Key Points
Question
Among people who smoke cigarettes, does providing medications and counseling in an opt-out manner improve treatment engagement, quit attempts, and smoking cessation compared with opt-in treatment?
Findings
In this randomized clinical trial of 739 adult patients that compared opt-in care with the opt-out approach (in which patients had to refuse treatment or they would receive it), among those in the opt-out group, medication/counseling use was doubled, quit attempts were increased, therapeutic alliance was improved, and patients’ sense of control over quitting was strengthened at relatively low cost.
Meaning
Opt-out paradigms could accelerate population health gains in tobacco use and potentially other chronic conditions, such as obesity.
Abstract
Importance
Tobacco use causes 7 million deaths per year; most national guidelines require people who use tobacco to opt in to care by affirming they are willing to quit. Use of medications and counseling is low even in advanced economy countries.
Objective
To evaluate the efficacy of opt-out care vs opt-in care for people who use tobacco.
Design, Setting, and Participants
In Changing the Default (CTD), a Bayesian adaptive population-based randomization trial, eligible patients were randomized into study groups, treated according to group assignment, and debriefed and consented for participation at 1-month follow-up. A total of 1000 adult patients were treated at a tertiary care hospital in Kansas City. Patients were randomized from September 2016 to September 2020; final follow-up was in March 2021.
Interventions
At bedside, counselors screened for eligibility, conducted baseline assessment, randomized patients to study group, and provided opt-out care or opt-in care. Counselors and medical staff provided opt-out patients with inpatient nicotine replacement therapy, prescriptions for postdischarge medications, a 2-week medication starter kit, treatment planning, and 4 outpatient counseling calls. Patients could opt out of any or all elements of care. Opt-in patients willing to quit were offered each element of treatment described previously. Opt-in patients who were unwilling to quit received motivational counseling.
Main Outcomes and Measures
The main outcomes were biochemically verified abstinence and treatment uptake at 1 month after randomization.
Results
Of a total of 1000 eligible adult patients who were randomized, most consented and enrolled (270 [78%] of opt-in patients; 469 [73%] of opt-out patients). Adaptive randomization assigned 345 (64%) to the opt-out group and 645 (36%) to the opt-in group. The mean (SD) age at enrollment was 51.70 (14.56) for opt-out patients and 51.21 (14.80) for opt-out patients. Of 270 opt-in patients, 123 (45.56%) were female, and of 469 opt-out patients, 226 (48.19%) were female. Verified quit rates for the opt-out group vs the opt-in group were 22% vs 16% at month 1 and 19% vs 18% at 6 months. The Bayesian posterior probability that opt-out care was better than opt-in care was 0.97 at 1 month and 0.59 at 6 months. Treatment use for the opt-out group vs the opt-in group was 60% vs 34% for postdischarge cessation medication (bayesian posterior probability of 1.0), and 89% vs 37% for completing at least 1 postdischarge counseling call (bayesian posterior probability of 1.0). The incremental cost-effectiveness ratio was $678.60, representing the cost of each additional quit in the opt-out group.
Conclusions and Relevance
In this randomized clinical trial, opt-out care doubled treatment engagement and increased quit attempts, while enhancing patients’ sense of agency and alliance with practitioners. Stronger and longer treatment could increase cessation.
Trial Registration
ClinicalTrials.gov Identifier: NCT02721082
This randomized clinical trial evaluates the efficacy of opt-out care vs opt-in care for people who use tobacco.
Introduction
Worldwide, tobacco causes 7 million deaths per year.1 Pharmacotherapy and counseling double or triple tobacco cessation rates compared with no treatment.2 In most countries, however, health care practitioners address tobacco among only 20% to 50% of patients who smoke.3 In the US, less than 5% of smokers get prescriptions for cessation medication during primary care visits; compared with tobacco use, the odds for receiving pharmacotherapy are 33:1 for hypertension, 21:1 for diabetes, and 17:1 for hyperlipidemia.4
Clinical guidelines for hypertension, diabetes, and hyperlipidemia identify and direct clinicians to provide evidence-based therapies, which patients by default receive unless they refuse or opt out of treatment.5,6,7 Most people who use tobacco, however, live in countries with guidelines that recommend opt-in treatment for tobacco dependence.8 These guidelines direct clinicians to ask tobacco users if they are willing to try to quit and only offer cessation medications and counseling to those who say yes. Moreover, guidelines direct clinicians to offer rather than provide treatments. Hence, the default for smokers is to not receive medications and counseling for their tobacco use disorder unless they state they are willing to quit and accede to 1 or more components of care.
These very defaults can have strong influences on health choices.9 Opt-out approaches, compared with opt-in approaches, lead to 2 to 4 times higher rates of engagement in human immunodeficiency virus (HIV) screening,10,11 vaccine safety surveillance,12 and colorectal cancer screening.13
Defaults are thought to influence behavior in several ways. People are more likely to opt for the status quo.14 The way health care practitioners present choices may leak information about their preferred course of action—an implied endorsement or recommendation.14,15 By asking smokers if they are willing to quit and offering rather than providing treatment, clinicians may signal that treatment is marginally useful or effective only for those motivated to quit. Current studies, however, suggest that unmotivated smokers quit if given treatment.16,17,18
National guidelines for most countries recommend an opt-in approach to tobacco treatment. Although most smokers state they want to quit at some point in time, only 6% to 20% state they are ready to quit in the near future.15,16,17 As long as current national guidelines are in place, most tobacco users will report that they are not yet ready to quit, and they will not receive medications or counseling. In advanced economy countries, the opt-in approach is likely a major rate-limiting step in delivering evidence-based tobacco treatment.
