Abstract
Phosphorus and carbon metabolism in Microlunatus phosphovorus was investigated by using a batch reactor to study the kinetics of uptake and release of extracellular compounds, in combination with 31P and 13C nuclear magnetic resonance (NMR) to characterize intracellular pools and to trace the fate of carbon substrates through the anaerobic and aerobic cycles. The organism was subjected to repetitive anaerobic and aerobic cycles to induce phosphorus release and uptake in a sequencial batch reactor; an ultrafiltration membrane module was required since cell suspensions did not sediment. M. phosphovorus fermented glucose to acetate via an Embden-Meyerhof pathway but was unable to grow under anaerobic conditions. A remarkable time shift was observed between the uptake of glucose and excretion of acetate, resulting in an intracellular accumulation of acetate. The acetate produced was oxidized in the subsequent aerobic stage. Very high phosphorus release and uptake rates were measured, 3.34 mmol g of cell−1 h−1 and 1.56 mmol g of cell−1 h−1, respectively, values only comparable with those determined in activated sludge. In the aerobic period, growth was strictly dependent on the availability of external phosphate. Natural abundance 13C NMR showed the presence of reserves of glutamate and trehalose in cell suspensions. Unexpectedly, [1-13C]glucose was not significantly channeled to the synthesis of internal reserves in the anaerobic phase, and acetate was not during the aerobic stage, although the glutamate pool became labeled via the exchange with intermediates of the tricarboxylic acid cycle at the level of glutamate dehydrogenase. The intracellular pool of glutamate increased under anaerobic conditions and decreased during the aerobic period. The contribution of M. phosphovorus for phosphorus removal in wastewater treatment plants is discussed on the basis of the metabolic features disclosed by this study.
Phosphorus contamination in wastewater causes eutrophication, a worldwide water pollution problem (23). Alga proliferation leading to the death of other organisms in surface waters cannot be stopped simply by minimizing the amount of phosphate (and nitrate) discharged into wastewater by industrial and agriculture activities, since the phosphate derived from human metabolism alone is sufficient to sustain the eutrophication process. Therefore, it is essential to remove phosphorus efficiently from wastewater.
The enhanced biological phosphorus removal (EBPR) process has gained a reputation as an economical and reliable option for the removal of phosphorus from wastewater (7). However, in many cases, full-scale EBPR plants show high variability in P removal efficiencies, and a full control of the process can be achieved only when the biological mechanisms involved are well understood (1).
The EBPR process works on the basis of anaerobic and aerobic cycles where substrates are supplied in the anaerobic stage (23). The resulting activated sludge becomes enriched in a bacterial population with the capability to take up phosphate in excess and store it as polyphosphate, under aerobic conditions. In this process, not only is phosphorus removed, but also the organic content in the waste stream is considerably reduced.
Under anaerobic conditions, the polyphosphate-accumulating organisms (PAO) are able to use the energy derived from polyphosphate hydrolysis for substrate uptake and conversion to internal carbon reserves (10). It is generally accepted that PAO take up short-chain fatty acids and store them as polyhydroxyalkanoates with a concomitant release of phosphorus. The reducing equivalents needed for this conversion were initially proposed to be derived from substrate degradation in the tricarboxylic acid (TCA) cycle–Comeau-Wentzel model (25) and later from the glycogen catabolism–Mino model (11). Recently, experimental evidence for the contribution of both glycogen metabolism and the TCA cycle has been provided (15).
In the aerobic stage, the stored polyhydroxyalkanoates are utilized for growth, for glycogen synthesis, and also for obtaining the energy required to restore the polyphosphate pool.
A considerable effort has been directed to the isolation of polyphosphate-accumulating bacteria capable of reproducing the general features of activated sludge in regard to phosphorus release and uptake. The first PAO isolated from activated sludge belonged to the genus Acinetobacter (5). However, subsequent studies questioned the importance of these organisms in the EBPR process: in fact, under anaerobic conditions they are unable to take up acetate, the phosphorus release rates are very low compared to activated sludge, and it has been shown that they do not dominate in the phosphorus removal process (12).
Other polyphosphate-accumulating bacteria, Pseudomonas spp. and Aeromonas spp., have been suggested as playing relevant roles in biological phosphorus removal (2, 8). However, the phosphorus uptake and release rates were also too low. A similar poor performance was shown by Lampropedia spp., originally reported as having a carbon metabolism representative of activated sludge (21).
Many organisms other than PAO are present in activated sludge (12). Some of them are able to store carbon substrates as polyhydroxyalkanoates, under anaerobic conditions, by using intracellular carbohydrates (glycogen), instead of polyphosphate, as an ATP source. These organisms are often referred to as glycogen-accumulating bacteria: in contrast to PAO, substrate uptake in the anaerobic stage is not dependent on polyphosphate hydrolysis, but their carbon metabolism is believed to be the same (4). It is generally accepted that polyphosphate-accumulating bacteria mainly use volatile fatty acids derived from the fermentation of organic substrates carried out by heterotrophic fermentative bacteria not included in the PAO group (25).
Microlunatus phosphovorus was isolated from activated sludge and reported to exhibit not only a high-level phosphorus-accumulating activity (up to more than 10% of its dry weight) but also phosphate uptake and release activities similar to those observed in activated sludge (13, 14). In this context, it is an interesting model organism to investigate biological phosphorus removal.
In this study, the phosphorus and carbon metabolism of M. phosphovorus was investigated by combining kinetic data obtained in a batch reactor and in vivo 31P and 13C nuclear magnetic resonance (NMR) with nongrowing cell suspensions.
MATERIALS AND METHODS
Organism and growth conditions.
M. phosphovorus JCM-9379 was obtained from the Japan Collection of Microorganisms. Stock cultures were maintained at −80°C in the medium described by Nakamura et al. (13) with 15% glycerol. The organism was adapted to grow in a less rich medium containing the following ingredients (per liter): MgSO4 · 7H2O (0.6 g), NH4Cl (0.16 g), CaCl2 · 2H2O (0.07 g), yeast extract (0.5 g), K2HPO4 (0.0923 g), KH2PO4 (0.0449 g), and glucose (0.25 g). The pH was adjusted to 7.1 before sterilization. Growth media as well as all the reactor components and solutions were autoclaved for 20 min at 121°C and 105 Pa. The carbon source and phosphate solutions were autoclaved separately. Plating and microscopic observation were used routinely to monitor the purity of the cultures.
