Abstract
Study Design
Systematic review.
Objectives
Understanding the prevalence and outcome of motor deficits in degenerative cervical radiculopathy is important to guide management. We compared motor radiculopathy outcomes after conservative and surgical management, a particular focus being painful vs painless radiculopathy.
Methods
MEDLINE and EMBASE databases were searched. We stratified each study cohort into 1 of 6 groups, I–VI, based on whether radiculopathy was painful, painless or unspecified, and whether interventions were surgical or non-surgical.
Results
Of 10 514 initial studies, 44 matched the selection criteria. Whilst 42 (95.5%) provided baseline motor radiculopathy data, only 22 (50.0%) provided follow-up motor outcomes. Mean baseline prevalence of motor deficits was 39.1% (9.2%–73.3%) in conservative cohorts and 60.5% (18.5%–94.1%) in surgical cohorts. Group VI, ‘surgically-managed motor radiculopathy with unclear pain status’ had the largest number of cohorts. Conversely, no cohorts were found in Group III, ‘conservatively-managed painless motor radiculopathy’. Large disparities in data quality made direct comparison of conservative vs operative management difficult.
Conclusions
Overall pre-intervention prevalence of motor deficits in degenerative cervical radiculopathy is 56.4%. Many studies fail to report motor outcomes after intervention, meaning statistical evidence to guide optimal management of motor radiculopathy is currently lacking. Our study highlights the need for more evidence, preferably from a prospective long-term study, to allow direct comparisons of motor outcomes after conservative and surgical management.
Keywords: degenerative disc disease, degenerative cervical radiculopathy, motor radiculopathy, motor outcomes
Introduction
Degenerative cervical radiculopathy is a common condition caused by compression of nerve roots in the cervical neural foramina. This may present as neck or arm pain, sensory deficits and/or motor deficits in corresponding myotomes. Degenerative radiculopathy may be secondary to disc herniation, osteophyte formation or spondylotic deformity occurring in the ageing spine. Initial management is usually conservative: analgesia, physiotherapy or corticosteroid injections. Improvement of arm pain occurs in up to 94% of cases,1,2 but surgery might be suggested if non-operative management fails. However, outcomes for motor radiculopathy after surgery are unclear.
It is a commonly held belief that a key indication for surgical management of radiculopathy is continuing or progressing motor deficit, which can result in significant disability. However, surgery is offered with the assumption that surgery is more effective at improving motor function compared to continued conservative management. Moreover, the risk of potential complications arising from surgery must be borne in mind. We therefore need a clearer understanding of evolving motor deficits in the clinical timeline of conservatively-managed radiculopathy, and of how surgery might influence this timeline.
We undertook a systematic review to ascertain the prevalence of motor deficits in degenerative radiculopathy and to investigate reported motor outcomes of conservative vs surgical management.
Methods
Search strategy and study selection
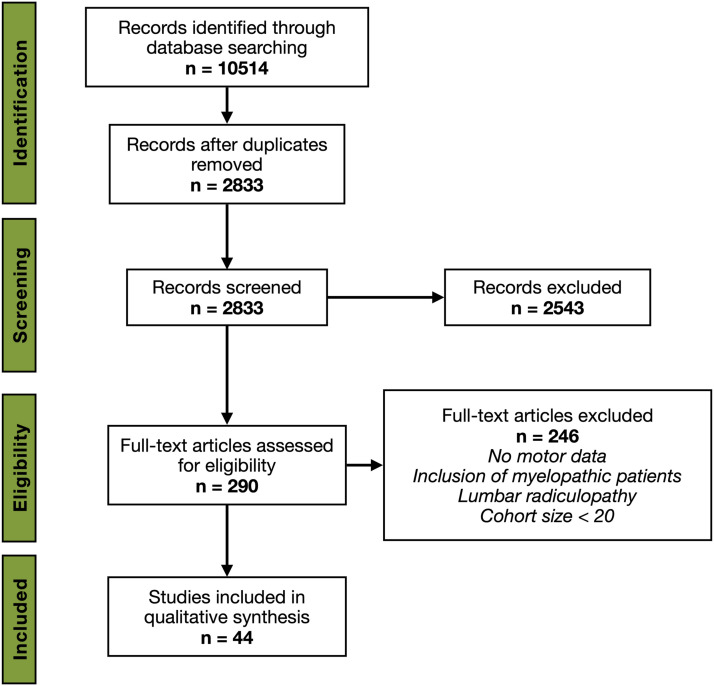
We conformed with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines 3 (Figure 1). The literature search was carried out by the junior authors (A.G. and M.E.), under supervision of the senior author (D.C.), using MEDLINE and EMBASE databases. An initial search strategy using the following terms was carried out up to January 2021: cervical radiculopathy; motor; painless; surgery; cervical radiculopathy AND surgery; cervical radiculopathy AND physiotherapy; cervical radiculopathy AND conservative; cervical radiculopathy AND natural history.
Figure 1.
PRISMA literature search strategy.
Eligibility criteria
The following inclusion criteria were stipulated: 1) human studies; 2) adult population; 3) degenerative aetiology for radiculopathy; 4) sample size > 20; and 5) clear motor data at baseline or post-intervention. Exclusion criteria were as follows: 1) animal or in-vitro studies; 2) paediatric population; 3) traumatic radiculopathy series; 4) radiculopathy secondary to malignancy or aneurysm; 5) myelopathy or spinal canal stenosis; 6) sample size < 20; 7) case reports; 8) studies not published in English; and 9) studies without clear motor data.
Risk of bias in individual studies
All included studies were assessed for quality of evidence using the National Institutes of Health (NIH) tools: ‘Quality Assessment of Controlled Intervention Studies’ for randomised controlled trials (RCTs) and ‘Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies’ for non-randomised cohorts. 4
Data collection process
During full text analysis, a spreadsheet with the following headings was populated: 1) first author and publication year; 2) study design; 3) inclusion/exclusion criteria for patient cohort; 4) cohort size; 5) intervention class; 6) baseline motor data; 7) post-intervention motor data; 8) other outcomes assessed (eg pain, sensory deficits).
Results
Search results
At initial screening, 10 514 citations were identified, from which 290 were selected for full-text analysis via title and abstract review. A total of 44 individual articles were included for data extraction (Table 1).
Table 1.
Characteristics of 44 studies included in final analysis.
