Abstract
Background
Acanthopanacis Cortex (AC) is a valuable Chinese medicine, which exerts beneficial effects on anti-fatigue, anti-stress, and inflammatory modulation in the periphery. However, the central nervous system (CNS) function of AC has not been clearly illustrated. As communication between the peripheral immune system and the CNS converges, it promotes a heightened neuroinflammatory environment that contributes to depression. We investigated the effect of AC against depression through neuroinflammatory modulation.
Methods
Network pharmacology was used to screen for target compounds and pathways. Mice with CMS-induced depression were used to evaluate the efficacy of AC against depression. Behavioral studies and detection of neurotransmitters, neurotrophic factors, and pro-inflammatory cytokines were performed. The IL-17 signaling cascade was involved to further investigate the underlying mechanism of AC against depression.
Results
Twenty-five components were screened by network pharmacology and the IL-17 mediated signaling pathway was associated with the antidepressant action of AC. This herb had a beneficial effect on CMS-induced depressive mice, including improvements in depressive behavior, modulation of neurotransmitter levels, neurotrophic factors, and pro-inflammatory cytokines.
Conclusions
Our results revealed that AC exhibits effects on anti-depression and one of the mechanisms was mediated by neuroinflammatory modulation.
Keywords: Acanthopanacis Cortex, anti-depression, neuroinflammatory modulation, IL-17
Introduction
Depression, also known as a major depressive disorder, is a serious mental illness characterized by a constant feeling of low self-esteem and loss of interest or pleasure. The pathogenesis of depression is complex and involves multiple genetic and environmental factors, resulting in inadequate treatment. Currently, mounting evidence indicates that the stress-induced communication between the peripheral immune system and central nervous system (CNS) converges to promote a heightened neuroinflammatory environment causing depression.1-3 The neuroinflammatory environment is manifested as the release of pro-inflammatory cytokines from peripheral immune cells, activation of glial cells, and consequently affecting neurotransmission, neuronal growth and synaptic plasticity.4 Amongst all the pro-inflammatory cytokines, interleukin-17 (IL-17) secreted by innate immune cells plays an important role in the neuroinflammatory environment.5 IL-17 induces macrophage inflammatory protein 1α (MIP-1α) expression in primary mouse astrocytes by extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK), mitogen-activated protein kinases (MAPKs) activation, which activates astrocytes and neuroinflammation in multiple sclerosis.3 It also causes (glycogen synthase kinase 3β) GSK-3β-dependent phosphorylation of C/EBPβ, which diminishes C/EBPβ binding to the endothelial locus 1 (Del-1) promoter and suppresses Del-1 expression in human endothelial cells, which induces pathological inflammation.6 Moreover, CNS-targeted production of IL-17A, one of the prominent members of the IL-17 family, induces astrocytes and microglial activation, microvascular pathology, and enhanced the neuroinflammatory response to brain diseases.7 Thus, it is likely to develop an antidepressant effect through neuroinflammatory modulation and this knowledge will deepen the understanding of preventing depression.
Acanthopanacis Cortex (AC, the dried cortex of Acanthopanax gracilistylus W. W. Smith) is a valuable Chinese herbal medicine, which has the effect of inflammatory modulation, anti-stress, anti-fatigue, sedation, and analgesia.8 Acanthopanacis Cortex belongs to the species of Eleutherococcus Maxim., and the chemicals of AC mainly consist of diterpenoids, triterpenoids, phenylpropanoids, and lignans, which are similar to other herbs in Eleutherococcus Maxim..9 The function of herbs from Eleutherococcus Maxim. involves neuroprotection, anti-fatigue, anti-stress and inflammatory modulation, and the peripheral function of which is consistent with AC.10-12 However, the CNS function of AC has not been clearly elucidated. It is reported that herbs from Eleutherococcus Maxim. present similar chemical composition and the majority of which are widely used in brain diseases.10-12 The main active ingredients of AC are terpenoids (lupinane-type triterpenes, shellacane-type diterpenes), sterols, fatty acids, phenylpropanoids, and flavonoids.13,14 Chlorogenic acid15 and syringin16 both exist in AC and Acanthopanax senticosus (Rupr.et Maxim.) Harms have been reported with neurological and potential anti-depression function. Chlorogenic acid could exert neuroprotective effects by inhibiting enzymes, like acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE) as an approach to suppressing neuronal damage.15 Syringin could upregulate Nrf2 protein levels and reduce the Aβ deposition which alleviates the AD progress.16 Thus it is reasonable to suggest that AC may be able to mediate resistance to brain disorders,17 for example, anti-depression, through neuroinflammatory modulation.
In this study, we aimed to investigate the effect of AC against depression as well as the underlying mechanism. Chemical candidates and their candidate targets in AC were obtained from the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Swiss Target Prediction. Network pharmacology analysis was carried out to explain the antidepressant effect of AC. Chronic mild stress (CMS)-induced depressive mouse was selected for its similarity with the true state of depressive patients.18 Thus, the CMS-induced depressive mouse model promises to be a stable and plausible approach in evaluating the effects of AC, and studies on tissue samples from depressive mice indicates the underlying mechanism of depression. We evaluated whether AC had a beneficial effect on mice with CMS-induced depression, including improvements in depressive behavior and modulation of the levels of neurotransmitters, neurotrophic factors and pro-inflammatory cytokine levels. We hypothesized that the AC-suppressed IL-17 signaling cascade was one of the molecular mechanisms responsible for relieving neuroinflammation and depression. Therefore, we investigated whether AC exerted an antidepressant-like effect and modulated IL-17 signaling in mice with CMS-induced depression. Our results could accelerate the development of therapies for depression, which will be useful for the clinical applications of AC.
