Abstract
Lower urinary tract symptoms (LUTS) secondary to benign prostrate hyperplasia (BPH) are common geriatric diseases, and its incidence rises with age. The treatment of BPH and LUTS is becoming a burden for health care. The meta-analysis was performed to evaluate the efficacy and safety of combination therapy (tamsulosin plus tadalafil) compared with tamsulosin alone in treatment of males with LUTS/BPH. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses were utilized to conduct this study. There were several databases available for literature retrieval, including Medline, Embase, PubMed, Scopus, Web of Science databases, and Cochrane Controlled Trials Register. To improve the comprehensiveness of the search, related references were also searched. Finally, six randomized controlled trials including 441 patients were included. The combination therapy had significant improvements in total International Prostate Symptom Score (p < .0001), quality of life score (p = .003), maximum urine flow rate (p < .00001), and International Index of Erectile Function (p < .00001) compared with the tamsulosin monotherapy, but there was no obvious difference in postvoid residual volume (p = .06). In terms of safety, the combination group had comparable rates of discontinuation due to adverse events (p = .19) than the monotherapy group except for pain symptoms (p < .0001). The combination of tamsulosin and tadalafil provided a preferable therapeutic effect compared with the tamsulosin alone in treating males with BPH/LUTS, and both therapy regimens were well tolerated by the patients.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, tadalafil, tamsulosin, meta-analysis, randomized controlled trial
Introduction
Lower urinary tract symptoms (LUTS) secondary to benign prostrate hyperplasia (BPH) are extremely common in aging men (Rosen et al., 2003). The negative impact of LUTS and BPH on the quality of life (QoL) of aging men has been thoroughly documented in the literature (Robertson et al., 2007; Rosen et al., 2003).
Tadalafil, a long-lasting phosphodiesterase type 5 inhibitor (PDE5i), is an established medication for patients with LUTS secondary to BPH (Madersbacher, 2017; Zhou, Chen et al., 2019). The level of evidence for this treatment is “1a,” and the strength grade of recommendation is “Strong” in the guideline of European Urological Association (EAU). Although tadalafil monotherapy is effective in treating males with LUTS, the efficacy of the combination of alpha-blockers and PDE5i is still emerging. There have been few studies that compared the combination of alpha-blockers and PDE5i with alpha-blocker monotherapy (often considered the first-line treatment in male with LUTS; Chua et al., 2015).
Tamsulosin has a considerable effect on relieving patients’ subjective symptoms, and it is an alpha-blocker approved to treat LUTS and the safest alpha-blocker to be used in the combination therapy with PDE5i (Kloner, 2004; Kloner et al., 2004). To the best of our knowledge, there have been few evidence-based medicine research concentrating on the combination therapy of two drugs with distinct mechanisms of action (Nagasubramanian et al., 2020; Zhou, Zheng et al., 2019). In this situation, combination therapy with tamsulosin plus tadalafil might be an option.
This meta-analysis was performed to assess the efficacy and safety of combination of tamsulosin and tadalafil compared with tamsulosin alone in treating males with LUTS secondary to BPH.
Materials and Methods
Search Strategy and Selection Criteria
This meta-analysis was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Moher et al., 2009). First, six online academic databases were searched for papers published in English, with no publication restrictions, including Medline, Embase, PubMed, Scopus, Web of Science, and Cochrane Controlled Trials Register until January 2022. The search keywords were a combination of free text and controlled vocabulary (i.e., MeSH terms) for each database, including “tamsulosin, tadalafil, LUTS, BPH and randomized controlled trial (RCT).”
Then, using Endnote X9 software (Clarivate, PA, USA), we deleted duplicates from the list of literatures retrieved in the first step. The following studies were excluded: reviews, editorials, book or book chapters, commentaries, conference papers, brief communications, articles with no complete text, studies that used qualitative methods only or interventional research. If multiple papers analyzed the same data set, the article with the most data was selected.
Third, articles were separately reviewed by two researchers who examined the title and abstract, followed by the full text if the paper matched the inclusion criteria. A third researcher would include or exclude papers in the event of a disagreement between the two researchers.
