Abstract
Membrane filtration processes have been used to treat landfill leachate. On the other hand, closing the leachate treatment loop and finding a final destination for landfill leachate membrane concentrate (LLMC) – residual stream of membrane systems – is challenging for landfill operators. The re-introduction of LLMC into the landfill is typical; however, this approach is critical as concentrate pollutants may accumulate in the leachate treatment facility. From that, leachate concentrate management based on resource recovery rather than conventional treatment and disposal is recommended. This work comprehensively reviews the state-of-the-art of current research on LLMC management from leachate treatment plants towards a resource recovery approach. A general recovery train based on the main LLMC characteristics for implementing the best recovery scheme is presented in this context. LLMCs could be handled by producing clean water and add-value materials. This paper offers critical insights into LLMC management and highlights future research trends.
Keywords: Concentrated leachate, landfill leachate, membrane concentrate, nanofiltration, reverse osmosis, resource recovery
Graphical Abstract
Introduction
Landfill leachate (LFL) contains a wide range of pollutants of varying concentrations, from high values for organic matter, nutrients and inorganics to low values for emerging contaminants (e.g. persistent organic pollutants, pharmaceuticals, personal care products and plastic additives) (Busch et al., 2010; Clarke et al., 2015; Ramakrishnan et al., 2015). Hence, its treatment remains a major socio-environmental and economic issue on the municipal solid waste management chain. Treatment of LFL must meet the wastewater disposal requirements established by regulatory authorities. Moreover, considering that the LFL physicochemical composition undergoes spatial and temporal variations, successful and efficient treatment must be ensured in both the active and post-closure landfill periods (Fan et al., 2006; Stegmann, 2018).
The conventional technologies for leachate treatment are biological and physicochemical processes or their combination in integrated or sequential schemes (Abbas et al., 2009; Kurniawan et al., 2010). Conventional biological systems alone cannot significantly treat methanogenic leachate, which contain contaminants resistant to biodegradation (Torretta et al., 2016). In addition, high ammonia concentrations cannot be removed successfully by biological treatment, such as activated sludge, aerated lagoons, sequence batch reactor or trickling filters (Mojiri et al., 2021). Therefore, the option of advanced technologies should be considered. In this regard, membrane-based technologies, that is, nanofiltration (NF) and reverse osmosis (RO), are considered the most reliable and effective methods for leachate treatment (Chaudhari and Murthy, 2010; Chianese et al., 1999; Cingolani et al., 2017; de Almeida et al., 2020a, 2020b; Linde et al., 1995; Šír et al., 2012).
In contrast, to close the loop of the LFL treatment and to find a final destination for the landfill leachate membrane concentrate (LLMC) – residual stream of the membrane filtration process – is a critical issue, and LLMC management is a challenging task. Several technologies have been proposed and investigated to manage the membrane concentrate, including recirculation (Calabrò et al., 2010; Chamem et al., 2020; He et al., 2015), natural evaporation (Cossu et al., 2018), solidification/stabilization (S/S) (Hunce et al., 2012), chemical coagulation (Long et al., 2017), electrocoagulation (EC) (Fernandes et al., 2019), ozonation (Shah et al., 2017), advanced oxidation processes (AOPs) (e.g. Fenton, photo-Fenton, anodic oxidation) (Fernandes et al., 2017; Hong et al., 2017; Soomro et al., 2020) and thermal treatment (Zhang et al., 2019). The recirculation of the LLMC onto landfill cells is the conventional approach, similar to leachate recycling to adjust moisture content and degrade organic pollutants in landfills (Calabrò and Mancini, 2012; Grossule and Lavagnolo, 2020; Sohoo et al., 2019). However, some critical issues such as failures of landfill stability and accumulation of pollutants in the leachate treatment facility can emerge as negative impacts linked to this practice. Before the study carried out by Henigin (1995), the consequences of the reinjection of concentrated leachate into the landfill body were under-discussed. In recent years, there has been an increasing amount of literature on the effects of this procedure (Calabrò et al., 2018; Chamem et al., 2020; Morello et al., 2016; Talalaj, 2015a; Talalaj and Biedka, 2015). Nonetheless, the published studies show contrasting conclusions. Therefore, further research in this area is still of high importance.
Several other reviews already exist, which do excellent work in describing LFL treatment processes (Abuabdou et al., 2020; Costa et al., 2019; Gao et al., 2014; Luo et al., 2020; Renou et al., 2008; Wiszniowski et al., 2006), utilization of membrane-based technologies for wastewater treatment (Kamali et al., 2019) and treatment technologies for membrane concentrate volume minimization (Joo and Tansel, 2015; Subramani and Jacangelo, 2014). Readers are guided towards these contributions for further background information. Recently, Keyikoglu et al. (2021) reviewed the state-of-the-art of technologies for the treatment of LLMCs. Among existing methods, they paid more attention to AOPs. However, these techniques cannot effectively handle the high salinity of the LLMC and, therefore, are mainly applied as a pre-treatment step rather than a stand-alone treatment. Besides, AOPs are associated with high installation and operational expenses, and the possible generation of intermediates with higher toxicity during the LLMC treatment also represents a limitation for their consolidation on a full-scale application.
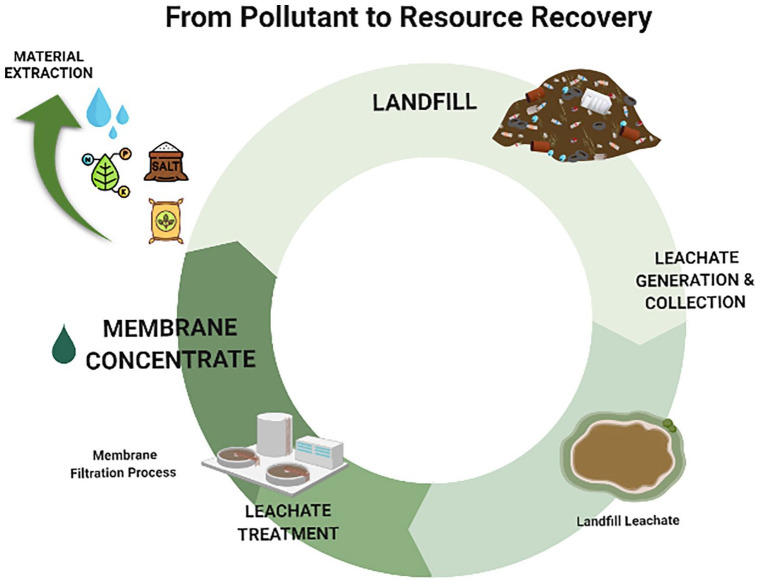
As aforementioned, several approaches could be adopted for the management of LLMCs. Considering the demands of efficient water reuse, carbon and nutrients from LLMCs or from LFL itself, efforts have been focused on extracting add-value products from leachate concentrates (e.g. inorganic salts and biofertilizers) (Gu et al., 2019; Kurniawan et al., 2021; Li et al., 2015). At present, a review dealing with the management of LLMCs focusing on resource recovery has not been published yet. This work comprehensively reviews the state-of-the-art of current research on membrane concentrates management from landfill leachate treatment plants (LLTPs) towards a resource recovery approach. Lastly, within a circular bioeconomy context, a general recovery train based on the main LLMC characteristics for implementing the best recovery route is presented.
Materials and methods
The databases, including Web of Science, Scopus and Engineering Village, were explored. The following keywords were combined to find the scientific literature: ‘landfill leachate’, ‘nanofiltration’, ‘reverse osmosis’, ‘membrane concentrate’ and ‘concentrated leachate’. The screening was undertaken using the eligibility and exclusion criteria to include the works relevant to the research topic. Eligibility criteria consisted of selecting articles that deal with NF concentrate (NFC) and RO concentrate (ROC) treatment and management options. In contrast, excluding articles published in a language other than English and papers that do not deal with LLMC management was part of the exclusion criteria.
The present article is structured as follows: first, we discussed the management of LFL emphasizing its treatment processes by NF and RO processes. Second, the main characteristics of NFC and ROC from LLTPs are introduced. Third, a critical analysis of LLMC destination practices and treatment systems are presented. Fourth, resource recovery from LLMCs covering water reuse technologies and material extraction, that is, organic fertilizers, nutrients, inorganic salts recovery and add-value products is comprehensively reviewed. Current trends and challenges are addressed. Last, a management diagram for the best resource recovery route from LLMCs is proposed.
LFL management
The composition of LFL varies depending on the gravimetric composition of landfilled waste, landfill age and mode of its operation, landfill status (i.e. active or closed), site conditions (e.g. climate, site geometry, soil properties and hydrogeology), among others (El-Fadel et al., 2002; Farquhar, 1989). The typical LFL contains a significant amount of biodegradable and non-biodegradable organics, inorganic compounds and xenobiotic organic compounds (Kulikowska and Klimiuk, 2008; Vaccari et al., 2019). Recent studies identified other pollutants such as pharmaceuticals, plasticizers and microplastics in untreated and treated leachate samples from both active and closed landfills (He et al., 2019; Su et al., 2019; van Praagh et al., 2019). Thus, a considerable amount of literature about this topic can be expected in the forthcoming years. Nevertheless, major concerns of the LFL are ammonia nitrogen (NH3-N), salts (e.g. chloride, sulphate, carbonate and bicarbonate) and organic matter – reported as 5-day biochemical oxygen demand (BOD5), chemical oxygen demand (COD) and total organic carbon (Ehrig and Robinson, 2010; Iskander et al., 2018b).
According to published studies (Costa et al., 2019; Kurniawan et al., 2006, 2010), the landfill age plays an important role in leachate characteristics; therefore, LFL can be classified into three categories on an age basis: young, intermediate and mature (Table 1). Overall, biodegradable organic matter (evaluated by BOD5) reduces over time, and leachate organic matter stabilizes (Kjeldsen et al., 2002; Luo et al., 2020). In other words, the BOD5/COD ratio (biodegradability index) decreases as the landfill age increases. The biodegradability index can even be more diminutive in tropical regions compared to others of temperate climate. The warmer conditions tend to boost microbial activity, which accelerates organic matter stabilization; thus, a high concentration of non-biodegradable compounds such as humic substances (HSs) can also be a concern in the short term for landfills located in tropical regions (Lebron et al., 2021). Besides, as stated earlier, the composition of landfilled residues can affect LFL characteristics; for instance, in regions where waste separation, pre-treatment and recycling of organic fraction are effective, inorganic leachate parameters such as total dissolved solids (TDS), conductivity and chloride may be more relevant (de Almeida and Campos, 2020).
Table 1.
Typical LFL physicochemical composition according to the landfill age in temperate and tropical regions. Adapted from Costa et al. (2019), Kurniawan et al. (2006, 2010) and Lebron et al. (2021).
| LFL age and leachate composition in temperate regions (years) | LFL age and leachate composition in tropical regions (years) | |||||
|---|---|---|---|---|---|---|
| Parameters | 0–5 (young) | 5–15 (intermediate) | >15 (mature) | 0.5–2 (young) | 1.7–2.1 (intermediate) | 7.2–14.4 (mature) |
| pH | 3–6 | 6–7.5 | 7.5–9.0 | 7.8–8.5 | 6.2–8.3 | 7.3–8.4 |
| BOD5 (mg L−1) | 10,000–25,000 | 500–4000 | <500 | 275–453 | 1–7068 | 1–12,766 |
| COD (mg L−1) | 15,000–40,000 | 1000–20,000 | <1000 | 1230–6027 | 164–17,440 | 576–21,137 |
| BOD5/COD | 0.5–1 | 0.1–0.5 | <0.1 | – | 0.006–0.3 | 0.002–0.3 |
| Biodegradability | Medium–high | Medium | Low | – | Low | Low |
| NH3-N (mg L−1) | 1500–4250 | 50–700 | <30 | 526–1787 | 21.1–1120 | 133–2808 |
| TDS (mg L−1) | 10,000–25,000 | 2000–10,000 | <1000 | – | 70–5885 | 310–3480 |
| Conductivity (mS cm−1) | 15–41.5 | 6–14 | – | 8.90–10.87 | 0.677–14.59 | 3.92–25.63 |
| Chloride (mg L−1) | 1000–3000 | 100–2000 | <100 | 2499–4204 | – | – |
| Sulphate (mg L−1) | 500–2000 | 50–1000 | <50 | – | – | – |
BOD5: 5-day biochemical oxygen demand; COD: chemical oxygen demand; LFL: landfill leachate; NH3-N: ammonia nitrogen; TDS: total dissolved solid.
Several methods for LFL treatment have been in use, such as co-treatment with sewage on wastewater treatment plants (Brennan et al., 2017; Dereli et al., 2021); recirculation of leachate into the landfill body (Bae et al., 2019; Beaven and Knox, 2018); constructed wetlands (Bakhshoodeh et al., 2020); physicochemical processes – coagulation-flocculation (C/F), chemical precipitation, chemical oxidation, air stripping, carbon adsorption and AOPs (Deng and Englehardt, 2006; Fernandes et al., 2015; Ferraz et al., 2013; Foo and Hameed, 2009; Lins et al., 2015); and biological processes – aerated lagoons, sequencing batch reactor process, activated sludge process, membrane bioreactor, biofilms in rotating biological contactors and trickling filters (Ahmed and Lan, 2012; Chelliapan et al., 2020; El-Gohary and Kamel, 2016; Robinson, 2019).
