Abstract
Background:
The phase 3 TERIKIDS study demonstrated efficacy and manageable safety for teriflunomide versus placebo in children with relapsing multiple sclerosis (RMS).
Objective:
Evaluate plasma neurofilament light chain (pNfL) concentrations in TERIKIDS.
Methods:
Patients received placebo or teriflunomide (14 mg adult equivalent) for up to 96 weeks in the double-blind (DB) period. In the open-label extension (OLE), all patients received teriflunomide until up to 192 weeks after randomization. pNfL was measured using single-molecule array assay (Simoa® NF-light™).
Results:
Baseline mean age was 14.5 years; 69.4% were female. Baseline geometric least square mean pNfL levels were similar for teriflunomide (n = 78) and placebo (n = 33) patients (19.83 vs 18.30 pg/mL). Over the combined DB and OLE periods, pNfL values were lower for teriflunomide versus placebo (analysis of variance p < 0.01; Week 192: 10.61 vs 17.32 pg/mL). Observed between-group pNfL differences were attenuated upon adjustment for gadolinium (Gd)-enhancing or new/enlarged T2 lesion counts at DB Week 24. Higher baseline pNfL levels were associated with shorter time since first MS symptom onset, higher baseline Gd-enhancing lesion counts and T2 lesion volume, and increased hazard of high magnetic resonance imaging activity or clinical relapse during the DB period.
Conclusion:
Teriflunomide treatment was associated with significantly reduced pNfL levels in children with RMS.
ClinicalTrials.gov identifier:
Keywords: Neurofilament light chain, pediatric MS, teriflunomide
Introduction
Pediatric multiple sclerosis (MS) accounts for 2%–5% of MS cases1 and is typically relapsing-remitting.2 Children with MS often have higher relapse rates early in the disease course compared with adults with MS.2 Although disease progression may be slower when MS onset occurs at a younger age, these patients often reach adult disability milestones earlier than patients with later MS onset.3 Multiple disease-modifying therapies (DMTs) have been used off-label to treat children with MS.4,5 Fingolimod is now approved for the treatment of children and adolescents aged ⩾10 years with relapsing forms of MS in the United States,6 European Union,7 and other geographies. However, the safety and efficacy of most DMTs are yet to be investigated in children with MS.
Teriflunomide is a DMT,8 taken orally once daily, and is approved in more than 80 countries for the treatment of relapsing MS (RMS) in adults. The phase 3 TERIKIDS trial was a 96 week, multicenter, multinational, randomized, double-blind (DB), placebo-controlled, parallel-group study investigating teriflunomide in pediatric RMS, followed by a 96-week open-label extension (OLE). Results from the DB period demonstrated efficacy and manageable safety for teriflunomide in children with RMS, with teriflunomide significantly reducing the combined risk of clinical relapse or high magnetic resonance imaging (MRI) activity compared with placebo.9 Teriflunomide has subsequently been approved for use in the European Union in children aged 10 years or older with relapsing-remitting MS (RRMS).10
Several potential biomarkers are currently being explored to detect neuroaxonal injury, which is an important correlate of long-term disability in MS, but is not well captured by routine MRI scans.11 Blood neurofilament light chain (NfL) concentrations have shown promise as a biomarker of axonal damage in many neurological diseases including MS.12 Blood NfL is emerging as a measurable, real-time, fluid biomarker of disease evolution and treatment response in patients with MS.13 –15
This post hoc analysis of the TERIKIDS trial aimed to evaluate changes in plasma NfL (pNfL) levels over time in children with RMS treated with teriflunomide or placebo, determine demographic or clinical characteristics associated with pNfL levels, and examine whether pNfL levels were associated with prospective outcomes.