| Reference | Study design | Intervention class | Intervention(s) | Motor data format |
|---|---|---|---|---|
| MacDowall et al, 2020 5 | Prospective cohort | Surgical | ACDF vs PCF a | Binary |
| Bacigaluppi et al, 2019 6 | Retrospective cohort | Surgical | open posterior decompression | Binary & quantified |
| MacDowall et al, 2019 7 | Retrospective cohort | Surgical | ACDF vs ACDR | Binary |
| Akkan & Gelecek, 2018 8 | RCT | Conservative | neck stabilisation exercises vs standard physiotherapy | Quantified |
| Siller et al, 2018 9 | Retrospective cohort | Surgical | ACDF vs open PCF | Binary & quantified |
| Lee et al, 2018 10 | Retrospective cohort | Surgical | endoscopic PCF + discectomy | Binary |
| Scholz et al, 2018 11 | Retrospective cohort | Surgical | ACDF vs MI PCF | Binary |
| Peto et al, 2017 12 | Retrospective cohort | Surgical | PCF (MI) | Binary |
| Kim et al, 2017 13 | Retrospective cohort | Surgical | ACDR vs MI PCF | Binary |
| ElAbed et al, 2016 14 | Retrospective cohort | Surgical | ACDF | Binary |
| Shiban et al, 2016 15 | Retrospective cohort | Surgical | ACDF | Binary |
| Xiao et al, 2015 16 | Retrospective cohort | Conservative | nerve root block + pulsed radiofrequency | Binary |
| Engquist et al, 2015 17 | RCT | Surgical vs conservative | ACDF vs physiotherapy | Binary |
| Lehmann et al, 2014 18 | Retrospective cohort | Surgical | ACDF | Binary |
| Kang et al, 2014 19 | Retrospective cohort | Surgical | open PCF | Binary |
| Church et al, 2014 20 | Retrospective cohort | Surgical | laminoforaminotomy | Binary |
| Park et al, 2013 21 | Retrospective cohort | Surgical | anterior foraminotomy | Binary |
| Peolsson et al, 2013 22 | RCT | Surgical vs conservative | ACDF vs physiotherapy | Quantified |
| Lee et al, 2012 23 | Prospective cohort | Conservative | epidural steroid injections | Binary |
| Lidar & Salame, 2011 24 | Retrospective cohort | Surgical | PCF (MI) + discectomy | Binary |
| Konstantinovic et al, 2010 25 | RCT | Conservative | laser therapy vs placebo | Binary |
| Kuijper et al, 2009 26 | RCT | Conservative | cervical collar vs physiotherapy vs ‘watch-and-wait’ | Binary |
| Kim & Kim, 2009 27 | RCT | Surgical | open vs MI PCF/discectomy | Binary |
| Balasubramanian et al, 2008 28 | Retrospective cohort | Surgical | anterior foraminotomy | Binary |
| Cornelius et al, 2007 29 | Retrospective cohort | Surgical | anterolateral foraminotomy | Binary |
| Xie & Hurlbert, 2007 30 | RCT | Surgical | ACD vs non-plated ACDF vs plated ACDF | Binary |
| Ruetten et al, 2007 31 | Prospective cohort | Surgical | PCF (MI) | Binary |
| Anderberg et al, 2007 32 | RCT | Conservative | transforaminal injections — steroid vs control | Binary |
| Lin et al, 2006 33 | Retrospective cohort | Conservative | epidural steroid injections | Binary |
| Korinth et al, 2006 34 | Retrospective cohort | Surgical | ACD vs open PCF | Binary |
| Aydin et al, 2005 35 | Retrospective cohort | Surgical | anterior contralateral discectomy | Binary |
| Joghataei et al, 2004 36 | RCT | Conservative | cervical traction vs electrotherapy/exercise | Quantified |
| Jho et al, 2002 37 | Retrospective cohort | Surgical | anterior foraminotomy | Binary |
| Rodrigues et al, 2001 38 | Retrospective cohort | Surgical | posterior approaches | Binary |
| Knight et al, 2001 39 | Prospective cohort | Surgical | percutaneous laser | Binary |
| Adamson, 2001 40 | Prospective cohort | Surgical | laminoforaminotomy (MI) | Binary |
| Hamburger et al, 2001 41 | Retrospective cohort | Surgical | ACDF | Binary |
| Heckmann et al, 1999 2 | Retrospective cohort | Surgical vs conservative | ACD vs conservative | Binary |
| Swezey, 1999 42 | Retrospective cohort | Conservative | conservative | Binary |
| Schneeberger et al, 1999 43 | Retrospective cohort | Surgical | ACDF | Binary |
| Persson et al, 1997 44 | RCT | Surgical vs conservative | ACDF/foraminotomy/ laminectomy vs physiotherapy vs cervical collar | Quantified |
| Bush & Hillier, 1996 45 | Prospective cohort | Conservative | steroid injections | Binary & quantified |
| Davis, 1996 46 | Retrospective cohort | Surgical | open posterior decompression | Binary |
| Bertalanffy & Eggert, 1988 47 | Retrospective cohort | Surgical | ACD | Binary |
Abbreviations: ACD, anterior cervical discectomy; ACDF, anterior cervical discectomy and fusion; ACDR, anterior cervical disc replacement; MI, minimally-invasive; PCF, posterior cervical foraminotomy; RCT, randomised controlled trial.
aPCFs in this study were mixed, including open, endoscopic and minimally-invasive (MI) techniques
The NIH quality of evidence assessments for 10 RCTs8,17,22,25–27,30,32,36,44 are summarised in Table 2, whilst those for 6 prospective5,23,31,39,40,45 and 28 retrospective2,6,7,9–16,18–21,24,28,29,33–35,37,38,41–43,46,47 studies can be found in Table 3.
Table 2.
Quality of evidence assessment for 10 included randomised studies.
| Reference | 1. Study clearly described as randomised? | 2. Adequate randomisation method? | 3. Concealed allocation? | 4. Participants & providers blinded? | 5. Outcome assessors blinded? | 6. Similar groups at baseline? | 7. Overall drop-out ≤20%? | 8. Differential drop-out ≤15% between groups? | 9. High adherence to protocols? | 10. Similar background interventions between groups? | 11. Outcomes assessed using valid & reliable measures? | 12. Sample size sufficiently large to detect difference with ≥80% power? | 13. Prespecified outcomes? | 14. Intention-to-treat analysis? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akkan & Gelecek, 2018 8 | Y | Y | NR | NR | NR | Y | N | N | N | Y | Y | NR | Y | N |
| Engquist et al, 2015 17 | Y | Y | N | N | NR | Y | Y | Y | Y | Y | Y | NR | Y | Y |
| Peolsson et al, 2013 22 | Y | Y | N | N | NR | Y | Y | Y | Y | Y | Y | N | Y | N |
| Konstantinovic et al, 2010 25 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y |
| Kuijper et al, 2009 26 | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | Y | NR |
| Kim & Kim, 2009 27 | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | NR | Y | NA |
| Xie & Hurlbert, 2007 30 | Y | Y | NR | NR | NR | Y | Y | N | Y | Y | Y | Y | Y | NA |
| Anderberg et al, 2007 32 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | NR | Y | NA |
| Joghataei et al, 2004 36 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | NR | Y | NA |
| Persson et al, 1997 44 | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | NR | Y | Y |
Abbreviations: N, no; NA, not applicable; NR, not reported; Y, yes.
Using National Institutes for Health (NIH) ‘Quality Assessment of Controlled Intervention Studies’.
Table 3.
Quality of evidence assessment for 34 included non-randomised studies.
| Reference | 1. Clearly-stated research question or objective? | 2. Study population clearly defined? | 3. ≥50% participation rate of eligible persons? | 4. Subjects recruited from similar populations with uniform inclusion & exclusion criteria? | 5. Sample size justification, or power calculation provided? | 6. Exposures of interest measured prior to outcomes being measured? | 7. Sufficient timeframe to see association between exposure & outcome? |
8. Outcomes compared amongst different levels of exposure? | 9. Exposures clearly defined, valid, reliable & implemented consistently across all study participants? | 10. Exposures assessed at more than 1 time point? | 11. Dependent variables clearly defined, valid, reliable & implemented consistently across all study participants? | 12. Outcome assessors blinded to exposure status of participants? | 13. ≤20% loss to follow-up? | 14. Key potential confounding variables measured & adjusted statistically? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MacDowall et al, 2020 5 | Y | Y | Y | Y | N | Y | Y | NA | Y | Y | Y | N | N | Y |
| Bacigaluppi et al, 2019 6 | Y | Y | Y | NA | N | Y | N | NA | Y | N | NA | NA | Y | NA |
| MacDowall et al, 2019 7 | Y | Y | Y | Y | N | Y | Y | NA | Y | Y | Y | N | N | Y |
| Siller et al, 2018 9 | Y | Y | Y | Y | N | Y | Y | NA | Y | Y | Y | N | N | N |
| Lee et al, 2018 10 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Scholz et al, 2018 11 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | N |
| Peto et al, 2017 12 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | N | NA |
| Kim et al, 2017 13 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | N |
| ElAbed et al, 2016 14 | Y | Y | Y | NA | N | Y | Y | Y | Y | Y | N | NA | Y | NA |
| Shiban et al, 2016 15 | Y | Y | Y | Y | N | Y | Y | Y | Y | N | NA | NA | Y | NA |
| Xiao et al, 2015 16 | Y | Y | Y | NA | N | Y | Y | N | Y | Y | Y | NA | Y | NA |
| Lehmann et al, 2014 18 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | N |
| Kang et al, 2014 19 | Y | Y | Y | N | N | Y | Y | N | Y | N | Y | N | N | N |
| Church et al, 2014 20 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Park et al, 2013 21 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Lee et al, 2012 23 | Y | Y | Y | Y | N | N | Y | Y | N | N | N | NA | Y | N |
| Lidar & Salame, 2011 24 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | Y | NA | Y | NA |
| Balasubramanian et al, 2008 28 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Cornelius et al, 2007 29 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Ruetten et al, 2007 31 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Lin et al, 2006 33 | Y | Y | Y | NA | N | Y | Y | N | Y | N | NA | NA | Y | NA |
| Korinth et al, 2006 34 | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | N | Y | N |
| Aydin et al, 2005 35 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Jho et al, 2002 37 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Rodrigues et al, 2001 38 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Knight et al, 2001 39 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Adamson, 2001 40 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | Y | NA |
| Hamburger et al, 2001 41 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | N | NA |
| Heckmann et al, 1999 2 | Y | Y | Y | Y | N | Y | Y | NA | N | N | Y | N | N | N |
| Swezey, 1999 42 | Y | Y | Y | NA | N | Y | Y | NA | N | Y | NA | NA | Y | NA |
| Schneeberger et al, 1999 43 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Bush & Hillier, 1996 45 | Y | Y | Y | NA | N | Y | Y | NA | N | N | NA | NA | Y | NA |
| Davis, 1996 46 | Y | Y | Y | NA | N | Y | Y | NA | Y | N | NA | NA | Y | NA |
| Bertalanffy & Eggert, 1988 47 | Y | Y | Y | NA | N | Y | Y | NA | Y | Y | NA | NA | N | NA |
Abbreviations: N, no; NA, not applicable; Y, yes.
