This cohort study assesses the association between serum neurofilament light chain (sNfL) levels and long-term worsening disability among patients with multiple sclerosis not treated with high-efficacy drugs.
Key Points
Question
Are serum neurofilament light chain (sNfL) levels associated with long-term disability worsening in patients with multiple sclerosis?
Findings
In this multicenter cohort study of 578 patients, sNfL values obtained within 12 months after disease onset were associated with higher risk of disability worsening and an Expanded Disability Status Scale score of 3 among patients who underwent their first demyelinating event. The risk mainly increased among patients with high sNfL concentrations who were not treated with high-efficacy disease-modifying treatments.
Meaning
The findings suggest that high levels of sNfL at disease onset may identify patients at high risk of disability worsening.
Abstract
Importance
The value of serum neurofilament light chain (sNfL) levels for predicting long-term disability in patients with multiple sclerosis (MS) remains controversial.
Objective
To assess whether high sNfL values are associated with disability worsening in patients who underwent their first demyelinating MS event.
Design, Setting, and Participants
This multicenter cohort study included patients who underwent their first demyelinating event suggestive of MS at Hospital Universitario Ramón y Cajal (development cohort; June 1, 1994, to September 31, 2021, with follow-up until August 31, 2022) and 8 Spanish hospitals (validation cohort; October 1, 1995, to August 4, 2020, with follow-up until August 16, 2022).
Exposures
Clinical evaluations at least every 6 months.
Main Outcomes and Measures
The main outcomes were 6-month confirmed disability worsening (CDW) and an Expanded Disability Status Scale (EDSS) score of 3. Levels of sNfL were measured in blood samples obtained within 12 months after disease onset using a single molecule array kit. The cutoffs used were sNfL level of 10 pg/mL and a standardized score (z score) of 1.5. Multivariable Cox proportional hazards regression models were used to evaluate outcomes.
Results
Of the 578 patients included in the study, 327 were in the development cohort (median age at sNfL analysis, 34.1 years [IQR, 27.2-42.7 years]; 226 female [69.1%]) and 251 patients were in the validation cohort (median age at sNfL analysis, 33.3 years [IQR, 27.4-41.5 years]; 184 female [73.3%]). The median follow-up was 7.10 years (IQR, 4.18-10.0 years). Levels of sNfL greater than 10 pg/mL were independently associated with higher risk of 6-month CDW and an EDSS of 3 in the development cohort (6-month CDW: hazard ratio [HR], 2.39; 95% CI, 1.39-4.12; P = .002; EDSS of 3: HR, 4.12; 95% CI, 2.18-7.77; P < .001) and the validation cohort (6-month CDW: HR, 1.61; 95% CI, 1.07-2.42; P = .02; EDSS of 3: HR, 2.03; 95% CI, 1.23-3.33; P = .005). Highly effective disease-modifying treatments were associated with lower risks of 6-month CDW and an EDSS of 3 in patients with high baseline sNfL values.
Conclusions and Relevance
This cohort study found that high sNfL values obtained within the first year of disease were associated with long-term disability worsening in MS, suggesting that sNfL level measurement may help identify optimal candidates for highly effective disease-modifying treatments.
Introduction
The accumulation of irreversible disability in multiple sclerosis (MS) is the main concern of the disease but is highly variable between patients. Predicting patient outcome is important for establishing accurate treatment decisions. Serum neurofilament light chain (sNfL) levels are indicative of axonal damage and have been associated with inflammation.1,2,3,4,5 Moreover, sNfL was found to predict disability accumulation in the short term,2,4,5,6 but more data are needed to explore the long-term prognostic value.3,7,8,9,10 This study investigated the value of sNfL levels for estimating long-term risks of disability worsening.
Methods
Study Design
This cohort study was performed at the University Hospital Ramón y Cajal MS Center and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. All patients who underwent their first demyelinating event suggestive of MS, presented to our MS unit with blood samples, and signed a written informed consent were consecutively included. Exclusion criteria are shown in the eAppendix in Supplement 1. A validation cohort was obtained from 8 other hospitals (eTable 1 in Supplement 1) and included patients with MS in whom blood samples were obtained within the first year of disease evolution. The same inclusion and exclusion criteria of the development cohort were applied to the validation cohort. Further details about how patients were recruited and how samples were collected from each center are shown in the eAppendix in Supplement 1. The study was approved by the ethics committee of the University Hospital Ramón y Cajal.