Methods
Chemicals and Reagents
HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Ultra-pure water was processed by a Milli-Q purification system (Millipore, Molsheim, France). The chemical standards of syringin, chlorogenic acid, isochlorogenic acid A, isochlorogenic acid B, and kaurenoic acid were purchased from Weikeqi-Biotech Co., Ltd (Chengdu, China). All the standards have a purity of at least 98% based on HPLC profile. Imipramine was from Yuanye Bio-Technology Co., Ltd (Shanghai, China) and other reagents came from Sigma-Aldrich (St Louis, MO).
Chemical Candidates and Target Chemicals in Acanthopanacis Cortex
Chemical candidates in AC were gathered from TCMSP (https://tcmspw.com/tcmsp.php) and relevant literature.19 The collected chemicals were screened according to the SWISS ADME to illustrate the proposed model. The candidate targets of the chemicals in AC were obtained from Swiss Target Prediction (http://www.swisstargetprediction.ch/). After removing duplicates, a total of 340 AC target candidates were collected.
Known Therapeutic Targets in the Treatment of Depression
The known therapeutic targets in depression were acquired from GeneCards database (http://www.genecards.org/). After filtering out low correlative targets (relevance score≤5), a total of 1589 targets related to depression were collected (as shown in Supplementary Table 1).
Network Construction and Analysis
Network construction was made by Cytoscape Software (http://www.cytoscape.org/). Compound-Target-Disease (C-T-D) network was established by connecting the targets with the compounds and depression.20 GO biological process and KEGG pathway analysis with FDR-adjusted P-values <0.05 was employed and the data were collected by RStudio 1.1.463 for R statistical computing (http://www.rstudio.com/).
Preparation of Acanthopanacis Cortex Extract
Acanthopanacis Cortex was obtained from the Bozhou Market in Anhui Province, China, and was morphologically authenticated by Dr Min Wei of the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences. The corresponding voucher specimens were deposited in the Research Center of Medicinal Plants of the Institute of Botany, Jiangsu Province and Chinese Academy of Science. The plant materials were tested for quality according to the requirements of Chinese Pharmacopeia (2020 Edition). In preparing of the AC extract, 100 g of the plant material was minced and soaked in 800 mL of water for 2 h and extracted twice. The extract was combined and spray-dried to obtain the extract of AC. For chemical analysis, the extract was weighed accurately and sonicated in 5 mL of 80% methanol for 45 min. After centrifugation (12 000 r/min at 4°C, 5 min), the supernatant was collected before HPLC analysis.
HPLC Analysis
Agilent rapid analysis LC 1200 series systems (equipped with a degasser, a binary pump, an auto-sampler, a DAD and a thermostated column compartment) was applied. A Waters XBridge C18 column (3.5 μm, 4.6 mm × 150 mm) was used for separation. For the quantification of syringin, chlorogenic acid, isochlorogenic acid A and isochlorogenic acid B (277 nm), the mobile phase condition was acetonitrile (A) and 0.1% phosphoric acid in water (B), flow rate of 0.8 mL/min, injection volume of 10 μL, and column temperature was 30°C. 0-10 min, linear gradient 86.0-70.0% (B); 10-15 min, linear gradient 70.0-60.0% (B); 15-20 min, linear gradient 60.0-5.0% (B). For the quantification of kaurenoic acid (202 nm), the mobile phase condition was acetonitrile (A) and 0.1% phosphoric acid in water (B), flow rate of 1.0 mL/min, injection volume of 10 μL, and column temperature was 30°C. 0-15 min, isocratic gradient 15.0% (B).21,22
Animal Experiments
Male C57BL/6J mice (8- to 10-week-old at the start of experiments) were obtained from Changzhou Cavens Laboratory Animal Co. Ltd (Changzhou, China). Animals were hosted on a 12 h light/dark cycle (lights on at 6:00 am and off at 6:00 pm) under controlled temperature (22 ± 2°C) and humidity (50 ± 10%), with standard diet and water ad libitum. Animals were acclimatized for 7 days. The experimental procedures had been approved by the Animal Experimentation Ethics Committee of China Pharmaceutical University and under the guidelines of “Principles of Laboratory Animal Care” (NIH publication No. 80-23, revised in 1996). All efforts were made to minimize suffering.
Sucrose adaptation and sucrose consumption assessment were performed to the CMS procedures at the beginning of the experiment trial. The CMS procedure was carried out with some adjustments. Briefly, a series of stressors were applied onto the animals: (1) water deprivation for 24 h, (2) stroboscopic illumination for 2 h, (3) cage tilt (45°) for 15 h, (4) noise for 2 h, (5) soiled cage (200 mL water in 100 g sawdust bedding) for 15 h, (6) body restraint for 1 h, (7) forced swimming at 8°C for 6 min, (8) tail-clipping restraint for 6 min, (9) food deprivation for 24 h, and (10) day and night reverse. These stressors were randomly arranged in 1 week and repeated for 6 weeks. At the end of the CMS procedure, a sucrose preference test was carried out to evaluate the CMS model.23
The mice were randomly divided into 5 groups (n = 12). The control and CMS model were given with saline. For the other 3 groups, AC at low dose (50.0 mg/kg/day), high dose (150 mg/kg/day), and imipramine (30 mg/kg/day) were intragastrically given 30 min before stress exposure for 6 weeks. Mouse body weight was recorded twice a week during CMS and AC treatment. The mice were weighed using an electronic scale and the average of two measurements was recorded.