Methods
Study design and participants
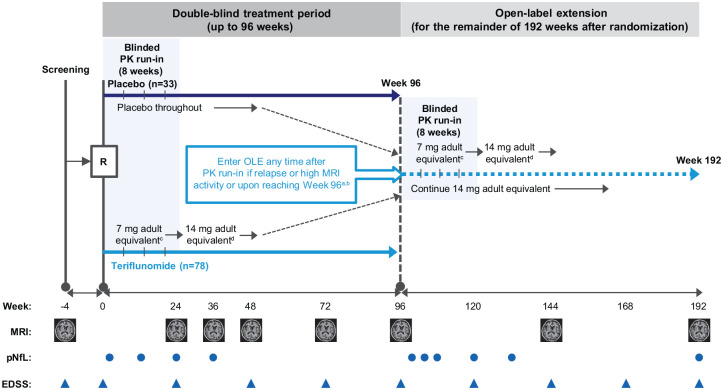
The TERIKIDS (NCT02201108) study design has been described previously.9 Briefly, patients with RMS aged 10–17 years were randomized 2:1 to teriflunomide or placebo at the start of the DB period, which lasted for up to 96 weeks (Figure 1). The first 8 weeks of the DB period included a run-in period in which teriflunomide patients received a half dose of teriflunomide, determined by body weight category. For the rest of the DB treatment period, teriflunomide patients received a dose equivalent to 14 mg in adults, determined by body weight category and patient pharmacokinetic parameters. Upon entering the OLE, patients from the DB placebo group received a half dose of teriflunomide during an 8-week run-in followed by the 14 mg adult equivalent teriflunomide dose; patients from the DB teriflunomide group continued through the OLE with the same previously adjusted teriflunomide dose from the DB period. Thus, for the OLE, the DB placebo group is referred to as the placebo/teriflunomide group and the DB teriflunomide group as the teriflunomide/teriflunomide group. Patients could enter the OLE early if they experienced a clinical relapse or high MRI activity (⩾9 new or enlarged T2 lesions at Week 36 or ⩾ 5 new/enlarged T2 lesions on each of two consecutive MRI scans at Weeks 36 and 48, or at Weeks 48 and 72).
Figure 1.
TERIKIDS study design.
aEntry to OLE any time after initial PK run-in; a new run-in period (8 weeks) starts after entry to extension.
bCriteria for high MRI activity to qualify for switch to the OLE treatment were ⩾9 new/enlarged T2 lesions at Week 36 or ⩾5 new/enlarged T2 lesions on each of two consecutive MRI scans at Weeks 36 and 48, or at Weeks 48 and 72.
cDetermined by body weight category: patients who weighed 20–40 kg received 3.5 mg/day; patients >40 kg received 7 mg/day.
dDetermined by a combination of body weight category and individually predicted PK parameters based on data collected during the run-in period: patients who weighed 20–40 kg received 7 mg/day if predicted PK parameters were equal to or less than the adult range of predicted parameters for a repeated dose of 7 mg, or received 3.5 mg/day if PK parameters were higher than the adult range; patients > 40 kg received 14 mg/day if PK parameters were equal to or less than the adult range of predicted parameters, or received 7 mg/day if PK parameters were higher than the adult range.
EDSS = Expanded Disability Status Scale; MRI = magnetic resonance imaging; OLE = open-label extension; PK = pharmacokinetic; pNfL = plasma neurofilament light chain; R = randomization.
Written informed consent to participate in this sub-study, additional to the consent provided for the main TERIKIDS trial, was obtained from the patient and patient’s legal representative (parents or guardians) according to local regulations. The study conduct was approved by local institutional ethics review boards or ethics committees, as required by local regulations.
Assessments
Sample collection
This study used ethylenediaminetetraacetic acid (EDTA) plasma samples from blood that had been taken for pharmacokinetic analysis at DB Weeks 2, 12, 24, and 36; OLE Weeks 4, 8, 12, 24, and 36; and Week 192/end of treatment (EOT; Figure 1). DB Week 2 was used as the baseline, since no blood samples were taken at DB Week 0. EDTA plasma samples were stored in accordance with local regulations and processed at Pharmaceutical Product Development bioanalytical lab (Richmond, VA, USA).
pNfL analysis
Plasma NfL was measured using a highly sensitive single-molecule array (Simoa® NF-light™) immunoassay, with a calibration range of 500–0.167 pg/mL. All samples were briefly centrifuged to pellet aggregates and the clear supernatant was diluted at least four-fold in sample diluent and plated into 96 well microtiter plates that were processed in a Quanterix HD-X Analyzer™ (Quanterix, Lexington, MA, USA).
Each analytical run consisted of a set of calibrators and two sets of buffer controls and one matrix quality control for run acceptance per United States Food and Drug Administration (FDA) guidance.16 Precision was evaluated by replicate analyses of buffer and human plasma quality control pools prepared at three concentrations spanning the calibration range, and was measured as the percent coefficient of variation (CV) of the set of values for each pool.