Conventional treatments of LFL are generally classified into three major groups: (1) biological processes (aerobic or anaerobic), (2) physicochemical processes and (3) a combination of biological and physicochemical processes (Luo et al., 2020). Biological treatment is often used to remove biodegradable organics and total nitrogen due to its reliability, simplicity and high cost-effectiveness (Ehrig et al., 2018). On the other hand, physical and chemical processes can be effective as a pre-treatment for leachate’s biological degradation since it helps to reduce the content of non-biodegradable substances, which can compromise the efficiency of the biological treatment (Mojiri et al., 2021). Recently, low-cost treatment schemes have been tested, including pre-treatment with biomass bottom ash (chemical precipitation) followed by microalgae remediation, which shows an excellent removal of leachate pollutants. Besides, it could enable the valorization of treatment residues (i.e. biomass and sludge) through the production of biofuels and add-value materials (Viegas et al., 2021). Figure 1 depicts the performance of different leachate treatment processes according to the landfill age, that is, young, intermediate and mature.
Figure 1.
Performance of different leachate treatment processes according to the landfill age. Based on Luo et al. (2020).
As mentioned before, several technologies are available for LFL treatment, and each of them has its merits and limitations. The selection of the best treatment route depends mainly on the LFL composition and economic feasibility. As leachate composition undergoes spatial and seasonal variation, treatment strategies cannot be standardized. Every scenario is unique, and treatment approaches should vary accordingly (Mukherjee et al., 2015).
From a techno-economic point of view, NF and RO seem to be the most promising and efficient methods among the existing technologies. NF and RO, either as a foremost or as a polishing step in a leachate treatment chain, have shown to be an essential technology to meet the most restrictive standards for water discharge or reuse (Chen et al., 2021b). NF and RO processes can (1) provide high-quality treated leachate (Chen et al., 2021b); (2) reduce the environmental footprint and size of the LLTP (modular design/installation) (Jamaly et al., 2014; Peter-Varbanets et al., 2009); (3) be automated and easily scaled (Peter-Varbanets et al., 2009); and (4) be easily moved from site to site (Kumano and Fujiwara, 2008). Since the late 1980s, the use of RO has become a proven technology in use for LFL treatment. In 2018, there were over 300 leachate treatment RO plants installed worldwide (Balkema et al., 2018). For this reason and considering the scope of the present article, the following section focuses on NF/RO processes applied to the treatment of LFL.
NF and RO
NF and RO are high-pressure-driven membrane filtration processes. NF/RO systems generate two output streams, which are named permeate and concentrate. The permeate is the treated liquid, and the concentrate, also known as retentate, is the residual stream (Baker, 2012).
NF membranes have pore sizes in the range of 0.5–2 nm, which corresponds to molecular weight cut-off (MWCO) of 100–500 Da. NF is also described as a process that removes particles and dissolved compounds smaller than 2 nm (Mohammad et al., 2015). Similarly, the RO process uses a physical mechanism, in which the operating pressure must be kept higher than the solution osmotic pressure. The transport mechanism across the RO membrane follows the dissolution/diffusion model, where both solvent and solute dissolve in the dense surface layer of the membrane and diffuse separately due to the chemical potential gradient of each species. In wastewater treatment systems, RO membranes are primarily used to remove low molecular mass solutes such as salts and heavy metals (Baker, 2012; Wilf, 2014).
NF process can remove colloids, low-molecular-weight organic matter and dissolved salts. However, NF membranes generally allow monovalent ions such as sodium and chloride to pass through while achieving high rejection for organic matter and suspended solids (Abdel-Fatah, 2018). On the other hand, RO membranes can separate monovalent/divalent ions and small neutral molecules. Both systems can separate organic and inorganic compounds from the influent, producing water with low levels of dissolved solids content (Yang et al., 2020). From the perspective of LFL treatment, they are the major membrane techniques used in LLTPs (Chen et al., 2021b).
The standard operating mode of full-scale NF/RO systems is cross-flow with high internal flow rate concentrate recirculation. Typical NF/RO system modules include tubular, spiral wound, hollow fibre and disc tube (Yang et al., 2020). Due to their modular configuration, these modules are available in standardized containers from various suppliers and adapted to each landfill site (ISWA, 2019). RO systems are generally used in a stand-alone mode, and depending on the effluent requirements, several steps can be combined, where leachate gets filtered in two or more stages before final discharge. On the other hand, the NF process is usually applied as a post-treatment step of biological or physicochemical processes into the LFL treatment chain (de Almeida et al., 2020c).
NF and RO have been widely applied in full-scale LFL treatment projects (Chen et al., 2021b; Di Maria et al., 2018; Lebron et al., 2021). A body of scientific research recognizes their importance for the LFL treatment (Anna Tałałaj et al., 2021; Dolar et al., 2016; Mariam and Nghiem, 2010; Ramaswami et al., 2018; Smol and Włodarczyk-Makuła, 2017), and several studies have operated NF/RO in pilot and full-scale application (Table 2), demonstrating the membrane technology maturity. For example, according to Argun et al. (2020), the NF process used as a final step of an LFL facility located in Turkey is essential to meet the local leachate disposal limits. The NF system is composed of 60 spiral wound modules, polyethersulfone membranes. It operates at pump pressure <18 bar, recovery rate of 85% and permeate flux of 16 L m−2 h−1. COD, NH3-N, TDS and colour removal efficiencies are, on average, 84%, 70%, 51% and >99%, respectively. Several full-scale RO systems are also documented in other studies that looked at the RO process (Cingolani et al., 2018; Rukapan et al., 2012; Theepharaksapan et al., 2011). In Poland, a 72 m3 d−1 LLTP has performed the disc-tube RO system at an operating pressure of 65 bar, permeate flux of up to 50 L m−2 h−1 and recovery of 75%. Treatability results showed removal efficiencies of BOD5, COD and NH3-N greater than 90% (Talalaj, 2015b).
Table 2.
NF and RO in the pilot- and full-scale application for LFL treatment.
| Treatment scheme | Location | Scale | Module type | Membrane | Operational conditions | Treatability (% removal) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Permeate flux (L m−2 h−1) | Recovery (%) | COD | NH3-N | ||||||
| NF | India | Pilot | Flat sheet membrane | NF-300 | ~53–266 | ~50–80 | 82.6–88.6 | 68–70 | Chaudhari and Murthy (2010) |
| Sedimentation + anoxic/aerobic tanks + UF + NF | Turkey | Full | Spiral wound | PVDF membrane | 17 | Data not available | 99 | >93 | Yaman et al. (2012) |
| MBR + NF | Brazil | Pilot | Spiral wound | NF90-2540 | 5.8–6.9 | 60 | 80–96 | 85–95 | Amaral et al. (2015) |
| MBR + NF | Turkey | Full | Spiral wound | PES membrane | 16 | 85 | 84 | 70 | Argun et al. (2020) |
| MBR + NF | China | Full | Data not available | Data not available | Data not available | Data not available | >80 | 70 | Shao et al. (2021) |
| MBR + RO | Korea | Full | Spiral wound | SW-4040 | Data not available | Data not available | 97 | 96 | Ahn et al. (2002) |
| ASP + flocculation/sedimentation + two-stage RO | German | Full | Novel thin open channel spiral wound | FT30 | 6.5–4.2 | 70 | 99.7 | 99.9 | Li et al. (2009) |
| Two-stage RO | German | Full | Novel thin open channel spiral wound | FT30 | 6.5–8.1 | 70 | 99.5 | 98.9 | Li et al. (2009) |
| Coagulation + sand filtration + MF + RO | Thailand | Full | Spiral wound | LFC3-LD | Data not available | 50 | 87.5 | >88 | Theepharaksapan et al. (2011) |
| Coagulation + sand filtration + RO | Thailand | Full | Spiral wound | LFC3-LD | Data not available | 50 | >98 | Data not available | Rukapan et al. (2012) |
| Two-stage RO | Czech Republic | Pilot | Spiral wound | SW30-4040 | 42 | 94 | 97.3 | 94.0 | Šír et al. (2012) |
| Sand and cartridge filtration + two-stage RO | Romania | Full | Disc tube | BIO-10058-v2 | 12–22 | Data not available | 94–96 | >94 | Şchiopu et al. (2012) |
| Buffer tank + RO | Poland | Full | Disc tube | CD9-RO | Up to 50 | 75 | 98.8 | 99.1 | Talalaj (2015b) |
| UF + three-stage RO | Italy | Full | Disc tube | Gel GPT-BW 30 | 13–32.5 | 91–95 | 92.4–99.2 | 46.2–95.8 | Cingolani et al. (2018) |
| Two stage RO + air stripping | China | Full | Disc tube | Data not available | Data not available | >83% | >99 | >99 | Wu and Li (2021) |
ASP: activated sludge process; COD: chemical oxygen demand; MBR: membrane bioreactor; MF: microfiltration; NF: nanofiltration; NH3-N: ammonia nitrogen; PES: polyethersulfone; PVDF: polyvinylidene fluoride; RO: reverse osmosis; UF: ultrafiltration.
However, two major issues can be identified as drawbacks for the implementation of membrane filtration processes: (1) membrane fouling, which decreases the permeate flux and water quality, and (2) concentrate stream management. Membrane fouling requires extensive pre-treatment or chemical cleaning of the membrane, resulting in a short lifespan of membranes. This fact is still the bottleneck problem in membrane promotion and application, as fouling can also increase operational costs. In general, before NF/RO filtration, there are pre-treatment steps to remove suspended solids and colloids and, consequently, prevent fouling and biofilm growth on the membrane surface (Jamaly et al., 2014).
Finally, the management of LLMCs is a challenge that must be deal with. The development of technologies and process breakthroughs in the water desalination field have helped to tackle the concentrate management issue in LLMC areas. There is a pool of patented technologies to improve the overall feed water recovery, aiming to reduce concentrate volume and achieve minimal liquid discharge (MLD) or zero liquid discharge (ZLD) (Joo and Tansel, 2015; Subramani and Jacangelo, 2014). However, their high costs linked mainly with energy requirements limit the implementation of full-scale ZLD systems. Furthermore, it is important to note that MLD/ZLD systems are associated with unintended environmental impacts as a result of their high energy demand and carbon footprint. Other major issues of MLD/ZLD systems include fouling, scaling and expensive metallic materials (Voutchkov and Kaiser, 2020). Therefore, future research to addresses these drawbacks are needed.
LFL membrane concentrate management
LLMC characteristics
Different factors can affect the composition of concentrate streams from LLTPs, including leachate characteristics, pre-treatment applied, additional chemicals used, that is, fouling/biofouling prevention chemicals or reagents used for pH control, and treatment configurations (Ladewig and Asquith, 2012; Van Der Bruggen et al., 2003). These influence factors have been found in the analysis of NFC and ROC from the LLTP in Xiamen (China), where concentrate streams were collected from two different leachate treatment configurations. The NFC contained a high amount of refractory organics; conversely, recalcitrant contaminants in the ROC were lower because most of these were removed by pre-treatment processes used in the treatment chain (Chu et al., 2020). From that, it is presumable that the LLMC composition can vary depending on the LLTP’s defined treatment scheme.
As NF and RO are the preferred membrane processes for leachate treatment, the main characteristics of NFC and ROC from LLTPs reported in various studies are summarized in Table 3. It must be emphasized that this table intends to highlight the characteristics of the main concentrate streams generated in LLTPs (i.e. NFC and ROC), focusing on the reported values instead of the treatment scheme that generated the concentrate stream. For instance, the values of some parameters like NH3-N and total Kjeldahl nitrogen (TKN) have a wide range since, in some cases, nitrogen can be removed by biological process in the leachate treatment chain, confirming what was discussed above.
Table 3.
The main characteristics of NFC and ROC from LLTPs.
BOD5: 5-day biochemical oxygen demand; COD: chemical oxygen demand; HS: humic substance; LLTPs: landfill leachate treatment plants; NFC: nanofiltration concentrate; NH3-N: ammonia nitrogen; pH: potential hydrogen; ROC: reverse osmosis concentrate; TDS: total dissolved solid; TKN: total Kjeldahl nitrogen; TOC: total organic carbon; UV254: absorbance at 254 nm.
As can be seen from Table 3, NFCs/ROCs are rich in some heavy metals such as copper, zinc, lead and nickel (up to 9.26 mg Cu L−1, 6,656 mg Zn L−1, 56.97 mg Pb L−1 and 3.182 mg Ni L−1), which can cause environmental pollution and potential bioaccumulation in living organisms and human body resulting in adverse effects (Briffa et al., 2020). Besides, priority pollutants such as toluene, ethylbenzene, chlorobenzene and dibutyl phthalate were also identified in LLMC samples. These chemicals are highly toxic pollutants, representing an environmental hazard (Zhang et al., 2013).