Sucrose preference test was conducted out at the end of the CMS procedure. In brief, mice in each group were learned to adapt to 2 bottles of 1% sucrose solution (w/v) 72 h before the test, and 24 h later, one bottle of 1% sucrose solution (w/v) was replaced with tap water for 24 h. Then, mice were deprived of water and food for 24 h. Sucrose preference test was conducted at 17:00 pm, where mice were kept in individual cages with 2 bottles, one with 100 mL of 1% sucrose solution (w/v) and the other with 100 mL of water. After 3 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated by the following formula: sucrose preference = sucrose consumption/ (water consumption + sucrose consumption) × 100%.24
Forced swimming test was carried out at the end of CMS procedures. Mice in each group were placed in large glass cylinders (50 cm height and 20 cm diameter) with 30 cm height water at 22 ± 2°C, so that mice were not able to support themselves by hind limbs. The test consists of 2 parts: the first 15 min was used for pre-swimming and then 24 h later, swimming behavior was observed for 5 min, and the latency to float was measured and analyzed.25
Real-Time Quantitative PCR
Total RNA was separated from mouse hippocampus by RNAprep pure Tissue Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The concentration of RNAs was detected by UV absorbance at 260 nm. cDNA was reverse transcribed from 1 μg sample of total RNA using RT SuperMix for qPCR (Vazyme, Nanjing, China), according to the protocol provided by the manufacturer. Real-time PCR was performed using SYBR Green Master Mix (Vazyme). The SYBR green signal was detected by qTOWER 2.0 (Analytic Jena AG, Germany). Primers used were: 18S-S: TGT GAT GCC CTT AGA TGT CC; 18S-AS: GAT AGT CAA GTT CGA CCG TC; IL-6-S: TAG TCC TTC CTA CCC CAA TTT CC; IL-6-AS: TTG GTC CTT AGC CAC TCC TTC; TNF-α-S: CAG GCG GTG CCT ATG TCT C; TNF-α-AS: CGA TCA CCC CGA AGT TCA GTA G; COX2-S: CGC ATC CTT TAC ATA ACA GAC G; COX2-AS: TAG GAG TTG AAG ATT AGT CCG C; MMP9-S: CAA AGA CCT GAA AAC CTC CAA C; MMP9-AS: GAC TGC TTC TCT CCC ATC.
SDS-PAGE and Immunoblotting
The mouse hippocampus was collected, and protein content was determined by the Bradford method. Proteins (∼20 μg) were separated on 8% SDS-polyacrylamide gels and transferred to a PVDF membrane. The PVDF membrane was blocked with 5% fat-free milk in tris-buffer saline/0.1% tween 20 (TBS-T), and then incubated in the primary antibodies diluted in 2.5% fat-free milk in TBS-T over night at 4°C. The primary antibodies were anti-phospho-JNK (Cell Signaling, Banvers, MA), anti-JNK (Cell Signaling), anti-phospho-Erk1/2 (Cell Signaling), anti-Erk1/2 (Cell Signaling), anti-phospho-GSK-3β (Cell Signaling), and anti-GSK-3β (Cell Signaling). After that, the PVDF membrane was rinsed with TBS-T and incubated for 2 h at room temperature in peroxidase (HRP)-conjugated anti-rabbit secondary antibody (Sangon, Shanghai, China), diluted in 2.5% fat-free milk in TBS-T. After intensive washing with TBS-T, the immune complexes were visualized using the Enhanced Chemiluminescence (ECL) method (Vazyme). The intensities of bands in the control and samples runs, both on the same gel and under strictly standardized ECL conditions, were compared on an image analyzer, using a calibration plot constructed from a parallel gel with serial dilutions of one of the samples.
Measurement of Neurotransmitters, Neurotrophic Factors, and Pro-inflammatory Cytokines
The levels of neurotransmitters, neurotrophic factors, and pro-inflammatory cytokines were determined by commercial ELISA kits (Lanpaibio, Shanghai, China; AbFrontier, Korea) according to the manufacturer’s instructions. In brief, the samples were added onto a 96-well plate with coating of anti-mouse serotonin (5-HT)/norepinephrine (NE)/dopamine (DA)/glutamate/nerve growth factor (NGF)/brain-derived neurotrophic factor (BDNF)/glial-cell derived neurotrophic factor (GDNF)/interleukin-1β (IL-1β)/interleukin-6 (IL-6)/IL-17/tumor necrosis factor-α (TNF-α) antibody, individually, and then incubated with HRP-labeled detection antibody at 37°C for 90 min. After washing 5 times with PBS, substrate solution was added at 37°C for another 15 min. At last, stop solution was added to stop the reaction and absorbance of 450 nm was measured immediately. Non-specific blinding absorbance was taken into consideration for sample analysis and each sample in duplicate was employed to minimize inter-assay variation.
Statistical Analysis
All data were analyzed using one-way ANOVA or Student’s t-test method. Differences with values of P < 0.05 were considered significant.
Results
Chemical Candidates and Targets
As 91 compounds were identified belonging to AC by TCMSP and relevant literature (as shown in Supplementary Table 2), 16 compounds were selected according to oral bioavailability ≥ 15% and drug-likeness ≥ 0.1. Removing 4 compounds (gracilistone A, B, C, and linalool) with no target information, 12 compounds with known target information were chosen for the following analysis. Another 13 compounds including caffeoylquinic acids, steroids and eleutherosides reported to have anti-inflammatory, anti-oxidative, anti-fatigue, and learning-enhancing effects were added additionally, and finally 25 compounds were analyzed (as shown in Table 1).26-28 These 25 identified active compounds interacted with 340 target proteins (as shown in Supplementary Table 3) based on a target fishing technique,29 that is, on average, each of the compounds interacted with 13.6 target genes, which did fully explain the multiple-target effects of pharmacology by AC.
Table 1.