Quality Assessment
We used the Cochrane risk of bias tool to evaluate the quality of the retrieved studies (Cumpston et al., 2019). The quality items were selective outcome reporting, random sequence generation, incomplete outcome data, allocation concealment, blinding, and other sources of bias. The risk of bias for each study was independently assessed by four reviewers. Any differences were settled through discussion. A graph summarizing the risk of bias was generated based on discussions among the authors. The studies were then classified qualitatively using guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.3.0 (Cumpston et al., 2019).
Data Extraction
The following data were collected for each study by different reviewers: (a) published time, (b) first author’s name, (c) patients’ managed treatment, (d) number of patients in each group, (e) medication management, (f) treatment period, (g) dosage of medication, and (h) obtained data: total International Prostate Symptom Score (IPSS), QoL score, IPSS storage, IPSS voiding, maximum urine flow rate (Qmax), postvoid residual volume (PVR), International Index of Erectile Function (IIEF), any adverse events (AEs), discontinuation due to AEs and pain (including headache, myalgia, backache, and bone pain). The study needed no ethical approval. The primary outcome was IPSS, and secondary outcomes were QoL, Qmax, IIEF, and PVR.
Statistical Analyses and Meta-Analysis
The abstracted data were analyzed with Review Manager Version 5.3.0 (Cochrane Collaboration, Oxford, UK; Cumpston et al., 2019). Differences between baseline (study entry) and study completion (end-point measure) were used to reflect changes in the primary and secondary outcomes. The mean difference (MD) with 95% confidence interval (CI) was utilized to analyze the continuous data, and the odds ratio (OR) with 95% CI was applied to analyze the dichotomous data. The chi-square test based on the Q statistic was performed to check the heterogeneity among the studies, and the result was determined to be significant at p < .05. I 2 statistic was applied to analyze inconsistent results, which can reflect the proportion of heterogeneity across trials. We used a random-effects model to reduce the impact of heterogeneity on the results.
Results
Characteristics of Each Study
The search and selection process was demonstrated in the PRISMA flow diagram (Figure 1). The initial literature search identified 206 potentially relevant articles. By scrutinizing all abstracts and titles, reviewers excluded 170 articles according to the inclusion and exclusion criteria. Through reading the full text, we excluded 30 articles due to the lack of useful information. Finally, six articles (Bechara et al., 2008; Karami et al., 2016; Nagasubramanian et al., 2020; Negoro et al., 2020; Regadas et al., 2013; Singh et al., 2014) with six RCTs were used to compare tamsulosin plus tadalafil with tamsulosin alone in treating males with LUTS secondary to BPH (Figure 1). The details of six articles and baseline characteristics of patients are listed in Tables 1 and 2, respectively.
Figure 1.
Flow Diagram of the Study Selection Process
Note. RCT = randomized controlled trial.
Table 1.
Details of Individual Study
| Study | Country | Study design | Therapy | Method | Time of therapy (weeks) | Dosage (mg/mg) | Main inclusion criteria | Outcome measures | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||||
| Bechara et al. (2008) | Argentina | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 6 | 0.4 mg + 20 mg/0.4 mg | Men ≥50 years old with a history of BPH, IPSS ≥12, PSA ≤4.0 ng/mL, Qmax >5 mL/s and <15 mL/s, MVV >125 mL | IPSS, QoL, VAS, GAQ, Qmax, PVR, IIEF |
| Regadas et al. (2013) | Brazil | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 4 | 0.4 mg + 5 mg/0.4 mg | Men ≥45 years old with a history of BPH/LUTS, BOOI ≥20, IPSS ≥14 | IPSS, PdetQmax, Qmax |
| Singh et al. (2014) | India | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 12 | 0.4 mg + 10 mg/0.4 mg | Men ≥45 years old with a history of LUTS secondary to BPH, IPSS >8, PSA ≤4.0 ng/mL, Qmax >5 mL/s, and <15 mL/s, MVV >125 mL | IPSS, QoL, Qmax, PVR, IIEF |
| Karami et al. (2016) | Iran | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 12 | 0.4 mg + 20 mg/0.4 mg | Men ≥45 years old with a history of BPH/LUTS, total IPSS ≥12 | PSA, PVR, IPSS, Qmax, IIEF |
| Negoro et al. (2020) | Japan | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 6 | 0.2 mg + 5 mg/0.2 mg | Men ≥50 years old with a history of BPE, PV ≥20 and ≤40 mL, total IPSS ≥8, QoL ≥3 | QoL, PVR, IPSS, Qmax, IIEF |
| Nagasubramanian et al. (2020) | India | RCT | Tamsulosin plus Tadalafil | Tamsulosin | Oral | 12 | 0.4 mg + 5 mg/0.4 mg | Men >45 years old with a history of LUTS, IPSS ≥8 and ≤19, Qmax ≥5 mL/s and ≤15 mL/s | IPSS, QoL, IIEF, Qmax, PVR |
Note. RCT = randomized controlled trial; BPH = benign prostatic hyperplasia; IPSS = international prostatic symptoms score; PSA = prostatic-specific antigen; Qmax = maximum flow rate; MVV = minimum voided volume; QoL = quality of life; VAS = visual analogue scale; GAQ = global assessment quality; PVR = post-void residual volume; IIEF = International Index of Erectile Function; LUTS = lower urinary tract symptoms; BOOI = bladder outlet obstruction index; BPE = benign prostatic enlargement; PV = prostate volume.
