300 or 350 μL, which may be attributed to the relative deficiency of calcium chloride at this dilution.
Third, we studied the metabolic changes of FPA, an FIB metabolite, during the coagulation process of PFP.41 FPA can be used to indicate thrombin activity and the rate of coagulation reaction.23,42 Our results show that there was almost no FPA production in the early stage of coagulation, and it significantly accelerated in the intermediate stage and overlapped those of the MPs-ACT assay. After removing most MPs, the production of FPA was slower and reduced. This also further confirms that MPs can accelerate the clotting process. Although what causes the plasma viscoelasticity to increase during this process was the mass production of fibrin, and FPA cannot explain this process. Therefore, we used HPICM to observe the fibrin production. Consistent with the results of FPA, fibrin production was also increased significantly in the intermediate stage. This observation indicates that the significant increase in plasma viscoelasticity derives from the massive production of fibrin. A previous study found that MPs are attached to fibers during fibrin production.43 We did not observe whether many MPs were embedded in fibrin, which would make the results more convincing. But this is listed as our future research plan.
For a reliable test, its reproducibility is also very important in addition to its sensitivity. We used high-value and low-value samples to simulate samples from healthy volunteers and patients and repeated the measurement 10 times. The CV of 10 tests was calculated and used to evaluate the stability of the test, namely its repeatability. The results show that the CV obtained was within a reasonable range (<15%). Subsequently, samples from 103 healthy donors were tested and the distribution of MPs-ACT was consistent with the normal distribution. Samples from patients with fractures, pre-eclampsia and lung cancer have significantly shorter MPs-ACT results compared to healthy donors. However, patients with lung cancer are in different stages of the disease and receive treatment, which may have influenced the results of the MPs-ACT assay. Strict inclusion and exclusion criteria are needed to obtain consistent MPs-ACT results.
There are other methods that have been used previously for the assessment of MPs procoagulant activity including clotting time by spectrophotometry,44 thrombin generation by chromogenic substrates,45 and “thrombodynamics” assay.46 Although these assays can determine the procoagulant activity of MPs, they require expensive equipment, complicated procedures, and long waiting times. The MPs-ACT can be performed at the bedside and the results are available in 10-15 min. Simple sampling and preparation involving the addition of only calcium chloride, makes the assay convenient for doctors to quickly determine the coagulation function status of patients.
Taken together, we have provided preliminary finding of a fast and efficient assay to detect the procoagulant activity of MPs. The assay detects the general coagulation time of PFP. The MPs-ACT assay is closely related to the function and quantity of MPs in plasma. However, in practice, we also found that MPs-ACT did not detect hypercoagulability in patients with cerebral hemorrhage, which may be a limitation of MPs-ACT. In the following studies, we will further clarify the limitations of MPs-ACT. And the number of red blood cells also affects MPs-ACT, so it may not be suitable for people with abnormal red blood cell counts. Although preliminary verification has been obtained in some clinical samples, its effect on monitoring hypercoagulability needs to be further verified and optimized. Additionally, we found that MPs may express certain coagulation factors, which further complicates the MPs-induced procoagulant activity. Furthermore, traditional coagulation assays that are currently available may not easily address clinically complex problems, which focus more on the detection of PS and TF, ignoring other pathways or coagulation factors.29
Conclusions
This study describes the development of the MPs-ACT assay to the procoagulant activity of MPs. We preliminarily demonstrated that MPs-ACT may indicate hypercoagulability in certain diseases, especially those with high plasma MPs levels. MPs-ACT might represent an important reference to evaluate the procoagulant activity of MPs in these patients.
Footnotes
Acknowledgement: We thank Century Yikang Medical Technology Development Co., Ltd. for providing us with equipment and technical guidance.
Author Contributions: YG and XL recruited patients, collected samples, performed experiments, analyzed data, and wrote the manuscript. YQ, JM, JR, XL, CX, QL, YiL, WC, LeL, SZ, and YaL recruited patients, collected samples, and performed experiments. JZ and LiL developed hypotheses, designed the study, analyzed the data, and wrote the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from the National Natural Science Foundation of China, grant nos. 81930031, 81901525, and Tianjin Municipal Education Commission, grant no. 19JCQNJC09500.