Sample quality control data acceptance criteria included ⩽20% CV between duplicate average enzymes per bead (AEB) for calibration standards, quality controls, and unknowns; and ⩽25% CV between duplicate AEB for upper and lower limits of quantification. The observed intra-assay CV ranged from 2.1% (mean 505 pg/mL) to 6.3% (0.6 pg/mL), and inter-assay CV ranged from 0.3% (mean 6.6 pg/mL) to 10.0% (150 pg/mL). All samples (N≈792) were analyzed at the Pharmaceutical Product Development bioanalytical lab in Richmond, VA, USA in duplicate. The fluorescence signal emitted from samples was captured with a built-in time-lapse molecular imager. Unknown sample concentrations were determined by interpolation from the standard curve, using a four-parameter logistic regression with 1/y2 weighting.
MRI assessment
Brain MRI acquisition in TERIKIDS has been described previously.9 Briefly, MRI with and without contrast was acquired using a standardized protocol on 1.5 or 3.0 T scanners, and scans were reviewed and interpreted independently by trained neuroradiologists with no access to treatment assignment at a central facility. The time points at which MRI was acquired are shown in Figure 1.
Assessment of disability progression
Expanded Disability Status Scale (EDSS) score was used to quantify disability.17,18 Disability progression was defined as a ⩾0.5-point increase from baseline EDSS score if baseline score was >5.5, or a ⩾1-point increase from baseline EDSS score if baseline score wasn ⩽5.5, sustained for ⩾24 weeks.9
Relapse assessment
Clinical relapses were defined as new or recurrent neurological symptoms that were not associated with fever or infection, lasted ⩾ 24 hours, and were accompanied by new objective neurological findings on examination by a neurologist; relapses were confirmed by an independent adjudication panel.9
Statistical analysis
We summarized baseline characteristics by study arm using mean (standard deviation) for continuous variables or n (%) for categorical variables.
Differences between pNfL levels by study arm over time were examined using a mixed effects model for repeated measures (MMRM) including treatment arm, study visit (modeled categorically), treatment-arm-by-visit interaction, and baseline age as fixed effects. As pNfL values were skewed and not normally distributed, we log-transformed pNfL (base e) and modeled pNfL on a log scale. Due to the log transformation and exponentiation of the resulting coefficients, values represent the geometric least square means (GLSM). To examine the difference between study treatment arms, we used the GLSM ratio (GLSMR). We used an analysis of variance (ANOVA) global F-test to assess the significance of the treatment-arm-by-visit interaction.
We conducted an exploratory analysis to evaluate whether observed differences in pNfL between study arms over time can be partially explained by early changes on MRI. For this analysis, we used the MMRM model described above, additionally adjusting for gadolinium (Gd)-enhancing lesion counts and, in a separate model, for new/enlarged T2 lesion counts; both MRI outcomes were measured at DB Week 24. In these analyses, we excluded pNfL values from DB Weeks 2 and 12.
We conducted two sensitivity analyses to test the robustness of our main findings. First, because of observed differences between treatment arms with respect to sex and region, we ran separate models additionally adjusting for these values. Second, because we observed a small, albeit non-significant, difference in pNfL levels between study arms at baseline, we modeled post-baseline pNfL values with adjustment for baseline pNfL values.
We examined the cross-sectional relationship at baseline between pNfL values and patient characteristics using multivariate linear regression. We examined the following baseline characteristics: age, sex, body mass index, EDSS score, time since first symptoms of MS, time since most recent relapse onset prior to randomization (< 120 days vs ⩾ 120 days), number of Gd-enhancing lesions, and T2 lesion volume (cube root transformed).
We examined the association between baseline pNfL and the following prospective outcomes using Cox proportional hazards regression adjusted for age: first confirmed clinical relapse during the DB period, high MRI activity or first confirmed clinical relapse during the DB period, and 24-week sustained disability progression during the OLE. In this analysis, we used log base 2 transformation so that the hazard ratio of pNfL would reflect the hazard of each outcome per doubling of baseline pNfL level. Given the expected association between treatment with teriflunomide and pNfL values over time, we conducted these analyses stratified by treatment arm.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). We report 95% confidence intervals (CIs) and all statistical tests were two-sided, with p-values < 0.05 considered statistically significant. The sample size of this analysis was lower than that of the main trial because participants had to opt into this sub-study, have available blood samples, and pass pNfL sample quality controls.
Results
From the original TERIKIDS trial patient cohort, 111 (67%) patients volunteered to participate in the pNfL sub-study and had available measurements (33 patients from the placebo arm and 78 patients from the teriflunomide arm). At baseline among the patients in this sub-study, mean age was 14.5 years, 69.4% of patients were female, mean number of relapses within the previous year was 1.5, and mean number of Gd-enhancing lesions was 3.7. Treatment groups were well balanced at baseline for demographic and disease characteristics (Table 1). The median (interquartile range) follow-up time among the patients in this analysis was 193.0 (191.1, 196.1) weeks. In general, baseline demographic and disease characteristics were similar to the overall TERIKIDS trial population.9
Table 1.