Table 2.
Baseline Characteristics of Individual Study
| Study | Group | Age (years) | BMI (kg/m2) | QoL | IIEF | PV (mL) | PSA (ng/mL) | IPSS | Qmax (mL/s) | PVR (mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total IPSS | IPSS storage | IPSS voiding | ||||||||||
| Bechara et al. (2008) | Combination | 63.7 (51–78) | NA | 4.1 (0–6) | 17 (1–29) | NA | ≤4.0 | 19.4 (12–34) | NA | NA | 9.6 (4–14) | 60 (0–100) |
| Tamsulosin | 63.7 (51–78) | 4.1 (0–6) | 17 (1–29) | ≤4.0 | 19.4 (12–34) | 9.6 (4–14) | 60 (0–100) | |||||
| Regadas et al. (2013) | Combination | 61.6 ± 1.7 | NA | NA | NA | 44.3 ± 1.8 | NA | 20.6 ± 3.9 | 7.3 ± 3.2 | 13.4 ± 3.7 | 6.2 ± 2.6 | NA |
| Tamsulosin | 59.2 ± 2.0 | 42.4 ± 2.1 | 20.4 ± 4.3 | 5.7 ± 3.4 | 14.1 ± 3.2 | 7.7 ± 2.6 | ||||||
| Singh et al. (2014) | Combination | 61.92 ± 6.291 | NA | 5.65 ± 0.56 | 10.61 ± 5.582 | NA | ≤4.0 | 21.73 ± 5.876 | NA | NA | 9.88 ± 3.581 | 126.31 ± 78.507 |
| Tamsulosin | 59.50 ± 6.048 | 5.59 ± 0.501 | 10.08 ± 5.064 | ≤4.0 | 20.93 ± 4.607 | 9.15 ± 3.022 | 79.11 ± 55.924 | |||||
| Karami et al. (2016) | Combination | 67.9 ± 8.8 | 27.1 ± 2.3 | 4.1 ± 1.2 | 10.6 ± 1.6 | 63.2 ± 12.1 | 2.1 ± 1.6 | 21 ± 7.5 | 6.6 ± 3.2 | 14.9 ± 4.1 | 12.4 ± 4.8 | 58.6 ± 60.2 |
| Tamsulosin | 68.5 ± 8.9 | 26.7 ± 2.4 | 3.9 ± 1.2 | 10.9 ± 1.6 | 61.1 ± 16.1 | 2.3 ± 1.9 | 20.6 ± 7.3 | 20.6 ± 3.9 | 14.2 ± 4.0 | 12.3 ± 4.8 | 57.2 ± 59.7 | |
| Negoro et al. (2020) | Combination | 73 (65–85) | 23.2 (16.9–28.4) | 4 (3–5) | NA | 30.0 (22.0–39.7) | NA | 17 (10–27) | 7 (2–13) | 7 (2–14) | 8.5 (3.1–21.2) | 66 (0–149) |
| Tamsulosin | 70 (50–80) | 23.2 (13.7–27.2) | 5 (3–6) | 17 (0–172) | 16 (10–24) | 7 (1–15) | 7 (1–14) | 12.6 (4.6–25.5) | 17 (0–172) | |||
| Nagasubramanian et al. (2020) | Combination | 58.87 ± 8.16 | 24.17 ± 3.50 | 4.55 ± 0.85 | 10.06 ± 3.34 | NA | NA | 16.26 ± 3.32 | NA | NA | 9.57 ± 2.17 | 48 (28–86) |
| Tamsulosin | 61.28 ± 8.23 | 24.39 ± 3.38 | 4.23 ± 0.90 | 9.93 ± 3.11 | 15.10 ± 3.96 | 9.89 ± 3.11 | 41 (26-64) | |||||
Note. Presented by mean ±SD/range. BMI = body mass index; QoL = quality of life; IIEF = International Index of Erectile Function; PV = prostate volume; PSA = prostatic-specific antigen; IPSS = international prostatic symptoms score; Qmax = maximum flow rate; PVR = post-void residual volume; NA = not available.