Using National Institutes for Health (NIH) ‘Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies’.
Study characteristics
The breakdown of intervention class in included studies was as follows: 10 (22.7%) conservative management only; 30 (68.2%) surgical management only; 4 (9.1%) comparing conservative and surgical management.
37 studies reported motor data in binary form (eg motor deficit ‘present’/‘absent’, or motor outcome ‘improved’/‘unchanged’: see Table 4). Four studies documented motor strength using quantified measures such as Medical Research Council (MRC) motor grading or grip strength in kilograms (Table 5). Three studies provided data in both binary and quantified forms.
Table 4.
Motor data from 40 studies reporting binary data.
| Reference | Cohort size | Baseline motor data | Motor data at latest follow-up | Additional motor data | Motor deterioration or complications | |||
|---|---|---|---|---|---|---|---|---|
| Motor deficit | Painless motor deficit | Motor deficit | Motor improvement | Average time | ||||
| MacDowall et al, 2020 5 | 4368 | 68.1% | — | No motor follow-up | Baseline motor deficit 69.01% in ACDF; 63.06% in PCF | — | ||
| Bacigaluppi et al, 2019 6 | 75 | 81.3% | — | 8.0% | 100% | 83.5 months | 50.7% MRC=4; 30.7% MRC < 4; 18.7% no weakness | None |
| MacDowall et al, 2019 7 | 3998 | 68.4% | — | No motor follow-up | Baseline motor deficit in ACDF 69.06%; 56.86% in ACDR | — | ||
| Siller et al, 2018 9 | 31 | All painless: see right | 3.93% a | — | 65.2% | 3.9 years | Baseline painless motor deficit 2.81% in ACDF; 5.88% in PCF | 2 pts (8.7%) |
| Lee et al, 2018 10 | 106 | 71.70% | — | 14.47% | 94.7% | 22.4 months | 49% MRC=4; 23% MRC < 4; 27% no weakness | Transient in 3 pts (2.8%) |
| Scholz et al, 2018 11 | 107 | 73.8% | — | — | 75.7% | 42 months | Baseline motor deficit 60.00% in ACDF; 82.09% in PCF | 1 pt (.9%) — new motor deficit at adjacent level in PCF group |
| Peto et al, 2017 12 | 34 | 58.8% | 11.76% | 25.0% | 80.0% | 30.4 months | Baseline motor deficit with radicular pain in 47.06% | None |
| Kim et al, 2017 13 | 35 | 57.1% | — | No motor follow-up | Baseline motor deficit 52.94% in ACDR; 61.11% in PCF | None | ||
| ElAbed et al, 2016 14 | 90 | 50.0% | — | 15.0% | 85.0% | 4.5 years | — | Transient in 1 pt (1.1%) |
| Shiban et al, 2016 15 | 133 | 61.7% | — | — | 84.2% | 21 months | — | 2 pts (1.5%) |
| Xiao et al, 2015 16 | 42 | 19.1% | — | No motor follow-up | — | None | ||
| Engquist et al, 2015 17 | 63 | 45.0% | — | No motor follow-up | — | — | ||
| Lehmann et al, 2014 18 | 118 | 55.1% | — | 16.9% | — | 3.8 years | — | 6 pts (5.1%) |
| Kang et al, 2014 19 | 135 | 18.5% | — | No motor follow-up | — | — | ||
| Church et al, 2014 20 | 319 | 78.1% b | — | 27.9% b | — | 10 years | ‘Subjective weakness’ in 53.5%; ‘objective weakness’ in 43.0% | 3 pts (.3%) |
| Park et al, 2013 21 | 50 | 36.0% | — | No motor follow-up | — | None | ||
| Lee et al, 2012 23 | 98 | 9.2% | — | No motor follow-up | — | None | ||
| Lidar & Salame, 2011 24 | 32 | — | — | — | 100% | 39mths | — | None |
| Konstantinovic et al, 2010 25 | 60 | 73.3% | — | No motor follow-up | — | None | ||
| Kuijper et al, 2009 26 | 205 | — | — | No motor follow-up | — | — | ||
| Kim & Kim, 2009 27 | 41 | 53.7% | — | No motor follow-up | — | None | ||
| Balasubramanian et al, 2008 28 | 34 | 38.2% | — | No motor follow-up | — | None | ||
| Cornelius et al, 2007 29 | 40 | 45.0% | 5.6% | 7.5% | 83.3% | 4.3 years | — | Transient in 1 pt (2.5%) — C5 palsy |
| Xie & Hurlbert, 2007 30 | 42 | 33.3% | — | No motor follow-up | Baseline motor deficit 25.0% in ACD; 26.7% in unplated ACDF; 46.7% in plated ACDF | None | ||
| Ruetten et al, 2007 31 | 100 | 43.0% | — | No motor follow-up | — | None | ||
| Anderberg et al, 2007 32 | 40 | — | — | — | 10.0% | 3 weeks | Follow-up motor status unchanged in 90.0% | None |
| Lin et al, 2006 33 | 70 | 47.1% | — | No motor follow-up | — | None | ||
| Korinth et al, 2006 34 | 292 | 67.8% | 8.2% | No motor follow-up | Baseline motor deficit 64.5% in ACD; 70.2% in PCF | Transient in 2 pts (.7%) — 1 paraparesis & 1 hemiparesis | ||
| Baseline painless motor deficit 5.7% in ACDF; 10.1% in PCF | ||||||||
| Aydin et al, 2005 35 | 182 | 80.2% | — | 7.1% | — | 18 months | — | None |
| Jho et al, 2002 37 | 104 | 61.5% | — | No motor follow-up | — | Transient in 1 pt (1.0%) —hemiparesis | ||
| Rodrigues et al, 2001 38 | 51 | 90.2% | — | 6.5% | 93.5% | 46 months | — | None |
| Knight et al, 2001 39 | 105 | 31.4% | — | 7.2% | — | 43 months | — | 2 pts (1.9%) — 1 transient, 1 persistent |
| Adamson, 2001 40 | 100 | 76.0% | — | No motor follow-up | — | None | ||
| Hamburger et al, 2001 41 | 249 | 85.1% | 1.2% | No motor follow-up | Painful motor deficit in 10.0%; painful motor and sensory deficit in 73.9% | 2 pts (.8%) — 1 transient hemiparesis, 1 persistent tetraparesis | ||
| Heckmann et al, 1999 2 | 60 | 51.7% | — | — | 74.2% | 5.5 years | Baseline motor deficit 43.6% in conservative; 66.7% in surgical | None in conservative; 1 pt (5.0%) in surgical |
| Swezey, 1999 42 | 83 | 42.2% | 6.0% | No motor follow-up | — | — | ||
| Schneeberger et al, 1999 43 | 35 | 31.4% | — | — | 100% | 54 months | — | None |
| Bush & Hillier, 1996 45 | 68 | 75.0% | — | — | 90.2% | 39 months | No change in 9.8% | None |
| Davis, 1996 46 | 170 | 94.1% | — | No motor follow-up | — | Transient in 2 pts (1.2%) — 1 axillary nerve injury, 1 C5 palsy | ||
| Bertalanffy & Eggert, 1988 47 | 146 | 77% | — | No motor follow-up | — | Transient in 1 pt (.7%) — paraparesis | ||
Abbreviations: ACD, anterior cervical discectomy; ACDF, anterior cervical discectomy and fusion; ACDR, anterior cervical disc replacement; MI, minimally-invasive; MRC, Medical Research Council grading; PCF, posterior cervical foraminotomy.
aThe 31 patients in this cohort are from a larger group of 788 patients with radiculopathy, which serves as a denominator for baseline prevalence of painless motor radiculopathy.
bBaseline and follow-up motor deficit percentages are provided for ‘subjective’ weakness. ‘Objective’ weakness was only reported as a separate percentage of the 1085 patients that met study inclusion criteria but did not have documented follow-up.