25 Identified Compounds in AC.

|

|

|

|
Network Construction and Analysis
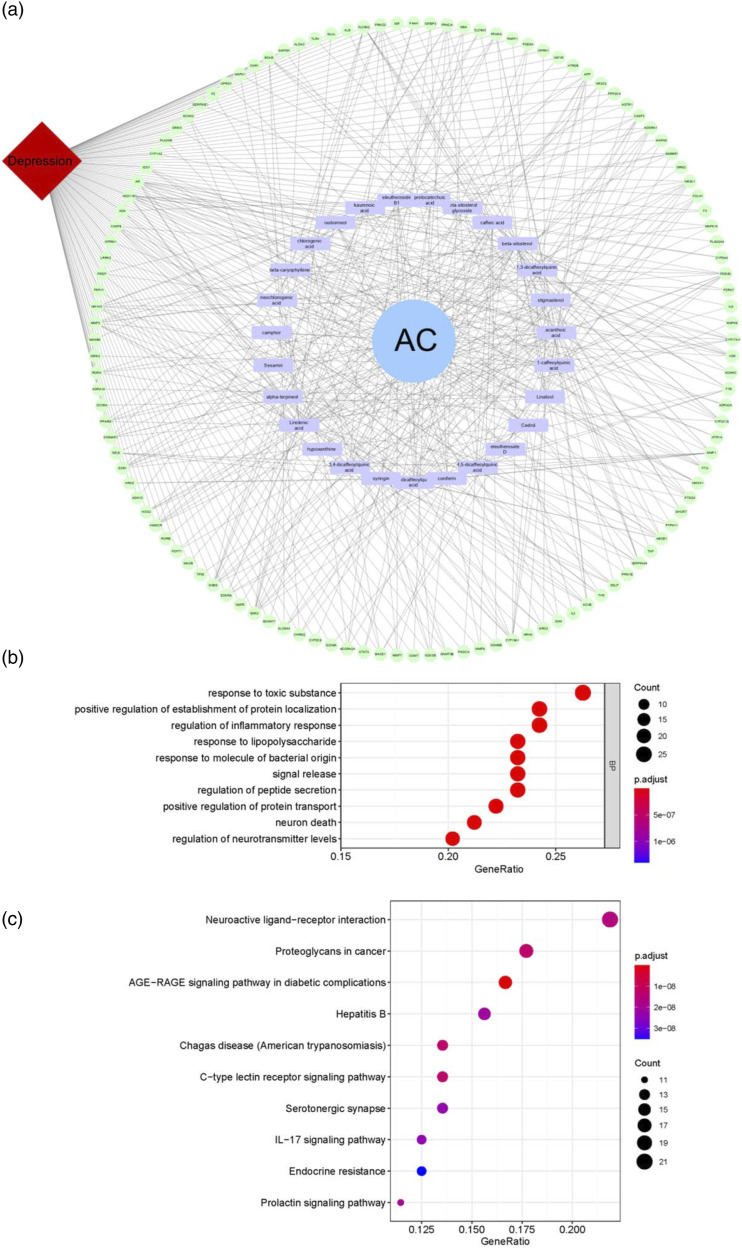
Among the 340 obtained targets and 1589 disease targets, 120 potential targets (as shown in Supplementary Table 4) were associated with depression, and they were reserved for further analysis. Network pharmacology provides a visual approach to understanding the complex relationship between disease and therapeutic spots.30 In the present study, 120 potential targets and 25 involved compounds were used to construct the C-T-D network for further cluster analysis (Figure 1(a)). All the compounds connected with more than 2 targets and all of the 120 targets interacted with more than 1 compound, indicating that the effect of AC on anti-depression was a result of multi-components, multi-targets, and multi-pathway interactions.
Figure 1.
Network analysis of Acanthopanacis Cortex. C-T-D network (a) was established by connecting the targets with the compounds and depression. GO biological process (b) and KEGG pathway analysis (c) were performed by RStudio 1.1.463 for R statistical computing, FDR-adjusted P-values <0.05.
With the C-T-D network, a macroscopic visualization of the relationship between AC, targets, and depression was obtained, but the underlying mechanism of AC against depression remained unclear. As a result, 120 potential targets for depression underwent GO biological process and KEGG pathway analysis. GO biological process showed that these targets were enriched to 10 biological process terms, and regulation of inflammatory response and neurotransmitter levels as well as neuron death may indicate the possible mechanism of AC against depression (Figure 1(b)). Twelve protein targets were mapped to IL-17 signaling pathway (Figure 1(c)). Depression is highly associated with neuroinflammation. IL-17 has a plethora of effects that could contribute to neuroinflammation, and consequently affect neurotransmission, neuronal growth, and synaptic plasticity, which may suggest the underlying mechanisms of AC against depression.
Standardization of Herbal Extracts
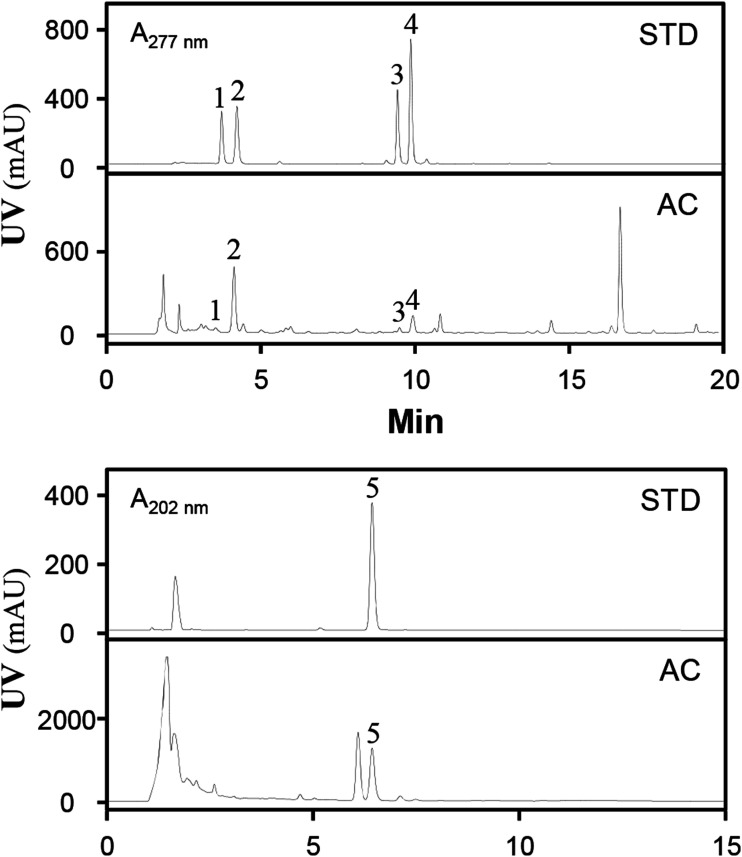
The AC extract was prepared according to the ancient preparation of Chinese herbs. In the study, we prepared AC extract using water, because water has been widely used in preparation of traditional Chinese medicine for years.31 The extraction efficiency was about 10.07 ± 1.25% (mean ± SD, n = 3). Five chemicals were chosen to control the quality of the extract: syringin, chlorogenic acid, isochlorogenic acid A, isochlorogenic acid B, and kaurenoic acid (Figure 2). The amount was about 0.12 ± 0.03 for syringin, 6.82 ± 0.46 for chlorogenic acid, 1.52 ± 0.14 for isochlorogenic acid A, 0.34 ± 0.07 for isochlorogenic acid B and 21.53 ± 2.07 for kaurenoic acid in mg/g of dried powder of extract (mean ± SD, n = 3). The established chemical parameters served as the control for repeatability of the below animal study.