The purpose of the Changing the Default (CTD) randomized clinical trial was to determine the population-based effect of providing opt-out treatment vs opt-in treatment for tobacco use disorder. To date, trials comparing treatment defaults have focused on one-off decisions linked to relatively simple but important behaviors, such as participating in health screening. This trial examines whether changing the treatment default can nudge patients into engaging in a chain of prohealth behaviors—including using counseling and pharmacotherapy—and lead to substantial behavior change, namely, quit attempts and cessation.
Methods
Trial Design
In this prospective, randomized, comparative effectiveness Bayesian adaptive design study,18 we used a delayed consent procedure to reduce potential bias from self-selection (Supplement 1). Study staff screened hospitalized patients for eligibility, collected baseline information, randomized patients to study group, administered the intervention, and called patients for follow-up assessment 1 month after randomization (eFigure in Supplement 2). At this time, staff debriefed patients and obtained consent for continued participation and use of their data.
The CTD trial procedures were approved by the University of Kansas Medical Center Human Subjects Committee. Recruitment occurred from September 2016 to September 2020; 6-month follow-up ended in March 2021. From April 2020 to September 2020, all study procedures occurred by phone due to the COVID-19 pandemic. The CTD trial used Research Electronic Data Capture (REDCap) (Vanderbilt University) to collect data and randomize patients to study group.19 The Consolidated Standards of Reporting Trials (CONSORT) reporting guideline was followed.
Participants
We identified all inpatients who currently smoked via the electronic health record and used a computer random function to select them into the study pool, which provided a full spectrum of participants in terms of interest in quitting. Staff used electronic health record data and bedside screening to determine eligibility. Inclusion criteria included participants who (1) were aged at least 18 years, (2) smoked 25 out of the past 30 days, (3) spoke English or Spanish, (4) had access to a phone, (5) were residents of Kansas or Missouri, (6) were medically eligible to use nicotine replacement therapy, and (7) were willing to provide a secondary phone number. Exclusion criteria included patients who (1) were pregnant and/or breastfeeding, (2) had a substantial comorbidity (life-threatening illness or altered mental status), (3) were incarcerated, (4) were receiving cessation pharmacotherapy, already treated for tobacco during the hospital stay, or currently enrolled in a cessation program, (5) were hospitalized for more than 3 days, (6) were in the process of being discharged, and/or (7) were previously screened ineligible for study. After screening, counselors collected baseline data, used random assignment functions on tablet computers to assign eligible participants to a study group, and administered the opt-in intervention or opt-out intervention as assigned.
Study staff called all patients 1 month after randomization to assess outcomes, debrief patients on their inclusion in the trial, and invite them to consent and enroll. Staff completed expanded 1-month data collection on all who consented and enrolled. For patients who refused consent, staff purged from the study record all but a subset of nonidentifying variables (age, sex, race and ethnicity, insurance) approved by the Human Subjects Committee. We included race and ethnicity as variables because they are known to be highly associated with tobacco use and cessation, and they are required by the study funder, the National Institutes of Health.
Randomization
The Bayesian trial design of CTD permitted more patients to be allocated to the better-performing study group and also provided for early stoppage of the trial if success criteria had been met (eMethods in Supplement 2).18 After 400 participants had been randomized in a 1:1 ratio, we conducted prespecified interim analyses every 13 weeks to determine the better-performing study group and reweight randomization to favor that study group. The 1-month study outcome drove the randomization probabilities using response-adaptive randomization. Randomization was performed using a pseudo-random number generator in blocks of size 4 in the 1:1 allocation and various block sizes for the response adaptive randomization depending on the allocation imbalance. Coauthors C.Z., B.G., and N.N. generated the allocation sequence. All other team members were blinded to study group assignment. Counselors assigned patients to a study group using a button in REDCap.
Intervention
All randomized into CTD received brief advice to quit and a cessation brochure.
Opt-Out Intervention Procedures—Inpatient Treatment
Staff arranged for inpatient medications to manage withdrawal, completed a treatment plan with patients, assisted patients to select a cessation medication for prescription on discharge, and described outpatient follow-up. Study staff used opt-out language designed to convey that the status quo for hospital practice is to provide medications and counseling to all patients to help smokers quit: “Now we’re going to write out a treatment plan to note your choices”; “Which sort of medication would you like a prescription for when you leave the hospital?”; “We will call you once you get home to see how you are doing.” Unless patients opted out of any or all elements of the treatment plan, they received all elements of care. Patients had to refuse twice; after 1 refusal, study staff provided a rationale for the care. If a patient refused again, he or she did not receive that component of care.
Opt-in Intervention Procedures—Inpatient Treatment
Patients were offered inpatient medications in an opt-in manner. Thereafter, in accordance with current treatment guidelines, staff delivered different interventions depending on patients’ willingness to try to quit.20 Those who indicated that they were unwilling to try to quit received a brief counseling session that addressed 5 Rs related to cessation (relevance, risks, rewards, roadblocks, and repetition).21 Those who were willing to try to quit were offered each component of care in an opt-in manner. Staff asked patients if they would like each component of care (“Would you like us to call you once you get home to see how you are doing?”). Patients received the elements of care to which they opted in.