Table 5.
Motor data from 7 studies reporting quantified data.
| Reference | Cohort size | Measurement unit | Intervention group | Baseline motor data | Latest follow-up | Significance from baseline | Inter-group difference at follow-up | |
|---|---|---|---|---|---|---|---|---|
| Data | Time | |||||||
| Bacigaluppi et al, 2019 6 | 75 | MRC grading | Surgery | 3.9 (mean) | 4.9 (mean) | 83.5 months | Yes (P < .001) | — |
| Siller et al, 2018 9 | 31 | MRC grading | Surgery | 3 (median) | 4 (median) | 3.9 years | Yes (P = .046) | — |
| Akkan & Gelecek, 2018 8 | 32 | Grip strength (kg) | Neck stabilisation exercises | 18.80 (mean) | 27.04 (mean) | 12 weeks | Yes (P < .001) | Not significant (P > .05) |
| Standard PT exercises | 19.07 (mean | 25.77 (mean) | Yes (P < .001) | |||||
| Peolsson et al, 2013 22 | 49 | Grip strength (kg) in L and R hand | Surgery | 34L/36R (both mean) | 37L/42R (both mean) | 24 months | Yes for R hand (P = .01); No for L hand (P = .20) | Not significant (P = .83 R hand; P = .71 L hand) |
| PT | 34L/34R (both mean) | 36L/38R (both mean) | Yes for R hand (P = .01); No for L hand (P = .20) | |||||
| Joghataei et al, 2004 36 | 30 | Grip strength (kPa) | Cervical traction | 14.17 (mean) | 22.83 (mean) | 3.3 weeks a | Yes (P < .01) | Not significant (P = .65) b |
| Exercise & electrotherapy | 17.64 (mean) | 24.91 (mean) | Yes (P < .01) | |||||
| Persson et al, 1997 44 | 81 | Ratio of muscle strength between affected and unaffected side c | Surgery | Pinch grip .78 | Pinch grip .83 | 16 months | No (P > .05) for all (except Yes (P < .05) for Elbow ext, Elbow flex, Shoulder Ab & Shoulder IR) | Surgery vs PT significant (P < .05) for Wrist ext, Elbow ext. Shoulder Ab, Shoulder IR |
| Elbow ext .75 | Elbow ext .86 | |||||||
| Elbow flex .87 | Elbow flex .95 | |||||||
| Shoulder Ab .85 | Shoulder Ab .97 | |||||||
| Shoulder IR .78 (all mean) | Shoulder IR .96 (all mean) | |||||||
| PT | Pinch grip .91 | Not provided | No (P > .05) for all except Hand grip | Surgery vs collar not significant (P > .05) | ||||
| Elbow ext .92 | ||||||||
| Elbow flex .93 | ||||||||
| Shoulder Ab .92 | ||||||||
| Shoulder IR .92 (all mean) | ||||||||
| Cervical collar | Pinch grip .87 | Not provided | No for all (P > .05) | PT vs collar not significant (P > .05) | ||||
| Elbow ext .85 | ||||||||
| Elbow flex .85 | ||||||||
| Shoulder Ab .86 | ||||||||
| Shoulder IR .91 (all mean) | ||||||||
| Bush & Hillier, 1996 45 | 68 | MRC grading | Steroid injections | 3 (median) | 5 (median) | 39 months | Significance not provided; calculated as P < .0001 (Wilcoxon rank) | — |
Abbreviations: L, left; MRC, Medical Research Council; PT, physiotherapy; R, right.
aThree sessions per week, corresponding with follow-up occurring at 5 and 10 sessions.
bThe inter-group difference was only significant (P = .04) at 5 sessions (1.6 weeks).
c12 muscle groups were tested, of which 5 with statistical significance have been selectively shown above. Shown: Pinch grip, Elbow extensors (Elbow ext), Elbow flexors (Elbow flex), Shoulder abductors (Shoulder Ab) and Shoulder internal rotators (Shoulder IR). Not shown: Hand grip, Wrist extensors, Wrist flexors, Shoulder adductors, Shoulder elevator, Shoulder extensors and Shoulder external rotators.
Motor deficits at baseline (pre-intervention)
42 of 44 (95.5%) studies presented baseline motor data. The remaining 2 studies documented only frequency of post-intervention motor improvement without baseline data.32,38 Another study provided detailed baseline motor deficit by individual muscle group, but no overall baseline prevalence of motor deficits, 26 whilst a further study documented only prevalence of painless motor deficits, but not presence of motor deficits as a whole. 18
Mean baseline prevalence of motor deficits was 56.4% (range 9.2%–94.1%) across all studies reporting binary motor deficit data at baseline: 39.1% (range 9.2%–73.3%) in 6 conservative cohorts and 60.5% (range 18.5%–94.1%) in 29 surgical cohorts.
Motor outcomes (post-intervention)
We categorised motor data from 22 studies (50%) that reported post-intervention outcome data into 6 groups according to whether motor radiculopathy was painful, painless or unspecified, and whether the intervention was conservative or surgical (see Table 6 for summary).
Table 6.
Stratification of studies into 6 categories (Groups I–VI).
| Group | No. of studies | Studies |
|---|---|---|
| I: conservatively-managed painful motor radiculopathy | 5 | • Akkan and Gelecek, 2018 8 |
| • Anderberg et al, 2007 32 | ||
| • Joghataei et al, 2004 36 | ||
| • Persson et al, 1997 44 | ||
| • Bush and Hillier, 1996 45 | ||
| II: surgically-managed painful motor radiculopathy | 6 | • Aydin et al, 2005 35 |
| • Rodrigues et al, 2001 38 | ||
| • Knight et al, 2001 39 | ||
| • Heckmann et al, 1999 2 | ||
| • Schneeberger et al, 1999 43 | ||
| • Persson et al, 1997 44 | ||
| III: conservatively-managed painless motor radiculopathy | 0 | |
| IV: surgically-managed painless motor radiculopathy | 1 | • Siller et al, 2018 9 |
| V: conservatively-managed motor radiculopathy with unspecified pain status | 2 | • Heckmann et al, 1999 2 |
| • Peolsson et al, 2013 22 | ||
| VI: surgically-managed motor radiculopathy with unspecified pain status | 11 | • Bacigaluppi et al, 2019 6 |
| • Lee et al, 2018 10 | ||
| • Scholz et al, 2018 11 | ||
| • Peto et al, 2017 12 | ||
| • ElAbed et al, 2016 14 | ||
| • Shiban et al, 2016 15 | ||
| • Lehmann et al, 2014 18 | ||
| • Church et al, 2014 20 | ||
| • Lidar and Salame, 2011 24 | ||
| • Cornelius et al, 2007 29 | ||
| • Peolsson et al, 2013 22 |
Group I: conservatively-managed painful motor radiculopathy
Five studies reported motor outcomes for cohorts in this category: 1 with binary data, 3 with quantified data and 1 with both forms.
Anderberg et al 32 reported motor improvements in 10% of patients undergoing steroid injections (90% unchanged) by three-week follow-up. No motor complications were reported.
Akkan and Gelecek 8 reported significantly (P < .001) increased grip strength over a 12-week period during which patients were randomised either to neck stabilisation exercises or standard physiotherapy: there were no significant inter-group differences (P>.05). Grip strength increased from a baseline mean of 18.8kg to 27.0kg at 12 weeks for the neck stabilisation exercise group, and from 19.1kg to 25.8kg in the standard physiotherapy group.
Joghataei et al 36 reported significantly (P < .01) increased grip strength after ten sessions of either cervical traction, or exercise and electrotherapy. Mean grip strength increased from 14.2kPa to 22.8kPa in the traction group, and 17.6kPa to 20.4kPa in the non-traction group.