The BOD5 and COD concentrations in LLMCs range from 2.6 to 17,000 mg L−1 and 1281 to 49,521 mg L−1, respectively. BOD5/COD ratios are in the range of 0.002–0.34, indicating low biodegradability. The differences between LFL and LLMC, such as non-biodegradable content and organic matter composition, were investigated in three LLTPs. HSs, including humic acid (HA) and fulvic acid (FA), accounted for the highest fraction of organic matter in LLMCs, ranging from 61.7% to 69.2% (Zhang et al., 2013). Chan et al. (2007) stand out that high-molecular-weight and non-biodegradable compounds are removed mainly by membrane processes and accumulate in the residual stream. He et al. (2015) reported that concentrates from a full-scale NF-plant had an average COD of 5357 mg L−1, a value about twofold higher than that found in the LFL (2623 mg L−1).
A COD of 6200 mg L−1 was found in concentrates from an NF full-scale plant in Odayeri Sanitary Landfill, Istanbul (Turkey). From the same site, concentrations of ammonia (110 mg L−1), TKN (1000 mg L−1) and chloride (10,000 mg L−1) were recorded (Top et al., 2011). Similar concentrations were found in NFCs from landfills in Shenzhen and Beijing (China) (3450 mg COD L−1, 80 mg TNK L−1 and 2519 mg Cl− L−1) (Li et al., 2016). Previous studies have also confirmed that NFC and ROC are heavily polluted by organic and inorganic compounds (Mojiri et al., 2017; Xiong et al., 2014; Xu et al., 2017).
Similarly, as shown in Table 3, a high concentration of salts (evaluated by conductivity) is found in ROCs – values range from 10,500 to 98,000 µS cm−1. Kallel et al. (2017) reported that ROCs from an LLTP located in Tunisia contained high levels of TDS (66,900 mg L−1), chloride (30,768 mg L−1), sodium (15,400 mg L−1) and potassium (9600 mg L−1). Similar findings have been reported by Hendrych et al. (2019). ROCs from Erzurum landfill (Turkey) were also characterized by high levels of BOD5 and COD, reaching values up to 4800 mg L−1 and 8882 mg L−1, respectively (Hunce et al., 2012).
Conventional management of LLMCs
Conventional membrane concentrate management from LLTPs can be categorized into two main groups: (1) disposal and (2) treatment. The former includes natural evaporation and recirculation to the landfill body, and the latter involves processes aiming at pollutants removal. These two approaches are critically discussed in the following items.
Disposal
The disposal of LLMC streams into the landfill body is the simplest and cheapest method. In general, LLMCs are accumulated in lagoons where physicochemical processes occur, and depending on climatic conditions, there is a moderate reduction of the concentrate volume. Afterwards, the concentrate is injected onto the waste mass by vertical and/ or horizontal drains (Calabrò et al., 2018; ISWA, 2019; Robinson, 2005). Before the work of Henigin (1995), the consequences of concentrate injection were under-discussed. Many scientific researches were published in the last two decades. However, literature findings have revealed contrasting conclusions; therefore, this practice’s sustainability is not a consensus.
Robinson (2005) presented monitoring data of a German landfill that operated a RO system for 1 year, returning the concentrate to the landfill. The study showed that ROC infiltration increased COD, ammonia and conductivity of the generated leachate, which immediately affected the RO performance. Similar results were found by Talalaj (2015a) and Talalaj and Biedka (2015). On the other hand, 15-year monitoring data of an Italian landfill revealed a moderate change in leachate composition (slight increase in NH4+, Cl− and SO4−2) and leachate quantity (i.e. leachate volume increased). However, RO treatment performance was not impacted (Calabrò et al., 2018). In a previous study at the same Italian site, Calabrò et al. (2010) observed a moderate rise in COD, nickel and zinc concentrations; on the other hand, no significant change in leachate quantity was identified in this case. Table 4 summarizes the main findings of LLMC infiltration monitoring studies found in the relevant literature.
Table 4.
Main findings of LLMC infiltration studies.
| Scale | Salient features | Main findings | References |
|---|---|---|---|
| Lab | German landfill Experimental landfill cells 20 months of monitoring |
The LFL quality remained equal with and without infiltration of LLMCs | Henigin (1995) |
| Full | German landfill 12 months of monitoring |
ROC infiltration increased COD, ammonia and conductivity of
the leachate Changes in leachate composition affected the RO performance |
Robinson (2005) |
| Full | Italian landfill Infiltration of 20 m3 LLMC d−1 30 months of monitoring |
No significant change in the leachate
quantity Moderate increase in COD, nickel and zinc of the generated leachate Possible reduction of methane content from the biogas stream |
Calabrò et al. (2010) |
| Full | Brazilian landfill Infiltration of 9.6 m3 ROC h−1 4 months of monitoring |
No significant change in the methane content from the biogas stream | Zanon et al. (2013) |
| Full | Polish landfill Infiltration of ~375 m3 ROC per month 8 months of monitoring |
ROC infiltration increased COD, ammonia, conductivity and
sulphates of the leachate The increase in leachate conductivity affected the COD removal via RO treatment |
Talalaj and Biedka (2015) |
| Lab | Italian landfill Infiltration of 17 LLMC litres during the study period 123 days of monitoring |
No change in the LFL quantity No consistent changes in COD emissions and methane production LLMC infiltration increased NH4+ of the generated leachate |
Morello et al. (2016) |
| Full | Landfill in Bosnia-Herzegovina 83 days of monitoring |
Increase in conductivity and decrease in pH
values Increased the landfill gas flow and methane content in the short term |
Dzolev and Vujic (2016) |
| Lab | Chinese landfill Simulated landfill cells filled with 1-, 5- and 15-year age wastes Infiltration of 72 ROC litres during the study period 24 days of monitoring |
Increase of organics and ammonia in leachate from the 1-year
waste landfill cell Moderate increase in organics, salinity and heavy metals in leachate from the 5-year waste landfill cell |
Wang et al. (2017) |
| Full | Italian landfill 15 years of monitoring The infiltrated LLMC corresponded to 30% of the generated leachate |
LFL quantity increase in 10 years of LLMC infiltration
monitoring Moderate increase in NH4+, Cl− and SO4−2 of the generated leachate Reduction of heavy metals concentration Changes in leachate composition did not affect RO treatment performance |
Calabrò et al. (2018) |
| Full | Tunisian landfill Infiltration of 8 m3 ROC d−1 during 5 years and 10 years of monitoring |
Reduction of biogas generation and methane content | Chamem et al. (2020) |
COD: chemical oxygen demand; LFL: landfill leachate; LLMC: landfill leachate membrane concentrate; RO: reverse osmosis; ROC: reverse osmosis concentrate.
In summary, the literature shows that the consequences of concentrate disposal to landfills are site specific. If adopted as a concentrate management strategy, proper engineering design must be done by monitoring site conditions. For example, in Rio de Janeiro State (Brazil), according to the recently sanctioned law number #9055/2020, landfill managers can recirculate the LLMC to the landfill body up to 1/3 of waste streams from the LLTP, that is, the by-products of the leachate treatment chain (e.g. sludge and LLMC) disposed of into the landfill body cannot exceed 1/3 in volume or mass (Rio de Janeiro State Governor, 2020). However, it should be stated that the concentrate recirculation may be only a temporary solution resulting in never-ending re-introduction of pollutants as concentrate contaminants may eventually accumulate in LLTPs. Moreover, this practice should be no longer acceptable within a circular and sustainable wastewater management system, if resources from LLMC streams can be extracted and recovered.
Treatment
LLMC treatment options include physicochemical processes (e.g. C/F, EC, adsorption, AOPs, ozonation and S/S) (An et al., 2012; Chen et al., 2019; He et al., 2021; Hong et al., 2017; Kallel et al., 2017; Ren et al., 2021; Top et al., 2011; Wang et al., 2020), biological methods (Yang et al., 2018) and thermal processes, including membrane distillation (MD) – a thermally driven membrane filtration technique (Chen et al., 2021a; Yue et al., 2007; Zhang et al., 2019, 2020) or even combinations of them (Ding et al., 2021; Woo et al., 2019). Several treatment technologies have been proposed and assessed in laboratory investigations; however, full-scale project data are scarce, and research is needed to cover this aspect. Some of LLMC treatment procedures and their salient features are summarized in Table 5.
Table 5.
LLMC treatment technologies and their salient features.
| Treatment technology | Important parameter | Salient features | Reference | |
|---|---|---|---|---|
| Physicochemical | C/F | Chemicals and pH | Low cost Insufficient removal efficiencies Requires addition of chemicals Requires sludge management |
Long et al. (2017) |
| EC | Electrode type, current intensity and time | Good removal efficiencies High-tech and automated system No chemical needed Requires energy input |
Top et al. (2011) | |
| Adsorption | Adsorbent dose and time | Low cost Insufficient removal efficiencies Adsorbent regeneration is needed |
Hong et al. (2017) | |
| Fenton oxidation | Chemicals, pH and time | Low cost Requires addition of chemicals Possible change in ecotoxicity Requires sludge management |
Yazici Guvenc and Varank (2021) | |
| Photo-Fenton | Chemicals dose, radiation intensity, pH and time | Increase concentrate biodegradability Requires addition of chemicals Requires sludge management Possible change in ecotoxicity |
Li et al. (2016) | |
| Ozonation | Ozone dose and time | Increase concentrate biodegradability High cost Possible changes in ecotoxicity |
Chen et al. (2019) | |
| S/S | Mixing ratio (LLMC/aggregate) | Low cost Time-consuming process Non-destructive technique Volume of treated concentrate increases |
Kallel et al. (2017) | |
| Biological | Co-bioevaporation | Mass ratio and aeration | Efficient removal of water and organics Requires energy input Time-consuming process Gaseous emissions |
Yang et al. (2018) |
| Algal treatment | Culture, aeration and light intensity | High nutrients removal Requires low energy Low cost Pre-treatment required Time-consuming process |
Woo et al. (2019) | |
| Incineration | LLMC’s properties, residence time, temperature and turbulence | High reduction of concentrate volume High energy demand Equipment corrosion Requires flue gas treatment system and management of the residual stream |
Ren et al. (2019), Tow et al. (2021) | |
| Thermal | Submerged combustion evaporation | Energy input and time | High reduction of concentrate volume High energy demand Equipment corrosion Requires management of the residual stream |
Zhang et al. (2019) |
| MD | Energy input and permeate flux | High water quality High reduction of concentrate volume High energy demand Equipment corrosion Membrane fouling susceptibility |
Chen et al. (2021a) |
C/F: coagulation/flocculation; EC: electro-coagulation; LLMC: landfill leachate membrane concentrate; MD: membrane distillation; S/S: solidification/stabilization.
Physicochemical processes are among the most investigated treatment route. C/F, EC and adsorption are low-cost techniques with good removal efficiencies. However, they are primarily applied as a pre-treatment step rather than a stand-alone technology because of their low salinity removal. In contrast, although AOPs and ozonation can produce a high-quality treated concentrate, these processes have high installation and operational costs, limiting a full-scale implementation. Similarly, thermal processes are associated with expensive operating costs, which contributes to few large-scale projects.
Biological techniques stand out in terms of simplicity and low cost. However, due to the high salinity and poor concentrate biodegradability, they are not recommended for the treatment of LLMCs. A novel biological method for LLMCs treatment has been investigated in a laboratory study. Yang et al. (2018) evaluated a named co-bioevaporation (coBE) process mixing LLMCs with food waste (FW). In coBE, the concentrate is evaporated by the metabolic heat released from aerobic microbial degradation of organic compounds. Under optimal operating conditions (1:1.1 (m/m) mixing ratio of LLMC and FW and 0.035 m3 h−1 airflow per kg TS), 96.7% of water was removed by consuming 96.5% of VS contained in the mixture during five cycles of operation (Yang et al., 2018). However, coBE is time-consuming and requires energy input for aeration, increasing the footprint and operational costs. Recently, the authors proposed intermittent aeration as an energy-saving strategy and found that at a regime of 10 min on/20 min off, more than 50% of energy could be saved with similar treatability results (Liu et al., 2021). Even though the coBE process seems promising, more research is needed to optimize the technique and assess its techno-economic feasibility in large-scale applications. Besides, air pollutant emissions from this process are a significant source of pollution; therefore, carbon footprint and related environmental impacts should also be considered in future investigations.
Resource recovery options
The development of sustainable and cost-effective methods for LLMCs treatment combining resource recovery processes is a promising field of research. LLMC components that would be infiltrated on the landfill waste mass could be extracted and transformed into valuable products such as organic fertilizer and solid salts with commercial value or used for energy purposes. This section summarizes the literature on concentrated leachate treatment techniques and their application in LLMCs’ resource recovery.
Reclaimed water
Typically, 50–80% of the NF/RO feed is recovered as water. The maximization of high-quality water recovery during the LFL treatment through membrane processes can reduce the concentrate volume and guarantee high reclaimed water production (Iskander et al., 2017b). As mentioned earlier, several patented technologies to improve feed water recovery based on MLD and ZLD strategies are available. However, these systems are high capital costs and energy-intensive, which hampers their implementation in LLTPs. For example, Panagopoulos and Haralambous (2020) assessed two different scenarios considering the MLD and ZLD framework. The MLD scheme comprises membrane-based technologies, and the ZLD is membrane and thermal-based. At the freshwater recovery of 84.60% (MLD system) and 98.15% (ZLD system), the energy consumption of MLD and ZLD systems was estimated at 5.40 and 10.43 kWh m−3, respectively.