Risk of Bias
This meta-analysis included six RCTs, and each RCT provided meticulous randomization methods in a double-blind manner. All RCTs had some calculation of sample size. Only one RCT performed intention-to-treat analysis (Nagasubramanian et al., 2020). The quality level of each included study was “A” (Table 3). A bias summary and graph are shown in Figure 2.
Table 3.
Quality Assessment of Individual Study
| Study | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow-up | Calculation of sample size | Statistical analysis | Level of quality | ITT analysis |
|---|---|---|---|---|---|---|---|---|
| Bechara et al. (2008) | A | A | A | 3 | Yes | ANOVA | A | No |
| Regadas et al. (2013) | A | A | A | 0 | Yes | t test; ANOVA | A | No |
| Singh et al. (2014) | A | A | A | 3 | Yes | t test; ANOVA | A | No |
| Karami et al. (2016) | A | A | A | 5 | Yes | t test; ANOVA | A | No |
| Negoro et al. (2020) | A | A | A | 0 | Yes | t test; ANOVA | A | No |
| Nagasubramanian et al. (2020) | A | A | A | 11 | Yes | ANOVA | A | Yes |
Note. A = almost all quality criteria met: low risk of bias; B = one or more quality criteria met: moderate risk of bias; C = one or more criteria not met: high risk of bias; ITT = intention-to-treat; ANOVA = analysis of variance.
Figure 2.
Summary of Risk of Bias
Efficacy
QoL Score
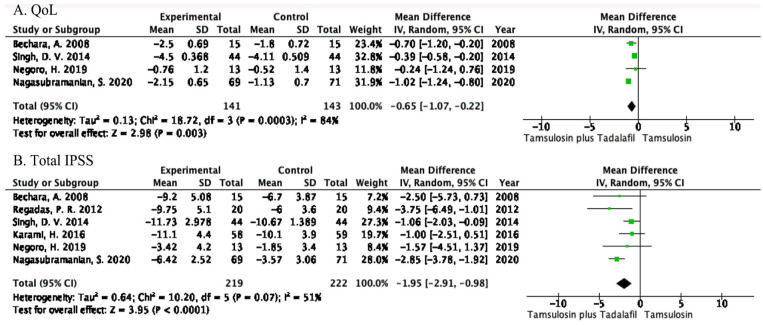
Four RCTs with a total of 284 patients (141 patients in the combination group and 143 patients in the tamsulosin group) included data on the change in QoL score. The combination group was significantly superior to the tamsulosin group in reducing QoL score (MD = −0.65, 95% CI = −1.07 to −0.22, p = .003; Figure 3A). This result suggested that the combination of tamsulosin and tadalafil can significantly improve the QoL of patients compared with tamsulosin monotherapy.
Figure 3.
Changes in (A) QoL Score and (B) Total IPSS
Note. QoL = quality of life; CI = confidence interval; IPSS = International Prostate Symptom Score.