Persson et al 44 presented an RCT comparing surgical with non-surgical management (see ‘Group II’ section for surgical cohort data). Whilst the authors provide motor data in ratio form (muscle strength in affected to non-affected side) at baseline, post-intervention motor outcomes are not provided for conservative cohorts. The authors reported that only hand grip strength increased significantly for the physiotherapy group at 16-month follow-up (P < .05).
Bush and Hillier 45 reported a significant (P < .0001) increase in median MRC grade from 3 at baseline to 5 after steroid injections, with an average follow-up duration of 39 months. Post-intervention motor improvement occurred in 90.2% of patients with baseline weakness, the remaining 9.8% having unchanged motor function. No motor complications were reported.
Group II: surgically-managed painful motor radiculopathy
Six studies reported motor outcomes for cohorts in this category: 5 with binary data and 1 with quantified data.
Aydin et al 47 reported motor deficits in 7.1% at 18-month follow-up after anterior cervical discectomy (ACD), compared to 80.2% at baseline. No motor complications were reported.
Rodrigues et al 10 reported motor deficits in 6.5% after posterior decompression, compared to 90.2% prevalence at baseline. Mean follow-up duration was 46 months (range 18-108 months). Improved motor function was reported in 93.5% of patients with baseline weakness. No motor complications were reported.
Knight et al 39 reported motor deficits in 7.2% after percutaneous anterior laser disc ablation, compared to a baseline prevalence of 31.4%. Mean follow-up duration was 43 months (range 24 months to 7 years). Motor complications arose in 2 patients (1.9%): 1 transient and 1 persistent.
Heckmann et al 2 reported improved motor function in 50.0% of patients with baseline weakness after anterior cervical discectomy and fusion (ACDF): weakness was unchanged in 21.4% and worse in 28.6%. Pre-operative prevalence of weakness was 66.7%. Average follow-up duration was 5.5 years (range 4.6 months to 10.6 years).
Schneeberger et al 13 reported 100% motor deficit improvement rate after ACDF, with average follow-up duration of 54 months (range 24-102 months). No motor complications were reported. Pre-operative prevalence of weakness was 31.4%.
Persson et al 44 presented 4- and 16-month follow-up motor data. Mean ratio of strength between affected and unaffected sides increased significantly from baseline to latest follow-up in 4 muscle groups: elbow extensors (.75 at baseline to .86 at 16 months, P < .05); elbow flexors (.87 to .95, P < .05); shoulder abductors (.85 to .97, P < .05); and shoulder internal rotators (.78 to .96, P < .01).
Group III: conservatively-managed painless motor radiculopathy
We found no studies reporting cohorts in this category.
Group IV: surgically-managed painless motor radiculopathy
One study, Siller et al, 18 reported motor outcomes this category, with either ACDF or open posterior cervical foraminotomy (PCF) as the surgical intervention. Median MRC grade increased significantly (P=.046) from baseline 3 to 4 at latest follow-up. Post-operative motor improvement occurred in 65.2% (27.3% ACDF; 16.7% PCF) of patients with baseline weakness, whilst 21.7% (27.3% ACDF; 16.7% PCF) remained stable and 13.0% (9.1% ACDF; 16.7% PCF) experienced motor deterioration. Mean follow-up duration was 3.9 years (range 1-10 years). Baseline prevalence of painless motor radiculopathy was 3.93%.
Group V: conservatively-managed motor radiculopathy with unspecified pain status
Two studies reported motor outcomes for cohorts in this category: 1 with binary data and 1 with quantified data.
Heckmann et al 2 reported motor improvement in 94.1% of conservatively-managed patients with baseline weakness. Motor function was unchanged in 5.9%. No patients experienced motor deterioration. Baseline prevalence of motor deficits was 43.6%. Average follow-up duration was 5.5 years (range 4.6 months to 10.6 years).
Peolsson et al 22 reported significantly increased mean grip strength for the right hand in the physiotherapy cohort, from 34kg at baseline to 38kg at final 24-month follow-up (P=.01), but not for the left hand (34kg to 36kg, P=.20).
Group VI: surgically-managed motor radiculopathy with unspecified pain status
Eleven studies reported motor outcomes for cohorts in this category: 9 with binary data, 1 with quantified data and 1 with both.
Bacigaluppi et al 6 reported motor deficits in 8% of the cohort after open posterior decompression, compared to 81.3% at baseline. Mean follow-up duration was 83.5 ± 48 months (no range given). Improved motor function was reported in 100% of patients with baseline weakness. Mean MRC grade increased significantly (P < .001) from 3.7 at baseline to 4.9 at follow-up. No motor complications were reported.
Lee et al 19 reported motor deficits in 14.5% after minimally-invasive PCF, compared to 71.7% at baseline. Average follow-up duration was 22.4 months (range 1-75 months). Improved motor function was reported in 94.7% of patients with baseline weakness. No persisting motor complications were reported.
Scholz et al 20 reported post-operative motor improvement in 75.7% (72.5% ACDF; 77.6% minimally-invasive PCF) of patients with baseline weakness. Baseline prevalence of weakness was 73.8% (60.0% ACDF; 82.1% PCF). Average follow-up duration was 42 months (range 6-89 months). One patient (.9%) experienced motor complications.
Peto et al 21 reported motor deficits in 25.0% after minimally-invasive PCF, compared to 58.8% at baseline. Average follow-up duration was 30.4 months (range 0-96 months). Improved motor function was reported in 80.0% of patients with baseline weakness. No motor complications were reported.
ElAbed et al 28 reported motor deficits in 15.0% after ACDF, compared to 50.0% at baseline. Average follow-up duration was 4.5 years (range 2-7 years). Improved motor function was reported in 85.0% of patients with baseline weakness. Transient neurological deterioration was reported in 1.1%, normalising by 1 year. No persisting motor complications were reported.
Shiban et al 29 reported post-ACDF motor improvement in 84.2% of patients reporting baseline weakness. Baseline prevalence of weakness was 61.7%. Average follow-up duration was 21 months (range 12-47 months). Motor deterioration occurred in 1.5%.
Lehmann et al 34 reported motor deficits in 16.9% of their cohort after ACDF, compared to 55.1% at baseline. The mean follow-up time was 3.8 ± 2.1 years (no range given). New post-operative motor deficits were reported in 5.4%.
Church et al 35 reported motor deficits in 27.9% of their cohort at 18-month follow-up after posterior decompression, compared to 78.1% at baseline. .3% experienced new ‘focal weakness’.
Lidar and Salame 38 reported 100% improvement rate of motor deficits after minimally-invasive PCF, with average follow-up duration of 39 months (range 20-39 months). No motor complications were reported. No pre-operative prevalence of weakness was given.
Cornelius et al 42 reported motor deficits in 7.5% after anterolateral foraminotomy, compared to 45.0% at baseline. Average follow-up duration was 4.3 years (range 2.7-7.4 years). Improved motor function was reported in 83.3% of patients with baseline weakness. One patient (2.5%) experienced transient C5 palsy. No persisting motor complications were reported.
Peolsson et al 22 reported significantly increased mean grip strength for the right hand in the ACDF cohort, from a baseline of 36kg to 42kg at final 24-month follow-up (P=.01), but not for the left hand (34kg at baseline to 37kg at 24 months, P=.20).
Motor outcomes after conservative management: Groups I, III and V
Seven studies reported motor outcomes in conservatively-managed cohorts (5 in Group I, none in Group III and 2 in Group V). Motor outcomes were highly variable in conservative cohorts, with Anderberg et al 32 reporting a modest 10.0% rate of motor improvement, compared to 94.1% in Heckmann et al, 2 and 90.2% in Bush and Hillier. 45 However, since Anderberg and colleagues 32 did not report baseline prevalence of motor deficits, it is impossible to deduce what proportion of the remaining 90.0% with ‘unchanged’ motor status had normal baseline function. Moreover, the follow-up of this study was 3 weeks post-intervention, 32 compared to mean follow-up durations of 39 months 45 and 5.5 years 2 for the others. No patients experienced motor complications in any conservatively-managed cohorts.