All patients presenting from June 1, 1994, to September 31, 2021, were included in the development cohort and were followed up until August 31, 2022. The recruitment period of patients in the validation cohort was from October 1, 1995, to August 4, 2020, and the last follow-up was August 16, 2022. Demographic, clinical, and radiological variables at baseline were collected. Data from all treatments were also collected. For analytical purposes, disease-modifying treatments (DMTs) were divided in 2 categories: high-efficacy DMTs (HE-DMTs) and others (Table 1). The end points assessed were the development of 6-month confirmed disability worsening (CDW) and an Expanded Disability Status Scale (EDSS) score of 3. (Scores range from 0 to 10 in 0.5-unit increments, with higher scores representing higher levels of disability.) Patients were evaluated prospectively with EDSS assessments at least every 6 months, with additional examinations in cases of relapses.
Table 1. Patient Characteristicsa.
| Characteristic | Development cohort (n = 327) | Validation cohort (n = 251) | Total (N = 578) |
|---|---|---|---|
| Sex | |||
| Female | 226 (69.1) | 184 (73.3) | 410 (70.9) |
| Male | 101 (30.9) | 67 (26.7) | 168 (29.1) |
| Age, median (IQR), y | |||
| At first symptom | 33.9 (27.1-42.4) | 33.2 (27.1-41.3) | 33.5 (27.1-42.1) |
| At sNfL analysis | 34.1 (27.2-42.7) | 33.3 (27.4-41.5) | 33.8 (27.3-42.2) |
| Time to analysis after first relapse, median (IQR), mo | 3.74 (1.21-6.36) | 2.26 (0.98-5.74) | 3.26 (1.12-6.03) |
| BMI, median (IQR) | 25 (22.4-26.0) | 24.3 (22.5-27.2) | 25 (22.5-26.7) |
| Topography of first relapse | |||
| Brain stem | 67 (20.5) | 60 (24.9) | 127 (22.4) |
| Optic nerve | 73 (22.3) | 49 (20.3) | 122 (21.5) |
| Spinal cord | 131 (40.1) | 76 (31.5) | 207 (36.4) |
| Multifocal | 9 (2.8) | 18 (7.5) | 27 (4.8) |
| Otherb | 47 (14.4) | 38 (15.8) | 85 (15) |
| Fulfillment of 2017 revised McDonald criteria at baseline11 | |||
| No | 45 (13.8) | 2 (0.8) | 47 (8.1) |
| Yes | 282 (86.2) | 249 (99.2) | 531 (91.9) |
| EDSS score at baseline, median (range) | 2 (0-6.5) | 1 (0-4) | 1.5 (0-6.5) |
| T2 lesions at baseline | |||
| 0-3 | 66 (17.1) | 25 (10.6) | 81 (13.4) |
| 4-9 | 92 (28.1) | 90 (38.1) | 182 (32.3) |
| 10-50 | 151 (46.2) | 118 (50) | 269 (47.8) |
| >50 | 28 (8.6) | 3 (1.3) | 31 (5.5) |
| Gadolinium-enhancing lesions, median (range) | 1 (0-36) | 0 (0-21) | 1 (0-36) |
| Patients with enhancing lesions, No. | 167/299 (55.9) | 97/212 (45.8) | 264/511 (51.7) |
| IgG oligoclonal bands | 267/327 (81.7) | 196/211 (92.9) | 460/538 (85.5) |
| DMT use during follow-up | |||
| None | 91 (27.8) | 32 (12.8) | 123 (21.3) |
| HE-DMTs exclusivelyc | 43 (13.1) | 18 (7.2) | 61 (10.6) |
| Other DMTs exclusivelyd | 160 (48.9) | 146 (58.2) | 306 (52.9) |
| Both HE-DMTs and other DMTs | 33 (10.1) | 55 (21.9) | 88 (15.2) |
| Time of follow-up, median (IQR), y | 5.43 (3.17-9.08) | 8.41 (5.83-10.85) | 7.10 (4.18-10.04) |
| Patients attaining 6-mo CDW during follow-upe | 72 (22.0) | 103 (41.0) | 175 (30.3) |
| Relapse-associated worsening | 34 (10.4) | 63 (25.1) | 97 (16.8) |
| Progression independent of relapse activity | 50 (15.3) | 69 (27.5) | 119 (20.6) |
| Patients reaching an EDSS score of 3 during follow-up | 61 (18.7) | 70 (27.9) | 131 (22.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CDW, confirmed disability worsening; DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale (scores range from 0 to 10 in 0.5-unit increments, with higher scores representing higher levels of disability); HE-DMT, highly effective DMT; IgG, immunoglobulin G; sNfL, serum neurofilament light chain.