Total IPSS, IPSS Storage, and IPSS Voiding
Six RCTs representing a cohort of 441 patients (219 patients in the combination group and 222 patients in the tamsulosin group) included data on the change in total IPSS. The forest plot demonstrated that compared with the tamsulosin group, the total IPSS of the combination group decreased significantly (MD = −1.95, 95% CI = −2.91 to −0.98, p < .0001; Figure 3B). This result indicated that the combination of tamsulosin plus tadalafil can significantly alleviate the subjective symptoms of patients with LUTS secondary to BPH.
In terms of IPSS storage and IPSS voiding, three RCTs representing a cohort of 183 patients (91 patients in the combination group and 92 patients in the tamsulosin group) were included in this study. For IPSS voiding, the fixed-effects measure of MD was −1.18, with a 95% CI of −1.89 to −0.47 (p = .001) (Figure 4A). For IPSS storage, the random-effects measure of MD was −0.94, and the 95% CI was −2.17 to 0.28 (p = .13) (Figure 4B). This result indicated that the difference in total IPSS might be represented primarily in the change of IPSS voiding.
Figure 4.
Changes of (A) IPSS Voiding and (B) IPSS Storage
Note. IPSS = International Prostate Symptom Score; CI = confidence interval.
PVR
Five RCTs with a sample of 401 patients (199 patients in the combination group and 202 patients in the tamsulosin group) evaluated data on PVR. The model showed no marked differences between the combination group and the tamsulosin group in the change of PVR (MD = −12.00, 95% CI = −24.38 to 0.38, p = .06; Figure 5A).
Figure 5.
Amount of (A) PVR, (B) IIEF, and (C) Qmax
Note. PVR = postvoid residual volume; CI = confidence interval; IIEF = International Index of Erectile Function; Qmax = maximum urine flow rate.
IIEF
There were five RCTs with a cohort of 401 patients (199 patients in the combination group and 202 patients in the tamsulosin group). The random-effects model showed that the combination group had a greater improvement than the tamsulosin group in the change of IIEF (MD = 3.23, 95% CI = 2.24 to 4.21, p < .00001; Figure 5B).
Qmax
Six RCTs with 441 patients (219 patients in the combination group and 222 patients in the tamsulosin group) contained data on the Qmax. The forest plots showed an MD of 1.38 and a 95% CI of 1.15 to 1.60 (p < .00001) (Figure 5C). This result suggested that the combination of tamsulosin and tadalafil had a significant improvement in terms of Qmax compared with tamsulosin monotherapy.
Safety
Discontinuation due to AEs
Six RCTs, representing a cohort of 441 patients (219 patients in the combination group and 222 patients in the tamsulosin group), assessed the incidence of discontinuation due to AEs. The OR was 2.08, and 95% CI was 0.70 to 6.22 with a p value of .19 (Figure 6A). The fixed model showed no statistical significance between the combination group and the tamsulosin group in the incidence in discontinuation due to AEs.
Figure 6.
Amount of (A) Discontinuation Due to AEs, (B) Pain (Including Headache, Myalgia, Back Pain, and Bone Pain), and (C) Any AEs
Note. AEs = adverse events; CI = confidence interval.
Pain (Including Headache, Myalgia, Back Pain, and Bone Pain)
Six RCTs with a sample of 441 patients (219 patients in the combination group and 222 patients in the tamsulosin group) analyzed the severity of pain after taking medicine. A fixed-effects model showed the combination group had a higher occurrence rate of pain compared with the tamsulosin group (OR = 8.55, 95% CI = 3.20 to 22.85, p < .0001; Figure 6B).
Any AEs
Six RCTs with a sample of 441 patients (219 in the combination group and 222 in the tamsulosin group) evaluated the incidence of AEs. The study showed a significant difference between the combination group and the tamsulosin group in the incidence of all AEs (OR = 4.17, 95% CI = 2.23 to 7.83, p < .00001; Figure 6C).