ORCID iD: Li Liu https://orcid.org/0000-0002-7286-4524
References
- 1.Zhao K, Zhang J, Li J, et al. Incidence of and risk factors for new-onset deep venous thrombosis after intertrochanteric fracture surgery. Sci Rep. 2021;11(1):17319. doi: 10.1038/s41598-021-96937-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han C, Chen YY, Dong JF. Prothrombotic state associated with preeclampsia. Curr Opin Hematol. 2021;28(5):323‐330. doi: 10.1097/moh.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau A, Van Dreden P, Khaterchi A, et al. Procoagulant microparticles derived from cancer cells have determinant role in the hypercoagulable state associated with cancer. Int J Oncol. 2017;51(6):1793‐1800. doi: 10.3892/ijo.2017.4170 [DOI] [PubMed] [Google Scholar]
- 4.Qiu F, Wu Y, Zhang A, et al. Changes of coagulation function and risk of stroke in patients with COVID-19. Brain Behav. 2021;11(6):e02185. doi: 10.1002/brb3.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noubouossie DF, Henderson MW, Mooberry M, et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135(10):755‐765. doi: 10.1182/blood.2019001643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campello E, Spiezia L, Radu CM, et al. Microparticles as biomarkers of venous thromboembolic events. Biomark Med. 2016;10(7):743‐755. doi: 10.2217/bmm-2015-0063 [DOI] [PubMed] [Google Scholar]
- 7.Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb. 2016;23(1):1‐9. doi: 10.5551/jat.32326 [DOI] [PubMed] [Google Scholar]
- 8.Condrat CE, Varlas VN, Duică F, et al. Pregnancy-related extracellular vesicles revisited. Int J Mol Sci. 2021;22(8):3904. doi: 10.3390/ijms22083904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen S, Kristensen AF, Falkmer U, et al. Increased activity of procoagulant factors in patients with small cell lung cancer. PLoS One. 2021;16(7):e0253613. doi: 10.1371/journal.pone.0253613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fröhlich M, Schäfer N, Caspers M, et al. Temporal phenotyping of circulating microparticles after trauma: a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2018;26(1):33. doi: 10.1186/s13049-018-0499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayers L, Harrison P, Kohler M, et al. Procoagulant and platelet-derived microvesicle absolute counts determined by flow cytometry correlates with a measurement of their functional capacity. J Extracell Vesicles. 2014;3(1). doi: 10.3402/jev.v3.25348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campello E, Spiezia L, Radu CM, et al. Evaluation of a procoagulant phospholipid functional assay as a routine test for measuring circulating microparticle activity. Blood Coagul Fibrinolysis. 2014;25(5):534‐537. doi: 10.1097/mbc.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 13.Connor DE, Exner T, Ma DD, et al. Detection of the procoagulant activity of microparticle-associated phosphatidylserine using XACT. Blood Coagul Fibrinolysis. 2009;20(7):558‐564. doi: 10.1097/MBC.0b013e32832ee915 [DOI] [PubMed] [Google Scholar]
- 14.Hisada Y, Alexander W, Kasthuri R, et al. Measurement of microparticle tissue factor activity in clinical samples: a summary of two tissue factor-dependent FXa generation assays. Thromb Res. 2016;139:90‐97. doi: 10.1016/j.thromres.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaydakov ME, Sigmon DF, Blebea J. Thromboelastography. StatPearls. StatPearls Publishing.Copyright © 2021, StatPearls Publishing LLC.; 2021.