Data are reported as number (percentage) of patients unless otherwise indicated.
Other topography of first relapse included cerebral hemisphere or paroxysmal symptoms.
HE-DMTs included natalizumab, alemtuzumab, ocrelizumab, rituximab, ofatumumab, and mitoxantrone.
Other DMTs included subcutaneous or intramuscular interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, fingolimod, oral cladribine, daclizumab, azathioprine, and tacrolimus.
Some patients may have experienced relapse-associated worsening, progression independent of relapse activity, or both events during their follow-up.
Patients were diagnosed with clinically isolated syndrome or MS based on the 2017 revisions of the McDonald criteria.11 Increases of EDSS scores of at least 1.5 points for baseline EDSS of 0, of 1 point for baseline EDSS of 1 to 5, and greater than 0.5 points for baseline EDSS of 5.5 or greater were required for CDW. Relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA) were defined according to the literature.12 Relapse-associated worsening was considered as an EDSS worsening within 90 days of a relapse and PIRA as a CDW at least 90 days after a relapse with no incident relapse within 30 days before or after confirmation 6 months later.12 The cutoff value for sNfL concentrations was established as 10 pg/mL based on previous studies.6,13,14 A standardized score (z score) reflecting the age and body mass index–adjusted SDs of sNfL levels from a normative data set of healthy controls was also considered. A value of 1.5 was also considered as a cutoff based on previous data.6
Statistical Analysis
The risk of 6-month CDW and an EDSS of 3 according to sNfL levels was analyzed using multivariable Cox proportional hazards regression models. The models were adjusted by sex, age at first relapse, baseline EDSS, T2 lesion load, time from first relapse to blood sample obtainment, and the proportion of time of DMT and HE-DMT receipt. Times to 6-month CDW and an EDSS of 3 were compared using Kaplan-Meier curves and a log-rank test. To further explore the mechanisms of disability worsening, additional models were used to test the associations of sNfL levels with risk of RAW and PIRA. Analyses were conducted using Stata, version 14 (StataCorp LLC). All tests were 2-tailed, and P < .05 was considered significant.
Results
We included 327 of 391 patients in the development cohort (83.6%) and 251 of 270 patients in the validation cohort (93.0%), for a total of 578 patients (eFigure in Supplement 1). Baseline characteristics of patients are summarized in Table 1. Briefly, the median age at sNfL analysis was 34.1 years (IQR, 27.2-42.7 years), 226 (69.1%) were female, and 101 (30.9%) were male in the development cohort. In the validation cohort, the median age at sNfL analysis was 33.3 years (IQR, 27.4-41.5 years), 184 (73.3%) were female, and 67 (26.7%) were male. In addition, 537 patients (92.9%) were younger than 51 years. Patients were followed up for a median of 5.43 years (IQR, 3.17-9.08 years) in the development cohort, 8.41 years (5.83-10.85 years) in the validation cohort, and 7.10 years (IQR, 4.18-10.0 years) overall. There were 48 patients (8.3%) with a follow-up of less than 24 months, representing a low percentage of the cohort. Levels of sNfL greater than 10 pg/mL and a z score greater than 1.5 were found in 151 patients (46.2%) and 149 (45.6%), respectively, in the development cohort and 115 (45.8%) and 119 (47.4%), respectively, in the validation cohort.
Patients with sNfL levels greater than 10 pg/mL showed higher risk of 6-month CDW independent of other covariates in both the development cohort (hazard ratio [HR], 2.39; 95% CI, 1.39-4.12; P = .002) and the validation cohort (HR, 1.61; 95% CI, 1.07-2.42; P = .02). Alternatively, we repeated all models using a cutoff value of 1.5 for the sNfL z score. Results mimicked those obtained with 10 pg/mL of sNfL as a cutoff (Table 2).