Four studies 8,22,36,44 demonstrated variable effects of non-surgical interventions on quantifiable muscle strength. Grip strength was shown to increase significantly (P < .01) in 2 RCTs, 1 investigating benefits of neck stabilisation vs home exercises.8,36 2 other RCTs, meanwhile, demonstrated equivocal results, Persson and colleagues 44 finding that of 12 muscle groups tested, only 1 showed significantly increased strength after physiotherapy, with no significant benefits in any muscles for the cervical collar group, whilst Peolsson et al 22 showed that physiotherapy significantly increased grip strength in the right hand but not the left.
Motor outcomes after surgical management: Groups II, IV and VI
18 studies reported motor outcomes in surgically-managed cohorts (6 in Group II, 1 in Group IV and eleven in Group VI).
Amongst Group II studies (surgically-managed painful motor radiculopathy), the proportion of patients with motor deficits decreased from a baseline mean of 67.3% (range 31.4%–90.2%) to 7.0% post-operatively (range 6.5%–7.2%) in 3 studies reporting this parameter.10,39,47 The mean proportion with improved post-operative motor function was 81.2% (range 50.0% to 100%) in 3 studies reporting rate of motor improvement.2,10,13 42,13,39,47 Group II studies utilised anterior surgical approaches for decompression, 1 10 used posterior approaches and 1 20 had a mixed cohort in which both approaches were used. No motor complications were reported by 4 studies,10,13,44,47 whilst 1 patient (1.0%) in Knight et al 39 experienced persistent motor complications. Heckmann et al 2 reported that of the 14 patients with baseline weakness, 4 (28.6%) had motor deterioration.
In the sole Group IV study (painless motor radiculopathy), Siller and colleagues 18 reported that median MRC grade increased significantly (P=.046) from 3 at baseline to 4 by latest follow-up, occurring at a mean of 3.9 years. Motor function improved in 65.2% of the 23 patients with baseline painless weakness, with comparable results amongst the 11 ACDF (63.6% improvement) and 12 PCF (66.7% improvement) patients. Weakness was unchanged in 21.7% (27.3% ACDF; 16.7% PCF) of the cohort, and worsened in 13.0% (9.1% ACDF; 16.7% PCF).
Amongst Group VI studies (motor radiculopathy with unclear pain status), the proportion of patients with motor deficits decreased from a mean of 62.9% at baseline (range 45.0%–81.3%) to 16.4% post-operatively (range 7.5%–27.9%) in 7 studies reporting post-operative motor deficit.6,19,21,28,34,35,42 The mean proportion with improved motor function at latest follow-up was 87.9% (range 75.7%–100%) in 8 studies reporting rate of motor improvement.6,19–21,28,29,38,42 Regarding surgical approach for decompression, 522,28,29,34,42 studies in Group VI used anterior approaches, 56,19,21,35,38 used posterior approaches and 1 20 compared both approaches. Seven studies in Group VI reported no persisting motor complications by latest follow-up.6,19,21,22,28,38,42 1 patient (.9%) in Scholz et al 20 had a new motor deficit after PCF. Shiban et al 29 reported 2 patients (1.5%) experiencing motor deterioration at follow-up. New motor deficits arose post-operatively in 6 patients (5.4%) in Lehmann et al, 34 and in 3 patients (.3%) in Church et al. 35
Discussion
Categorisation of studies into 6 groups
We have stratified study cohorts into 6 categories according to a), whether cohorts contain patients with motor radiculopathy that is painful, painless or unspecified and b), whether cohorts are conservatively- or surgically-managed. The majority of cohorts (eleven) fall into Group VI: ‘surgically-managed motor radiculopathy with unknown or mixed pain status’. By contrast, no studies report cohorts belonging to Group III, ‘conservatively-managed painless motor radiculopathy’, so it is not possible to clarify the natural history of this condition without surgery.
We speculate that the reasons for this are two-fold: firstly, painless motor radiculopathy is a significantly less common entity than painful motor radiculopathy, as evidenced by 6 studies that specified baseline prevalence of painless motor radiculopathy, with a mean of 6.1%.11,12,18,21,42,46 Hamburger and colleagues 11 reported that of 249 radiculopathy patients undergoing ACDF, 1.2% presented with painless motor radiculopathy: 10.0% had painful motor radiculopathy without sensory impairment, but the majority (73.9%) had painful motor radiculopathy with sensory impairment such as hypoaesthesia. Painless motor radiculopathy featured in 1 patient of 40 (5.6%) in Cornelius et al, 42 and in 24 of 292 patients (8.2%) in Korinth et al. 46 Swezey 12 reported painless motor radiculopathy in 5 of 83 patients (6.0%). Peto’s group 21 reported the highest rate of painless motor radiculopathy (11.8%) in a small cohort of 34 patients undergoing PCF, with 47.1% having painful motor radiculopathy. Siller and colleagues recently described the largest cohort of painless motor radiculopathy, with 31 patients (of 788 patients undergoing surgery for radiculopathy, representing 3.9%). 18
The second factor that may explain the absence of patients for inclusion in Group III is the belief that conservative management is more efficacious at addressing the pain component—rather than motor deficits—in radiculopathy. Successful relief of radiculopathic pain, at which conservative treatment is highly effective,1,2 is often accompanied by corresponding improvements in motor function, indicating resolution of ‘subjective weakness’ (in which perceived motor function is limited by pain) rather than true ‘objective weakness’. 19 This is a distinction made by Church et al, 35 who noted that of 580 patients reporting subjective weakness, only 467 were found to have weakness on examination. Notably, the authors used subjective rather than objective weakness when reporting pre- and post-operative motor impairment in their cohort, 35 perhaps to capture the positive effect on perceived motor function in cases with successful post-operative relief of pain. The implication of this is that patients presenting with painless motor radiculopathy (generally—though not always 18 —thought to represent a more advanced stage of radiculopathy) may be more likely to be offered surgical treatment in lieu of conservative options, hence explaining the lack of Group III studies.
Heterogeneity of motor data reporting
The reporting of motor deficits and outcomes is highly variable: a large number of studies were excluded during full-text screening for not reporting motor impairment, often assessing combined ‘neurological deficit’ instead, or using overall measures of outcome, such as Odom’s criteria. 48 Even amongst the 44 studies included for final analysis, half report only baseline motor data without reporting follow-up motor outcomes. Where motor follow-up does occur, average length of time post-intervention ranges considerably from 3 weeks 32 to ten years.18,35 This heterogeneity is perhaps a reflection of the diverse range of disciplines from which the studies originate, from physiotherapists8,22,44 to neurologists 26 and spinal surgeons,13,18,19,21,28 leading to differences in selection criteria, treatment priorities, follow-up duration and reporting style of motor outcomes.
For instance, quantitative studies of muscle strength8,22,36,44 provide no clear ‘cut-off’ values for motor impairment, making it difficult to derive clinically-meaningful conclusions from these data, apart from evidencing the benefit of conservative interventions in motor rehabilitation of cervical radiculopathy. This is in contrast to MRC grading used in other quantitative studies,6,18,45 which demonstrate motor improvement using a validated and clinically-orientated scale.
Comparisons between conservative and surgical management
By stratifying motor radiculopathy studies into 6 categories according to intervention type and presence or absence of associated radicular pain, we had hoped to provide comparable numerical data relating to pre- and post-intervention prevalence of motor deficits, as well as improvement in motor function after operative and non-operative management. However, the published data does not allow direct comparison of conservative vs surgical management in addressing motor deficits in degenerative cervical radiculopathy. This is because of heterogeneity in the reporting of motor deficits, and the paucity of studies investigating motor outcomes in conservative cohorts, where the focus was commonly on pain relief and global (as opposed to motor-specific) outcome measures. Bush and Hillier 45 showed that conservative management resulted in 90.2% of patients with painful motor radiculopathy experiencing motor improvements, a stark contrast to the 10.0% reported by Anderberg et al, 32 who however omitted to state the baseline prevalence of motor deficits and only reported short-term follow-up. Heckmann et al 2 directly compared conservative and surgical management of radiculopathy, demonstrating a superior rate of motor improvement in the conservatively-managed group (94.1%) over the surgical cohort (50.0%), albeit with small cohort sizes of 17 and 14 patients, respectively. The probable selection bias of milder deficits being more likely to be treated conservatively must also be considered. Amongst twelve surgical cohorts (including Heckmann et al 2 ) reporting motor improvement rates, a mean of 83.5% patients experienced improved motor function.