Figure 2.
HPLC chromatograms of Acanthopanacis Cortex extract. The chromatographic method was described in method session. syringing (1, 277 nm), chlorogenic acid (2, 277 nm), isochlorogenic acid A (4, 277 nm), isochlorogenic acid B (3, 277 nm), and kaurenoic acid (5, 202 nm) was detected by a HPLC couple with a DAD detector. The detected wavelength was indicated. Representative chromatograms are shown, n = 3.
Acanthopanacis Cortex Relieves the Depression-like Behavior in CMS-Induced Depressive Mice
Two animal behavior tests including sucrose preference and forced swimming were employed to evaluate the effect of AC against depression in mice. After the treatment of herbal extract for 6 weeks, AC (low dose: 50 mg/kg/day and high dose: 150 mg/kg/day) alleviated sucrose preference of CMS-induced depressive mice (Figure 3(a)). In forced swimming test, the CMS-induced depressive mice doubled cumulative immobility time, while AC restored the cumulative immobility time (Figure 3(b)). In body weight evaluation, CMS-induced depressive mice showed a decrease of body weight, while AC relieved the body loss (Figure 3(c)). Imipramine (30 mg/kg/day) was set as a positive control.
Figure 3.
Acanthopanacis Cortex relieves the depression-like behavior in CMS-induced depressive mice. The CMS-induced depressive mice were randomly divided into 5 groups: control (Con), CMS, imipramine (Imi, 30 mg/kg/day), AC low dose (AC-L, 50 mg/kg/day) and AC high dose (AC-H, 150 mg/kg/day). The Imi, AC-L and AC-H three groups were given CMS procedure. After drug administration, sucrose preference tests (a), forced swimming tests (b), body weight c were carried out, as described in the method session. Data are expressed as mean ± SEM, where n = 8, *P < 0.05, **P < 0.01, ***P < 0.001 compared with CMS.
Acanthopanacis Cortex Restores the Levels of Neurotransmitters, Neurotrophic Factors, and Pro-inflammatory Cytokines in CMS-Induced Depressive Mice
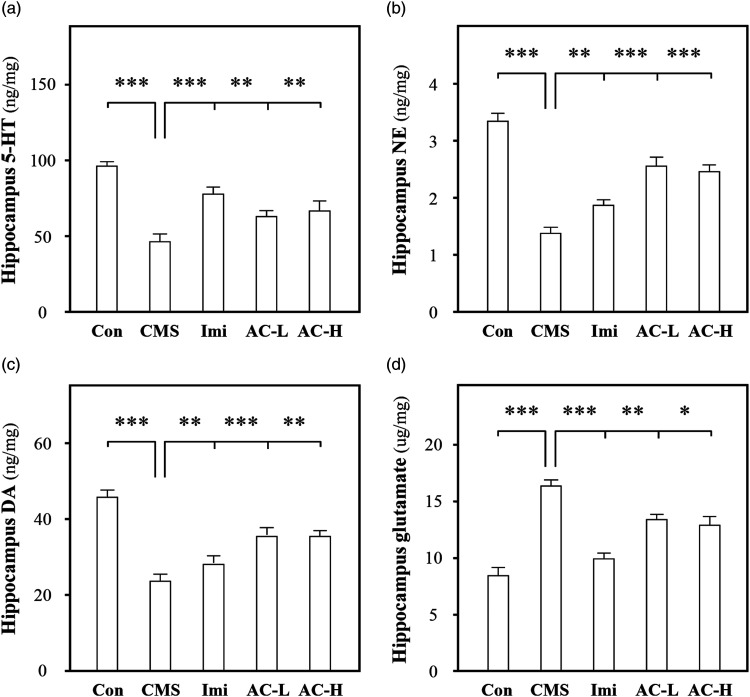
According to a previous study, a systematic method was used to evaluate the anti-depressive efficiency of AC. The detected targets included: 5-HT, NE, DA, glutamate, NGF, BDNF, GDNF, IL-1β, IL-6, TNF-α, and IL-17. In CMS-induced depressive mouse hippocampus, the amounts of 5-HT, NE, and DA levels were decreased to ∼50%, ∼30%, and ∼50%, while glutamate level was increased to ∼300%, respectively. The treatment of AC (low dose: 50 mg/kg/day and high dose: 150 mg/kg/day) and imipramine (30 mg/kg/day) restored the levels of these neurotransmitters (Figure 4). The amounts of neurotrophic factors (NGF, BDNF, and GDNF) were reduced to ∼30%, which were up-regulated under AC and imipramine administration. The levels of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-17) were increased to ∼200%, which were restored by AC and imipramine treatment (Figure 5). The stress-induced neuroinflammation contributes to neuronal dysfunction and depression, while the modulation of pro-inflammatory cytokines, neurotrophic factors and neurotransmitters could prevent the pathological change. In addition, stress-induced neuroinflammation is related with the activation of astrocytes and microglia,32 which is consistent with our result that AC inhibits the activation of astrocytes and microglia. (Supplementary Figure 2) These results indicated that AC might mediate resilience to stress-induced depression through modulating the levels of pro-inflammatory cytokines, neurotransmitters, and neurotrophic factors.