Baseline demographic and disease characteristics.
| Characteristic | Placebo (n = 33) | Teriflunomide (n = 78) |
|---|---|---|
| Age, years | 14.6 (2.0) | 14.5 (2.1) |
| Female, n (%) | 25 (75.8) | 52 (66.7) |
| Pubertal status, n (%) | ||
| Prepubertal (Tanner stage 1) | 2 (6.1) | 5 (6.4) |
| Pubertal (Tanner stage > 1) | 31 (93.9) | 73 (93.6) |
| Number of relapses within past 1 year | 1.4 (0.7) | 1.6 (0.7) |
| Number of relapses within past 2 years | 1.9 (1.0) | 2.1 (1.0) |
| MS disease duration, years | 2.5 (2.3) | 2.3 (2.1) |
| Patients receiving MS medication in past 2 years, n (%) | 8 (24.2) | 10 (12.8) |
| Patients with Gd-enhancing lesions, n (%) | 14 (42.4) | 41 (53.9) |
| Number of Gd-enhancing lesions | 2.6 (6.3) | 4.1 (8.1) |
| T2 lesion volume, cm3 | 11.1 (11.1) | 13.1 (19.0) |
| Normalized brain volume, cm3 | 1556.7 (82.1) | 1542.5 (80.0) |
Values are mean (standard deviation) unless otherwise indicated. Disease duration was calculated as the difference between date of first MS symptoms and randomization date.
Gd = gadolinium; MS = multiple sclerosis.
The cross-sectional associations of baseline pNfL with demographic and clinical characteristics are shown in Table 2. Shorter MS disease duration, higher Gd-enhancing lesion counts, and higher T2 lesion volume were associated with higher baseline pNfL levels (Table 2).
Table 2.
Associations between demographic and clinical factors and baseline pNfL values.
| Beta (95% CI) (N = 111) | Percentage change in pNfLa(95% CI) (N = 111) | p-value | |
|---|---|---|---|
| Ageb | −0.043 (−0.113, 0.027) | −4.2 (−10.7, 2.7) | 0.23 |
| Male sexc | −0.036 (−0.354, 0.281) | −3.6 (−29.8, 32.4) | 0.82 |
| Body mass index | −0.018 (−0.058, 0.021) | −1.8 (−5.6, 2.1) | 0.36 |
| EDSS score | 0.109 (−0.057, 0.274) | 11.5 (−5.5, 31.5) | 0.20 |
| Relapse < 120 days agod | 0.091 (−0.218, 0.399) | 9.5 (−19.6, 49.1) | 0.56 |
| MS disease duration relative to randomizationb | −0.068 (−0.135, −0.001) | −6.5 (−12.6, −0.1) | 0.048 |
| Number of Gd-enhancing lesions | 0.048 (0.025, 0.071) | 4.9 (2.6, 7.4) | <0.01 |
| T2 lesion volumee | 0.347 (0.143, 0.551) | 41.5 (15.3, 73.6) | <0.01 |
Multivariable linear regression analysis of log-transformed pNfL concentration values was used to examine association with the variables listed above. Numbers in bold indicate statistical significant association (p < 0.05) between the variable and the baseline pNFL value.
Beta coefficients were back-transformed and converted to a percentage for interpretation; values represent percentage increase/decrease in pNfL per unit change of the independent variable for continuous variables or relative to the reference group for categorical variables. Disease duration was calculated as the difference between date of first MS symptoms and randomization date.
Per year.
Reference is female sex.
Reference group is relapsed ⩾120 days ago.
Cubic root.
CI = confidence interval; EDSS = Expanded Disability Status Scale; Gd = gadolinium; MS = multiple sclerosis; pNfL = plasma neurofilament light chain.