Membrane reactor setup and operation.
In order to simulate anaerobic and aerobic alternating conditions that are used in wastewater treatment plants and because the exposure to these conditions may improve the phosphorus removal activity of microorganisms (14), a sequential batch reactor was operated continuously in periods of time from 1 to 4 months. An ultrafiltration membrane module was coupled to the reactor to overcome the difficulty originating from the fact that M. phosphovorus cell suspensions do not sediment. This procedure allows a high cell concentration to be reached inside the reactor and avoids mass transfer restrictions that occur in an immobilized cell reactor. After coupling the bioreactor to the membrane module, the cell suspension was pumped tangentially along the membrane surface, generating two streams, a retentate stream (with cells) and a permeate stream (free of cells), which were both recirculated to the reactor except when the liquid phase was withdrawn (see below). The reactor was operated with three cycles a day. Each cycle consisted of 3 h of anaerobiosis, followed by 4 h of aeration and 1 h of withdrawal where one-third of the liquid phase was replaced with fresh medium. The withdrawal of the liquid phase was achieved by discarding the permeate stream out of the reactor during the first 0.5 h of this stage. The addition of fresh medium was done in the final 0.25 h. The reactor was operated with a 600-ml working volume, controlled temperature (27°C), agitation (magnetic stirring, 125 rpm), and pH 7.0. The pH was controlled by the addition of 0.2 M NaOH or 0.2 M HCl. Anaerobic and aerobic conditions were maintained by argon gassing or by the use of an air pump, at a flow rate of 0.2 volume of gas per volume of reactor per minute. The redox potential was measured and taken as an indication of the anaerobic and aerobic conditions. The microorganism retention time was controlled to 6 days by purging 100 ml of cell suspension per day at the end of an aerobic phase.
An ultrafiltration module (Fresenius module SPS 9005-M) with a hollow fiber polysulfone membrane was used. The internal diameter of the fiber was 500 μm, and the molecular weight cutoff was 1,000 K. The fiber’s total length was 8.4 cm, the number of fibers was 608, and the surface area was 0.1 m2. The module was operated with a constant permeate flux (J = 4 liters m−2 h−1); the cross flow velocity was 0.12 m s−1. Operation of the membrane cell recycle reactor with a controlled permeate flux gave the best results in preventing membrane fouling.
A preculture was grown aerobically for 2 days at 27°C with agitation. This culture was used to inoculate the reactor (5%, vol/vol), which was then operated in a batch mode under aerobic conditions for 2 days. Both the preculture and this starting culture were grown on glucose (0.5 g liter−1). After this starting period of 2 days, the reactor was operated with anaerobic and aerobic cycles and the glucose concentration was changed to 0.25 g liter−1.
Reactor setup for kinetic studies.
Cell adhesion to the membrane was responsible for unreliable cell density determinations. Therefore, in order to allow quantification of cell mass, the kinetic studies were performed in a batch reactor without a coupled membrane. A defined volume of cell suspension was removed from the membrane cell recycle reactor and centrifuged (5,000 × g for 10 min at 4°C) under sterile conditions. The cells were suspended in an aliquot of fresh growth medium (the same as used in the membrane reactor) without phosphorus or carbon sources and transferred to the batch reactor containing 300 ml of medium previously supplemented with phosphorus and carbon. This batch reactor was submitted to one anaerobic and aerobic cycle and operated under the conditions described above for the membrane bioreactor, except that the temperature was kept at 25°C. The cell mass concentration was determined for each experiment, and an initial value of 1.87 g liter−1, on average, was used.
Determination of cell mass concentration.
The cell mass concentration was determined by measurement of optical density at 600 nm and comparison with an optical density versus dry weight calibration curve.
Quantification of phosphorus, glucose, acetate, and glutamate levels.
The inorganic phosphate concentration was determined by segmented flow analysis (Skalar Analytical B. V., Breda, The Netherlands) using the colorimetric method based on the reduction of the phosphomolybdate complex with ascorbic acid (1). Glucose and acetate levels were determined by high-performance liquid chromatography with a reverse-phase column (SHODEX SH1011) coupled to a refractive index detector. l-Glutamate levels in cell extracts were determined enzymatically by using the test combination kit 139092 (Boehringer Mannheim GmbH, Mannheim, Germany), based on the colorimetric Farb-test method.
Sample preparation for in vivo NMR spectroscopy.
A sample cell suspension was taken from the reactor (∼200 ml); cells were harvested by centrifugation (6,400 × g for 10 min at 4°C), washed once with fresh growth medium without glucose or phosphate, and suspended in the same medium supplemented with 10 mM KCl and 2 mM MgSO4 · 7H2O (medium for NMR). 2H2O was added (5%, vol/vol) in order to provide a lock signal, and the pH was adjusted to 7.2. A final volume of 5 ml of cell suspension was used for the experiments, corresponding to a cell mass concentration of approximately 40 g liter−1. Anaerobic and aerobic conditions in the NMR tube were achieved by using an air lift system (17), with bubbling of argon or air, respectively (0.13 liters min−1). At the end of each anaerobic or aerobic phase, cells were centrifuged and suspended in the medium for NMR as described above.
Preparation of cell extracts.
After the NMR experiments, cells were harvested by centrifugation and boiled in 80% ethanol for 13 min. The extraction with ethanol was repeated. Ethanol from the combined supernatants was removed by rotary evaporation. The aqueous residue was then extracted with chloroform (water/chloroform ratio, 2:1). After centrifugation, the chloroform phase was washed with water and centrifuged. The combined water phases were lyophilized, and the residue was dissolved in 2H2O for further NMR analysis.