Discussion
LUTS secondary to BPH is a prevalent and chronic disease of aging males (Chitale et al., 2007; Feldman et al., 1994); therefore, physicians should be able to manage this condition actively. In many cases, alpha-blockers are regarded as the most effective monotherapy for LUTS secondary to BPH; meanwhile, some researchers evaluated the role of PDE5i in alleviating LUTS symptoms (McVary et al., 2007; Mulhall et al., 2006). The coadministration of alpha-blockers and PDE5i to treat LUTS secondary to BPH, demonstrating a significant efficacy and acceptable safety, has recently gained an increase in popularity (Bechara et al., 2008). Tamsulosin was the only alpha-blocker approved by the Food and Drug Administration to be used in combination therapy with tadalafil (Kloner, 2004). Many researchers assessed the role of alpha-blockers and PDE5i in vitro and in vivo for showing similarities in the pathophysiology and comorbidity in improving LUTS (Bechara et al., 2008; Kallidonis et al., 2020; Sun et al., 2020; Zhou, Zheng et al., 2019).
The meta-analysis was performed from six RCTs including 441 participants to compare the combination of tamsulosin and tadalafil with tamsulosin alone in treating LUTS secondary to BPH. The study identified that the combination therapy had a greater decrease compared with tamsulosin monotherapy in terms of total IPSS, IPSS voiding, QoL score, and IIEF. Compared with the tamsulosin group, six RCTs including data on Qmax showed a significant improvement in the combination therapy. However, as far as IPSS storage and PVR were concerned, there were no apparent differences among the two therapeutic regimens.
This study suggested that the combination therapy of tamsulosin plus tadalafil significantly improved subjective LUTS compared with the tamsulosin monotherapy. The difference of total IPSS was mostly represented in the change in IPSS voiding, indicating that tadalafil may enhance the total IPSS through reliving symptoms during urination. The study demonstrated that the combination of tamsulosin and tadalafil was determined to be safe, effective, and well tolerated in the subjects investigated, suggesting that the fixed-dose regimen can provide clinically relevant benefits for patients with LUTS secondary to BPH (Kim et al., 2017).
Although the mechanism and pharmacological action of combination therapy to produce greater improvements than monotherapy are not well understood, several theories (NOS/NO pathway, autonomic overactivity, alpha adrenoreceptors, and Rho-kinase activity) have been proposed (Bing et al., 2003; Deedwania, 2003). According to the previous studies, it was speculated that both alpha-blockers and PDE5i, acting by two different mechanisms on common urogenital target organs, might have a synergistic impact on BPH-LUTS. Increased smooth muscle tension in the prostate or vasculature around lower urinary tract may play a contributing role. Mulhall et al. (2006) have reported that alpha-1-adrenergic receptors can enhance the NO-mediated relaxant effect of PDE5i. Similarly, one study reported that PDE5i improved the inhibitory action of alpha-1-blockers on neurogenic contractions of prostate/bladder neck (Angulo et al., 2012). Furthermore, the mechanism of tadalafil (a long-acting PED5i) in treatment of males with BPH-LUTS is more appropriate for prolonged duration of action to alleviate some psychological disorders.
The safety indexes included in the study suggested that both groups were well tolerated. The combination group had a higher incidence of some adverse reactions including any AEs and pain (including headache, myalgia, back pain, and bone pain) compared with the tamsulosin group. However, according to the data from six RCTs, those AEs would resolve swiftly with standard medical care. Simultaneously, one RCT reported that the complications of combination therapy were myalgia, headache, back pain, and nasopharyngitis dizziness. The complication rate in the combination therapy group was considerably greater than that in the monotherapy group. Before adopting this medication, patients should be informed of the potential serious side effects of long-term combined use of tamsulosin and tadalafil.
The reader must be aware of the limitations of this meta-analysis. The quality of these studies is flawed, particularly in terms of study design, patient selection, blinding, and outcome data (discarding of such data to increase the positive rate, etc.). In addition, selection factors and subjective factors should also be taken into consideration. RCTs with adequate sample sizes, corrected information, and lengthy follow-up should be employed to validate our results.
Conclusion
The combination of tamsulosin and tadalafil provides a preferable therapeutic effect in IPSS voiding, QoL, IIEF, and Qmax compared with the tamsulosin alone in treating men with BPH/LUTS, and both therapy regimens were well tolerated by the patients.
Acknowledgments
All author has no acknowledgments to disclose.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Young Innovation Topic of Sichuan Province Medical Association, Code: Q21087.
Ethics Approval and Consent to Participate: The authors have no ethical conflicts to disclose.