Table 2. Multivariable Cox Proportional Hazards Regression Models to Test Associations Between sNfL Levels and Risk of 6-Month CDW and EDSS of 3 Using the Cutoff Values of 10 pg/mL and a z Score of 1.5a.
| Variables | HR (95% CI) | |||||
|---|---|---|---|---|---|---|
| 6-mo CDW | EDSS of 3 | |||||
| Development cohort (n = 327) | Validation cohort (n = 251) | Total (N = 578) | Development cohort (n = 327) | Validation cohort (n = 251) | Total (N = 578) | |
| sNfL cutoff of 10 pg/mL | ||||||
| sNfL>10 pg/mL | 2.39 (1.39-4.12)b | 1.61 (1.07-2.42)c | 1.88 (1.37-2.60)d | 4.12 (2.18-7.77)d | 2.03 (1.23-3.33)b | 2.48 (1.69-3.64)d |
| Age at first relapse | 1.01 (0.98-1.03) | 1.01 (0.99-1.03) | 1.01 (0.99-1.02) | 1.01 (0.98-1.04) | 1.01 (0.98-1.03) | 1.01 (0.99-1.03) |
| Male sex | 2.02 (1.22-3.32)b | 1.21 (0.78-1.86) | 1.50 (1.10-2.07)c | 1.58 (0.88-2.84) | 1.47 (0.87-2.47) | 1.38 (0.95-2.02) |
| Baseline EDSS score | 1.44 (1.18-1.75)d | 1.18 (0.91-1.53) | 1.20 (1.03-1.39)c | 1.66 (1.37-2.05)d | 2.08 (1.55-2.79)d | 1.55 (1.35-1.78)d |
| T2 lesion load | 1.42 (1.07-1.88)c | 1.09 (0.82-1.44) | 1.24 (1.03-1.49)c | 1.35 (1.01-1.16)c | 1.05 (0.74-1.49) | 1.27 (1.04-1.57)c |
| Time from first relapse to sample obtainment, mo | 1.06 (0.99-1.13) | 1.00 (0.95-1.06) | 1.01 (0.98-1.05) | 1.08 (1.01-1.16)c | 1.02 (0.96-1.09) | 1.04 (0.99-1.09) |
| Proportion of time of DMT usee | 0.38 (0.17-0.78)b | 0.63 (0.37-1.08) | 0.58 (0.38-0.87)b | 0.25 (0.11-0.56)b | 0.31 (0.16-0.59)d | 0.35 (0.22-0.58)d |
| Proportion of time of HE-DMT usef | 0.26 (0.09-0.77)c | 0.19 (0.04-0.89)c | 0.26 (0.11-0.61)b | 0.05 (0.01-0.21)d | 0.26 (0.06-1.09) | 0.11 (0.04-0.30)d |
| AIC | 671 | 1020 | 1938 | 543 | 682 | 1415 |
| C statistic | 0.72 | 0.63 | 0.65 | 0.82 | 0.72 | 0.75 |
| z Score cutoff of 1.5 | ||||||
| z Score >1.5 | 2.06 (1.21-3.52)b | 1.56 (1.03-2.38)c | 1.80 (1.30-2.50)d | 2.99 (1.65-5.40)d | 1.94 (1.16-3.23)c | 2.13 (1.46-3.11)d |
| Age at first relapse | 1.01 (0.99-1.04) | 1.01 (0.99-1.03) | 1.01 (0.99-1.03) | 1.02 (0.99-1.05) | 1.01 (0.99-1.04) | 1.02 (1.00-1.04)c |
| Male sex | 1.90 (1.15-3.13)c | 1.25 (0.81-1.93) | 1.49 (1.08-2.05)c | 1.43 (0.80-2.58) | 1.59 (0.94-2.69) | 1.36 (0.93-1.98) |
| Baseline EDSS score | 1.45 (1.19-1.77)d | 1.20 (0.93-1.55) | 1.20 (1.03-1.39)c | 1.67 (1.36-2.04)d | 2.11 (1.57-2.84)d | 1.55 (1.34-1.78)d |
| T2 lesion load | 1.46 (1.11-1.94)b | 1.09 (0.82-1.45) | 1.26 (1.05-1.52)c | 1.39 (1.04-1.87)c | 1.07 (0.75-1.51) | 1.31 (1.07-1.61)c |
| Time from first relapse to sample obtainment, mo | 1.06 (0.99-1.13) | 1.00 (0.95-1.06) | 1.02 (0.98-1.06) | 1.08 (1.01-1.16)c | 1.02 (0.96-1.09) | 1.04 (0.99-1.08) |
| Proportion of time of DMT usee | 0.39 (0.19-0.79)b | 0.61 (0.35-1.04) | 0.56 (0.37-0.85)b | 0.26 (0.12-0.59)b | 0.28 (0.14-0.54)d | 0.34 (0.21-0.56)d |
| Proportion of time of HE-DMT usef | 0.27 (0.09-0.79)c | 0.19 (0.04-0.91)c | 0.26 (0.11-0.61)b | 0.06 (0.01-0.25)d | 0.27 (0.07-1.12) | 0.12 (0.04-0.32)d |
| AIC | 674 | 1021 | 1941 | 552 | 683 | 1422 |
| C statistic | 0.72 | 0.63 | 0.65 | 0.82 | 0.72 | 0.75 |
Abbreviations: AIC, Akaike information criterion; CDW, confirmed disability worsening; DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale (scores range from 0 to 10 in 0.5-unit increments, with higher scores representing higher levels of disability); HE-DMT, high-efficacy DMT; HR, hazard ratio; sNfL, serum neurofilament light chain.