For glutamate level quantification, cell samples from the reactor used in the kinetic studies were lyophilized and subjected to ethanol extraction as described above. The complete evaporation of ethanol was achieved by using an inert gas current. The residue obtained after lyophilization was dissolved in water.
NMR spectra.
31P and 13C NMR spectra were recorded on a Bruker model DRX500 spectrometer, with a quadruple nucleus probe head (10-mm diameter), operating at 202.45 MHz for phosphorus and 125.77 MHz for carbon. Phosphorus spectra were acquired without proton decoupling with the following parameters: spectral width, 12 kHz; repetition delay, 1.7 s; pulse width, 15 μs (corresponding to a flip angle of 65°); and data size, 16 K. Phosphorus resonances were referenced with respect to external 85% phosphoric acid designated at 0 ppm. Carbon spectra were acquired with the following parameters: spectral width, 30 kHz; repetition delay, 1.5 s; pulse width, 10 μs (corresponding to a flip angle of 45°); and data size, 32K. Proton decoupling was continuously applied by using the wide-band alternating phase low-power technique for zero residue splitting (WALTZ) sequence. Resonances were referenced with respect to external methanol (designated at 49.3 ppm). The probe head temperature was kept at 30°C in all experiments.
13C NMR spectra of extracts were recorded by using a selective probe head (5-mm diameter). The following acquisition conditions were used: spectral width, 31 kHz; repetition delay, 61 s; pulse width, 6 μs (corresponding to a flip angle of 60°); data size, 65 K; and probe temperature, 25°C. Proton broadband decoupling was applied during the acquisition time only.
Chemicals.
[1-13C]glucose (99 atom% 13C enrichment), [2-13C]acetate (sodium salt, with 99 atom% 13C enrichment), and 2H2O (99.9 atom% 2H) were purchased from Sigma Chemical Co. (St. Louis, Mo.). All other chemicals were reagent grade.
Determination of kinetic parameters.
Initial rates were determined by adjusting a curve to the experimental data and calculating the first derivative at time zero.
RESULTS
Kinetic studies in a batch bioreactor.
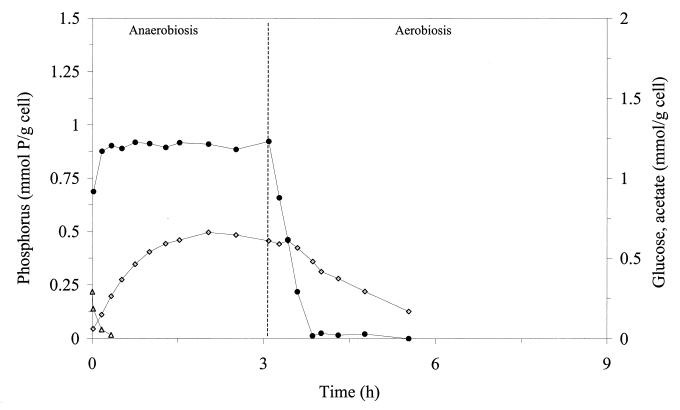
The ability of M. phosphovorus to release phosphate and utilize glucose under anaerobic conditions and to take up phosphate under aerobic conditions was evaluated in a batch reactor by using glucose and phosphorus concentrations of 0.29 mmol of glucose g of cell−1 and 0.69 mmol of P g of cell−1, respectively. The concentrations of glucose, phosphorus, and acetic acid in the medium were monitored over the anaerobic and aerobic cycles (Fig. 1). Under anaerobic conditions, phosphorus was released at the very high rate of 2.59 mmol of P g of cell−1 h−1 (Table 1), and glucose was fermented to acetic acid. It is interesting to note that although glucose was exhausted after 0.75 h of fermentation, acetic acid production continued for up to 2 h. Under aerobic conditions, the uptake of phosphorus also occurred at a very high rate (1.40 mmol of P g of cell−1 h−1), and the acetic acid produced in the previous anaerobic stage was consumed (Table 1). Under these conditions, the net phosphorus accumulation (difference between the final and initial amounts of phosphorus in the medium) was 0.69 mmol of P g of cell−1. It is important to stress that all the phosphorus present in the medium in the beginning of the aerobic stage (0.92 mmol of P g of cell−1) was consumed after 50 min.
FIG. 1.
Time courses for phosphorus (●), glucose (▵), and acetate (◊) in a batch reactor submitted to anaerobiosis (3 h) and an aerobic period. Concentrations of these metabolites were determined in the supernatant of the culture. A glucose concentration of 0.29 mmol g of cell−1 was used in this experiment.
TABLE 1.
Kinetic parameters determined for experiments in sequential batch reactors performed with different glucose concentrations
| Initial amt of glucose (mmol g of cell−1) | Initial amt of P (mmol g of cell−1) | Anaerobiosis
|
Aerobiosis
|
||
|---|---|---|---|---|---|
| P release rate (mmol P g of cell−1 h−1) | YP/Sa | Initial acetate (mmol g of cell−1) | P uptake rate (mmol g of cell−1 h−1) | ||
| 0.71 | 0.54 | 3.34 | 0.82 | 1.69 | 1.56 |
| 0.29 | 0.69 | 2.59 | 0.81 | 0.61 | 1.40 |
| 0.00 | 0.69 | 1.65 | 0.19 | 0.87 | |
YP/S is the yield coefficient determined with the experimental points obtained during phosphorus release (mole of P/mole of glucose).