ORCID iD: Zhongbao Zhou  https://orcid.org/0000-0002-9810-8145
https://orcid.org/0000-0002-9810-8145
References
- Angulo J., Cuevas P., Fernández A., La Fuente J. M., Allona A., Moncada I., Sáenz de Tejada I. (2012). Tadalafil enhances the inhibitory effects of tamsulosin on neurogenic contractions of human prostate and bladder neck. Journal of Sexual Medicine, 9(9), 2293–2306. 10.1111/j.1743-6109.2012.02821.x [DOI] [PubMed] [Google Scholar]
- Bechara A., Romano S., Casabé A., Haime S., Dedola P., Hernández C., Rey H. (2008). Comparative efficacy assessment of tamsulosin vs. Tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. Journal of Sexual Medicine, 5(9), 2170–2178. 10.1111/j.1743-6109.2008.00940.x [DOI] [PubMed] [Google Scholar]
- Bing W., Chang S., Hypolite J. A., DiSanto M. E., Zderic S. A., Rolf L., . . .Chacko S. (2003). Obstruction-induced changes in urinary bladder smooth muscle contractility: A role for Rho kinase. American Journal of Physiology-Renal Physiology, 285(5), F990–F997. 10.1152/ajprenal.00378.2002 [DOI] [PubMed] [Google Scholar]
- Chitale S., Collins R., Hull S., Smith E., Irving S. (2007). Is the current practice providing an integrated approach to the management of LUTS and ED in primary care? An audit and literature review. Journal of Sexual Medicine, 4(6), 1713–1725. 10.1111/j.1743-6109.2007.00598.x [DOI] [PubMed] [Google Scholar]
- Chua M. E., Mendoza J., See M., Esmena E., Aguila D., Silangcruz J. M., . . .Morales M. (2015). A critical review of recent clinical practice guidelines on the diagnosis and treatment of non-neurogenic male lower urinary tract symptoms. CUAJ-Canadian Urological Association Journal, 9(7–8), E463–E470. 10.5489/cuaj.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., Thomas J. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database of Systematic Reviews, 10, Article Ed000142. 10.1002/14651858.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deedwania P. C. (2003). Mechanisms of endothelial dysfunction in the metabolic syndrome. Current Diabetes Reports, 3(4), 289–292. 10.1007/s11892-003-0019-8 [DOI] [PubMed] [Google Scholar]
- Feldman H. A., Goldstein I., Hatzichristou D. G., Krane R. J., McKinlay J. B. (1994). Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. Journal of Urology, 151(1), 54–61. 10.1016/s0022-5347(17)34871-1 [DOI] [PubMed] [Google Scholar]
- Kallidonis P., Adamou C., Kotsiris D., Ntasiotis P., Verze P., Athanasopoulos A. (2020). Combination therapy with alpha-blocker and phosphodiesterase-5 inhibitor for improving lower urinary tract symptoms and erectile dysfunction in comparison with monotherapy: A systematic review and meta-analysis. European Urology Focus, 6(3), 537–558. 10.1016/j.euf.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Karami H., Hassanzadeh-Hadad A., Fallah-Karkan M. (2016). Comparing monotherapy with tadalafil or tamsulosin and their combination therapy in men with benign prostatic hyperplasia: A randomized clinical trial. Urology Journal, 13(6), 2920–2926. [PubMed] [Google Scholar]
- Kim S. W., Park N. C., Lee S. W., Yang D. Y., Park J. K., Moon D. G., . . .Hyun J. S. (2017). Efficacy and safety of a fixed-dose combination therapy of tamsulosin and tadalafil for patients with lower urinary tract symptoms and erectile dysfunction: Results of a randomized, double-blinded, active-controlled trial. Journal of Sexual Medicine, 14(8), 1018–1027. 10.1016/j.jsxm.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Kloner R. A. (2004). Cardiovascular effects of the 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation, 110(19), 3149–3155. 10.1161/01.Cir.0000146906.42375.D3 [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Jackson G., Emmick J. T., Mitchell M. I., Bedding A., Warner M. R., Pereira A. (2004). Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. Journal of Urology, 172(5 Pt.1), 1935–1940. 10.1097/01.ju.0000142687.75577.e4 [DOI] [PubMed] [Google Scholar]