Multivariable models were adjusted by age at first relapse, sex, T2 lesion load, time from first relapse to blood sample obtainment, proportion of time that the patient received DMT, excluding HE-DMT (obtained by dividing the time of DMT use by the time of disease evolution until the outcome of interest), and proportion of time of HE-DMT use.
P < .01.
P < .05.
P < .001.
DMTs, excluding HE-DMTs, included subcutaneous or intramuscular interferon-beta, glatiramer acetate, teriflunomide, dimethyl fumarate, fingolimod, oral cladribine, daclizumab, azathioprine, and tacrolimus.
HE-DMTs included natalizumab, alemtuzumab, ocrelizumab, rituximab, ofatumumab, and mitoxantrone.
We found that high sNfL values were associated with higher risk of both RAW and PIRA (eTable 2 in Supplement 1). Patients with high sNfL levels also showed higher risk of an EDSS of 3 in the development cohort (HR, 4.12; 95% CI, 2.18-7.77; P < .001). This was confirmed in the validation group (HR, 2.03; 95% CI, 1.23-3.33; P = .005). The models using sNfL values stratified by a z score of 1.5 showed similar results (Table 2).
HE-DMTs were associated with a decrease in risk of disability worsening (Table 2); thus, we explored the estimated value of sNfL greater than 10 pg/mL in patients receiving or not receiving these treatments before the preestablished outcomes. High sNfL levels were not associated with worse disease evolution when HE-DMTs were used (Figure). Sensitivity analyses are shown in eTables 3 to 5 and the eAppendix in Supplement 1.
Figure. Kaplan-Meier Curves of Risk of 6-Month Confirmed Disability Worsening (CDW) and an Expanded Disability Status Scale Score (EDSS) of 3 by Serum Neurofilament Light Chain (sNfL) Levels Stratified by Highly Effective Disease-Modifying Treatment (HE-DMT) Use.
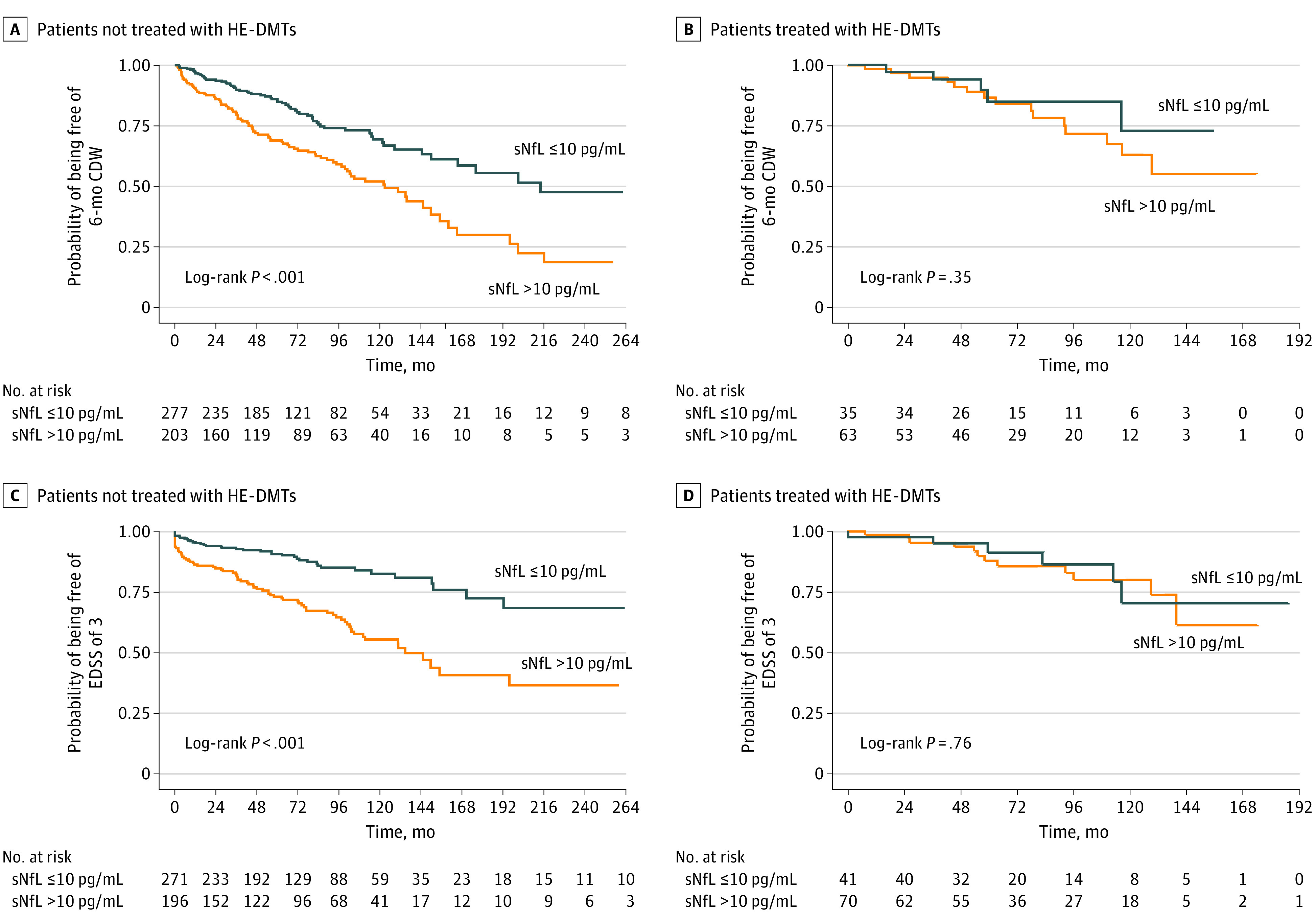
The stratification of patients was made according to HE-DMT use before each outcome (6-month CDW and EDSS of 3).
Discussion