Figure 4.
Acanthopanacis Cortex restores the levels of neurotransmitters in CMS-induced depressive mice. The CMS-induced depressive mice were randomly divided into 5 groups as described above. The Imi, AC-L and AC-H three groups were given CMS procedure. After drug administration, the hippocampus was collected. The amounts of 5-HT (a), NE (b), DA (c), and glutamate (d) in the extracts of hippocampus were detected using ELISA kits. Data are expressed in ng/mg or μg/mg, mean ± SEM, n = 7, *P < 0 .05, **P < 0.01, ***P < 0.001 compared with CMS.
Figure 5.
Acanthopanacis Cortex restores the levels of neurotrophic factors and pro-inflammatory cytokines in CMS-induced depressive mice. The CMS-induced depressive mice were randomly divided into 5 groups as described above.The Imi, AC-L and AC-H three groups were given CMS procedure. After drug administration, the hippocampus was collected. The amounts of NGF (a), BDNF (b), GDNF (c), IL-1β (d), IL-6 (e), TNF-α (f), and IL-17 (g) in the extracts of hippocampus were detected using ELISA kits. Data are expressed in pg/mg, mean ± SEM, n = 7, *P < 0.05, **P < 0.01, ***P < 0.001 compared with CMS.
Acanthopanacis Cortex Regulates the Phosphorylation of JNK, Erk1/2, and GSK-3β in CMS-Induced Depressive Mice
For the underlying mechanism of AC against depression, IL-17 signaling molecules based on KEGG pathway analysis (Supplementary Figure 1), that is, MAPKs, Erk1/2, and GSK-3β, were involved. JNK is another subtype of MAPKs and JUN-AP-1 is involved in intervention on neuroinflammation.33 Thus, the phosphorylation levels of JNK, Erk1/2, and GSK-3β were determined. In CMS-induced depressive mouse hippocampus, the phosphorylation of Erk1/2 and GSK-3β was reduced to ∼60%, while the phosphorylation of JNK was increased to ∼240%, respectively. The application of AC (low dose: 50 mg/kg/day and high dose: 150 mg/kg/day) and imipramine (30 mg/kg/day) restored the pathological change (Figure 6). These results suggested that AC was able to suppress IL-17 signaling cascade, which may be one of the molecular mechanisms for relieving neuroinflammation and depression.34,35
Figure 6.
Acanthopanacis Cortex regulates the phosphorylation of JNK, Erk1/2 and GSK-3β in CMS-induced depressive mice. The CMS-induced depressive mice were randomly divided into 5 groups as described above. The Imi, AC-L and AC-H three groups were given CMS procedure. After drug administration, the hippocampus was collected. The phosphorylation of JNK (a), Erk1/2 (b) and GSK-3β c were revealed by using specific antibodies. Data are expressed as fold of control, and in mean ± SEM, n = 4, *P < 0.05, **P < 0.01 compared with CMS.
For the underlying mechanism of AC against depression, the downstream targets of IL-17 signaling based on KEGG pathway analysis (Supplementary Figure 1), that is, IL-6, TNF-α, cyclooxygenase 2 (COX2) and matrix metallopeptidase 9 (MMP9), were involved. The mRNA levels of IL-6, TNF-α, COX2, and MMP9 were determined. In CMS-induced depressive mouse hippocampus, the mRNA levels of IL-6, TNF-α, and COX2 were increased to ∼240%, ∼160%, and ∼350%, respectively. The application of AC (low dose: 50 mg/kg/day and high dose: 150 mg/kg/day) and imipramine (30 mg/kg/day) restored the pathological change (Figure 7(a)–(c)). Furthermore, AC induced the expression of MMP9 (Figure 7(d)), which could help tissue remodeling and repair following neuroinflammation. These results were consistent with our previous results that AC could modulate neuroinflammation to mediate resilience to stress-induced depression through IL-17 signaling cascade.
Figure 7.
Acanthopanacis Cortex regulates the expression of IL-6, TNF-α, COX2 and MMP9 in CMS-induced depressive mice. The CMS-induced depressive mice were randomly divided into 5 groups as described above. The Imi, AC-L and AC-H three groups were given CMS procedure. After drug administration, the hippocampus was collected. The mRNA amount of IL-6 (a), TNF-α (b), COX2 (c) and MMP9 (d) were determined. Data are expressed as fold of control, and in mean ± SEM, n = 4, *P < 0.05, **P < 0.01 compared with CMS.
Discussion
Depression is a serious mental illness and has been recognized as one of the most disabling diseases worldwide. The stress-induced communication between peripheral immune activation and CNS inflammation contributes to the onset of depression.36 Astrocytes are the most abundant glial cells in CNS, which could sense and respond to IL-17 secreted by innate immune cells, thereby modulating the responses of neighboring cells throughout the CNS.37 Astrocytes secrete neurotrophic factors to regulate synaptogenesis, neuronal differentiation, and neuronal survival and actively modulate synaptic transmission through the release and clearance of neurotransmitters.38,39 Under inflammatory environment, sustained increased level of IL-17 activates astrocytes and microglia which contribute to the impairment of microvascular pathology and enhance the neuroinflammation through JNK, ERK1/2, GSK-3β inducing depression.
Acanthopanacis Cortex, a valuable Chinese herb, has been used to treat inflammatory diseases, fatigue, and weakness for years. In China, Acanthopanax gracilistylus wine, which is made of AC in liquor, is considered as a health supplement for the treatment of inflammatory diseases due to its clinical application.40 As a therapeutic candidate, AC particularly exerts anti-inflammation and hepatoprotection, showing potential for inflammatory modulation.41 However, current study on the pharmacological activity of AC has mainly focuses on peripheral inflammatory regulation, and the effect of AC on the CNS needs further study.