- Madersbacher S. (2017). Re: Efficacy and safety of tadalafil 5mg once daily in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia in men aged ≥75 years: Integrated analyses of pooled data from multinational, randomized, placebo-controlled clinical studies. European Urology, 71(6), 990. 10.1016/j.eururo.2017.01.027 [DOI] [PubMed] [Google Scholar]
- McVary K. T., Monnig W., Camps J. L., Young J. M., Tseng L. J., van den Ende G. (2007). Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: A randomized, double-blind trial. Journal of Urology, 177(3), 1071–1077. 10.1016/j.juro.2006.10.055 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Medicine, 3(3), e123–e130. [PMC free article] [PubMed] [Google Scholar]
- Mulhall J. P., Guhring P., Parker M., Hopps C. (2006). Assessment of the impact of sildenafil citrate on lower urinary tract symptoms in men with erectile dysfunction. Journal of Sexual Medicine, 3(4), 662–667. 10.1111/j.1743-6109.2006.00259.x [DOI] [PubMed] [Google Scholar]
- Nagasubramanian S., John N. T., Antonisamy B., Mukha R. P., Jeyachandra Berry C. S., Kumar S., . . .Kekre N. S. (2020). Tamsulosin and placebo vs. tamsulosin and tadalafil in male lower urinary tract symptoms: A double-blinded, randomised controlled trial. BJU International, 125(5), 718–724. 10.1111/bju.15027 [DOI] [PubMed] [Google Scholar]
- Negoro H., Goto T., Akamatsu S., Terada N., Kobayashi T., Matsui Y., . . .Ogawa O. (2020). Add-on effects of tadalafil in tamsulosin-treated patients with small benign prostatic enlargement: A randomized, placebo-controlled, double-blind, crossover study. Neurourology and Urodynamics, 39(1), 237–242. 10.1002/nau.24175 [DOI] [PubMed] [Google Scholar]
- Regadas R. P., Reges R., Cerqueira J. B., Sucupira D. G., Josino I. R., Nogueira E. A., . . .Silva L. F. (2013). Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A randomized, placebo-controlled clinical trial. International Urology and Nephrology, 45(1), 39–43. 10.1007/s11255-012-0317-7 [DOI] [PubMed] [Google Scholar]
- Robertson C., Link C. L., Onel E., Mazzetta C., Keech M., Hobbs R., . . .McKinlay J. B. (2007). The impact of lower urinary tract symptoms and comorbidities on quality of life: The BACH and UREPIK studies. BJU International, 99(2), 347–354. 10.1111/j.1464-410X.2007.06609.x [DOI] [PubMed] [Google Scholar]
- Rosen R., Altwein J., Boyle P., Kirby R. S., Lukacs B., Meuleman E., . . .Giuliano F. (2003). Lower urinary tract symptoms and male sexual dysfunction: The multinational survey of the aging male (MSAM-7). European Urology, 44(6), 637–649. 10.1016/j.eururo.2003.08.015 [DOI] [PubMed] [Google Scholar]
- Singh D. V., Mete U. K., Mandal A. K., Singh S. K. (2014). A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Journal of Sexual Medicine, 11(1), 187–196. 10.1111/jsm.12357 [DOI] [PubMed] [Google Scholar]
- Sun Y., Peng B., Lei G. L., Wei Q., Yang L. (2020). Study of phosphodiesterase 5 inhibitors and α-adrenoceptor antagonists used alone or in combination for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Minerva Urologica e Nefrologica, 72(1), 13–21. 10.23736/s0393-2249.19.03408-8 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Chen H., Wu J., Wang J., Zhang X., Ma J., Cui Y. (2019). Meta-analysis of the long-term efficacy and tolerance of tadalafil daily compared with tadalafil on-demand in treating men with erectile dysfunction. Sexual Medicine, 7(3), 282–291. 10.1016/j.esxm.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Zheng X., Wu J., Gao Z., Xu Z., Cui Y. (2019). Meta-analysis of efficacy and safety of tadalafil plus tamsulosin compared with tadalafil alone in treating men with benign prostatic hyperplasia and erectile dysfunction. American Journal of Men’s Health, 13(5). 10.1177/1557988319882597 [DOI] [PMC free article] [PubMed] [Google Scholar]