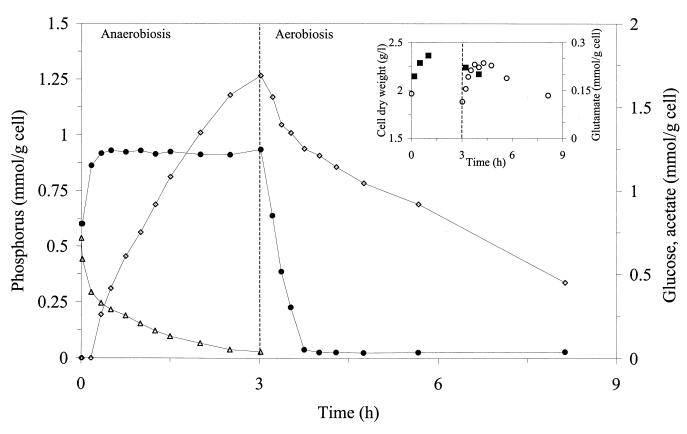
To evaluate the effect of glucose concentration on the pattern of phosphorus uptake and release, the initial glucose concentration was increased to 0.71 mmol g of cell−1, while a similar value for the phosphorus concentration was used (0.54 mmol g of cell−1) (Fig. 2). Carbon and phosphorus evolution followed a pattern similar to that observed for a lower carbon concentration. Nevertheless, the initial phosphorus release rate was clearly higher (3.34 mmol of P g of cell−1 h−1), while the initial phosphorus uptake rate (1.56 mmol of P g of cell−1 h−1) was only slightly higher (Table 1). Glucose was consumed at a very high rate at the beginning of the anaerobic phase concomitantly with phosphorus release, but glucose consumption was drastically reduced when the release of phosphorus stopped. Thus, the uptake of glucose is clearly stimulated by phosphorus release, although it is not strictly dependent on it.
FIG. 2.
Time courses for phosphorus (●), glucose (▵), and acetate (◊) in a batch reactor submitted to anaerobiosis (3 h) and an aerobic period. Concentrations of these metabolites were determined in the supernatant of the culture. The intracellular concentration of glutamic acid (■) and cell dry weight (○) are depicted in the inset. A glucose concentration of 0.71 mmol g of cell−1 was used in this experiment.
For both concentrations of glucose used, the ratio of phosphorus release versus glucose consumed (YP/S) has the same value (Table 1).
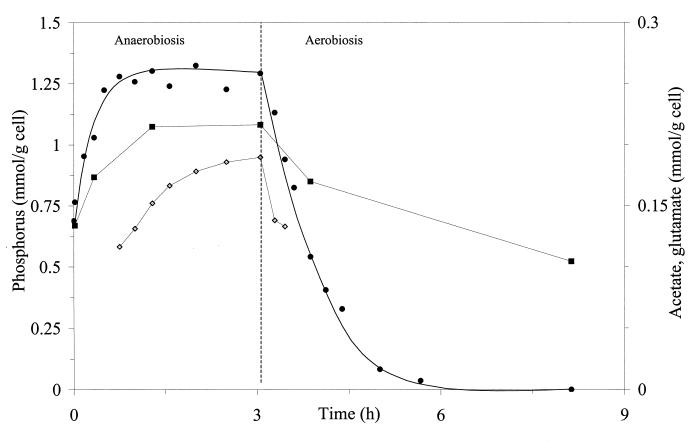
A control experiment without the addition of glucose was performed (Fig. 3). The culture was able to release and take up phosphorus, although at rates lower than those achieved in the experiments in which glucose was supplied (Table 1). Despite the absence of added glucose, acetic acid was still produced during the anaerobic phase. The fermentation of internal reserves and/or diffusion limitation of internal acetic acid to the extracellular medium are possible mechanisms to explain this result.
FIG. 3.
Time courses for phosphorus (●) and acetate (◊) in a batch reactor submitted to anaerobiosis (3 h) and an aerobic period. Concentrations of these metabolites were determined in the supernatant of the culture. The intracellular concentration of glutamate (■) is also shown. No glucose was added to the growth medium.
It has been reported that M. phosphovorus is an obligate aerobe (13). Growth was observed at the initial stage of all the aerobic phases, except for the control experiment. Growth ceased when external phosphorus was exhausted (Fig. 2, inset); however, acetic acid consumption continued beyond this point. Therefore, acetic acid is probably used for cell maintenance and/or for storage of internal reserves. A maximum specific growth rate of 0.33 h−1 was determined when the initial glucose concentration was 0.71 mmol of glucose g of cell−1.
Intracellular glutamate levels were also determined. Glutamate accumulated in the anaerobic phase and was consumed under aerobiosis (Fig. 2 and 3). When 13C NMR was used, glutamate and trehalose concentrations, quantified in a cell extract performed at the end of the anaerobic period, were 0.23 and 0.09 mM, respectively. The concentration of glutamate in the growth medium originating from the yeast extract was 0.24 mM.
In vivo NMR measurements.
NMR experiments were performed to provide further insight into the phosphorus and carbon metabolism of M. phosphovorus. Cell suspensions were subjected to alternating anaerobic and aerobic cycles (3 to 5 h each) to simulate the conditions used in the bioreactor. Typically, cells were harvested at the end of an aerobic phase, and the sample for NMR was prepared as described above. Anaerobic conditions were created by bubbling argon, and a pulse of glucose (5 to 20 mM final concentration) was added. 13C and 31P spectra were acquired alternately in time, starting with carbon. Each spectrum was acquired for 7.0 or 10.7 min (carbon) and 4.0 min (phosphorus).
Anaerobic stage.