In the study, the effects of AC on CNS, especially depression, were investigated. Acanthopanacis Cortex had a plethora of effects against depression, including improvements in depressive behavior and modulation of the levels of neurotransmitters, neurotrophic factors, and pro-inflammatory cytokines. It is interesting that AC was able to suppress the IL-17 signaling cascade and neuroinflammation in depressive mice. The results were consistent with the network pharmacology analysis that AC mediated resilience to depression, with one of mechanism was mediated by neuroinflammation modulation via the IL-17 signaling cascade. Furthermore, much more effort is required to elucidate the underlying mechanism of AC on IL-17 signaling as well as the chemical components responsible for neuroinflammatory modulation.
Considering the major ingredients in the AC should be crucial for neuroinflammatory modulation. The majority of the identified 25 chemical components exhibit a variety of biological activities experimentally. In our study, syringin accounts for 0.12 ± 0.03 mg/g of dried powder of extract, which have anti-inflammatory and sleep-potentiating effect in mice and human beings.42 The amount of chlorogenic acid was about 6.82 ± 0.46 mg/g of dried powder of extract, and it is reported that chlorogenic acid possesses anti-oxidation, inflammatory modulation, anti-depression, hepatoprotection, and cardioprotection.43 These results indicate they might be potential active components. Moreover, caffeic acid,44 protocatechuic acid,45 eleutheroside B1,46 camphor,47 β-caryophyllene,48 and β-sitosterol49 with neurological functions have been reported, which indicate these components could be potential active targets. In fact, AC is a multi-component extract and many pathological factors are involved in the development of depression. In addition, although AC plays a critical role in anti-depression, the potent implications for active components or molecular mechanisms are an important topic that remains to be revealed. Therefore, a comprehensive study considering different aspects of systemic regulation is needed to fully understanding of the anti-depressant role of AC.
Conclusions
In this study, the anti-depressant effect of AC was investigated. Acanthopanacis Cortex had a beneficial effect on CMS-induced depressive mice, including improvements in depressive behavior and modulation of the levels of neurotransmitters, neurotrophic factors and pro-inflammatory cytokines. Moreover, AC was able to suppress the IL-17 signaling cascade and thereby inhibiting neuroinflammation. These findings provide an insight into depression treatment that will be useful in the development of clinical application of AC. Further research should focus on validating the active components of AC in cell and animal models.
Supplemental Material
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response
Author Contributions: LY and MW designed research; LY, CL, and YQ conducted research; PS collected data; TW analyzed data; LY and CL wrote the paper; LY and CL revised the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by National Natural Science Foundation of China (grant number: 81803758) and Natural Science Foundation of Jiangsu Province (grant number: BK20150553, BK20200296). The funders played no role in the design of this study, and collection, analysis and interpretation of data and writing the manuscript. A preprint of the draft has previously been published (Yan et al, 2021).
Ethical Approval and Consent to Participate: The study was approved by the Animal Experimentation Ethics Committee of China Pharmaceutical University and under the guidelines of “Principles of Laboratory Animal Care” (NIH publication No. 80-23, revised in 1996). The study was carried out in compliance with the ARRIVE guidelines.
Data Availability: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Supplemental Material: Supplementary material for this article is available on the online.
ORCID iD
Chuhan Liu https://orcid.org/0000-0003-1162-3986
References
- 1.Tubbs JD, Ding J, Baum L, Sham PC. Immune dysregulation in depression: Evidence from genome-wide association. Brain Behav Immun Health. 2020;7:100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: Monocytes carry the signal. Neuropsychopharmacology. 2017;42:46-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reale M, Iarlori C, Thomas A, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55-63. [DOI] [PubMed] [Google Scholar]
- 5.Milovanovic J, Arsenijevic A, Stojanovic B, et al. Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol. 2020;11:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maekawa T, Hosur K, Abe T, et al. NEW-Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann J, Krauthausen M, Hofer MJ, Heneka MT, Campbell IL, Muller M. CNS-targeted production of IL-17A induces glial activation, microvascular pathology and enhances the neuroinflammatory response to systemic endotoxemia. PLoS One. 2013;8:e57307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Dong J, Liu M, et al. Therapeutic effects of cortex acanthopanacis aqueous extract on bone metabolism of ovariectomized rats. Evid-Based Compl Alt. 2012;2012:492627-492628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing Z, Guijun Z, Min W, et al. Research progress of original base, chemical componets and pharmacological effects on cortex acanthopanacis. J Liaoning Univer Traditi Chinese Med. 2015;17:104-107. [Google Scholar]
- 10.Jin ML, Park SY, Kim YH, Park G, Lee SJ. Acanthopanax senticosus exerts neuroprotective effects through HO-1 signaling in hippocampal and microglial cells. Environ Toxicol Pharmacol. 2013;35:335-346. [DOI] [PubMed] [Google Scholar]
- 11.Lau KM, Yue GG, Chan YY, et al. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin Med-Uk. 2019;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wang Z, Wang C, et al. Comprehensive phytochemical analysis and sedative-hypnotic activity of two Acanthopanax species leaves. Food Funct. 2021;12:2292-2311. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Cai W, Li M, et al. Progress in chemical and pharmacological reasearch of acanthopanax gracilistylus. Mod Chinese Med. 2020;22:652-662. [Google Scholar]