Postdischarge Treatment
All patients in the opt-out group (unless they refused) and patients in the opt-in group who accepted postdischarge counseling received up to 4 weekly proactive counseling calls. Counselors helped participants develop problem-solving and coping skills, secure social support, and implement a plan for long-term abstinence. Initial calls lasted approximately 30 minutes, and follow-up calls lasted approximately 15 minutes. Among participants who quit smoking, counselors discussed high-risk situations, coping strategies, and stress management to prevent relapse. When participants slipped, counselors reviewed relapse situations and encouraged new quit attempts.
For all patients in the opt-out group (unless they refused) and patients in the opt-in group who accepted a postdischarge medication prescription, staff requested a prescription and provided a 2-week starter kit of nicotine patches and nicotine gum or nicotine lozenges. Study staff helped patients select medications based on personal preferences, quitting history, and medical contraindications. Study staff texted patients’ resident physicians and floor pharmacists to request prescriptions for preferred medications at discharge. Patients’ hospital physicians made the final determination regarding whether to provide a prescription and which medication to prescribe.
Fidelity
Counselors audio-recorded inpatient encounters. Coauthor T.H. coded for fidelity a purposive sample of 426, representing willing-to-quit patients and not-willing-to-quit patients in both study groups.
Retention
Participants received telephone, text, and postcard reminders before each assessment and up to 4 messages to reschedule missed assessments. Remuneration included $10 at hospital bedside, $25 at week 4, an additional $25 at week 4 for an extended survey among consenters, $25 at month 6, and $150 for each salivary cotinine sample returned. To reduce enrollment bias, staff assessed smoking status prior to collecting consent or describing reimbursement.
Measures
The CTD trial staff collected self-reported measures verbally. Baseline assessment included demographics and measures of tobacco use (Table 1). Assessments at week 4 included tobacco use and, among those reporting abstinence, biochemical verification.25,26 Measures of the psychological effects of opt-in care vs opt-out care included smokers’ perceptions of the degree to which their practitioner recommended tobacco treatment,27 perceptions of being coerced into treatment,28 and the quality of the patient-practitioner therapeutic alliance.27,29,30,31 For study costs, counseling time for inpatient and postdischarge calls was calculated from time stamps for counseling onset and offset in REDCap. Personnel time was valued at counselors’ wages plus benefits. Starter packs were evaluated for cost based on purchase price.
Table 1. Participant Characteristics.
| Baseline characteristic | No. (%) | Cohen da | Total (n = 739), No. (%) | |
|---|---|---|---|---|
| Opt-in group (n = 270 [36.5%]) | Opt-out group (n = 469 [63.5%]) | |||
| Age at enrollment, mean (SD), y | 51.70 (14.56) | 51.21 (14.80) | 0.03 | 51.39 (14.71) |
| Sex | ||||
| Female | 123 (45.56) | 226 (48.19) | 0.06 | 349 (47.23) |
| Male | 147 (54.44) | 243 (51.81) | 0.06 | 390 (52.77) |
| Race and ethnicityb | ||||
| Non-Hispanic | ||||
| Black | 49 (18.15) | 83 (17.70) | 0.02 | 132 (17.86) |
| Multiracial | 51 (18.89) | 75 (15.99) | 0.11 | 126 (17.05) |
| White | 158 (58.52) | 270 (57.57) | 0.02 | 428 (57.92) |
| Otherc | 1 (0.37) | 9 (1.92) | 0.92d | 10 (1.35) |
| Hispanic | 11 (4.07) | 32 (6.82) | 0.30 | 43 (5.82) |
| Emergency admissions | 140 (51.85) | 248 (52.88) | 0.02 | 388 (52.50) |
| Length of stay, mean (SD), d | 5.10 (3.82) | 5.09 (4.68) | <0.01 | 5.10 (4.38) |
| Primary hospital diagnosis (ICD-10) | ||||
| Diseases of the circulatory system | 35 (12.96) | 59 (12.58) | 0.02 | 94 (12.72) |
| Diseases of the respiratory system | 16 (5.93) | 24 (5.12) | 0.09 | 40 (5.41) |
| Neoplasms | 28 (10.37) | 61 (13.01) | 0.14 | 89 (12.0) |
| Mental, behavioral, neurodevelopmental disorders | 4 (1.48) | 14 (2.99) | 0.39 | 18 (2.44) |
| Other | 187 (69.26) | 311 (66.31) | 0.07 | 498 (67.39) |
| Primary insurance | ||||
| Medicare | 92 (34.07) | 166 (35.39) | 0.03 | 258 (34.91) |
| Private | 84 (31.11) | 134 (28.57) | 0.07 | 218 (29.50) |
| Medicaid | 51 (18.89) | 99 (21.11) | 0.08 | 150 (20.30) |
| VA | 11 (4.08) | 6 (1.28) | 0.65d | 17 (2.30) |
| Other public | 5 (1.85) | 9 (1.92) | 0.02 | 14 (1.89) |
| Self-pay/none | 27 (10.00) | 55 (11.73) | 0.10 | 82 (11.10) |
| Age of smoking initiation [n = 736], mean (SD), y | 17.62 (5.95) | 16.90 (6.03) | 0.12 | 17.17 (6.01) |
| Cigarettes smoked per day | 13.31 (8.57) | 12.57 (8.24) | 0.09 | 12.84 (8.36) |