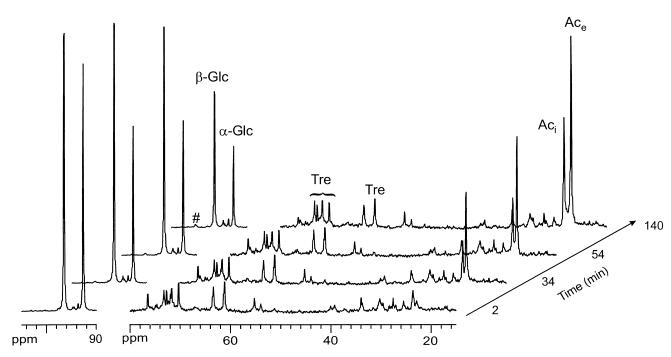
A selection of 13C NMR spectra acquired in the first anaerobic stage following glucose addition is shown in Fig. 4. The major resonances in the initial spectrum acquired prior to glucose addition were assigned to the natural abundance 13C in the six carbons of trehalose; lower-intensity resonances could also be detected and were assigned to glutamate (55.0, 33.7, and 27.3 ppm) and acetate (23 ppm). Anomeric α- and β-carbon atoms of [1-13C]glucose create strong resonances at 92.6 and 96.2 ppm, respectively. Glucose was converted to acetic acid that appears labeled in the methyl group. Two resonances, at approximately 22 and 23 ppm, were assigned to the extracellular and intracellular pools of acetate, respectively. The chemical shift separation between these resonances reflects the different pH values of the intracellular and extracellular compartments, primarily caused by acidification of the external medium. Acetate was labeled only in the methyl group as confirmed by analysis of the 13C and 1H spectra of the supernatant resulting from the centrifugation of the cell suspension at the end of the anaerobic stage (not shown). On the other hand, labeled CO2 was not detected in the anaerobic stage. Therefore, the metabolism of glucose in this organism is likely to proceed via an Embden-Meyerhof-type glycolytic pathway (6, 19). The percentage of isotopic enrichment in C-2 of acetate was 35%, whereas a value of 50% would be expected if acetate was derived only from the supplied [1-13C]glucose. This deficit in the isotopic enrichment of the acetate pool is explained by the presence of this compound inside the cells at the beginning of the experiment and/or by the utilization of carbon reserves.
FIG. 4.
In vivo 13C NMR spectra of an M. phosphovorus cell suspension in anaerobic conditions. [1-13C]glucose was added at time zero to a final concentration of 20 mM. Spectra were started at the times indicated. Each spectrum was acquired for 10.7 min. The region of the spectrum between 90 and 105 ppm is plotted with a threefold decrease in the vertical scale in order to fit the glucose peaks. Tre, trehalose; Glc, glucose; Aci, internal acetate; Ace, external acetate; #, glycogen C-1.
Besides labeled acetate, a very low intensity new resonance was barely detected at 100.2 ppm assigned to carbon C-1 of glycogen; the intensity of the C-1 resonance in trehalose at 93.5 ppm increased only slightly. This shows that glucose was largely fermented to acetate and negligible amounts were channeled to reserves. Increase in the C-1 resonance of trehalose was hardly detected in vivo, but this was confirmed by the analysis of an ethanol cell extract performed in a separate experiment with cells collected at the end of the anaerobic stage.
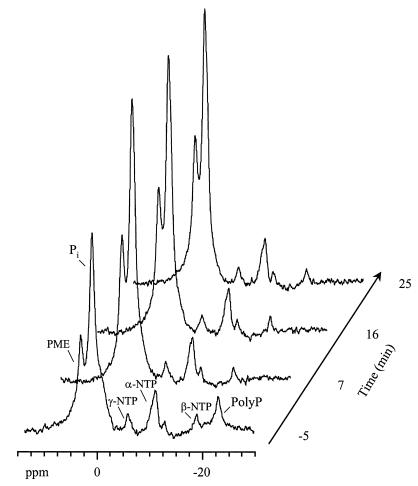
31P NMR allowed phosphorylated metabolites in the cell to be monitored, as well as polyphosphate (Fig. 5). The initial spectrum of an M. phosphovorus cell suspension under anaerobic conditions showed strong resonances due to inorganic phosphate, phosphomonoesters, and nucleoside triphosphates (NTP), as well as a resonance at approximately −22 ppm assigned to the internal phosphate groups in polyphosphate. Upon glucose addition the polyphosphate peak disappeared and the level of inorganic phosphate increased, denouncing the hydrolysis of polyphosphate. These phenomena were very rapid, since polyphosphate could no longer be detected in the first spectrum following glucose addition, and the level of inorganic phosphate remained constant thereafter. It is interesting to point out that the energetic status of the cells was already high before glucose addition, showing that NTP was formed from the catabolism of internal reserves, such as trehalose, and/or polyphosphate. Evidence for a contribution of sugar degradation was provided by the presence of a reasonably intense sugar phosphate resonance prior to the supplying of glucose (Fig. 5).
FIG. 5.
In vivo 31P NMR spectra of an M. phosphovorus cell suspension under anaerobic conditions. [1-13C]glucose (5 mM) was added at time zero, and spectra were acquired at regular time intervals. This pulse of glucose was exhausted after approximately 67 min. Each spectrum was acquired for 4 min. Pi, inorganic phosphate; PolyP, polyphosphate; PME, phosphomonoesters.
Aerobic stage.
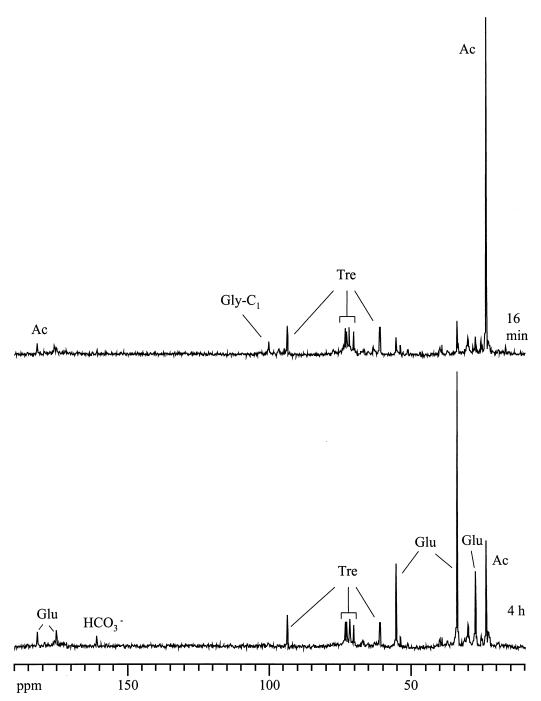
Cells used in the experiment depicted in Fig. 4 were centrifuged and resuspended in medium for NMR, and aerobic conditions were established. 13C NMR spectra acquired at 16 min and 4 h after the starting of the aerobic stage were selected as an illustration (Fig. 6). In the earlier spectrum, a resonance due to intracellular acetate remaining after centrifugation was apparent and decreased with time. Resonances due to C-4 of glutamate (33.7 ppm) and due to C-2 (55.0 ppm) and C-3 (27.3 ppm) with lower similar intensity appeared and grew concomitantly with acetate consumption. The relative isotopic enrichment of C-4 and C-2 (or C-3) in glutamate was determined by 13C NMR in cell extracts and was approximately 5:1. A clear peak assigned to labeled bicarbonate was also detected. The small glycogen peak present at the beginning of this aerobic phase rapidly disappeared. The observed labeling pattern in glutamate is easily explained: from acetyl coenzyme A labeled on C-2, glutamate labeled on C-4 was produced in the first turn of the TCA cycle. C-2 and C-3 of glutamate became labeled to a similar extent due to the scrambling at the levels of succinate dehydrogenase and fumarase, after a second turn in the TCA cycle. A third turn led to the release of labeled CO2.