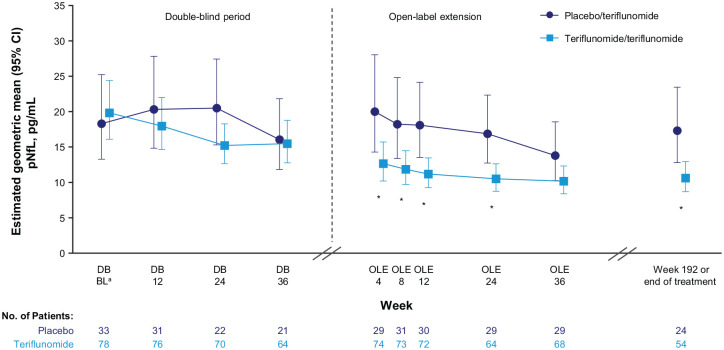
Estimated GLSM concentrations of pNfL over time are shown in Figure 2 and Supplemental Table 1. At DB Week 2, GLSM (95% CI) pNfL levels were 18.30 (13.27, 25.25) pg/mL in the placebo arm and 19.83 (16.10, 24.42) pg/mL in the teriflunomide arm. In the DB period, pNfL levels for placebo increased to Week 24 before decreasing at Week 36, by which time 10 of the 33 placebo patients (30.3%), compared with 12 of the 78 teriflunomide patients (15.4%), had transferred early to the OLE after experiencing relapse or high MRI activity (Supplemental Table 2). For teriflunomide, pNfL levels decreased to Week 24 and then remained stable to Week 36. Over the OLE, pNfL levels decreased in both treatment arms from Week 4 (GLSM (95% CI) pNfL at OLE Week 4: placebo/teriflunomide arm, 20.02 (14.30, 28.04) pg/mL; teriflunomide/teriflunomide arm, 12.66 (10.19, 15.72) pg/mL), reaching their lowest levels at OLE Week 36 (placebo/teriflunomide, 13.80 (10.26, 18.57) pg/mL; teriflunomide/teriflunomide, 10.16 (8.38, 12.31) pg/mL).
Figure 2.
The effect of teriflunomide versus placebo on pNfL levels over time in the TERIKIDS study
*p < 0.05 between treatment groups.
Geometric least square means and 95% CIs were obtained by exponentiating estimates from the mixed model for repeated measures analysis, which modeled the log-transformed pNfL concentration as response variable, and had treatment, visit, treatment-by-visit interaction, and age as covariates.
aBL is DB Week 2.
BL = baseline; CI = confidence interval; DB = double blind; OLE = open-label extension; pNfL = plasma neurofilament light chain.
Over the combined DB and OLE periods, pNfL values were significantly lower for teriflunomide compared with placebo (global ANOVA F-test for interaction: p < 0.01). In the DB period, the greatest difference between treatment arms was observed at Week 24, with a GLSMR (95% CI) for teriflunomide versus placebo of 0.74 (0.53, 1.05; p = 0.09). At five of the six OLE time points, pNfL values differed significantly between the treatment groups, with GLSMRs for teriflunomide/teriflunomide versus placebo/teriflunomide ranging from 0.61–0.65; at OLE Week 36, the reduction with teriflunomide/teriflunomide was not significant (GLSMR (95% CI): 0.74 (0.52, 1.05); p = 0.09; Supplemental Table 1).
In exploratory analyses that included adjustment for MRI lesion counts at DB Week 24, the GLSMR at DB Week 24 for teriflunomide versus placebo was attenuated from 0.74 in the main analysis, to 0.98 and 1.00, after including Gd-enhancing lesion and new/enlarged T2 lesion counts as covariates, respectively. During the OLE, GLSMRs were attenuated at each time point, ranging from 0.74–0.94 and 0.76–0.96 after adjustment for DB Week 24 Gd-enhancing lesion and new/enlarged T2 lesion counts, respectively; all p-values for these GLSMRs were not significant (Supplemental Table 3). In sensitivity analyses adjusting the mixed models for sex (Supplemental Table 4) and region (Supplemental Table 5), the results were similar to those from the main analysis. A final sensitivity analysis examining post-baseline pNfL values with adjustment for baseline pNfL yielded slightly more favorable GLSMRs, compared with the main analysis, for teriflunomide versus placebo in the DB period (p-value at DB Week 24: < 0.01) and teriflunomide/teriflunomide versus placebo/teriflunomide in the OLE (significant p-values at all OLE time points; Supplemental Table 6).
In analyses of prospective outcomes, each doubling of baseline pNfL was associated with an increased hazard of high MRI activity or clinical relapse for all patients during the DB period (hazard ratio [HR] = 1.22, 95% CI: 1.01, 1.48; p = 0.04); statistical significance was not reached for associations between baseline pNfL level and the other prospective outcomes for the overall study population, nor when any of these prospective outcomes were assessed by treatment arm (Table 3).
Table 3.