| Smoke within 30 min of waking (n = 736)e | 187 (69.52) | 308 (65.95) | 0.09 | 495 (67.26) |
| Heaviness of smoking index (HSI), mean (SD)f | 2.47 (1.47) | 2.23 (1.42) | 0.17 | 2.32 (1.45) |
| Willing to stay quit after discharge22 | 173 (64.07) | 311 (66.31) | 0.05 | 484 (65.49) |
| Interest in quitting smoking (range, 0-10; n = 731), mean (SD) | 6.80 (3.01) | 6.60 (3.21) | 0.06 | 6.67 (3.14) |
| Confidence to quit/stay quit (range, 0-10; n = 722), mean (SD) | 7.11 (2.91) | 6.87 (3.04) | 0.08 | 6.95 (3.00) |
| Use e-cigarettes in the past 30 d | 13 (4.81) | 38 (8.10) | 0.31 | 51 (6.90) |
| Use other forms of tobacco, past 30 d | 20 (7.41) | 41 (8.74) | 0.10 | 61 (8.25) |
| Live with other smoker (n = 738) | 134 (49.63) | 235 (50.21) | 0.05 | 369 (50.00) |
| Past use of medication/counseling | 151 (55.93) | 276 (58.85) | 0.07 | 427 (57.78) |
| Ban smoking in home | 106 (39.26) | 199 (42.43) | 0.07 | 305 (41.27) |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; VA, Veterans Affairs.
Cohen d, effect size interpretation: <0.2 negligible, 0.2-0.5 small, >0.5-0.8 medium, >0.8 large.23
Race and ethnicity variables were included because they are known to be highly associated with tobacco use and cessation, and they are required by the study funder, the National Institutes of Health.
American Indian or Alaska Native, Asian, Native Hawaiian, or other Pacific Islander.
Medium/large effect size, Cohen d.
Smoking within 30 minutes of waking indicates clinically substantial nicotine dependence.
HSI is a 2-item measure combining cigarettes per day with time to first cigarette: 0-2 = low addiction, 3-4 = moderate addiction, and 5-6 = high addiction.24
Outcomes
The primary outcome for CTD was biochemically verified 7-day point prevalence abstinence at Week 4, confirmed by salivary cotinine results of no more than 56.75 μg/L (to convert to nmol/L, multiply by 5.675).26 For participants reporting abstinence and current use of nicotine replacement therapy, we used in-person expired carbon monoxide measurements (CO, confirmed at ≤10 parts per million) or mailed salivary anabasine (a tobacco-specific marker, confirmed at <1 ng/mL).32 Secondary outcomes included verified abstinence at month 6; population outcome analysis of all 1000 randomized (with nonconsenters counted as smokers); and medication and counseling engagement. Tertiary outcomes include perceived recommendation and coercion; therapeutic alliance; and the Incremental Cost-effectiveness Ratio (ICER)—the added cost per additional verified quit at 1 month.
Sample Size
The CTD trial was powered using a Bayesian model for a dichotomous variable of abstinence. Based on the prespecified design, this study had 90% power with approximately 5% type I error to detect the best study group; this calculation was revised, after the trial was completed, to 80% power after accounting for the participants that did not enroll or dropped out after randomization.
Statistical Methods
We used a Bayesian adaptive design because it can be halted early—yielding quicker results—and it benefits more patients by increasing randomization to the better-performing study group. Thirteen-week interim analyses were prespecified to test for trial success, based on 1-month outcomes, after 500 participants. The 6-month end point was added as an early stopping criterion after trial commencement but before 500 participants. The rationale for this change was to assure enough data was collected to detect effects at the 6-month secondary end point. The decision to stop the trial or continue enrolling participants was based on a prespecified calculation of the Bayesian posterior probability (BPP) that the opt-out group outperformed the opt-in group by 0.9925—for both 1-month outcomes and 6-month outcomes. After trial completion, regardless of stopping early or at 1000 randomized, the criterion was 0.99 for month 1 or month 6 outcomes. All criteria were established through simulation. Abstinence rates by study group for both 1-month and 6-month outcomes were modeled as treatment-specific binomial distributions with uninformative priors.
The posterior probability represents the probability that the abstinence rate from opt-out is higher by any amount than opt-in among enrolled participants. Similarly, the 95% credible interval represents the abstinence rate interval having 0.95 probability given the trial data. When enrollment continued, the randomization schedule was updated in REDCap and interim analyses continued every 13 weeks. We used RStudio, version 1.2.5019 (Posit, PBC) and OpenBUGS (Bayesian inference Using Gibbs Sampling), version 3.2.3, revision 1012 (OpenBUGS) for all Bayesian analyses. All interims and final analyses were fitted using 1 000 000 draws of Markov chain Monte Carlo (using Bayesian bootstrap) for inference.