As might be expected due to differences in invasiveness, no reported motor deterioration occurred in any conservative studies, whereas 7 surgical cohorts2,18,20,29,34,35,39 reported persistent motor impairment, the highest rate being 28.6% in Heckmann et al’s 2 ACDF cohort.
The aetiology of degenerative radiculopathy may have prognostic significance in motor outcomes, as well as influencing the effectiveness of conservative measures. Radiculopathy arising from soft disc (non-osteophytic) rather than hard disc (osteophytic) disease is generally associated with improved global11,14,35 and motor-specific35,49 post-operative outcomes. This may be due to the increased likelihood of resorption of a soft disc prolapse, single-level pathology, shorter symptom duration and younger age with soft disc disease,15,49 or possibly decreased operative time. 47 The pre-operative distinction between soft and hard disc is 1 made via imaging. Most studies in our analysis required magnetic resonance imaging (MRI) and/or computerised tomography (CT) to radiologically demonstrate evidence of nerve root pathology as part of the inclusion criteria, though a minority8,33 stipulated only clinical diagnosis of pure radiculopathy in the study inclusion criteria.
Study limitations
Our selection criteria were intentionally strict such that all included studies had to either clearly exclude myelopathic patients, or to analyse patients with myelopathy or combined radiculomyelopathy in a separate cohort. Whilst this methodology has the potential to miss data in studies providing motor data in radiculopathy patients, assessing outcomes of pure radiculopathy cohorts is important since cervical myelopathy is clinically and pathophysiologically distinct, with differing motor outcomes. 15
Conversely, certain studies list ‘severe’ or ‘profound’ weakness (most commonly defined as MRC < 4) as an exclusion criterion, perhaps in an effort to rigorously exclude patients with myelopathy or canal stenosis. Though these studies are included in our review, a degree of outcome bias is to be expected, since a significant proportion of radiculopathy patients are known to present with severe (MRC < 4) rather than mild (MRC=4) weakness, Bacigaluppi et al 6 reporting a figure of 30.7%, Lee et al 19 reporting 23%, and Heckmann et al 2 reporting 10%.
Conclusion
Our aim was to assess the literature to determine if and when surgery should be performed for motor radiculopathy. The data does not allow clear conclusions or guidance. Many studies fail to report motor data entirely, particularly for post-intervention follow-up. No studies document the natural history of untreated painless motor radiculopathy. Future large-scale studies comparing pre- and post-intervention motor data for conservative and surgical cohorts would be highly beneficial.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Axumawi Mike Hailu Gebreyohanes https://orcid.org/0000-0001-8181-2139
References
- 1.Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy. Brain. 1994;117(2):325-335. doi: 10.1093/brain/117.2.325 [DOI] [PubMed] [Google Scholar]
- 2.Heckmann JG, Lang CJG, Zöbelein I, Laumer R, Druschky A, Neundörfer B. Herniated cervical intervertebral discs with radiculopathy: An outcome study of conservatively or surgically treated patients. Journal of Spinal Disorders. 1999;12(5):396-401. doi: 10.1097/00002517-199912050-00008 [DOI] [PubMed] [Google Scholar]
- 3.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine. 2009;6:e1000100. Published online. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health (NIH) . Study Quality Assessment Tools; 2013. Published 2013 https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed May 24, 2022 [Google Scholar]
- 5.MacDowall A, Heary RF, Holy M, Lindhagen L, Olerud C. Posterior foraminotomy versus anterior decompression and fusion in patients with cervical degenerative disc disease with radiculopathy: Up to 5 years of outcome from the national Swedish Spine Register. Journal of Neurosurgery: Spine. 2020;32(3):344-352. doi: 10.3171/2019.9.SPINE19787 [DOI] [PubMed] [Google Scholar]
- 6.Bacigaluppi S, Bragazzi NL, Zella S, Prada F, Zavanone ML, Rampini P. Reappraisal of the posterior approach for cervical decompressive herniectomy. Journal of Neurosurgical Sciences. 2019;63:30-35. Published online. doi: 10.23736/S0390-5616.16.03774-7 [DOI] [PubMed] [Google Scholar]
- 7.Kang MS, Choi KC, Lee CD, Shin YH, Hur SM, Lee SH. Effective cervical decompression by the posterior cervical foraminotomy without discectomy. Journal of Spinal Disorders and Techniques. 2014;27(5):271-276. doi: 10.1097/BSD.0b013e3182a35707 [DOI] [PubMed] [Google Scholar]
- 8.Akkan H, Gelecek N. The effect of stabilization exercise training on pain and functional status in patients with cervical radiculopathy. Journal of Back and Musculoskeletal Rehabilitation. 2018;31:247-252. Published online. doi: 10.3233/BMR-169583 [DOI] [PubMed] [Google Scholar]
- 9.Jho HD, Kim WK, Kim MH. Anterior microforaminotomy for treatment of cervical radiculopathy: Part 1 - Disc-preserving “functional cervical disc surgery. Neurosurgery; 2002. Published online. 10.1097/00006123-200211002-00007 [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues MA, Hanel RA, Prevedello DMS, Antoniuk A, Araújo JC. Posterior approach for soft cervical disc herniation: A neglected technique? Surgical Neurology. 2001;55(1):17-22. doi: 10.1016/S0090-3019(00)00349-9 [DOI] [PubMed] [Google Scholar]
- 11.Hamburger C, Festenberg F v., Uhl E. Ventral discectomy with PMMA interbody fusion for cervical disc disease: Long-term results in 249 patients. Spine (Phila Pa 1976). 2001;26(3):249-255. doi: 10.1097/00007632-200102010-00009 [DOI] [PubMed] [Google Scholar]
- 12.Swezey RL. Conservative treatment of cervical radiculopathy. Journal of Clinical Rheumatology. 1999;5:65-73. Published online. doi: 10.1097/00124743-199904000-00006 [DOI] [PubMed] [Google Scholar]
- 13.Schneeberger AG, Boos N, Schwarzenbach O, Aebi M. Anterior cervical interbody fusion with plate fixation for chronic spondylotic radiculopathy: A 2− to 8-year follow-up. Journal of Spinal Disorders and Techniques. 1999;12(3):215-220. Published online. doi: 10.1097/00002517-199906000-00008 [DOI] [PubMed] [Google Scholar]
- 14.Davis RA. A long-term outcome study of 170 surgically treated patients with compressive cervical radiculopathy. Surgical Neurology. 1996;46(6):523-533. doi: 10.1016/S0090-3019(96)00278-9 [DOI] [PubMed] [Google Scholar]
- 15.Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy without fusion for treatment of cervical radiculopathy and myelopathy - A follow-up of 164 cases. Acta Neurochirurgica; 1988. Published online. 10.1007/BF01560567 [DOI] [PubMed] [Google Scholar]
- 16.MacDowall A, Skeppholm M, Lindhagen L, Robinson Y, Lofgren H, Michaelsson K, et al. Artificial disc replacement versus fusion in patients with cervical degenerative disc disease with radiculopathy: 5-year outcomes from the National Swedish Spine Register. Journal of Neurosurgery: Spine. Published online. 2019;30:159-167. doi: 10.3171/2018.7.SPINE18657 [DOI] [PubMed] [Google Scholar]
- 17.Engquist M, Löfgren H, Öberg B, Holtz A, Peolsson A, Soderlund A, et al. Factors affecting the outcome of surgical versus nonsurgical treatment of cervical radiculopathy: A randomized, controlled study. Spine (Phila Pa 1976). 2015;40(20):1553-1563. doi: 10.1097/BRS.0000000000001064 [DOI] [PubMed] [Google Scholar]
- 18.Siller S, Kasem R, Witt TN, Tonn JC, Zausinger S. Painless motor radiculopathy of the cervical spine: Clinical and radiological characteristics and long-term outcomes after operative decompression. Journal of Neurosurgery: Spine. 2018;28:621-629. Published online. doi: 10.3171/2017.10.SPINE17821 [DOI] [PubMed] [Google Scholar]