Hazard ratio of the association between baseline pNfL level and prospective outcomes.
| Placebo | Teriflunomide | Overall | |
|---|---|---|---|
| First confirmed clinical relapse during DB period | |||
| n/N (%) | 15/33 (45%) | 27/78 (35%) | 42/111 (38%) |
| Hazard ratio (95% CI) | 1.00 (0.66, 1.50) | 1.22 (0.91, 1.62) | 1.10 (0.88, 1.38) |
| p value | 0.98 | 0.18 | 0.41 |
| High MRI activity or first confirmed clinical relapse during DB period | |||
| n/N (%) | 23/33 (70%) | 35/78 (45%) | 58/111 (52%) |
| Hazard ratio (95% CI) | 1.21 (0.90, 1.64) | 1.27 (0.99, 1.63) | 1.22 (1.01, 1.48) |
| p value | 0.21 | 0.06 | 0.04 |
| 24-week sustained disability progression in OLE | |||
| n/N (%) | 8/32 (25%) | 11/74 (15%) | 19/106 (18%) |
| Hazard ratio (95% CI) | 1.01 (0.56, 1.80) | 1.40 (0.95, 2.06) | 1.26 (0.92, 1.73) |
| p value | 0.98 | 0.09 | 0.15 |
Hazard ratios (95% CIs) were estimated using Cox proportional hazards regression with adjustment for age. pNfL was modeled using log base 2 transformation so that the hazard ratio of pNfL would reflect the hazard of each outcome per doubling of baseline pNfL level. Analyses of 24 week sustained disability progression extend to the OLE; the 5 patients who did not enter the OLE were excluded from the disability progression analyses.
CI = confidence interval; DB = double blind; MRI = magnetic resonance imaging; OLE = open-label extension; pNfL = plasma neurofilament light chain.
Discussion
In this post hoc analysis of the TERIKIDS trial, in the combined DBP and OLE, we found that pNfL levels were lower among children with RMS treated with teriflunomide compared with placebo. We also found that pNfL was prognostic of relapse or MRI activity. Thus, our analysis supports pNfL as a useful biomarker of the risk of acute focal inflammatory activity in pediatric MS.
Previous studies of DMTs for MS have reported similar effects on NfL levels in adults. In an analysis of 246 patients with MS, adjusted for demographic and disease characteristics, use of a DMT was significantly associated with approximately 18% lower serum NfL levels.15 In another cohort study (N = 286) of treatment-naïve patients with MS, there was an 11% reduction in median serum NfL levels upon initiation of a DMT; switching to a higher efficacy therapy resulted in a 30% reduction in NfL levels.19 These findings have been further supported by studies of patients from clinical trials. In an analysis of patients with RRMS from two phase 3 trials (N = 589), fingolimod treatment was associated with significantly reduced blood NfL levels compared with intramuscular interferon beta (IFNB)-1a (ratio of means (95% CI): 0.794 (0.705–0.894) over 1 year and compared with placebo (0.628 (0.552–0.714) over 2 years (p < 0.001 for both comparisons).20 Similarly, over 2 years among treatment-naïve RRMS patients in the phase 3 CARE-MS I study, median serum NfL levels reduced to a significantly greater extent with alemtuzumab treatment (from 31.7 to 13.2 pg/mL) compared with subcutaneous IFNB-1a (from 31.4 to 18.7 pg/mL; p < 0.0001 between groups); the lower levels with alemtuzumab also remained stable over longer-term follow-up (12.7 pg/mL at Year 7).21
Our study found associations between higher baseline pNfL levels and shorter MS disease duration, higher baseline Gd-enhancing lesion counts, and higher baseline T2 lesion volume, as well as increased hazard of high MRI activity or clinical relapse during the DB period. Several previous studies in adult patients have yielded similar correlations between higher pNfL levels and worsening disability, increased number of relapses, and increased MRI disease activity.13,19 –23 Of note, recent cohort studies have extended these findings to pediatric MS, with associations reported between higher serum NfL levels and recent relapse,24 future relapses,25 increased MRI activity,24,25 and higher EDSS scores.24 The previous reports of elevated pNfL levels for up to 3 months after a relapse19,24 may explain the trajectory of pNfL levels observed in the placebo arm in our study. pNfL levels increased in this placebo arm from DB Week 2 to 24, and decreased at Week 36. These findings likely reflect the higher frequency of relapses in these patients and therefore the higher proportion switching early to the OLE, compared with the DB teriflunomide group. This higher proportion of patients switching to the OLE early due to relapse or high MRI activity may also explain the higher pNfL levels observed at OLE Week 4 in the placebo/teriflunomide arm compared with teriflunomide/teriflunomide.