- 14.Xian L, Qian S, Li Z. Studies on the chemical constituents from the stems of acanthopanax gracilistylus. J Chin Med Mater. 2010;33:538-542. [PubMed] [Google Scholar]
- 15.Lu H, Tian Z, Cui Y, Liu Z, Ma X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci F. 2020;19:3130-3158. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Zhang Q, Xun Z, et al. Increases of iASPP-Keap1 interaction mediated by syringin enhance synaptic plasticity and rescue cognitive impairments via stabilizing Nrf2 in Alzheimer’s models. Redox Biol. 2020;36:101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nam Y, Lee D. Ameliorating effects of constituents from cortex acanthopanacis radicis on memory impairment in mice induced by scopolamine. J Tradit Chin Med. 2014;34:57-62. [DOI] [PubMed] [Google Scholar]
- 18.Yan L, Hu Q, Mak MS, et al. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci Rep-Uk. 2016;6:30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu SY, Li XY, Heng X, et al. Analysis of antidepressant activity of huang-lian jie-du decoction through network pharmacology and metabolomics. Front Pharmacol. 2021;12:619288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu XK, Chen ZY, Liao LP, Zhang Z, Wang Z. [Determination of scopolin, chlorogenic acid, scopoletin, isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C in plants of Erycibe]. Zhongguo Zhongyao Zazhi. 2015;40:1119-1122. [PubMed] [Google Scholar]
- 22.Oliveira BH, Sant’Ana AE, Bastos DZ. Determination of the diterpenoid, kaurenoic acid, in annona glabra by HPLC. Phytochem Anal. 2002;13:368-371. [DOI] [PubMed] [Google Scholar]
- 23.Agudelo LZ, Femenia T, Orhan F, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33-45. [DOI] [PubMed] [Google Scholar]
- 24.Liu MY, Yin CY, Zhu LJ, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686-1698. [DOI] [PubMed] [Google Scholar]
- 25.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology. 2005;177:245-255. [DOI] [PubMed] [Google Scholar]
- 26.Wu QZ, Zhao DX, Xiang J, Zhang M, Zhang C, Xu X. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm Biol. 2016;54:1117-1124. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, Sha F, Zhao C, et al. Synthesis and anti-inflammatory activity of novel steroidal chalcones with 3beta-pregnenolone ester derivatives in RAW 264.7 cells in vitro. Steroids. 2021;171:108830. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Yang X. Eleutheroside E decreases oxidative stress and NF-kappaB activation and reprograms the metabolic response against hypoxia-reoxygenation injury in H9c2 cells. Int Immunopharm. 2020;84:106513. [DOI] [PubMed] [Google Scholar]
- 29.Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357-W364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110-120. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Ross J. Theories and Concepts in the Composition of Chinese Herbal Formulas. London, UK: Churchill Livingstone; 2010:1-34. [Google Scholar]
- 32.Picard K, St-Pierre MK, Vecchiarelli HA, Bordeleau M, Tremblay ME. Neuroendocrine, neuroinflammatory and pathological outcomes of chronic stress: A story of microglial remodeling. Neurochem Int. 2021;145:104987. [DOI] [PubMed] [Google Scholar]
- 33.Vukic V, Callaghan D, Walker D, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nothdurfter C, Milenkovic VM, Sarubin N, et al. The cytokine IL-17A as a marker of treatment resistance in major depressive disorder? Eur J Neurosci. 2021;53:172-182. [DOI] [PubMed] [Google Scholar]
- 35.Tsuboi H, Sakakibara H, Minamida Y, et al. Elevated levels of serum IL-17A in community-dwelling women with higher depressive symptoms. Behav Sci-Basel. 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trager U, Tabrizi SJ. Peripheral inflammation in neurodegeneration. J Mol Med. 2013;91:673-681. [DOI] [PubMed] [Google Scholar]
- 37.Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler MA, Quintana FJ. Regulation of astrocyte functions in multiple sclerosis. Csh Perspect Med. 2019;9:a029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben HL, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31-41. [DOI] [PubMed] [Google Scholar]
- 40.Zhao S, Chen X, Song J, Pang X. Internal transcribed spacer 2 barcode: A good tool for identifying acanthopanacis cortex. Front Plant Sci. 2015;6:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanting Y, Yingying W, Desheng L. The protective effects of cortex acanthopanacis decoction on acute liver injury induced by CCl4 in mice. J Hubei Univer Sci and Tech (Med Sci). 2014;28:4-6. [Google Scholar]
- 42.Cui Y, Zhang Y, Liu G. Syringin may exert sleep-potentiating effects through the NOS/NO pathway. Fund Clin Pharmacol 2015;29:178-184. [DOI] [PubMed] [Google Scholar]
- 43.Zeng A, Liang X, Zhu S, et al. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NFkappaB signaling pathway. Oncol Rep 2021;45:717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Wu Q, Zhang L, et al. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res 2019;150:104538. [DOI] [PubMed] [Google Scholar]
- 45.Song J, He Y, Luo C, et al. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol Res 2020;161:105109. [DOI] [PubMed] [Google Scholar]
- 46.Zhang B, Chang HS, Hu KL, et al. Combination of Geniposide and Eleutheroside B Exerts Antidepressant-like Effect on Lipopolysaccharide-Induced Depression Mice Model. Chin J Integr Med 2021;27:534-541. [DOI] [PubMed] [Google Scholar]
- 47.Salama A, Mahmoud HA, Kandeil MA, et al. Neuroprotective role of camphor against ciprofloxacin induced depression in rats: modulation of Nrf-2 and TLR4. Immunopharm Immunot 2021;43:309-318. [DOI] [PubMed] [Google Scholar]
- 48.Machado K, Islam MT, Ali ES, et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother Res 2018;32:2376-2388. [DOI] [PubMed] [Google Scholar]
- 49.Yin Y, Liu X, Liu J, et al. The effect of beta-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. Rsc Adv 2018;8:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response
Supplemental Material for The Extract of Acanthopanacis Cortex Relieves the Depression-Like Behavior and Modulates IL-17 Signaling in Chronic Mild Stress-Induced Depressive Mice by Chuhan Liu, Lu Yan, Yiyun Qian, Pingping Song, Tao Wang, and Min Wei in Dose-Response