On the other hand, multi-stage RO has proved to improve permeate recovery and reduce specific energy demand when less than five stages are used (Judd, 2017). Cingolani et al. (2018) proposed a three-stage RO system (RO1, RO2 and RO3) to maximize water recovery of LFL treatment and reach standards for water discharge or reuse. The water recovery was optimized to >90% following RO1 and RO2 stages, while the RO3 step was needed to achieve boron and nitrogen local requirements for reuse.
Another approach beyond concentrate minimization would be treating LLMC streams to accomplish minimal concentrate disposal producing high-quality water. In this regard, a microbial desalination cell (MDC) was studied to treat the concentrated leachate from a forward osmosis (FO) system. The FO concentrate was desalinated in the MDC and the treated concentrate was returned to the FO for further water extraction (Figure 2). FO water recovery increased from 51.5% to 83.5% when operated along with the MDC system at the hydraulic retention time of 10 h (Iskander et al., 2018a).
Figure 2.
Schematic diagram of the FO–MDC system. Reprinted from Iskander et al. (2018a) with permission from Elsevier (license number: 5117181379011).
AEM: anion exchange membrane; CEM: cation exchange membrane; FO: forward osmosis; MDC: microbial desalination cell.
It should be highlighted that FO technology has received increased attention for water recovery due to its low energy requirements and the low fouling propensity of FO membranes (Li et al., 2019). The FO technology uses the osmotic pressure gradient to transport freshwater through the membrane. In this process, a draw solution (DS) with high salinity creates an osmotic pressure difference, leading water to flow from the feed effluent across the semipermeable FO membrane. Further separation of the diluted DS is required after the FO process to obtain reclaimed water as a product (Shaffer et al., 2015).
CaCl2, Ca(NO3)2, NaCl and thermolytic solutes based on ammonia and carbon dioxide, similar to NH4HCO3, are the most DS employed for FO applications (Achilli et al., 2010). At the end of the FO filtration, the DS is recovered and recycled back to the FO unit while the permeate may need further treatment for its direct discharge or reuse (Wu et al., 2018). The energy consumption of FO systems without DS regeneration is notably lower (0.2–0.9 vs 10–14 kWh m−3) (Voutchkov and Kaiser, 2020); therefore, from an energy-efficient and resource recovery point of view, the selection of a fertilizer draw solute for FO application can be an attractive strategy. Li et al. (2017) investigated a FO system using NH4HCO3 (3 mol L−1) as the DS to treat LFL. FO recovery was higher than 90%, and the water product met the regulatory standards for agricultural fertigation. In another study, Qin et al. (2016) proposed to use the recovered NH4HCO3 (2 mol L−1) as DS in a hybrid-FO system for water recovery from LFL.
Humic substances
As discussed earlier, LLMCs are rich in refractory organic compounds, mainly consisting of HSs – high molecular weight compounds (300–10,000 Da) with phenolic, carboxylic and alkoxy groups along with the occasional presence of esters and quinones (Gu et al., 2019; Lima et al., 2017; Xu et al., 2017). In agriculture, HSs are used as organic fertilizer and play a key role in improving soil proprieties (e.g. soil physical structure, nutrient retention and water-holding capacity), increase soil organic content and microbial diversity, and boost fertilizer efficiency. Due to its high salinity, the LLMC cannot be applied directly as an HS-containing liquid fertilizer (Ye et al., 2019). Thus, the enrichment and extraction of HSs from LLMCs have raised strong interest.
To date, the main methods for HS extraction from LFL are chemical precipitation and membrane filtration. Chemical precipitation requires low pH (pH < 2), and therefore, the recovered product is not suitable for direct soil applications. Membrane processes have been used for the efficient extraction of HSs (Gu et al., 2019). Overall, ultrafiltration (UF) and NF membranes with MWCO ranges of 200–1000 Da and 1–10 kDa are the primary techniques to fractionate these compounds (Xu et al., 2017; Ye et al., 2019, 2020). However, tight NF membranes can jeopardize the purification process for producing liquid fertilizer. The high salt rejection of NF membranes can result in excessive salts in the target product (i.e. concentrate stream) when the NF process is applied for desalination. On the other hand, UF membranes have wide pore sizes and enable almost unrestricted passage of inorganic salts, failing to efficiently fractionate salts and humic compounds due to inadequate rejection of substances of low molecular weight (300–3500 Da) such as FAs (Ye et al., 2019).
Xu et al. (2017) employed two-stage tight UF (MWCO = 1000 Da) for extracting HSs from leachate concentrate. At the end of the concentration process, organic content was 45,370 mg HS L−1. With the addition of nutrient macroelements in the obtained liquor, the recovered stream could be used as liquid fertilizer. The authors estimated the economic benefit of the HS-containing soluble fertilizer production at 4672 USD m−3. This value could offset production costs, including operating and purchase costs of macronutrients and generates an attractive profit margin.
To efficient fractionating and desalting of NFCs, Ye et al. (2019) propose to use loose NF membrane with MWCO of 860 Da, which takes the merits of both NF and UF membranes. At a concentration factor of 9.6, the HS content was enriched from 1765 to 15,287 mg L−1 with about 86% desalting efficiency. As a water-soluble fertilizer, the recovered liquor stimulated the seed germination and enhanced the growth of green mungbean plants, presenting no phytotoxicity. In recent work, the same research group proposed an integrated bio-inspired self-polymerization procedure to tailor loose NF proprieties for efficient fractionation of HSs and desalination. Using the modified loose NF (298 Da), the LLMC was preconcentrated by a factor of 10.0 without permeate recirculation into the feed. Subsequently, a diafiltration step was performed to demineralize the pre-treated LLMC. The bio-inspired membrane showed superior selectivity between HSs and inorganic salts. The concentration of HSs was enriched from 1779.4 to 17,247.1 mg L−1 and desalting efficiency of 99.5% was achieved, resulting in high HS purity (i.e. 98.3%) for potential liquid fertilizer applications (Ye et al., 2020).
Table 6 depicts recovering schemes for organic components extraction of concentrated leachate.
Table 6.
Recovery schemes for HSs extraction from concentrated leachate.
| Recovery scheme | Target resource | Product applications | Main findings | Reference |
|---|---|---|---|---|
| UF | HSs | Liquid fertilizer | High fractioning of HS, salts and heavy metals | Yue et al. (2011) |
| Coagulation + centrifugation + sun drying | HA and FA* | Soil conditioning | Recovered material enhanced germination and growth of soybeans | Yang and Li (2016) |
| Two-stage tight UF | HSs | Liquid fertilizer | Economic benefit/attractive margin profit | Xu et al. (2017) |
| Loose NF | HSs | Water-soluble fertilizer | Liquid fertilizer application promoted plant growth with no phytotoxicity | Ye et al. (2019) |
| FO + chemical precipitation | Humic acid | Fertilizer component | Possible application as soil stabilizer or fertilizer component | Iskander et al. (2019) |
| Bio-inspired loose NF + diafiltration | HSs | Liquid organic fertilizer | High HS concentration and desalting efficiency | Ye et al. (2020) |
Recovery from the dewatering effluent of thermally treated sludge (raw effluent: 2180 ± 82 mg HA L−1 and 1317 ± 96 mg FA L−1).
FA: fulvic acid; FO: forward osmosis; HA: humic acid; HS: humic substance; NF: nanofiltration; UF: ultrafiltration.
It should be underlined that membrane concentrates may have a certain level of toxic pollutants (e.g. heavy metals and xenobiotic organic compounds), which may hinder the direct reuse of the recovered HS as organic fertilizer; thus, further purification will be needed. An interesting work investigated the use of encapsulated HAs in alginate beds extracted from anaerobic sewage sludge to allow controlled and slow release of HSs in the soil. The agronomic tests showed that the dry biomass of the treated plants was remarkably higher than that for non-treated plants. The encapsulation of HAs within alginate beads could immobilize toxic compounds as well as reduce the amount of added product, ensuring a proper dose of HAs in the soil at lower operational costs (Cristina et al., 2020).
Following another recovery approach, the recalcitrant substances in LLMC streams could be reused for energetic proposes. Ben Hassen-Trabelsi et al. (2020) investigated the co-pyrolysis of ROC and sewage sludge to recycle organics as biofuels. At optimum conditions (mixture of 30:70 ROC/sewage sludge, 550°C and 10°C min−1 as heating rate), the process produced high energy gas (12.29 MJ kg−1), which could be at least employed as an energy source for pyrolytic conversion.
Nutrients
Nitrogen (N) and phosphorus (P) are the major nutrients that are present in LFL. Ammonia nitrogen is one of the main LFL pollutants and therefore most of the LLTPs are designed to remove N. As contaminants, N and P stimulate excessive plant and algal growth, leading to waterbody eutrophication and associated adverse impacts. NH3-N removal of LFL has been extensively studied (Antwi et al., 2020; Costa et al., 2021; de Almeida et al., 2019; Genethliou et al., 2021). As resources, N and P are critical macronutrients for crops and hence are key components for fertilizer production. Besides, phosphate shortage linked to food security has intensified interest in P recovering of wastewater. In NFC and ROC, the ammonia nitrogen concentration is generally high, reaching levels of 3273–8300 mg L−1 (Table 3); such levels are high enough to shift the focus from removal to recovery.
Stripping technology and chemical precipitation are the main methods for nutrients recovery from wastewater. Stripping technology is based on the ammonia gas–liquid equilibrium in an aqueous solution. In an alkaline environment, usually pH from 10.5 to 11.5 at 25°C, the balance of ammoniacal nitrogen in leachate tends to produce more ammonia than ammonium (NH4+(aq) + OH−(aq) ↔ NH3(g)↑ + H2O(l)) (Campos et al., 2013). Ammonia is a water-soluble gas. Thus, passing an air stream through the wastewater, the ammonia concentration reaches the gas–liquid equilibrium in the system and ammonia can be recovered from the stripped gas. The primary factors affecting ammonium stripping are pH, airflow and temperature. High-temperature water vapour can be used as the air stream to boost the ammonia mass transfer. The stripping gas must be allocated into an acid solution for recovery purposes, so ammonia is recovered as an ammonium salt like NH4Cl and (NH4)2SO4. The recovered salt can be reused in different industrial and commercial applications (Campos et al., 2013; Kurniawan et al., 2021; Xiang et al., 2020).
However, full-scale applications of ammonia stripping may be costly, making nitrogen recovery from membrane concentrates unfeasible. dos Santos et al. (2020) estimated the total cost of ammonia recovery from concentrated leachate at 51.64 USD m−3. This cost was mainly dependent on the price of the tower (in which the mass transfer occurs), chemicals for absorption and electricity consumption. In each scenario, a detailed techno-economic evaluation should be performed, considering the process energy demand, system robustness, product quality and local market demands (Kurniawan et al., 2021).
In the precipitation method, nutrients are recovered via struvite precipitation. Struvite (MgNH4PO4 ⋅ 6H2O) is a phosphate mineral and can be used as a slow-release fertilizer or raw material for the chemical industry. In struvite precipitation, an alkaline solution is obtained either by the addition of alkali solution or aeration stripping of carbon dioxide, followed by the introduction of magnesium salts for MgNH4PO4 ⋅ 6H2O formation, which has a 1:1:1 molar ratio of ammonium (NH4+), phosphate (PO4−3) and magnesium (Mg+2). When the concentrations of NH4+, PO4−3 and Mg+2 exceed their solubility limit, struvite formation recovers both N and P from leachate (Li et al., 2019). The main drawback is that, in general, struvite precipitation requires external magnesium and phosphorus to promote struvite crystallization. As leachate contain less magnesium and phosphate ammonium, a large amount of chemicals may be required. Besides, P recovery is ideal at a pH higher than 9.5, requiring the addition of an alkaline solution (Kurniawan et al., 2021).
Moreover, struvite precipitation is susceptible to interference by heavy metals and some inorganic ions such as Ca+2, K+, Fe+3 and CO3-2, which also reduces the purity of the recovered mineral. Therefore, a compromise between process control and cost-effectiveness is needed to ensure the product quality and the process sustainability with more economic benefits (Li et al., 2019; Xiang et al., 2020). According to Kurniawan et al. (2021), P recovery from waste streams is hardly carried out because the cost of the recovered P is higher than that of natural rock-phosphate and the current technologies have a long cost recovery time of up to 7 years.
Recently, techniques such as microbial electrolysis, MD and FO have been proposed for nutrients recovery of LFL (Qin et al., 2016; Xie et al., 2016; Zico et al., 2021). Considering the demerits of each technology, the hybridization of these processes with existing precipitation methods could benefit nutrients reclamation from waste streams. Hybrid systems could improve nutrient recovery efficiency and integrate different resource platforms, making nutrient recovery cost-effective and more attractive to be an option for valorization of LLMC streams (Xie et al., 2016).