To describe study participants, we used frequencies and percentages for categorical variables and means and SDs for continuous variables. We calculated Cohen d values to evaluate differences between study groups. Cohen d values from 0 to 0.2 imply negligible effect size; 0.2 to 0.5 imply small effect size; 0.5 to 0.8 imply medium effect size; and 0.8 to infinity imply large effect size.23
Results
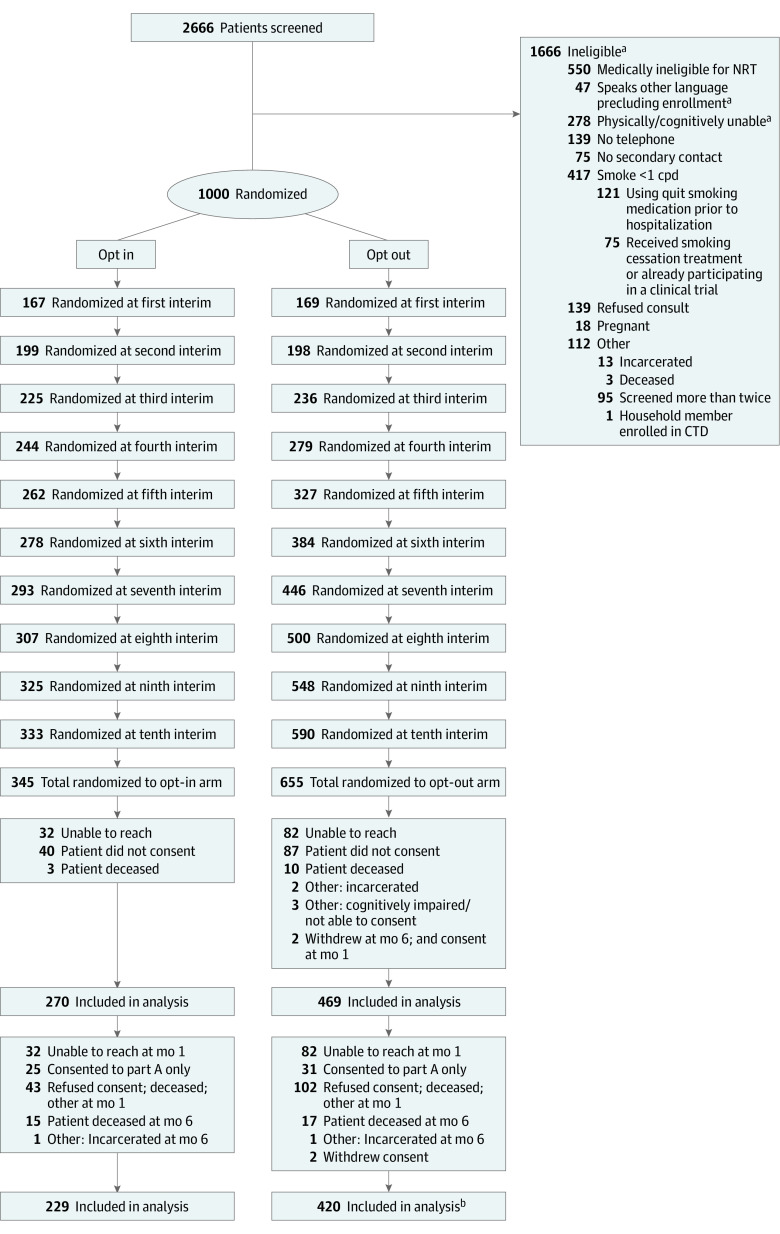
Of 2666 patients screened, 1000 adult patients were eligible and randomized. Adaptive randomization ultimately assigned 345 patients to the opt-in group and 645 to the opt-out group; 739 consented and were enrolled in the trial (270 in the opt-in group, 469 in the opt-out group; Figure 1). Study groups had similar baseline characteristics (Table 122,23,24). The mean (SD) age at enrollment was 51.70 (14.56) for opt-out patients and 51.21 (14.80) for opt-out patients. Of 270 opt-in patients, 123 (45.56%) were female, and of 469 opt-out patients, 226 (48.19%) were female. At baseline, among enrollees assigned to the opt-in group, 173 (64%) reported they were willing to quit and received opt-in offers of care, and 97 (36%) reported they were not willing to quit and received motivational intervention. Opt-out interventions and opt-in interventions were delivered with fidelity (eTables 1 and 2 in Supplement 2).
Figure 1. Recruitment, Randomization, and Participant Flow in the Changing the Default (CTD) Trial.
cpd indicates cigarettes per day; NRT, nicotine replacement therapy.
aPatients could be ineligible for more than 1 reason.
bSome patients were lost to follow-up at 6 months due to multiple reasons.
Primary Outcome
Among the 739 participants enrolled in the trial, the BPP that opt-out care was better than opt-in care was 0.97 for biochemically verified abstinence 1 month after randomization (abstinence rate, 21.5%; 95% credible interval, 0.18-0.25 vs 15.8%; 95% credible interval, 0.12-0.21) (Table 2). The difference in abstinence between the opt-out group and the opt-in group at 1 month was 5.7% (95% credible interval, 0.00-0.11).