FIG. 6.
In vivo 13C NMR spectra of an M. phosphovorus cell suspension under aerobiosis. Following the experiment whose results are shown in Fig. 4, air was provided at time zero and spectra were acquired at regular time intervals. The upper spectrum was acquired in the beginning of this aerobic phase, and the lower spectrum was acquired towards the end of this phase. Tre, trehalose; Glu, glutamate; Ac, acetate; Gly-C1, glycogen C-1.
A similar labeling pattern was observed in a different experiment, where cells were directly subjected to aerobic conditions and [2-13C]acetate was supplied (results not shown). When labeled acetate was exhausted, the resonance of C-4 in glutamate rapidly decreased, but the intensities of C-2 and C-3 did not change significantly. From the NMR labeling experiments described above, it is not possible to conclude whether the increase in the glutamate peaks was due to a net synthesis of glutamate from labeled acetate or instead resulted from labeling of the existing glutamate pool in the cell; this exchange of label between intermediates of the TCA cycle and glutamate occurs due to the well-known glutamate dehydrogenase activity. Evidence for the net utilization of the glutamate pool in the aerobic phase was obtained from the in vitro analysis of the glutamate intracellular pools during the course of fermentation in the batch reactor (Fig. 2, insert).
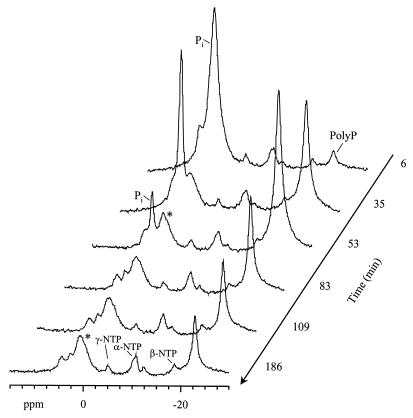
The time course for the changes in the levels of phosphorus-containing metabolites during the aerobic phase as monitored by 31P NMR is illustrated in Fig. 7. Uptake of inorganic phosphate coupled to polyphosphate synthesis was clearly seen from the sharp decrease in the intensity of the corresponding resonance at approximately 1.5 ppm and the increase in the polyphosphate resonance. The resonance of the terminal phosphate groups in polyphosphate was not detected, suggesting that the polyphosphate pool was composed of long chains. The subsequent intensity loss in the polyphosphate resonance reflects the decrease in the NMR detectability of this polymer due to immobilization. A similar effect was previously observed in Acinetobacter spp. (16). Furthermore, previous studies have shown that polyphosphate is totally invisible by NMR in phosphorus-accumulating activated sludge (15).
FIG. 7.
In vivo 31P NMR spectra of an M. phosphovorus cell suspension under aerobic conditions. Cells were previously subjected to an anaerobic phase, and labeled glucose (10 mM) was supplied. After approximately 2.5 h, the gas atmosphere was changed to air at a time designated zero, and spectra were acquired. Pi, inorganic phosphate; PolyP, polyphosphate; ∗, unidentified phosphodiester.
Second anaerobic phase.
To monitor the fate of labeled glutamate resulting from the aerobic metabolism of acetate, cells were submitted to a second anaerobic stage in the absence of external substrate, and 13C NMR spectra were acquired over time. No change was detected in the resonances due to glutamate (data not shown). A similar result was obtained in experiments where labeled glucose was added at the beginning of the second anaerobic stage. It is possible that trehalose has been utilized to a small extent, despite the fact that no significant alteration in the trehalose resonances was observed. Small changes in the pool of internal trehalose would be undetectable by 13C NMR due to the low natural abundance of 13C (1.1%). During this second anaerobic period phosphorus was released (data not shown).
DISCUSSION
Phosphorus and carbon metabolism in M. phosphovorus was investigated by using a batch reactor to study the kinetics of uptake and release of extracellular compounds, in combination with 31P and 13C NMR to characterize intracellular pools and to trace the fate of carbon substrates through the anaerobic and aerobic cycles.
Like other PAO present in activated sludge, M. phosphovorus releases inorganic phosphate in the anaerobic stage and accumulates phosphorus, as polyphosphate, in the subsequent aerobic phase. However, M. phosphovorus shows an outstanding ability to take up and release phosphorus, only comparable to that found in activated sludge (8), with rates of phosphorus uptake and release being about 1 order of magnitude higher than those reported for other pure cultures of polyphosphate-accumulating bacteria, such as Lampropedia spp. (21) and Acinetobacter spp. (only release) (22).
The rate of glucose consumption by M. phosphovorus was stimulated by phosphate release, even though glucose was still fermented when phosphorus release stopped. Reciprocally, the availability of glucose caused a sharp increase in the rate of phosphate release (compare Fig. 2 and 3). Phosphorus release was also observed in the absence of glucose, although at a lower rate (Table 1). These results suggest that phosphorus and carbon metabolism are not tightly coupled, as reported for other PAO (11, 20). On the other hand, in the aerobic phase, the rate of acetate consumption did not change significantly when external phosphorus was exhausted, suggesting that these processes are uncoupled. Conversely, growth always stopped when phosphorus was depleted. It is rather surprising that phosphorus is the limiting nutrient for growth in a bacterium that is able to accumulate polyphosphate. It seems as though, for an unknown reason, M. phosphovorus cannot mobilize the internal reserve of phosphorus and use it for growth.