A submerged FO process linked to struvite precipitation was proposed to focus on both water and nutrient extraction. Three arrangements were tested to determine the optimal configuration about effects of struvite recovery on the FO performance (Figure 3): FO – calcium pre-treatment – struvite precipitation (A1), calcium pre-treatment – FO – struvite precipitation (A2) and calcium pre-treatment – struvite precipitation – FO (A3). The A2 system was the optimum arrangement in terms of FO performance. Calcium pre-treatment mitigated FO membrane fouling and improved the purity of the obtained struvite. The submerged FO system efficiently recovered water from the leachate and reduced its volume by 37%. The recovered mineral in chemical precipitation had a similar crystal structure and composition to that of standard struvite. The proposed system recovered about 4.34 kg struvite and 366 kg of water per m3 of treated leachate in optimum conditions. The net profit was estimated at 0.80 USD m−3 (Wu et al., 2018).
Figure 3.
Schematic diagram of three systems for different arrangements of FO process, calcium pre-treatment and struvite precipitation. A1: FO – calcium pre-treatment – struvite precipitation, A2: calcium pre-treatment – FO – struvite precipitation and A3: calcium pre-treatment – struvite precipitation – FO. Reprinted from Wu et al. (2018) with permission from Elsevier (license number: 5121960273804).
FO: forward osmosis.
Inorganic salts
NFC and ROC from LLTPs contain valuable inorganic ions such as Na+, K+ and Cl−, often located at levels higher than 890, 210 and 1280 mg L−1, respectively (Table 3). Some of these inorganic species like potassium ions are scarce and therefore could exist a driving force behind their possible extraction. Other elements like chloride ions could be recovered as mixed salts or even transformed into high add-value products. Therefore, in this scenario, mineral reclamation of waste streams could make leachate treatment more sustainable and potentially economical (Huang et al., 2020; Le Dirach et al., 2005; Li et al., 2015).
At present, few published studies focus on mineral recovery from concentrated leachate. Most of the researches has investigated mineral extraction from sea-water brines. For example, Mohammadesmaeili et al. (2010) applied RO and isothermal evaporation to manage lima-soda pre-treated concentrate from desalination plants. The proposed system recovered freshwater and produced mixed solid salt (i.e. Na2SO4 and NaCl) with potential resale value. However, it should be noted that the current energy demand of thermal-based technologies (7.7–72 kWh m−3) can hinder field applications (Panagopoulos, 2021). Therefore, more studies are still needed to evaluate the process economics considering the influence factors of each situation.
A combined process composed of cation-exchange membrane electrolysis and chemical precipitation was developed to simultaneously treat NFCs and recover K+ and Cl− ions as commercial by-products. The combined system exhibited excellent treatability results and allowed efficient recovery of gaseous chlorine and potassium-containing struvite. However, a preliminary economic analysis showed that the net profit of products resale would not offset the system’s operating costs, which were most associated with the high electricity consumption of the electrochemical process (Li et al., 2015).
In another work, through a simple chemical precipitation method, antichlors of Bi(III) containing oxides with quantum dots or two-dimensional structures were synthesized and then mixed with spinel ferrites (M-Fe3O4) and titanium dioxide (TiO2) to combine with Bi2O3 for magnetic recycling and photocatalysis improvement. The constructed antichlor was then used to treat concentrated leachate. Under optimum conditions, Cl− removal efficiencies ranged from 60% to 90%. The treatment of concentrated leachate with the antichlors led to the formation of Bi-precipitates that contained coated BiOCl on the residual Bi2O3/TiO2 structure. This by-product was recovered and further used to treat the dechlorinated leachate. It was concluded that due to the excellent photocatalytic activity of the recovered material under UV–vis-near-infrared irradiation, better than that of commercial BiOCl in the mineralization of methyl orange, they could be used as a photocatalyst for the degradation of organic compounds of dechlorinated wastewater (Huang et al., 2020). Figure 4 shows a schematic illustration of the overall experiment. This research provides insights into chlorine removal techniques and the potential production of commercial photocatalytic materials. Further research in this field could help finding alternatives for the valorization of membrane concentrates and other chlorinated effluents.
Figure 4.
Illustration of the concentrated leachate treatment with constructed antichlors along with the recovery of Cl-related by-products. Reprinted from Huang et al. (2020) with permission from Elsevier (license number: 5122441160351).
Future perspectives and management diagram for resource recovery
Environmental concerns and resources depletion are expected to accelerate greener and sustainable practices. Most of the literature findings are based on laboratory studies, showing that LLMC resource recovery systems are at the embryonic stage. At present, few technologies appear to be techno-economically applicable at a commercial scale, and some critical aspects (e.g. energy demand and process robustness) still need to be solved. Considering that future researches are required, we propose a recovery train to select the best recovery route. The proposed diagram can be helpful to define and test the overall performance of the selected recovery route. In general, a sequential scheme for concentrate valorization should include volume reduction with the recovery of clean water, followed by extraction of add-value materials. As discussed earlier, different types of LFL and applied membrane treatment will result in different organic, salt and nutrient contents in the LLMC. Figure 5 shows the recovery train for implementing the best management route based on the main characteristics of LLMCs.
Figure 5.
General recovery train for the selection of resource recovery route.
FO: forward osmosis; LLMC: landfill leachate membrane concentrate; NF: nanofiltration; RO: reverse osmosis; UF: ultrafiltration.
The recovery scheme’s first step incorporates FO for water reclamation. The FO process appears the most promising technology for volume reduction and water recovery from membrane concentrates. As FO permeate stream is not clean water but a diluted DS, a regeneration step is required. The hybrid FO-RO process is more energy efficient than standalone FO; therefore, coupling these processes helps save energy and reduce operational costs (Singh et al., 2021).
After volume reduction, a membrane-based process, that is, tight UF or loose NF, can fractionate heavily organic streams, recovering HSs as liquid fertilizer. A desalting process should be employed if either FO concentrate stream or UF/NF permeate has high salinity. Thermal-based technologies such as thermal evaporation and crystallization are the preferred systems for the valorization of saline effluents (Panagopoulos, 2021). However, high energy consumption and operational expenses can hamper its implementation in LLTPs. A renewable energy source such as biogas, commonly available in landfills, could produce energy input for desalting systems. In addition, mixed salts (e.g. NaCl, Na2SO4, CaCl2 and MgCl2) resale can generate net profit and make them more economically attractive.
The tertiary step is optional if the quality of the concentrate stream is acceptable to be recycled back to the beginning of LLTP or sent to the FO-RO system for further water recovery. On the other hand, high nutrient loads imply that instead of being moved back to the beginning of LLTP, struvite could be precipitated out and sold as fertilizer. The treated effluent could then be recycled to the beginning of LLTP, reducing the effluent’s nutrient load.
The proposed recovery train intends to recommend LLMC management based on resource recovery approaches rather than treatment and disposal. Recommended technologies in the diagram are not limited to those described here. For example, emerging resource recovery systems have been tested in laboratory studies, and further research on pilot scale is expected for its implications and economic feasibility. Bioelectrochemical systems (BESs) have grabbed attention for simultaneous nutrient reclamation and energy production from leachate (Iskander et al., 2018a; Qin et al., 2016). Iskander et al. (2017a) demonstrated that a BES producing 0.123 kWh m−3 could treat LFL. Hybrid processes involving BESs coupled to FO might be promising to recover both water and chemicals, improving FO efficiency with less energy consumption. Low-cost phytoremediation systems can be implemented as a polishing step for nutrients and heavy metals removal depending on the concentrate composition. Harvested biomasses are useful for bioenergy production (biogas, biofuels, combustion for energy recuperation and heating) (Gomes, 2012; Wijekoon et al., 2021). Thus, energy recovery can further improve the proposed system’s overall economic and commercial viability.
The following aspects are recommended during the assessment of the defined resource recovery framework: (1) Material balance aiming to describe the resource recovery route in a quantitative way before its implementation; (2) detailed analysis of product quality, applicability and local market demand for the recovered material and (3) evaluation of potential impacts of the management route through a life cycle analysis perspective.
Conclusions
The state-of-the-art of current research about membrane concentrates from LLTPs was critically examined in this article. Although LLMC recycling into the landfill body is the convenient management option, a more sustainable strategy is recommended. A general recovery train for implementing the best LLMC recovery route was proposed within this context. Low energy demand and membrane fouling propensity have made FO indispensable for volume reduction and water reclamation of concentrate streams. Material extraction (e.g. fertilizers and inorganic salts) from the FO-treated effluent could generate net profit and increase the system’s economic feasibility. However, few technologies appear to be techno-economically applicable at a commercial scale, and some critical aspects (e.g. energy requirements and process robustness) still need to be solved. Future studies should focus on developing novel integrated systems combining benefits of each recovering technology, scale-up, techno-economic evaluation of recovering processes and assessment based on a life cycle perspective (i.e. environmental impacts and carbon footprint). Besides, the extraction of non-conventional add-value products (e.g. catalysts and bio-fuels) via existing or novel technologies is a promising area for future investigations.
Footnotes
Author contributions: Almeida, R.: Conceptualization, Investigation and data analysis; Methodology; Writing – Original draft preparation; Writing – Reviewing and Editing. Porto, R. F.: Involved in investigation. Quintaes, B. R.: Supervision. Bila, D. M.: Writing – Reviewing and Editing; Supervision. Lavagnolo, M. C.: Writing – Reviewing and Editing; Supervision. Campos, J. C.: Writing – Reviewing and Editing, Supervision; Funding acquisition.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The authors gratefully acknowledge the financial support received from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj) (Grant Number 200.065/2020 and 202.923/2018) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant Number 165018/2018-6).
ORCID iDs: Ronei de Almeida  https://orcid.org/0000-0002-3798-634X
https://orcid.org/0000-0002-3798-634X
Raphael Ferreira Porto  https://orcid.org/0000-0003-4789-9837
https://orcid.org/0000-0003-4789-9837
Juacyara Carbonelli Campos  https://orcid.org/0000-0002-3988-5649
https://orcid.org/0000-0002-3988-5649
References
- Abbas AA, Jingsong G, Ping LZ, et al. (2009) Review on landfill leachate treatments. American Journal of Applied Sciences 6: 672–684. [Google Scholar]
- Abdel-Fatah MA. (2018) Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Engineering Journal 9: 3077–3092. [Google Scholar]
- Abuabdou SMA, Ahmad W, Aun NC, et al. (2020) A review of anaerobic membrane bioreactors (AnMBR) for the treatment of highly contaminated landfill leachate and biogas production: Effectiveness, limitations and future perspectives. Journal of Cleaner Production 255: 120215. [Google Scholar]
- Achilli A, Cath TY, Childress AE. (2010) Selection of inorganic-based draw solutions for forward osmosis applications. Journal of Membrane Science 364: 233–241. [Google Scholar]
- Ahmed FN, Lan CQ. (2012) Treatment of landfill leachate using membrane bioreactors: A review. Desalination 287: 41–54. [Google Scholar]
- Ahn W-Y, Kang M-S, Yim S-K, et al. (2002) Advanced landfill leachate treatment using an integrated membrane process. Desalination 149(1–3): 109–114. [Google Scholar]
- Amaral MCS, Pereira HV, Nani E, et al. (2015) Treatment of landfill leachate by hybrid precipitation/microfiltration/nanofiltration process. Water Science and Technology 72: 269–276. [DOI] [PubMed] [Google Scholar]
- An ZY, Xu XJ, Xu XJ. (2012) Treatment of concentrated liquid of landfill leachate by coagulation and micro-electrolysis strengthened by H2O2. Adv Mater Res 573–574: 492–496. [Google Scholar]
- Anna Tałałaj I, Bartkowska I, Biedka P. (2021) Treatment of young and stabilized landfill leachate by integrated sequencing batch reactor (SBR) and reverse osmosis (RO) process. Environmental Nanotechnology, Monitoring & Management 16: 100502. [Google Scholar]
- Antwi P, Zhang D, Su H, et al. (2020) Nitrogen removal from landfill leachate by single-stage anammox and partial-nitritation process: Effects of microaerobic condition on performance and microbial activities. Journal of Water Process Engineering 38: 101572. [Google Scholar]
- Argun ME, Akkuş M, Ateş H. (2020) Investigation of micropollutants removal from landfill leachate in a full-scale advanced treatment plant in Istanbul city, Turkey. Science of the Total Environment 748: 141423. [DOI] [PubMed] [Google Scholar]
- Bae W, Kim S, Lee J, Chung J. (2019) Effect of leachate circulation with ex situ nitrification on waste decomposition and nitrogen removal for early stabilization of fresh refuse landfill. Journal of Hazardous Materials 371: 721–727. [DOI] [PubMed] [Google Scholar]
- Baker RW. (2012) Membrane Technology and Applications, Membrane Technology and Applications. Newark, California: Wiley, p. 575. [Google Scholar]
- Bakhshoodeh R, Alavi N, Oldham C, et al. (2020) Constructed wetlands for landfill leachate treatment: A review. Ecological Engineering 146: 105725. [Google Scholar]
- Balkema R, Tollenaere K, Pat Stanford (2018) Reverse Osmosis to the Rescue [WWW Document]. Available at: https://www.mswmanagement.com/home/article/13035294/reverse-osmosis-to-the-rescue (accessed 26 July 2020).