The present study has some limitations. The statistical power of the study, which is already limited in a post hoc analysis, was further reduced due to many patients in the DB placebo arm experiencing relapses or meeting the high MRI activity criteria and therefore switching early to the OLE. This potentially attenuated the observed differences in pNfL between the teriflunomide and placebo arms. In addition, we had limited power to quantify the associations between baseline pNfL level and prospective outcomes since only a small number of the children experienced those outcomes. Furthermore, while it was necessary to set the baseline for this analysis at 2 weeks after the start of treatment, previous studies suggest meaningful changes in NfL levels would not be expected within this time frame post-DMT initiation.26,27 Finally, as the Week 192/EOT time point included a range of treatment exposures, this limited direct comparisons with the OLE Week 36 data. Nonetheless, TERIKIDS was a multinational study conducted in 57 clinical centers in 22 countries, potentially increasing generalizability of the findings. The measurements in this study, including for pNfL, MRI outcomes, and relapses, were performed in a rigorous and standardized manner, to minimize measurement error, and the study arms in this post hoc analysis were well balanced at baseline.
The findings of this post hoc analysis of the TERIKIDS trial suggest that pNfL levels decrease over time among children treated with teriflunomide compared with placebo, in parallel with the impact of treatment on inflammatory activity observed on MRI. Further investigation is warranted on the utility of pNfL as a biomarker for monitoring disease activity and treatment effects in clinical practice.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585221144742 for Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: A post hoc analysis of the randomized TERIKIDS trial by Jens Kuhle, Tanuja Chitnis, Brenda Banwell, Marc Tardieu, Douglas L Arnold, Andreea M Rawlings, Svend S Geertsen, Alex L Lublin, Stephane Saubadu, Philippe Truffinet and Ludwig Kappos in Multiple Sclerosis Journal
Acknowledgments
The authors and Sanofi thank the patients for their participation in the trial, as well as the TERIKIDS investigators. Medical writing assistance was provided by Richard J. Hogan, PhD, and Renee E. Granger, PhD, of Elevate Scientific Solutions, a division of Envision Pharma Group, and editorial and graphics assistance was provided by Elevate Scientific Solutions. This support was funded by Sanofi.
Footnotes
Authorship Contributions: All authors participated in study design, or data acquisition, analysis or interpretation; drafted or critically revised the manuscript; and approved the final version before submission. AMR performed the statistical analyses.
Data Sharing Statement: Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.K.: Institution (University Hospital Basel) received and used exclusively for research support; consulting fees (Biogen, Novartis, Protagen AG, Roche, and Teva), speaker fees (Biogen, Novartis, Roche, Sanofi, and Swiss MS Society), travel expenses (Merck Serono, Novartis, and Roche), and grants (Bayer AG, Biogen, Celgene, ECTRIMS Research Fellowship Programme, Merck, Novartis, Roche, Sanofi, Swiss MS Society, Swiss National Research Foundation (320030_160221) and University of Basel). T.C.: Consulting fees (Biogen, Novartis Pharmaceuticals, Roche Genentech, and Sanofi) and research support (National Institutes of Health, National MS Society, U.S. Department of Defense, EMD Serono, I-Mab Biopharma, Novartis Pharmaceuticals, Octave Bioscience, Genentech, Sanofi, and Tiziana Life Sciences). B.B.: Consulting fees (Novartis Pharmaceuticals, Roche, Sanofi, and UCB), nonremunerated advisory input (Biogen, EMD Serono, Novartis Pharmaceuticals, and Teva), and speaker fees (Medscape). M.T.: Research support (Novartis Pharmaceuticals and Sanofi). D.L.A.: Consulting fees (Biogen, Celgene, Frequency Therapeutics, Genentech, Merck, Novartis, Race to Erase MS, Roche, Sanofi, Shionogi, and Xfacto Communications), grants (Immunotec and Novartis), and equity interest (NeuroRx). A.M.R., S.S.G., S.S., and P.T.: Employees of Sanofi, and may hold shares and/or stock options in the company. ALL: Employee of Sanofi and owns shares in Sanofi. L.K.: Institution (University Hospital Basel) has received the following exclusively for research support in the past 3 years: steering committee, advisory board, and consultancy fees (Abbvie, Actelion, AurigaVision AG, Biogen, Celgene, Desitin, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Janssen, Japan Tobacco, Merck, Minoryx, Novartis, Roche, Sanofi, Santhera, Senda, Shionogi, Teva, and Wellmera); speaker fees (Celgene, Janssen, Merck, Novartis, and Roche); support of educational activities (Biogen, Desitin, Novartis, Sanofi, and Teva); license fees for Neurostatus products; and grants (European Union, Innosuisse, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Sanofi.