Table 2. Probabilities That Opt-out Care Achieved Higher Quit Rates and Quit Attempts Than Opt-in Care.
| Time | Abstinent, No. abstinent/total in group (%) | Posterior mean proportion (95% credible interval) | Posterior mean difference (95% credible interval) | Bayesian posterior probabilitya | ||
|---|---|---|---|---|---|---|
| Opt-in group | Opt-out group | Opt-in group | Opt-out group | |||
| Main outcome (all enrolled) | ||||||
| At 1 mo | 43/270 (15.93) | 101/469 (21.54) | 0.158 (0.118 to 0.205) | 0.215 (0.179 to 0.254) | 0.057 (0.00 to 0.11) | 0.971 |
| At 6 mo | 41/229 (17.90) | 78/420 (18.57) | 0.178 (0.132 to 0.231) | 0.185 (0.150 to 0.224) | 0.007 (−0.06 to 0.070) | 0.591 |
| Population outcomes (all randomized) | ||||||
| At 1 mo | 43/345 (12.46) | 101/655 (15.42) | 0.124 (0.092 to 0.161) | 0.154 (0.128 to 0.183) | 0.030 (−0.016 to 0.073) | 0.902 |
| At 6 mo | 41/345 (11.88) | 78/655 (11.91) | 0.118 (0.087 to 0.155) | 0.119 (0.095 to 0.145) | 0.001 (−0.041 to 0.041) | 0.512 |
| Quit attempts (among continued smokers)b | ||||||
| At 1 mo | 125/211 (59.2) | 245/354 (69.2) | 0.592 (0.526 to 0.657) | 0.691 (0.642 to 0.738) | 0.101 (0.018 to 0.181) | 0.990 |
Bayesian posterior probability that opt-out group performed better than opt-in group.
Included in this group were those participants who answered "yes" to the following question: "Since you left the hospital on [day_0_arm_1], have you ever tried to quit smoking and made it for 24 hours or more?”
Secondary Outcomes
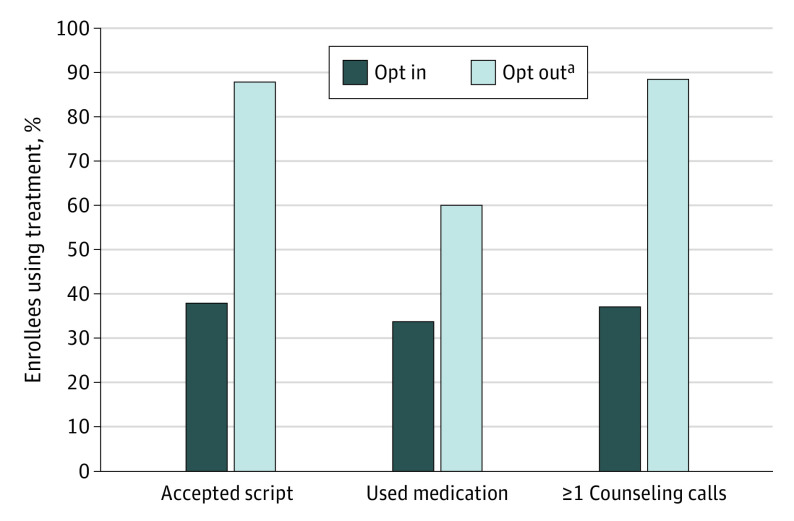
The BPP was lower (0.59) at 6 months after randomization (abstinence rate 18.5%; 95% credible interval, 0.20-0.22 vs 17.8%; 95% credible interval, 0.13-0.23). The difference at 6 months was 0.7% (95% credible interval, −0.06 to 0.07). Results were similar for the population outcome analyses (Table 2). Among patients continuing to smoke at 1 month, the BPP that opt-out care produced more quit attempts than opt-in care was 0.99 (Table 2). Compared with patients in the opt-in group, those in the opt-out group had higher rates of receiving a prescription for medication at discharge (87.7% vs 37.8%), reporting they used a cessation medication at the 1-month follow-up (59.9% vs 33.8%), and engaging in 1 or more counseling calls after discharge (88.7% vs 37.1%) (Figure 2; BPP 1.0 for each).
Figure 2. Medication and Counseling Use by Study Group .
A total of 739 participants were included in the analysis.
aThe Bayesian posterior probability that the opt-out group outperformed the opt-in group was 1.00 for all 3 outcomes.
Participants in the opt-out group rated the working alliance with their tobacco counselor higher than participants randomized to the opt-in group (3.37 on a 4-point scale; 95% credible interval, 3.32-3.42 vs 3.20; 95% credible interval, 3.12-3.29; BPP 1.0) (eTable 3 in Supplement 2). Similarly, opt-out participants rated their clinicians’ overall implied recommendation for tobacco treatment higher than opt-in participants (3.34 on a 4-point scale; 95% credible interval, 3.29-3.39 vs 3.17; 95% credible interval, 3.09-3.25; BPP 1.0). More than three-quarters of participants in both study groups agreed that they had a lot of control over whether they tried to quit and that they did not feel forced to try to quit (eTable 3 in Supplement 2).
The mean cost of providing treatment to each patient in the opt-out group was greater than providing treatment to each patient in the opt-in group. Cost was evaluated on the basis of counseling costs and medication use (Table 3). This yielded an ICER of $678.60, which represents the cost of each additional quit in the opt-out condition at 1 month.