Neurofilament light is one of the most promising biomarkers in MS.1 Higher levels reflect acute axonal damage and have been associated with inflammation observed as relapses and gadolinium-enhancing T1 or new T2 lesions.1,2,3,4,5,13 These levels are also associated with disease progression, prognosis, and response to treatment.1,4
Increased sNfL levels may predict future disease activity and EDSS worsening in the short term5,6 as these levels are the first evidence of inflammation before the clinical threshold is reached. However, studies of long-term outcomes provided less consistent results.3,7,8,9,10 This difference could be due to the different cutoff levels, times of blood sample obtainment, and effects of different DMTs. Additionally, sNfL levels are affected by age and body mass index, which were controlled using standardized scores in a previous study.6
We studied the long-term value of sNfL in blood samples obtained from patients who underwent their first demyelinating MS event within the first year of disease. Levels of sNfL greater than 10 pg/mL or a z score greater than 1.5 were associated with higher risk of disability worsening (either RAW or PIRA) and of an EDSS of 3. Results were more specific when blood samples were obtained at least 60 or 90 days after a relapse. These results were validated in a multicenter cohort.
The equivalence of both cutoffs may be explained by the demographic characteristics of the cohort since 92.9% of patients were younger than 51 years, the age group in which sNfL levels less than 10 pg/mL are physiological.14 However, using z scores may help discriminate patients with higher risk of disability worsening independent of age at sampling since they counteract the increase of sNfL levels associated with aging.
All DMTs, especially HE-DMTs, are associated with prevention of disability worsening even in patients with high sNfL levels but only when HE-DMTs are administered early during the course of the disease15 when acute inflammation generally predominates. Thus, sNfL levels may help identify patients with MS with the most favorable benefit-to-risk ratio for early HE-DMT.