- Beaven RP, Knox K. (2018) Leachate Recirculation, Solid Waste Landfilling. Amsterdam, Netherlands: Elsevier Inc, pp. 703–727. [Google Scholar]
- Ben Hassen-Trabelsi A, Kallel A, Ben Amor E, et al. (2020). Up-grading biofuel production by co-pyrolysis of landfill leachate concentrate and sewage sludge mixture. Waste and Biomass Valorization 11: 291–301. [Google Scholar]
- Brennan RB, Clifford E, Devroedt C, et al. (2017) Treatment of landfill leachate in municipal wastewater treatment plants and impacts on effluent ammonium concentrations. Journal of Environmental Management 188: 64–72. [DOI] [PubMed] [Google Scholar]
- Briffa J, Sinagra E, Blundell R. (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6: e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch J, Ahrens L, Sturm R, et al. (2010) Polyfluoroalkyl compounds in landfill leachates. Environmental Pollution 158: 1467–1471. [DOI] [PubMed] [Google Scholar]
- Calabrò PS, Gentili E, Meoni C, et al. (2018) Effect of the recirculation of a reverse osmosis concentrate on leachate generation: A case study in an Italian landfill. Waste Management 76: 643–651. [DOI] [PubMed] [Google Scholar]
- Calabrò PS, Mancini G. (2012) Possible interactions between recirculated landfill leachate and the stabilized organic fraction of municipal solid waste. Waste Management and Research 30: 551–557. [DOI] [PubMed] [Google Scholar]
- Calabrò PS, Sbaffoni S, Orsi S, et al. (2010) The landfill reinjection of concentrated leachate: Findings from a monitoring study at an Italian site. Journal of Hazardous Materials 181: 962–968. [DOI] [PubMed] [Google Scholar]
- Campos JC, Moura D, Costa AP, et al. (2013) Evaluation of pH, alkalinity and temperature during air stripping process for ammonia removal from landfill leachate. Journal of Environmental Science and Health, Part A 48: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Chamem O, Fellner J, Zairi M. (2020) Ammonia inhibition of waste degradation in landfills – A possible consequence of leachate recirculation in arid climates. Waste Management & Research: The Journal for a Sustainable Circular Economy 38: 1078–1086. [DOI] [PubMed] [Google Scholar]
- Chan GYS, Chang J, Kurniawan TA, et al. (2007) Removal of non-biodegradable compounds from stabilized leachate using VSEPRO membrane filtration. Desalination 202: 310–317. [Google Scholar]
- Chaudhari LB, Murthy ZVP. (2010) Treatment of landfill leachates by nanofiltration. Journal of Environmental Management 91: 1209–1217. [DOI] [PubMed] [Google Scholar]
- Chelliapan S, Arumugam N, Md. Din MF, et al. (2020) Anaerobic treatment of municipal solid waste landfill leachate. In: Singh L, Yousuf A, Mahapatra DM. (eds) Bioreactors: Sustainable Design and Industrial Applications in Mitigation of GHG Emissions. Amsterdam, The Netherlands: Elsevier, pp.175–193. [Google Scholar]
- Chen L, Chen Z, Wang Y, et al. (2021. a) Effective treatment of leachate concentrate using membrane distillation coupled with electrochemical oxidation. Separation and Purification Technology 267: 118679. [Google Scholar]
- Chen W, Gu Z, Ran G, et al. (2021. b) Application of membrane separation technology in the treatment of leachate in China: A review. Waste Management 121: 127–140. [DOI] [PubMed] [Google Scholar]
- Chen W, Gu Z, Wen P, et al. (2019) Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 217: 411–422. [DOI] [PubMed] [Google Scholar]
- Chianese A, Ranauro R, Verdone N. (1999) Treatment of landfill leachate by reverse osmosis. Water Research 33: 647–652. [Google Scholar]
- Chu D, Ye ZL, Chen S. (2020) Interactions among low-molecular-weight organics, heavy metals, and Fe(III) during coagulation of landfill leachate nanofiltration concentrate. Waste Management 104: 51–59. [DOI] [PubMed] [Google Scholar]
- Cingolani D, Eusebi AL, Battistoni P. (2017) Osmosis process for leachate treatment in industrial platform: Economic and performances evaluations to zero liquid discharge. Journal of Environmental Management 203: 782–790. [DOI] [PubMed] [Google Scholar]
- Cingolani D, Fatone F, Frison N. (2018) Pilot-scale multi-stage reverse osmosis (DT-RO) for water recovery from landfill leachate. Waste Management 76: 566–574. [DOI] [PubMed] [Google Scholar]
- Clarke BO, Anumol T, Barlaz M, et al. (2015) Investigating landfill leachate as a source of trace organic pollutants. Chemosphere 127: 269–275. [DOI] [PubMed] [Google Scholar]
- Cossu R, Ehrig H-J, Muntoni A. (2018) Physical–chemical leachate treatment. In: Solid Waste Landfilling. Elsevier, pp.575–632. [Google Scholar]
- Costa AM, Alfaia RG, de SM, Campos JC. (2019) Landfill leachate treatment in Brazil – An overview. Journal of Environmental Management 232: 110–116. [DOI] [PubMed] [Google Scholar]
- Costa AM, dos Santos Valentim MR, da Silva LF, et al. (2021) Comparison between Aliivibrio fischeri and activated sludge microorganisms in the evaluation of the toxic pollutants of leachates from Brazilian landfills. Environmental Science and Pollution Research 29: 1546–1558. [DOI] [PubMed] [Google Scholar]
- Cristina G, Camelin E, Ottone C, et al. (2020) Recovery of humic acids from anaerobic sewage sludge: Extraction, characterization and encapsulation in alginate beads. International Journal of Biological Macromolecules 164: 277–285. [DOI] [PubMed] [Google Scholar]
- de Almeida R, Bila DM, Quintaes BR, et al. (2020. a) Cost estimation of landfill leachate treatment by reverse osmosis in a Brazilian landfill. Waste Management & Research: The Journal for a Sustainable Circular Economy 38: 1087–1092. [DOI] [PubMed] [Google Scholar]
- de Almeida R, Campos JC. (2020) Análise tecnoeconômica do tratamento de lixiviado de aterro sanitário. Revista Ineana 8: 6–27. [Google Scholar]
- de Almeida R, Campos J, de Oroski F. (2020. b) Techno-economic evaluation of landfill leachate treatment by hybrid lime application and nanofiltration process. Detritus 10: 170–181. [Google Scholar]
- de Almeida R, de Souza Couto JM, Gouvea RM, et al. (2020. c) Nanofiltration applied to the landfill leachate treatment and preliminary cost estimation. Waste Management & Research: The Journal for a Sustainable Circular Economy 38: 1119–1128. [DOI] [PubMed] [Google Scholar]
- de Almeida R, Moraes Costa A, de Almeida Oroski F, et al. (2019) Evaluation of coagulation–flocculation and nanofiltration processes in landfill leachate treatment. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering 54: 1091–1098. [DOI] [PubMed] [Google Scholar]
- Deng Y, Englehardt JD. (2006) Treatment of landfill leachate by the Fenton process. Water Research 40: 3683–3694. [DOI] [PubMed] [Google Scholar]
- Dereli RK, Clifford E, Casey E. (2021) Co-treatment of leachate in municipal wastewater treatment plants: Critical issues and emerging technologies. Critical Reviews in Environmental Science and Technology 51(11): 1079–1128. [Google Scholar]
- Di Maria F, Sisani F, Contini S, et al. (2018) Impact of different schemes for treating landfill leachate. Waste Management 71: 255–266. [DOI] [PubMed] [Google Scholar]
- Ding J, Jiang M, Zhao G, et al. (2021) Treatment of leachate concentrate by electrocoagulation coupled with electro-Fenton-like process: Efficacy and mechanism. Separation and Purification Technology 255: 117668. [Google Scholar]
- Dolar D, Košutić K, Strmecky T. (2016) Hybrid processes for treatment of landfill leachate: Coagulation/UF/NF-RO and adsorption/UF/NF-RO. Separation and Purification Technology 168: 39–46. [Google Scholar]
- dos Santos HAP, de Castilhos Júnior AB, Nadaleti WC, et al. (2020) Ammonia recovery from air stripping process applied to landfill leachate treatment. Environmental Science and Pollution Research 27: 45108–45120. [DOI] [PubMed] [Google Scholar]
- Dzolev N, Vujic G. (2016) Impact assessment of concentrate recirculation on the landfill gas production. Thermal Science 20: 1283–1294. [Google Scholar]
- Ehrig H-J, Robinson H. (2010) Landfilling: Leachate treatment. In: Christensen TH. (ed). Solid Waste Technology & Management. Chichester, UK: John Wiley & Sons, Ltd, pp.858–897. [Google Scholar]
- Ehrig H-J, Stegmann R, Robinson T. (2018) Biological Leachate Treatment, Solid Waste Landfilling. Elsevier Inc. [Google Scholar]
- El-Fadel M, Bou-Zeid E, Chahine W, et al. (2002) Temporal variation of leachate quality from pre-sorted and baled municipal solid waste with high organic and moisture content. Waste Management 22: 269–282. [DOI] [PubMed] [Google Scholar]
- El-Gohary FA, Kamel G. (2016) Characterization and biological treatment of pre-treated landfill leachate. Ecological Engineering 94: 268–274. [Google Scholar]
- El Kateb M, Trellu C, Darwich A, et al. (2019) Electrochemical advanced oxidation processes using novel electrode materials for mineralization and biodegradability enhancement of nanofiltration concentrate of landfill leachates. Water Research 162: 446–455. [DOI] [PubMed] [Google Scholar]
- Fan HJ, Shu HY, Yang HS, et al. (2006) Characteristics of landfill leachates in central Taiwan. Science of the Total Environment 361: 25–37. [DOI] [PubMed] [Google Scholar]
- Farquhar GJ. (1989) Leachate: Production and characterization. Canadian Journal of Civil Engineering 16: 317–325. [Google Scholar]
- Fernandes A, Chamem O, Pacheco MJ, et al. (2019) Performance of electrochemical processes in the treatment of reverse osmosis concentrates of sanitary landfill leachate. Molecules 24: 2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A, Labiadh L, Ciríaco L, et al. (2017) Electro-Fenton oxidation of reverse osmosis concentrate from sanitary landfill leachate: Evaluation of operational parameters. Chemosphere 184: 1223–1229. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Pacheco MJ, Ciríaco L, et al. (2015) Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Applied Catalysis B: Environmental 176–177: 183–200. [Google Scholar]
- Ferraz FM, Povinelli J, Vieira EM. (2013) Ammonia removal from landfill leachate by air stripping and absorption. Environmental Technology (United Kingdom) 34: 2317–2326. [DOI] [PubMed] [Google Scholar]
- Foo KY, Hameed BH. (2009) An overview of landfill leachate treatment via activated carbon adsorption process. Journal of Hazardous Materials 171: 54–60. [DOI] [PubMed] [Google Scholar]
- Gao J, Oloibiri V, Chys M, et al. (2014) The present status of landfill leachate treatment and its development trend from a technological point of view. Reviews in Environmental Science and Bio/Technology 14: 93–122. [Google Scholar]
- Genethliou C, Triantaphyllidou IE, Giannakis D, et al. (2021) Simultaneous removal of ammonium nitrogen, dissolved chemical oxygen demand and color from sanitary landfill leachate using natural zeolite. Journal of Hazardous Materials 406: 124679. [DOI] [PubMed] [Google Scholar]
- Gomes HI. (2012) Phytoremediation for bioenergy: Challenges and opportunities. Environmental Technology Reviews 1: 59–66. [Google Scholar]
- Grossule V, Lavagnolo MC. (2020) Lab tests on semi-aerobic landfilling of MSW under varying conditions of water availability and putrescible waste content. Journal of Environmental Management 256: 109995. [DOI] [PubMed] [Google Scholar]
- Gu N, Liu J, Ye J, et al. (2019) Bioenergy, ammonia and humic substances recovery from municipal solid waste leachate: A review and process integration. Biomass and Biofuels Association 293: 122159. [DOI] [PubMed] [Google Scholar]
- He L, Chen H, Wu L, et al. (2021) Synergistic heat/UV activated persulfate for the treatment of nanofiltration concentrated leachate. Ecotoxicology and Environmental Safety 208: 111522. [DOI] [PubMed] [Google Scholar]
- He P, Chen L, Shao L, et al. (2019) Municipal solid waste (MSW)landfill: A source of microplastics? Evidence of microplastics in landfill leachate. Water Research 159: 38–45. [DOI] [PubMed] [Google Scholar]
- He R, Wei X-M, Tian B-H, et al. (2015) Characterization of a joint recirculation of concentrated leachate and leachate to landfills with a microaerobic bioreactor for leachate treatment. Waste Management 46: 380–388. [DOI] [PubMed] [Google Scholar]
- Hendrych J, Hejralová R, Kroužek J, et al. (2019) Stabilisation/solidification of landfill leachate concentrate and its residue obtained by partial evaporation. Waste Management 95: 560–568. [DOI] [PubMed] [Google Scholar]
- Henigin PLA. (1995) Recirculation of leachate concentrate from reverse osmosis treatment. In: Proceedings of the Fifth International Landfill Symposium, Sardinia 95. S. Margherita di Pula, Cagliari, Italy, pp. 2–6. [Google Scholar]