Table 3. Cost of Each Additional Quit Among Participants in Opt-out Group Compared With Those in Opt-in Group.
| Study group | No. | Counseling costs (95% CI)a,b | Starter pack costs (95% CI)a,b | Sum (95% CI)a,b | Verified quit, No. (%) (95% CI)b |
|---|---|---|---|---|---|
| Opt-in | 270 | 15.35 (13.82-16.99) | 21.46 (17.80-24.94) | 36.81 (31.95-41.29) | 43 (15.9) (11.7-20.2) |
| Opt-out | 469 | 25.19 (24.27-26.19) | 49.62 (47.67-51.63) | 74.81 (72.60-77.16) | 101 (21.5) (17.8-25.2) |
| Difference | NA | 9.84 | 28.16 | 38 (33.22-42.93) | 5.6% (5.0-6.1) |
| ICER | NA | NA | NA | NA | 678.6 (664.4-703.8) |
Abbreviation: ICER, incremental cost-effectiveness ratio; NA, not applicable.
Mean cost in dollars per person.
CIs for costs and quit rates were estimated by bootstrapping using samples of 1000.
Discussion
This randomized clinical trial with high treatment fidelity demonstrated that opt-out treatment achieved high rates of medication and counseling use, higher quit attempts, and a posterior probability of 0.97 higher quit rates 1 month after randomization. Compared with opt-in care, opt-out care doubled evidence-based treatment use without diminishing patient autonomy and control. Rather than offending patients—which could be an outcome of presumptively providing tobacco treatment33—patients’ perceived alliance with their practitioners was higher in the opt-out group.
Opt-out care resulted in costs-per-quit similar to other cessation interventions but vastly lower than cancer treatments. The ICER for a tobacco cessation intervention among parents in pediatric primary care was $762 per cotinine-confirmed quit.34 Median ICERs for cancer treatments, including colorectal cancer and hematologic cancers ranged from $22 000 to $48 000 (in 2008 US$).32
The findings of this trial confirm and extend other trials. Opt-out HIV testing doubled participation rates compared with opt-in HIV testing (65.9% vs 38%, respectively).11 Outpatients provided with mailed fecal immunochemical tests (FIT) unless they opted out had higher rates of being mailed (93%) and completing a test (29.1%) compared with patients receiving opt-in offers of FIT (23.1% and 9.6%, respectively).13
None of these trials evaluated mechanisms of behavior change or health outcomes. We found that perceived implied recommendation for tobacco treatment was higher in the opt-out group, which had higher rates of treatment engagement and abstinence. Hence opt-out care doubles treatment engagement and markedly improves target behaviors, such as participating in screening and making tobacco quit attempts. It potentially does so by implying a preferred course of action, strengthening the patient-practitioner relationship, and maintaining patients’ sense of control.
Limitations
We used opt-out framing for multiple aspects of care; we cannot discern if any aspect(s) were more effective. Consent differed across study groups (78% vs 72%); however, characteristics of enrollees differed little. The difference between the groups, though marked (the opt-out group had a 36% higher quit rate than the opt-in group), was not statistically significant. Tobacco use disorder is a chronic, relapsing condition.35 Future research should test whether stronger treatment options—for example, providing postdischarge medications rather than just prescriptions36—and longer treatment, such as 26 weeks of care,37 could translate engagement and quit attempts into substantial, long-term cessation for all.
Conclusions
Most smokers want to quit15; nudging all in the direction of their desire could translate to major changes in individual lives and the health of the public. In addition, as demonstrated in this randomized clinical trial, opt-out approaches could increase evidence-based treatment for other chronic conditions, such as obesity,38 that many practitioners assume require patient willpower to be effective.
Trial protocol
eFigure 1. Study organization
eTable 1. Fidelity ratings for baseline inpatient session by treatment group
eTable 2. Fidelity to treatment components by treatment group
eTable 3. Probabilities that the opt-out group achieved better psychological outcomes than the opt-in group
eMethods. Changing the default Bayesian analysis and statistical approach
Data sharing statement
References
- 1.World Health Organization . WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. 2017. Accessed March 27, 2022. https://apps.who.int/iris/handle/10665/255874
- 2.US Department of Health and Human Services . Interventions for smoking cessation and treatments for nicotine dependence. 2020. Accessed January 21, 2023. https://www.cdc.gov/tobacco/sgr/2020-smoking-cessation/pdfs/2020-cessation-sgr-chapter-6-508c.pdf
- 3.Borland R, Li L, Driezen P, et al. Cessation assistance reported by smokers in 15 countries participating in the International Tobacco Control (ITC) policy evaluation surveys. Addiction. 2012;107(1):197-205. doi: 10.1111/j.1360-0443.2011.03636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein SL, Yu S, Post LA, Dziura J, Rigotti NA. Undertreatment of tobacco use relative to other chronic conditions. Am J Public Health. 2013;103(8):e59-e65. doi: 10.2105/AJPH.2012.301112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi: 10.1097/HJH.0000000000002453 [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation . IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. Accessed January 21, 2023. https://idf.org/our-activities/care-prevention/type-2-diabetes.html
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter KP, Ellerbeck EF. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381-386. doi: 10.1111/add.12734 [DOI] [PubMed] [Google Scholar]