A typical PAO in an EBPR system will utilize the substrates available during the anaerobic stage to synthesize carbon reserves (often polyhydroxyalkanoates) at the expense of the energy derived from the hydrolysis of polyphosphate; the carbon reserves will subsequently be oxidized during the aerobic stage to obtain energy for growth and for the polyphosphate accumulation. In contrast with this metabolic scenario, M. phosphovorus ferments glucose to acetate during the anaerobic stage without significant channeling of this substrate to internal reserves. Furthermore, the acetate produced is respired during the aerobic stage, again without formation of carbon reserves. Therefore, the energy harvested in this stage is mainly directed to growth and polyphosphate accumulation. At this stage of our knowledge, the fate of the energy obtained during fermentation remains obscure, since the organism is unable to grow under anaerobic conditions.
Internal reserves of trehalose and glutamate were clearly detected in vivo by natural abundance 13C NMR (Fig. 4), but their pools did not change significantly throughout the experiments. In fact, only a negligible increase in the C-1 resonance of trehalose was detected when labeled glucose was provided. Moreover, high-intensity resonances due to glutamate were detected when labeled acetate was available at the beginning of the aerobic stage, but we concluded that this resulted from the labeling of the glutamate pool at the level of glutamate dehydrogenase and not from de novo synthesis of glutamate. Nevertheless, it is interesting that the glutamate pool increased during the anaerobic phase and was partially consumed during the subsequent aerobic phase (Fig. 2 and 3). The NMR experiments also showed that glutamate was not synthesized from glucose during anaerobiosis. These observations suggest that glutamate was probably taken up from the medium during anaerobiosis and stored until used under aerobic conditions. The yeast extract present in the culture medium is a suitable source for both glutamate (7% [wt/wt]) and trehalose (11% [wt/wt]) (26), which is also accumulated by the organism (Fig. 4 and 6). The role played by glutamate in the metabolism of M. phosphovorus is not fully understood at this stage and requires further investigation.
One of the most interesting features of the kinetics of substrate consumption and product formation by this organism is the clear shift between the glucose depletion and the release of acetate during anaerobiosis. While glucose (0.29 mmol g of cell−1) was totally consumed in about 30 min, only 0.26 mmol g of cell−1 of acetate was excreted, an amount corresponding to less than half of the total acetate released at the end of the anaerobic period (0.61 mmol g of cell−1) (Fig. 1). The same behavior was observed when a higher concentration of glucose was used (Fig. 2). The observation of acetate release following glucose deprivation could be due to the fermentation of internal reserves and/or control of acetate release by mass transfer resistance through the cell membrane. Evidence for the utilization of internal reserves, even in the presence of external glucose, was obtained from the isotopic enrichment determined in acetate produced from [1-13C]glucose (35% instead of the expected 50%). Despite the fact that internal reserves are not synthesized from external glucose or acetate, the energetic status of the cells was always high, even in the absence of added substrates, showing that internal reserves were necessarily being mobilized (Fig. 5).
The second hypothesis is supported by the observation in the 13C NMR spectra of a high-intensity resonance assigned to internal acetate, when labeled glucose was metabolized under anaerobic conditions (Fig. 4). The delay in the excretion of acetate could be due to the ability of this organism to accumulate acetate. It is conceivable that this represents a survival strategy, enabling M. phosphovorus to store significant amounts of a carbon substrate that would otherwise be used by competing organisms.
The presence of label in the methyl group of acetate (derived from [1-13C]glucose) and the observed stoichiometry for the glucose-acetate conversion (approximately 0.5 mol/mol) indicate that glucose is metabolized in M. phosphovorus via the Embden-Meyerhof pathway.
The fermentation of glucose to volatile fatty acids, such as acetate, is usually ascribed to non-polyphosphate-accumulating heterotrophs (25). M. phosphovorus has a peculiar metabolism, insofar as it ferments glucose to acetic acid without producing significant amounts of internal reserves, yet displays a phosphorus metabolism similar to that of typical PAO. The metabolism of M. phosphovorus is also different from other PAO that are able to consume glucose. The utilization of glucose by PAO was postulated by Tracy and Flammino (24) and was recently corroborated experimentally by Carrucci et al. (3). The metabolism proposed for these microorganisms involves the generation of energy from polyphosphate hydrolysis, which is used for glucose uptake and storage in the form of glycogen. This reserve is subsequently used in the aerobic phase as an energy source for growth and phosphorus uptake. While these microorganisms grow and take up phosphorus at the expense of energy from internal glycogen, M. phosphovorus seems to obtain energy mainly from the external acetate released in the previous anaerobic phase.
In order to understand the mechanisms of biological phosphorus removal it is necessary to characterize in depth the metabolism of the different organisms contributing to the global process. Several microorganisms with the ability of enhanced phosphorus uptake have been isolated from EBPR systems (2, 5, 8, 21), but thus far, none of them was able to present the behavior exhibited by activated sludge. Unlike all the other isolates studied, M. phosphovorus shows high phosphorus release and uptake rates that are comparable to those of activated sludge. At this stage it is, however, difficult to understand how this fermentative bacterium will be able to compete efficiently with typical PAO, which store high levels of internal reserves in the anaerobic phase. Nevertheless, the ability of M. phosphovorus to accumulate acetate intracellularly could represent an alternative strategy of this interesting organism to retain the carbon substrate required for growth in aerobic conditions and, therefore, to compete successfully with typical PAO.
Further isolates from activated sludge and knowledge on their metabolism are required to gain insight into the relative contributions of the several microbial components in enhanced phosphorus removal processes.
ACKNOWLEDGMENTS
We acknowledge the financial support of Fundação para a Ciência e Tecnologia (FCT) under project PEAM/C/SEL//494/95.