- Hong M, Lu G, Hou C, et al. (2017) Advanced treatment of landfill leachate membrane concentrates: Performance comparison, biosafety and toxic residue analysis. Water Science & Technology 76: 2949–2958. [DOI] [PubMed] [Google Scholar]
- Huang J, Chen J, Xie Z, et al. (2015) Treatment of nanofiltration concentrates of mature landfill leachate by a coupled process of coagulation and internal micro-electrolysis adding hydrogen peroxide. Environmental Technology (United Kingdom) 36: 1001–1007. [DOI] [PubMed] [Google Scholar]
- Huang S, Li L, Zhu N, et al. (2020) Removal and recovery of chloride ions in concentrated leachate by Bi(III) containing oxides quantum dots/two-dimensional flakes. Journal of Hazardous Materials 382: 121041. [DOI] [PubMed] [Google Scholar]
- Hunce SY, Akgul D, Demir G, et al. (2012) Solidification/stabilization of landfill leachate concentrate using different aggregate materials. Waste Management 32: 1394–1400. [DOI] [PubMed] [Google Scholar]
- Iskander SM, Novak JT, Brazil B, et al. (2017. a) Simultaneous energy generation and UV quencher removal from landfill leachate using a microbial fuel cell. Environmental Science and Pollution Research 24: 26040–26048. [DOI] [PubMed] [Google Scholar]
- Iskander SM, Novak JT, He Z. (2019) Reduction of reagent requirements and sludge generation in Fenton’s oxidation of landfill leachate by synergistically incorporating forward osmosis and humic acid recovery. Water Research 151, 310–317. 10.1016/j.watres.2018.11.089 [DOI] [PubMed] [Google Scholar]
- Iskander SM, Novak JT, He Z. (2018. a) Enhancing forward osmosis water recovery from landfill leachate by desalinating brine and recovering ammonia in a microbial desalination cell. Bioresource Technology 255: 76–82. [DOI] [PubMed] [Google Scholar]
- Iskander SM, Zhao R, Pathak A, et al. (2018. b) A review of landfill leachate induced ultraviolet quenching substances: Sources, characteristics, and treatment. Water Research 145, 297–311. [DOI] [PubMed] [Google Scholar]
- Iskander SM, Zou S, Brazil B. (2017. b) Energy consumption by forward osmosis treatment of landfill leachate for water recovery. Waste Management 63: 284–291. [DOI] [PubMed] [Google Scholar]
- ISWA (2019) Landfill Operational Guidelines. A Report from ISWA’s Working Group on Landfill 2019. Region: International. ISWA. [Google Scholar]
- Jamaly S, Darwish NN, Ahmed I, et al. (2014) A short review on reverse osmosis pretreatment technologies. Desalination 354: 30–38. [Google Scholar]
- Joo SH, Tansel B. (2015) Novel technologies for reverse osmosis concentrate treatment: A review. Journal of Environmental Management 150: 322–335. [DOI] [PubMed] [Google Scholar]
- Judd SJ. (2017) Membrane technology costs and me. Water Research 122: 1–9. [DOI] [PubMed] [Google Scholar]
- Kallel A, Ellouze M, Trabelsi I. (2017) Co-management of landfill leachate concentrate with brick waste by solidification/stabilization treatment. Arabian Journal of Geosciences 10: 81. [Google Scholar]
- Kamali M, Suhas DP, Costa ME, et al. (2019) Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chemical Engineering Journal 368: 474–494. [Google Scholar]
- Keyikoglu R, Karatas O, Rezania H, et al. (2021) A review on treatment of membrane concentrates generated from landfill leachate treatment processes. Separation and Purification Technology 259: 118182. [Google Scholar]
- Kjeldsen P, Barlaz MA, Rooker AP, et al. (2002) Present and long-term composition of MSW landfill leachate: A review. Critical Reviews in Environmental Science and Technology 32: 297–336. [Google Scholar]
- Kulikowska D, Klimiuk E. (2008) The effect of landfill age on municipal leachate composition. Bioresource Technology 99: 5981–5985. [DOI] [PubMed] [Google Scholar]
- Kumano A, Fujiwara N. (2008) Cellulose Triacetate Membranes for Reverse Osmosis. In: Li NN, Fane AG, Winston Ho WS, et al. (eds) Advanced Membrane Technology and Applications. Hoboken, New Jersey: John Wiley & Sons, Inc, pp.21–46. [Google Scholar]
- Kurniawan TA, Lo W, Chan G, et al. (2010) Biological processes for treatment of landfill leachate. Journal of Environmental Monitoring 12: 2032. [DOI] [PubMed] [Google Scholar]
- Kurniawan TA, Lo WH, Chan GYS. (2006) Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. Journal of Hazardous Materials 129: 80–100. [DOI] [PubMed] [Google Scholar]
- Kurniawan TA, Singh D, Avtar R, et al. (2021) Resource recovery from landfill leachate: An experimental investigation and perspectives. Chemosphere 274: 129986. [DOI] [PubMed] [Google Scholar]
- Labiadh L, Fernandes A, Ciríaco L, et al. (2016) Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. Journal of Environmental Management 181: 515–521. [DOI] [PubMed] [Google Scholar]
- Ladewig B, Asquith B. (2012) Desalination Concentrate Management, Emerging Compounds Removal from Wastewater, SpringerBriefs in Molecular Science. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Le Dirach J, Nisan S, Poletiko C. (2005) Extraction of strategic materials from the concentrated brine rejected by integrated nuclear desalination systems. Desalination 182: 449–460. [Google Scholar]
- Lebron YAR, Moreira VR, Brasil YL, et al. (2021) A survey on experiences in leachate treatment: Common practices, differences worldwide and future perspectives. Journal of Environmental Management 288: 112475. [DOI] [PubMed] [Google Scholar]
- Li B, Boiarkina I, Yu W, et al. (2019) Phosphorous recovery through struvite crystallization : Challenges for future design. Science of the Total Environment 648: 1244–1256. [DOI] [PubMed] [Google Scholar]
- Li F, Wichmann K, Heine W. (2009) Treatment of the methanogenic landfill leachate with thin open channel reverse osmosis membrane modules. Waste Management 29: 960–964. [DOI] [PubMed] [Google Scholar]
- Li J, Niu A, Lu C-J, et al. (2017) A novel forward osmosis system in landfill leachate treatment for removing polycyclic aromatic hydrocarbons and for direct fertigation. Chemosphere 168: 112–121. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao L, Qin L, et al. (2016) Removal of refractory organics in nanofiltration concentrates of municipal solid waste leachate treatment plants by combined Fenton oxidative-coagulation with photo – Fenton processes. Chemosphere 146: 442–449. [DOI] [PubMed] [Google Scholar]
- Li L, Shi W, Yu S. (2019) Research on forward osmosis membrane technology still needs improvement in water recovery and wastewater treatment. Water 12, 107. 10.3390/w12010107 [DOI] [Google Scholar]
- Li X, Zhu W, Wu Y, et al. (2015) Recovery of potassium from landfill leachate concentrates using a combination of cation-exchange membrane electrolysis and magnesium potassium phosphate crystallization. Separation and Purification Technology 144: 1–7. [Google Scholar]
- Lima LSMS, De Almeida R, Quintaes BR, et al. (2017) Evaluation of humic substances removal from leachates originating from solid waste landfills in Rio de Janeiro State, Brazil. Journal of Environmental Science and Health Part A 52: 1–9. [DOI] [PubMed] [Google Scholar]
- Linde K, Jönsson AS, Wimmerstedt R. (1995) Treatment of three types of landfill leachate with reverse osmosis. Desalination 101: 21–30. [Google Scholar]
- Lins CMMS, Alves MCM, Campos JC, et al. (2015) Removal of ammonia nitrogen from leachate of Muribeca municipal solid waste landfill, Pernambuco, Brazil, using natural zeolite as part of a biochemical system. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering 50: 980–988. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Q, Zhou X, et al. (2021) Evaporation efficiency and important microorganisms under different ventilation strategies of co-bioevaporation process. Environmental Technology & Innovation 21: 101374. [Google Scholar]
- Long Y, Xu J, Shen D, et al. (2017) Effective removal of contaminants in landfill leachate membrane concentrates by coagulation. Chemosphere 167: 512–519. [DOI] [PubMed] [Google Scholar]
- Luo H, Zeng Y, Cheng Y, et al. (2020) Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Science of the Total Environment 703: 135468. [DOI] [PubMed] [Google Scholar]
- Mariam T, Nghiem LD. (2010) Landfill leachate treatment using hybrid coagulation-nanofiltration processes. Desalination 250: 677–681. [Google Scholar]
- Mohammad AW, Teow YH, Ang WL, et al. (2015) Nanofiltration membranes review: Recent advances and future prospects. Desalination 356: 226–254. [Google Scholar]
- Mohammadesmaeili F, Badr MK, Abbaszadegan M, et al. (2010) Mineral recovery from inland reverse osmosis concentrate using isothermal evaporation. Water Research 44, 6021–6030. 10.1016/j.watres.2010.07.070 [DOI] [PubMed] [Google Scholar]
- Mojiri A, Zhou JL, Ratnaweera H, et al. (2021) Treatment of landfill leachate with different techniques: An overview. Journal of Water Reuse and Desalination 11: 66–96. [Google Scholar]
- Mojiri A, Ziyang L, Hui W, et al. (2017) Concentrated landfill leachate treatment with a combined system including electro-ozonation and composite adsorbent augmented sequencing batch reactor process. Process Safety and Environmental Protection 111: 253–262. 10.1016/j.psep.2017.07.013 [DOI] [Google Scholar]
- Morello L, Cossu R, Raga R, et al. (2016) Recirculation of reverse osmosis concentrate in lab-scale anaerobic and aerobic landfill simulation reactors. Waste Management 56: 262–270. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Mukhopadhyay S, Hashim MA, et al. (2015) Contemporary environmental issues of landfill leachate: Assessment and remedies. Critical Reviews in Environmental Science and Technology 45: 472–590. [Google Scholar]
- Panagopoulos A. (2021) Beneficiation of saline effluents from seawater desalination plants: Fostering the zero liquid discharge (ZLD) approach – A techno-economic evaluation. Journal of Environmental Chemical Engineering 9: 105338. [Google Scholar]
- Panagopoulos A, Haralambous K-J. (2020) Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery – Analysis, challenges and prospects. Journal of Environmental Chemical Engineering 8: 104418. [Google Scholar]
- Peter-Varbanets M, Zurbrügg C, Swartz C, et al. (2009) Decentralized systems for potable water and the potential of membrane technology. Water Research 43: 245–265. [DOI] [PubMed] [Google Scholar]
- van Praagh M, Hartman C, Brandmyr E, 2019. Microplastics in Landfill Leachates in the Nordic Countries, TemaNord NV – 2018:557, TemaNord. Nordic Council of Ministers, Copenhagen. 10.6027/TN2018-557 [DOI] [Google Scholar]
- Qin M, Molitor H, Brazil B, et al. (2016) Recovery of nitrogen and water from landfill leachate by a microbial electrolysis cell–forward osmosis system. Bioresource Technology 200: 485–492. 10.1016/j.biortech.2015.10.066 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Blaney L, Kao J, et al. (2015) Emerging contaminants in landfill leachate and their sustainable management. Environmental Earth Sciences 73: 1357–1368. [Google Scholar]
- Ramaswami S, Behrendt J, Otterpohl R. (2018) Comparison of NF-RO and RO-NF for the treatment of mature landfill leachates: A guide for landfill operators. Membranes (Basel). 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Song K, Chen W, et al. (2021) Treatment of membrane concentrated leachate by two-stage electrochemical process enhanced by ultraviolet radiation: Performance and mechanism. Separation and Purification Technology 259: 118032. [Google Scholar]
- Ren X, Song K, Xiao Y, et al. (2019) Constituent transformation mechanism of concentrated leachate after incineration at different temperatures. Environmental Science and Pollution Research 26: 34613–34621. [DOI] [PubMed] [Google Scholar]
- Renou S, Givaudan JG, Poulain S, et al. (2008) Landfill leachate treatment: Review and opportunity. Journal of Hazardous Materials 150: 468–493. [DOI] [PubMed] [Google Scholar]
- Robinson AH. (2005) Landfill leachate treatment. Membrane Technology 2005: 6–12. [Google Scholar]
- Robinson T. (2019) aerobic biological treatability studies on landfill leachate with nitrification and denitrification. Detritus 7: 1. [Google Scholar]
- Rukapan W, Khananthai B, Chiemchaisri C, et al. (2012) Short- and long-term fouling characteristics of reverse osmosis membrane at full scale leachate treatment plant. Water Science and Technology 65: 127–134. [DOI] [PubMed] [Google Scholar]
- Şchiopu AM, Piuleac GC, Cojocaru C, et al. (2012) Reducing environmental risk of landfills: Leachate treatment by reverse osmosis. Environmental Engineering and Management Journal 11: 2319–2331. [Google Scholar]