- 9.Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Yale University Press; 2008. [Google Scholar]
- 10.Walmsley S. Opt in or opt out: what is optimal for prenatal screening for HIV infection? CMAJ. 2003;168(6):707-708. [PMC free article] [PubMed] [Google Scholar]
- 11.Montoy JC, Dow WH, Kaplan BC. Patient choice in opt-in, active choice, and opt-out HIV screening: randomized clinical trial. BMJ. 2016;532:h6895. doi: 10.1136/bmj.h6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry JG, Ryan P, Gold MS, Braunack-Mayer AJ, Duszynski KM; Vaccine Assessment Using Linked Data (VALiD) Working Group . A randomised controlled trial to compare opt-in and opt-out parental consent for childhood vaccine safety surveillance using data linkage. J Med Ethics. 2012;38(10):619-625. doi: 10.1136/medethics-2011-100145 [DOI] [PubMed] [Google Scholar]
- 13.Mehta SJ, Khan T, Guerra C, et al. A randomized controlled trial of opt-in versus opt-out colorectal cancer screening outreach. Am J Gastroenterol. 2018;113(12):1848-1854. doi: 10.1038/s41395-018-0151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahneman D, Knetsch JL, Thaler RH. Anomalies: the endowment effect, loss aversion, and status quo bias. J Econ Perspect. 1991;5(1):193-206. doi: 10.1257/jep.5.1.193 [DOI] [Google Scholar]
- 15.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 16.Lifestyles Statistics team, Health and Social Care Information Centre . Statistics on smoking: England 2015. May 2015. Accessed January 21, 2023. https://untobaccocontrol.org/impldb/wp-content/uploads/uk_2016_annex1_statstics_on_smoking_2015.pdf
- 17.Lin H, Chen M, Yun Q, Zhang L, Chang C. Tobacco dependence affects determinants related to quitting intention and behaviour. Sci Rep. 2021;11(1):20202. doi: 10.1038/s41598-021-99766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry SM, Carlin BP, Lee JJ, Muller P. Bayesian Adaptive Methods for Clinical Trials. CRC Press; 2011. [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services . Smoking cessation: a report of the Surgeon General. 2020. Accessed January 21, 2023. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf [PubMed]
- 21.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 22.Riley WT, Stevens VJ, Zhu SH, Morgan G, Grossman D. Overview of the consortium of hospitals advancing research on tobacco (CHART). Trials. 2012;13:122. doi: 10.1186/1745-6215-13-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; 1977. [Google Scholar]
- 24.de Leon J, Diaz FJ, Becoña E, Gurpegui M, Jurado D, Gonzalez-Pinto A. Exploring brief measures of nicotine dependence for epidemiological surveys. Addict Behav. 2003;28(8):1481-1486. doi: 10.1016/S0306-4603(02)00264-2 [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13-25. doi: 10.1080/1462220031000070552 [DOI] [PubMed] [Google Scholar]
- 26.SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149-159. doi: 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- 27.McKenzie CR, Liersch MJ, Finkelstein SR. Recommendations implicit in policy defaults. Psychol Sci. 2006;17(5):414-420. doi: 10.1111/j.1467-9280.2006.01721.x [DOI] [PubMed] [Google Scholar]
- 28.Gardner W, Hoge SK, Bennett N, et al. Two scales for measuring patients’ perceptions for coercion during mental hospital admission. Behav Sci Law. 1993;11(3):307-321. doi: 10.1002/bsl.2370110308 [DOI] [PubMed] [Google Scholar]
- 29.Knoll MA. The role of behavioral economics and behavioral decision making in Americans’ retirement savings decisions. Soc Secur Bull. 2010;70(4):1-23. [PubMed] [Google Scholar]
- 30.Johnson EJ, Steffel M, Goldstein DG. Making better decisions: from measuring to constructing preferences. Health Psychol. 2005;24(4S)(suppl):S17-S22. doi: 10.1037/0278-6133.24.4.S17 [DOI] [PubMed] [Google Scholar]
- 31.Warlick C, Richter KP, Catley D, Gajewski BJ, Martin LE, Mussulman LM. Two brief valid measures of therapeutic alliance in counseling for tobacco dependence. J Subst Abuse Treat. 2018;86:60-64. doi: 10.1016/j.jsat.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg D, Earle C, Fang CH, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective: a systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst. 2010;102(2):82-88. doi: 10.1093/jnci/djp472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashcroft RE. The ethics of an opt-out default in tobacco treatment. Addiction. 2015;110(3):389-390. doi: 10.1111/add.12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drouin O, Sato R, Drehmer JE, et al. Cost-effectiveness of a smoking cessation intervention for parents in pediatric primary care. JAMA Netw Open. 2021;4(4):e213927. doi: 10.1001/jamanetworkopen.2021.3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695. doi: 10.1001/jama.284.13.1689 [DOI] [PubMed] [Google Scholar]
- 36.Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719-728. doi: 10.1001/jama.2014.9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlam TR, Fiore MC, Smith SS, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111(1):142-155. doi: 10.1111/add.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Befort CA, Greiner KA, Hall S, et al. Weight-related perceptions among patients and physicians: how well do physicians judge patients’ motivation to lose weight? J Gen Intern Med. 2006;21(10):1086-1090. doi: 10.1111/j.1525-1497.2006.00567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure 1. Study organization
eTable 1. Fidelity ratings for baseline inpatient session by treatment group
eTable 2. Fidelity to treatment components by treatment group
eTable 3. Probabilities that the opt-out group achieved better psychological outcomes than the opt-in group
eMethods. Changing the default Bayesian analysis and statistical approach
Data sharing statement