Limitations
This study has limitations. First, patients with a short follow-up may not attain 6-month CDW and/or an EDSS of 3, which could potentially have yielded biased results. However, people with a follow-up of less than 24 months represented a low percentage (8.3%) of the cohort. Second, the only HE-DMTs under consideration were monoclonal antibodies and mitoxantrone. We preferred to define HE-DMTs as those treatments most frequently considered as highly efficacious in previous studies.15
Conclusions
In this cohort study, high sNfL levels detected during the first year of disease were associated with long-term disability worsening in MS, suggesting that high sNfL level may help identify patients with MS who are at increased risk of long-term worsening. High sNfL levels were not associated with worse disease evolution when HE-DMTs were used, suggesting that when administered early during the disease course, HE-DMTs may have the potential to counteract this detrimental evolution. Further studies are needed to confirm these results.
eAppendix. Eligibility Criteria and Samples Management in the Validation Cohort, Exclusion Criteria, Sensitivity Analyses
eTable 1. Study Design. Selection of Cohorts
eFigure. Flowchart of Patients Included
eTable 2. Multivariable Cox Regression Models to Test Associations Between High sNfL Levels and the Risk of RAW and PIRA (n = 578)
eTable 3. Multivariable Cox Regression Models to Test Associations Between High sNfL Levels and the Risk of 6-Month CDW and EDSS Score of 3 Stratifying by the Time of Sampling With Respect to First Relapse
eTable 4. Multivariable Cox Regression Models to Test Associations Between sNfL Levels and the Risk of 6-Month CDW and EDSS of 3 Using the Cut-Off Values of 10 pg/mL and z Score of 1.5 and Including Contrast-Enhancing Lesions
eTable 5. Multivariable Cox Regression Models to Test Associations Between sNfL Levels and the Risk of Sustained Disability Worsening Using the Cut-Off Values of 10 pg/mL and z Score of 1.5
Data Sharing Statement
References
- 1.Thebault S, Bose G, Booth R, Freedman MS. Serum neurofilament light in MS: the first true blood-based biomarker? Mult Scler. 2022;28(10):1491-1497. doi: 10.1177/1352458521993066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thebault S, Abdoli M, Fereshtehnejad S-M, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. doi: 10.1038/s41598-020-67504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826-831. doi: 10.1212/WNL.0000000000003653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benkert P, Meier S, Schaedelin S, et al. ; NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 7.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359-1366. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5(12):1478-1491. doi: 10.1002/acn3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457-e2467. doi: 10.1212/WNL.0000000000009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uphaus T, Steffen F, Muthuraman M, et al. NfL predicts relapse-free progression in a longitudinal multiple sclerosis cohort study. EBioMedicine. 2021;72:103590. doi: 10.1016/j.ebiom.2021.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 12.Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145(9):3147-3161. doi: 10.1093/brain/awac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comabella M, Sastre-Garriga J, Carbonell-Mirabent P, et al. Serum neurofilament light chain levels predict long-term disability progression in patients with progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. Published online April 29, 2022. doi: 10.1136/jnnp-2022-329020 [DOI] [PubMed] [Google Scholar]
- 14.Simrén J, Andreasson U, Gobom J, et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5-90 years. Brain Commun. 2022;4(4):fcac174. doi: 10.1093/braincomms/fcac174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He A, Merkel B, Brown JWL, et al. ; MSBase study group . Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307-316. doi: 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Eligibility Criteria and Samples Management in the Validation Cohort, Exclusion Criteria, Sensitivity Analyses
eTable 1. Study Design. Selection of Cohorts
eFigure. Flowchart of Patients Included
eTable 2. Multivariable Cox Regression Models to Test Associations Between High sNfL Levels and the Risk of RAW and PIRA (n = 578)
eTable 3. Multivariable Cox Regression Models to Test Associations Between High sNfL Levels and the Risk of 6-Month CDW and EDSS Score of 3 Stratifying by the Time of Sampling With Respect to First Relapse
eTable 4. Multivariable Cox Regression Models to Test Associations Between sNfL Levels and the Risk of 6-Month CDW and EDSS of 3 Using the Cut-Off Values of 10 pg/mL and z Score of 1.5 and Including Contrast-Enhancing Lesions
eTable 5. Multivariable Cox Regression Models to Test Associations Between sNfL Levels and the Risk of Sustained Disability Worsening Using the Cut-Off Values of 10 pg/mL and z Score of 1.5
Data Sharing Statement