- Scantamburlo E. (2015) Reinjection of Leachate Reverse Osmosis Concentrate in the Landfill Body. University of Padova. [Google Scholar]
- Shaffer DL, Werber JR, Jaramillo H, et al. (2015) Forward osmosis: Where are we now? Desalination 356: 271–284. [Google Scholar]
- Shah TM, Ramaswami S, Behrendt J, et al. (2017) Simultaneous removal of organics and ammonium-nitrogen from reverse osmosis concentrate of mature landfill leachate. Journal of Water Process Engineering 19: 126–132. [Google Scholar]
- Shao L, Deng Y, Qiu J, et al. (2021) DOM chemodiversity pierced performance of each tandem unit along a full-scale “MBR+NF” process for mature landfill leachate treatment. Water Research 195: 117000. [DOI] [PubMed] [Google Scholar]
- Singh SK, Sharma C, Maiti A. (2021) A comprehensive review of standalone and hybrid forward osmosis for water treatment: Membranes and recovery strategies of draw solutions. Journal of Environmental Chemical Engineering 9: 105473. [Google Scholar]
- Šír M, Podhola M, Patočka T, et al. (2012) The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. Journal of Hazardous Materials 207–208: 86–90. [DOI] [PubMed] [Google Scholar]
- Smol M, Włodarczyk-Makuła M. (2017) Effectiveness in the removal of organic compounds from municipal landfill leachate in integrated membrane systems: Coagulation–NF/RO. Polycyclic Aromatic Compounds 37: 456–474. [Google Scholar]
- Sohoo I, Ritzkowski M, Kuchta K. (2019) Evaluation of behavior of waste disposal sites in Karachi, Pakistan and effects of enhanced leaching on their emission potential. Detritus 7: 1. [Google Scholar]
- Soomro GS, Qu C, Ren N, et al. (2020) Efficient removal of refractory organics in landfill leachate concentrates by electrocoagulation in tandem with simultaneous electro-oxidation and in-situ peroxone. Environmental Research 183: 109249. [DOI] [PubMed] [Google Scholar]
- Rio de Janeiro State Governor (2020) Law number #9055. Mandatory control and treatment of leachate in solid waste disposal systems, dumps, controlled landfills and sanitary landfills, as well as the remediation of dumps in the State of Rio de Janeiro and other measures. Diário Oficial do Estado do Rio de Janeiro (DOERJ), Brazil, 8th October 2020. Available at: https://leisestaduais.com.br/rj/lei-ordinaria-n-9055-2020-rio-de-janeiro-institui-a-obrigatoriedade-do-controle-e-tratamento-do-chorume-nos-sistemas-de-destinacao-final-de-residuos-solidos-vazadouros-aterros-controlados-e-aterros-sanitarios-bem-como-a-remediacao-de-vazadouros-no-estado-do-rio-de-janeiro-e-da-outras-providencias [In Portuguese] (accessed April 2022). [Google Scholar]
- Stegmann R. (2018) Strategic Issues in Leachate Management, Solid Waste Landfilling. Amsterdam, Netherlands: Elsevier Inc, pp. 501–509. [Google Scholar]
- Su Y, Zhang Z, Wu D, et al. (2019) Occurrence of microplastics in landfill systems and their fate with landfill age. Water Research 164: 114968. [DOI] [PubMed] [Google Scholar]
- Subramani A, Jacangelo JG. (2014) Treatment technologies for reverse osmosis concentrate volume minimization: A review. Separation and Purification Technology 122: 472–489. [Google Scholar]
- Talalaj IA. (2015. a) Mineral and organic compounds in leachate from landfill with concentrate recirculation. Environmental Science and Pollution Research 22: 2622–2633. [DOI] [PubMed] [Google Scholar]
- Talalaj IA. (2015. b) Removal of nitrogen compounds from landfill leachate using reverse osmosis with leachate stabilization in a buffer tank. Environmental Technology 36: 1091–1097. [DOI] [PubMed] [Google Scholar]
- Talalaj IA, Biedka P. (2015) Impact of concentrated leachate recirculation on effectiveness of leachate treatment by reverse osmosis. Ecological Engineering 85: 185–192. [Google Scholar]
- Theepharaksapan S, Chiemchaisri C, Chiemchaisri W, et al. (2011) Removal of pollutants and reduction of bio-toxicity in a full scale chemical coagulation and reverse osmosis leachate treatment system. Bioresource Technology 102: 5381–5388. [DOI] [PubMed] [Google Scholar]
- Top S, Sekman E, Hoşver S, et al. (2011) Characterization and electrocaogulative treatment of nanofiltration concentrate of a full-scale landfill leachate treatment plant. Desalination 268: 158–162. [Google Scholar]
- Torretta V, Ferronato N, Katsoyiannis I, et al. (2016) Novel and conventional technologies for landfill leachates treatment: A review. Sustainability 9: 9. [Google Scholar]
- Tow EW, Ersan MS, Kum S, et al. (2021) Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Science 3: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Tudor T, Vinti G. (2019) Characteristics of leachate from landfills and dumpsites in Asia, Africa and Latin America: An overview. Waste Management 95: 416–431. [DOI] [PubMed] [Google Scholar]
- Van Der Bruggen B, Lejon L, Vandecasteele C. (2003) Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes. Environmental Science and Technology 37: 3733–3738. [DOI] [PubMed] [Google Scholar]
- Varank G, Yazici Guvenc S, Dincer K, et al. (2020) Concentrated leachate treatment by electro-fenton and electro-persulfate processes using central composite design. International Journal of Environmental Research 14: 439–461. [Google Scholar]
- Viegas C, Nobre C, Mota A, et al. (2021) A circular approach for landfill leachate treatment: Chemical precipitation with biomass ash followed by bioremediation through microalgae. Journal of Environmental Chemical Engineering 9: 105187. [Google Scholar]
- Voutchkov N, Kaiser GN. (2020). Zero-liquid discharge concentrate disposal systems, In: Management of Concentrate from Desalination Plants. Amsterdam, Netherlands: Elsevier, pp. 187–208. [Google Scholar]
- Wang D, Liu D, Tao L, et al. (2017) The impact on the effects of leachate concentrates recirculation for different fill age waste. Journal of Material Cycles and Waste Management 19: 1211–1219. [Google Scholar]
- Wang H, Wang Y, Li X, et al. (2016) Removal of humic substances from reverse osmosis (RO) and nanofiltration (NF) concentrated leachate using continuously ozone generation-reaction treatment equipment. Waste Management 56,: 271–279. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li X, Zhen L, et al. (2012) Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. Journal of Hazardous Materials 229–230: 115–121. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou C, Meng G, et al. (2020) Treatment of landfill leachate membrane filtration concentrate by synergistic effect of electrocatalysis and electro-Fenton. Journal of Water Process Engineering 37: 101458. [Google Scholar]
- Wijekoon P, Wickramasinghe C, Athapattu BCL, et al. (2021) Biomass valorization and phytoremediation as integrated Technology for Municipal Solid Waste Management for developing economic context. Biomass Conversion and Biorefinery 11: 363–382. [Google Scholar]
- Wilf M. (2014) The Reverse Osmosis process. In: Kucera J. (ed.) Desalination. Hoboken, NJ: John Wiley & Sons, Inc., pp.155–204. [Google Scholar]
- Wiszniowski J, Robert D, Surmacz-Gorska J, et al. (2006) Landfill leachate treatment methods: A review. Environmental Chemistry Letters 4: 51–61. [Google Scholar]
- Woo H, Yang HS, Timmes TC, et al. (2019) Treatment of reverse osmosis concentrate using an algal-based MBR combined with ozone pretreatment. Water Research 159: 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Li Q. (2021) Characteristics of organic matter removed from highly saline mature landfill leachate by an emergency disk tube-reverse osmosis treatment system. Chemosphere 263: 128347. [DOI] [PubMed] [Google Scholar]
- Wu S, Zou S, Liang G, et al. (2018) Enhancing recovery of magnesium as struvite from landfill leachate by pretreatment of calcium with simultaneous reduction of liquid volume via forward osmosis. Science of the Total Environment 610–611: 137–146. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou S, Qin F, et al. (2010) Removal of humic substances from landfill leachate by Fenton oxidation and coagulation. Process Safety and Environmental Protection 88: 276–284. [Google Scholar]
- Xiang S, Liu Y, Zhang G, et al. (2020) New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World Journal of Microbiology and Biotechnology 36: 144. [DOI] [PubMed] [Google Scholar]
- Xie M, Shon HK, Gray SR, et al. (2016) Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Research 89: 210–221. [DOI] [PubMed] [Google Scholar]
- Xiong C, Li G, Zhang Z, et al. (2014) Technique for advanced electrochemical oxidation treatment of nanofiltration concentrate of landfill leachate. Wuhan University Journal of Natural Sciences 19: 355–360. [Google Scholar]
- Xu Y, Chen C, Li X, et al. (2017) Recovery of humic substances from leachate nanofiltration concentrate by a two-stage process of tight ultrafiltration membrane. Journal of Cleaner Production 161: 84–94. [Google Scholar]
- Xue W-J, Cui Y-H, Liu Z-Q, et al. (2020) Treatment of landfill leachate nanofiltration concentrate after ultrafiltration by electrochemically assisted heat activation of peroxydisulfate. Separation and Purification Technology 231: 115928. [Google Scholar]
- Yaman C, Ozcan HK, Demir G, et al. (2012) Landfill leachate treatment: A case study for Istanbul City. Clean Soil, Air, Water 40: 706–711. [Google Scholar]
- Yang B-Q, Yang J-M, Yang H, et al. (2018) Co-bioevaporation treatment of concentrated landfill leachate with addition of food waste. Biochemical Engineering Journal 130: 76–82. [Google Scholar]
- Yang J, Monnot M, Ercolei L, et al. (2020) Membrane-based processes used in municipal wastewater treatment for water reuse: State-of-the-art and performance analysis. Membranes (Basel) 10: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li H. (2016) Recovering humic substances from the dewatering effluent of thermally treated sludge and its performance as an organic fertilizer. Frontiers of Environmental Science & Engineering 10: 578–584. [Google Scholar]
- Yazici Guvenc S, Varank G. (2021) Degradation of refractory organics in concentrated leachate by the Fenton process: Central composite design for process optimization. Frontiers of Environmental Science & Engineering 15: 2. [Google Scholar]
- Ye W, Liu H, Jiang M, et al. (2019) Sustainable management of landfill leachate concentrate through recovering humic substance as liquid fertilizer by loose nanofiltration. Water Research 157: 555–563. [DOI] [PubMed] [Google Scholar]
- Ye W, Liu R, Lin F, et al. (2020). Elevated nanofiltration performance via mussel-inspired co-deposition for sustainable resource extraction from landfill leachate concentrate. Chemical Engineering Journal 388: 124200. [Google Scholar]
- Yue D, Han B, Qi G, et al. (2011) Recovery of humic substances from landfill leachate via 2500 Da ultrafiltration membrane. In: Brebbia CA, Popov V. (eds). WIT Transactions on Ecology and the Environment. Ashurst, UK: IT Press, pp. 737–746. [Google Scholar]
- Yue D, Xu Y, Mahar RB, et al. (2007) Laboratory-scale experiments applied to the design of a two-stage submerged combustion evaporation system. Waste Management 27: 704–710. [DOI] [PubMed] [Google Scholar]
- Zanon T, Ferrari A, Lima L, et al. (2013) Implantation of Leachate treatment by reverse Osmosis and the influence of controlled infiltration of retentate on biogas generation in a sanitary landfill – A Case Study. In: Proceedings Sardinia 2013, Fourteenth International Waste Management and Landfill Symposium. S. Margherita di Pula, Cagliari, Italy, 30 September–October. CISA Publisher. [Google Scholar]
- Zhang L, Lavagnolo MC, Bai H, et al. (2019). Environmental and economic assessment of leachate concentrate treatment technologies using analytic hierarchy process. Resources, Conservation and Recycling 141: 474–480. [Google Scholar]
- Zhang L, Wang X, Yue D. (2020) Effect of submerged combustion evaporation on Cd complexation potential of organic matter in municipal solid waste landfill leachate. Environmental Pollution 267: 115573. [DOI] [PubMed] [Google Scholar]
- Zhang Q-Q, Tian B-H, Zhang X, et al. (2013) Investigation on characteristics of leachate and concentrated leachate in three landfill leachate treatment plants. Waste Management 33: 2277–2286. [DOI] [PubMed] [Google Scholar]
- Zico MM, Ricci BC, Reis BG, et al. (2021) Sustainable ammonia resource recovery from landfill leachate by solar-driven modified direct contact membrane distillation. Separation and Purification Technology 264: 118356. [Google Scholar]