We thank João Crespo from FCT/Universidade Nova de Lisboa for useful suggestions concerning the operation of the membrane cell recycle reactor.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 17th ed. Washington, D.C: American Public Health Association; 1989. [Google Scholar]
- 2.Brodisch K E U, Joyner S J. The role of micro-organisms other than Acinetobacter in biological phosphate removal in activated processes. Water Sci Technol. 1983;15:117–125. [Google Scholar]
- 3.Carucci A, Lindrea K, Majone M, Ramadori R. Different mechanisms for the anaerobic storage of organic substrates and their effect on enhanced biological phosphate removal (EBPR) Water Sci Technol. 1999;39(6):21–28. [Google Scholar]
- 4.Cech J B, Hartman P. Competition between polyphosphate and polysaccharide accumulating bacteria in enhanced biological phosphate removal systems. Water Res. 1993;27:1219–1225. [Google Scholar]
- 5.Fuhs G W, Chen M. Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb Ecol. 1975;2:119–138. doi: 10.1007/BF02010434. [DOI] [PubMed] [Google Scholar]
- 6.Gottschalk G. Bacterial metabolism. 2nd ed. New York, N.Y: Springer-Verlag Inc.; 1986. [Google Scholar]
- 7.Henze M. Biological phosphorus removal from wastewater: processes and technology. Water Qual Int. 1996;1996:32–36. [Google Scholar]
- 8.Hiraishi A, Morishima Y. Capacity for polyphosphate accumulation of predominant bacteria in activated sludge showing enhanced biological phosphate removal. J Ferment Bioeng. 1990;69:368–371. [Google Scholar]
- 9.Lemos P C, Viana C, Carrondo M J T, Crespo J P S G, Reis M A M. Proceedings of the IAWQ International Conference on the Advanced Wastewater Treatment: Nutrient Removal and Anaerobic Processes. Amsterdam, The Netherlands: (Aquatech ’96); 1996. Effect of carbon source on the production of polyhydroxyalkanoates in a biological phosphorus removal system; pp. 395–397. [Google Scholar]
- 10.Loosdrecht M C M, van, Hooijmans C M, Brdjanovic D, Heijnen J J. Biological phosphorus removal processes. Appl Microbiol Biotechnol. 1997;48:289–296. [Google Scholar]
- 11.Mino T, Tsuzuki Y, Matsuo T. Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process. In: Ramadori R, editor. Biological phosphate removal from wastewaters: proceedings from the International Conference of Advanced Water Pollution Control (IAWPRC). Oxford, England: Pergamon Press; 1987. pp. 27–38. [Google Scholar]
- 12.Mino T, van Loosdrecht M C M, Heijnen J J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998;32:3193–3207. [Google Scholar]
- 13.Nakamura K, Hiraishi A, Yoshimi Y, Kawaharasaki M, Masuda K, Kamagata Y. Microlunatus phosphovorus gen. nov., sp. nov., a new gram-positive polyphosphate-accumulating bacterium isolated from activated sludge. Int J Syst Bacteriol. 1995;45:17–22. doi: 10.1099/00207713-45-1-17. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Ishikawa S, Kawaharasaki M. Phosphate uptake and release activity in immobilized polyphosphate-accumulating bacterium Microlunatus phosphovorus strain NM-1. J Ferment Bioeng. 1995;80:377–382. [Google Scholar]
- 15.Pereira H, Lemos P C, Reis M A M, Crespo J P G, Carrondo M J T, Santos H. Model for carbon metabolism in biological phosphorus removal processes based on in vivo13C-NMR labeling experiments. Water Res. 1996;30:2128–2138. [Google Scholar]
- 16.Pereira H. Ph.D. thesis. Lisbon, Portugal: Instituto de Tecnologia Química e Biológica; 1997. [Google Scholar]
- 17.Santos H, Turner D L. Characterization of the improved sensitivity obtained using a flow method for oxygenation and mixing cell suspensions in NMR. J Magn Reson. 1986;68:345–349. [Google Scholar]
- 18.Satoh H, Ramey W D, Koch F A, Oldham W K, Mino T, Matsuo T. Anaerobic substrate uptake by the enhanced biological phosphorus removal activated sludge treating real sewage. Water Sci Technol. 1996;34:9–16. [Google Scholar]
- 19.Selig M, Xavier K B, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- 20.Smolders G J F, van der Meij J, van Loosdrecht M C M, Heijnen J J. A structured metabolic model for anaerobic and aerobic stoichiometry and kinetics of the biological phosphorus removal process. Biotechnol Bioeng. 1995;47:277–287. doi: 10.1002/bit.260470302. [DOI] [PubMed] [Google Scholar]
- 21.Stante L, Cellamare C, Malaspina F, Bortone G, Tilche A. Biological phosphorus removal by pure culture Lampropedia spp. Water Res. 1997;31:1317–1324. [Google Scholar]
- 22.Tandoi V, Majone M, May J, Ramadori R. The behaviour of phosphate accumulating Acinetobacter isolates in an anaerobic-aerobic chemostat. Water Res. 1999;32:2903–2912. [Google Scholar]
- 23.Torien D F, Gerbe A, Lötter L H, Cloete T E. Enhanced biological phosphorus removal processes in activated sludge systems. In: Marshall K C, editor. Advances in microbial ecology. Vol. 11. New York, N.Y: Plenum Publishing Corporation; 1990. pp. 173–230. [Google Scholar]
- 24.Tracy K D, Flammino A. Biochemistry and energetics of biological phosphorus removal. In: Ramadori R, editor. Biological phosphate removal from wastewaters: proceedings from the International Conference of Advanced Water Pollution Control (IAWPRC). Oxford, England: Pergamon Press; 1987. pp. 15–26. [Google Scholar]
- 25.Wentzel M C, Lötter L H, Loewenthal R E, Marais G v R. Metabolic behavior of Acinetobacter spp. in enhanced biological phosphate removal—a biochemical model. Water S A. 1986;12:209–244. [Google Scholar]
- 26.Xavier K B, Martins L O, Peist R, Kossmann M, Boos W, Santos H. High-affinity maltose/trehalose transport system in the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1996;178:4773–4777. doi: 10.1128/jb.178.16.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]