ORCID iDs: Tanuja Chitnis  https://orcid.org/0000-0002-9897-4422
https://orcid.org/0000-0002-9897-4422
Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Jens Kuhle, MS Center, Neurology and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Head, Spine and Neuromedicine, Biomedicine and Clinical Research, University Hospital Basel and University Basel, Basel, Switzerland.
Tanuja Chitnis, Massachusetts General Hospital for Children, Boston, MA, USA.
Brenda Banwell, Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA, USA.
Marc Tardieu, Hôpitaux Universitaires Paris-Sud, Paris, France.
Douglas L Arnold, McGill University, Montréal, QC, Canada NeuroRx Research, Montréal, QC, Canada.
Andreea M Rawlings, Sanofi, Cambridge, MA, USA.
Svend S Geertsen, Sanofi, Cambridge, MA, USA.
Alex L Lublin, Sanofi, Cambridge, MA, USA.
Stephane Saubadu, Sanofi, Cambridge, MA, USA.
Philippe Truffinet, Sanofi, Cambridge, MA, USA.
Ludwig Kappos, MS Center, Neurology and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Head, Spine and Neuromedicine, Biomedicine and Clinical Research, University Hospital Basel and University Basel, Basel, Switzerland.
References
- 1. Ness JM, Chabas D, Sadovnick AD, et al. Clinical features of children and adolescents with multiple sclerosis. Neurology 2007; 68: S37–S45. [DOI] [PubMed] [Google Scholar]
- 2. Waldman A, Ness J, Pohl D, et al. Pediatric multiple sclerosis: Clinical features and outcome. Neurology 2016; 87: S74–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007; 356: 2603–2613. [DOI] [PubMed] [Google Scholar]
- 4. Ghezzi A, Moiola L, Pozzilli C, et al. Natalizumab in the pediatric MS population: Results of the Italian registry. BMC Neurol 2015; 15: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krysko KM, Graves JS, Rensel M, et al. Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol 2020; 88(1): 42–55. [DOI] [PubMed] [Google Scholar]
- 6. Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022527s008lbl.pdf (accessed 22 March 2022).
- 7. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf (accessed 25 July 2022).
- 8. Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014; 74(6): 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chitnis T, Banwell B, Kappos L, et al. Safety and efficacy of teriflunomide in paediatric multiple sclerosis (TERIKIDS): A multicentre, double-blind, phase 3, randomised, placebo-controlled trial. Lancet Neurol 2021; 20(12): 1001–1011. [DOI] [PubMed] [Google Scholar]
- 10. Aubagio, https://www.ema.europa.eu/en/medicines/human/EPAR/aubagio (accessed 5 July 2022).
- 11. Yang J, Hamade M, Wu Q, et al. Current and future biomarkers in multiple sclerosis. Int J Mol Sci 2022; 23: 5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14(10): 577–589. [DOI] [PubMed] [Google Scholar]
- 13. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–2391. [DOI] [PubMed] [Google Scholar]
- 14. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol 2022; 21(3): 246–257. [DOI] [PubMed] [Google Scholar]
- 15. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. U.S. and Department of Health Human Services. Bioanalytical method validation: Guidance for industry, https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed 6 October 2022).
- 17. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 18. Neurostatus.net. Version 04/10.2, https://neurostatus.net/scoring/index.php (accessed October 2022).
- 19. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuhle J, Daizadeh N, Benkert P, et al. Sustained reduction of serum neurofilament light chain over 7 years by alemtuzumab in early relapsing-remitting MS. Mult Scler 2022; 28(4): 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Håkansson I, Tisell A, Cassel P, et al. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflammation 2018; 15: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol 2020; 7(6): 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reinert MC, Benkert P, Wuerfel J, et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020; 7(4): e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wendel EM, Bertolini A, Kousoulos L, et al. Serum neurofilament light-chain levels in children with monophasic myelin oligodendrocyte glycoprotein-associated disease, multiple sclerosis, and other acquired demyelinating syndrome. Mult Scler 2022; 28(10): 1553–1561. [DOI] [PubMed] [Google Scholar]
- 26. Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry 2019; 90(12): 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2018; 5(1): e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585221144742 for Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: A post hoc analysis of the randomized TERIKIDS trial by Jens Kuhle, Tanuja Chitnis, Brenda Banwell, Marc Tardieu, Douglas L Arnold, Andreea M Rawlings, Svend S Geertsen, Alex L Lublin, Stephane Saubadu, Philippe Truffinet and Ludwig Kappos in Multiple Sclerosis Journal