- 16.Whiting D, DiNardo J. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89(2):228‐232. doi: 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 17.Johansson PI, Stissing T, Bochsen L, et al. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsantes AG, Trikoupis IG, Papadopoulos DV, et al. Higher coagulation activity in hip fracture patients: a case-control study using rotational thromboelastometry. Int J Lab Hematol. 2021;43(3):477‐484. doi: 10.1111/ijlh.13409 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Huang P, Han C, et al. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: a clinical observational study. BJOG. 2021;128(6):1037‐1046. doi: 10.1111/1471-0528.16552 [DOI] [PubMed] [Google Scholar]
- 20.Dong X, Li M, Li Q, et al. Effects of cryopreservation on microparticles concentration, procoagulant function, size distribution, and morphology. Med Sci Monitor. 2019;25:6675‐6690. doi: 10.12659/msm.917962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li SS, Gao S, Chen Y, et al. Platelet-derived microvesicles induce calcium oscillations and promote VSMC migration via TRPV4. Theranostics. 2021;11(5):2410‐2423. doi: 10.7150/thno.47182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian Y, Salsbery B, Wang M, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125(13):2151‐2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin M, Lassman ME, Weiner R, et al. Development and fit-for-purpose validation of a LC-MS/MS assay for fibrinogen peptide A quantitation in human plasma. Bioanalysis. 2014;6(13):1759‐1766. doi: 10.4155/bio.14.148 [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Li Y, Zhu H, et al. Use of non-contact hopping probe ion conductance microscopy to investigate dynamic morphology of live platelets. Platelets. 2015;26(5):480–485.. doi: 10.3109/09537104.2014.940888 [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Zhou Y, Tian Y, et al. Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell. 2017;8(11):801‐810. doi: 10.1007/s13238-017-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iba T, Ogura H. Role of extracellular vesicles in the development of sepsis-induced coagulopathy. J Intensive Care. 2018;6:68. doi: 10.1186/s40560-018-0340-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189‐197. [PubMed] [Google Scholar]
- 28.Mooberry MJ, Bradford R, Hobl EL, et al. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J Thromb Haemost. 2016;14(5):1031‐1042. doi: 10.1111/jth.13285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooberry MJ, Key NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. 2016;89(2):111‐122. doi: 10.1002/cyto.a.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53(8):1744‐1754. doi: 10.1111/trf.12008 [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Wang M, Tian Y, et al. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. 2016;127(22):2763‐2772. doi: 10.1182/blood-2015-12-688838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacroix R, Plawinski L, Robert S, et al. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012;97(12):1864‐1872. doi: 10.3324/haematol.2012.066167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Festoff BW, Smirnova IV, Ma J, et al. Thrombin, its receptor and protease nexin I, its potent serpin, in the nervous system. Semin Thromb Hemost. 1996;22(3):267‐271. doi: 10.1055/s-2007-999018 [DOI] [PubMed] [Google Scholar]
- 34.Shikamoto Y, Morita T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett. 1999;463(3):387‐389. doi: 10.1016/s0014-5793(99)01657-9 [DOI] [PubMed] [Google Scholar]
- 35.Krenzlin H, Lorenz V, Alessandri B. The involvement of thrombin in the pathogenesis of glioblastoma. J Neurosci Res. 2017;95(10):2080‐2085. doi: 10.1002/jnr.24049 [DOI] [PubMed] [Google Scholar]
- 36.Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemostasis. 2008;100(4):576‐581. [PubMed] [Google Scholar]
- 37.Zhou Y, Cai W, Zhao Z, et al. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood. 2018;131(5):563‐572. doi: 10.1182/blood-2017-08-801738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta SK, Le A, Chavakis T, et al. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125(13):1664‐1672. doi: 10.1161/circulationaha.111.068833 [DOI] [PubMed] [Google Scholar]
- 39.Frey B, Gaipl US. The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Semin Immunopathol. 2011;33(5):497‐516. doi: 10.1007/s00281-010-0228-6 [DOI] [PubMed] [Google Scholar]
- 40.Getrajdman C, Sison M, Lin HM, et al. The effects of hemodilution on coagulation in term parturients: an in vitro study utilizing rotational thromboelastometry. J Matern Fetal Neonatal Med. 2020;35(10):1‐9. doi: 10.1080/14767058.2020.1776250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shainoff JR, Smejkal GB, DiBello PM, et al. Isolation and characterization of the fibrin intermediate arising from cleavage of one fibrinopeptide A from fibrinogen. J Biol Chem. 1996;271(39):24129‐24137. doi: 10.1074/jbc.271.39.24129 [DOI] [PubMed] [Google Scholar]
- 42.Emanuele RM, Fareed J, Walenga JM, et al. Evaluation of thrombin activity by immunoquantitation of fibrinopeptide A generation. Semin Thromb Hemost. 1986;12(4):318‐323. doi: 10.1055/s-2007-1003573 [DOI] [PubMed] [Google Scholar]
- 43.Zong Y, Pruner I, Antovic A, et al. Phosphatidylserine positive microparticles improve hemostasis in in-vitro hemophilia A plasma models. Sci Rep. 2020;10(1):7871. doi: 10.1038/s41598-020-64686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aleman MM, Gardiner C, Harrison P, et al. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9(11):2251‐2261. doi: 10.1111/j.1538-7836.2011.04488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripisciano C, Weiss R, Eichhorn T, et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7(1):6522. doi: 10.1038/s41598-017-03262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipets E, Vlasova O, Urnova E, et al. Circulating contact-pathway-activating microparticles together with factors IXa and XIa induce spontaneous clotting in plasma of hematology and cardiologic patients. PLoS One. 2014;9(1):e87692. doi: 10.1371/journal.pone.0087692 [DOI] [PMC free article] [PubMed] [Google Scholar]