- 19.Lee U, Kim CH, Chung CK, et al. The Recovery of Motor Strength after Posterior Percutaneous Endoscopic Cervical Foraminotomy and Discectomy. World Neurosurgery. 2018;22:131. Published online. doi: 10.1016/j.wneu.2018.04.090 [DOI] [PubMed] [Google Scholar]
- 20.Scholz T, Geiger MF, Mainz V, et al. Anterior Cervical Decompression and Fusion or Posterior Foraminotomy for Cervical Radiculopathy: Results of a Single-Center Series. Journal of Neurological Surgery, Part A: Central European Neurosurgery. 2018;79(3):211-217. Published online. doi: 10.1055/s-0037-1607225 [DOI] [PubMed] [Google Scholar]
- 21.Peto I, Scheiwe C, Kogias E, Hubbe U. Minimally Invasive Posterior Cervical Foraminotomy: Freiburg Experience With 34 Patients. Clin Spine Surg. 2017;30(10):E1419-E1425. http://www.ncbi.nlm.nih.gov/pubmed/28234772 [DOI] [PubMed] [Google Scholar]
- 22.Peolsson A, Söderlund A, Engquist M, Lind B, Lofgren H, Vavruch L, et al. Physical function outcome in cervical radiculopathy patients after physiotherapy alone compared with anterior surgery followed by physiotherapy: A prospective randomized study with a 2-year follow-up. Spine (Phila Pa 1976). 2013;38(4):300-307. doi: 10.1097/BRS.0b013e31826d2cbb [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim KT, Kim DH, Lee BJ, Son ES, Kwack YH. Clinical outcomes of cervical radiculopathy following epidural steroid injection: A prospective study with follow-up for more than 2 years. Spine (Phila Pa 1976). 2012;37(12):1041-1047. doi: 10.1097/BRS.0b013e31823b4d1f [DOI] [PubMed] [Google Scholar]
- 24.Kim KT, Cho DC, Sung JK, Kim YB, Kim DH. Comparative analysis between total disc replacement and posterior foraminotomy for posterolateral soft disc herniation with unilateral radiculopathy: Clinical and biomechanical results of a minimum 5 years follow-up. J Korean Neurosurg Soc. 2017;60(1):30-39. doi: 10.3340/jkns.2015.0506.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstantinovic LM, Cutovic MR, Milovanovic AN, Jovic SJ, Dragin AS, Letic MD, et al. Low-level laser therapy for acute neck pain with radiculopathy: A double-blind placebo-controlled randomized study. Pain Medicine (United States). 2010;11(8):1169-1178. doi: 10.1111/j.1526-4637.2010.00907.x [DOI] [PubMed] [Google Scholar]
- 26.Kuijper B, JTJ Tans, Beelen A, Nollet F, De Visser M. Cervical collar or physiotherapy versus wait and see policy for recent onset cervical radiculopathy: Randomised trial. BMJ (Online). 2009;339(7727):952. doi: 10.1136/bmj.b3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KT, Kim YB. Comparison between open procedure and tubular retractor assisted procedure for cervical radiculopathy: Results of a randomized controlled study. Journal of Korean Medical Science. 2009;24(4):649-653. doi: 10.3346/jkms.2009.24.4.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ElAbed K, Shawky A, Barakat M, Ainscow D. Anterior cervical discectomy and fusion with stand-alone trabecular metal cages as a surgical treatment for cervical radiculopathy: Mid-term outcomes. Asian Spine Journal. 2016;10(2):245-250. doi: 10.4184/asj.2016.10.2.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiban E, Gapon K, Wostrack M, Meyer B, Lehmberg J. Clinical and radiological outcome after anterior cervical discectomy and fusion with stand-alone empty polyetheretherketone (PEEK) cages. Acta Neurochirurgica. 2016;158(2):349-355. doi: 10.1007/s00701-015-2630-2 [DOI] [PubMed] [Google Scholar]
- 30.Xie JC, Hurlbert RJ. Discectomy versus discectomy with fusion versus discectomy with fusion and instrumentation: A prospective randomized study. Neurosurgery. 2007;61(1):107-116. doi: 10.1227/01.neu.0000279730.44016.da [DOI] [PubMed] [Google Scholar]
- 31.Ruetten S, Komp U, Merk H, Godolias G. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: Prospective 2-year results of 87 patients. Minimally Invasive Neurosurgery. 2007;50(4):219-226. doi: 10.1055/s-2007-985860 [DOI] [PubMed] [Google Scholar]
- 32.Anderberg L, Annertz M, Persson L, Brandt L, Säveland H. Transforaminal steroid injections for the treatment of cervical radiculopathy: A prospective and randomised study. European Spine Journal. 2007;16(3):321-328. doi: 10.1007/s00586-006-0142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao L, Li J, Li D, Yan D, Yang J, Wang D, et al. A posterior approach to cervical nerve root block and pulsed radiofrequency treatment for cervical radicular pain: A retrospective study. Journal of Clinical Anesthesia. 2015;27:486-491. Published online. doi: 10.1016/j.jclinane.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 34.Lehmann CL, Buchowski JM, Stoker GE, Riew KD. Neurologic Recovery after Anterior Cervical Discectomy and Fusion. Global Spine Journal. 2014;4(1):041-046. doi: 10.1055/s-0033-1360723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Church EW, Halpern CH, Faught RW, Balmuri U, Attiah M, Hayden S, et al. Cervical laminoforaminotomy for radiculopathy: Symptomatic and functional outcomes in a large cohort with long-term follow-up. Surgical Neurology International. 2014;5(15):S536-S543. doi: 10.4103/2152-7806.148029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joghataei MT, Arab AM, Khaksar H. The effect of cervical traction combined with conventional therapy on grip strength on patients with cervical radiculopathy. Clinical Rehabilitation. 2004;18(8):879-887. doi: 10.1191/0269215504cr828oa [DOI] [PubMed] [Google Scholar]
- 37.Park YK, Moon HJ, Kwon TH, Kim JH. Long-term outcomes following anterior foraminotomy for one- or two-level cervical radiculopathy. European Spine Journal. 2013;22(7):1489-1496. doi: 10.1007/s00586-013-2712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lidar Z, Salame K. Minimally invasive posterior cervical discectomy for cervical radiculopathy: Technique and clinical results. Journal of Spinal Disorders and Techniques. 2011;24(8):521-524. doi: 10.1097/BSD.0b013e31820679e3 [DOI] [PubMed] [Google Scholar]
- 39.Knight MTN, Goswami A, Patko JT. Cervical percutaneous laser disc decompression: Preliminary results of an ongoing prospective outcome study. Journal of Clinical Laser Medicine and Surgery. 2001;19(1):3-8. doi: 10.1089/104454701750066875 [DOI] [PubMed] [Google Scholar]
- 40.Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: Results of a new technique in 100 cases. Journal of Neurosurgery. 2001;95:51-57. Published online. doi: 10.3171/spi.2001.95.1.0051 [DOI] [PubMed] [Google Scholar]
- 41.Balasubramanian C, Price R, Brydon H. Anterior cervical microforaminotomy for cervical radiculopathy - Results and review. Minimally Invasive Neurosurgery. 2008;51(5):258-262. doi: 10.1055/s-0028-1082320 [DOI] [PubMed] [Google Scholar]
- 42.Cornelius JF, Bruneau M, George B. Microsurgical cervical nerve root decompression via an anterolateral approach: Clinical outcome of patients treated for spondylotic radiculopathy. Neurosurgery. 2007;61(5):972-980. doi: 10.1227/01.neu.0000303193.64802.8f [DOI] [PubMed] [Google Scholar]
- 43.Lin EL, Lieu V, Halevi L, Shamie AN, Wang JC. Cervical epidural steroid injections for symptomatic disc herniations. Journal of Spinal Disorders and Techniques. 2006;19(3):183-186. doi: 10.1097/01.bsd.0000190558.13248.e1 [DOI] [PubMed] [Google Scholar]
- 44.Persson LCG, Moritz U, Brandt L, Carlsson CA. Cervical radiculopathy: Pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery, physiotherapy or cervical collar: A prospective, controlled study. European Spine Journal. 1997;6(4):256-266. doi: 10.1007/BF01322448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush K, Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: A prospective study with independent clinical review. European Spine Journal. 1996;5(5):319-325. doi: 10.1007/BF00304347 [DOI] [PubMed] [Google Scholar]
- 46.Korinth MC, Krüger A, Oertel MF, Gilsbach JM. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: Results in 292 patients with monoradiculopathy. Spine. 2006;31(11):1207-1214. Published online. doi: 10.1097/01.brs.0000217604.02663.59 [DOI] [PubMed] [Google Scholar]
- 47.Aydin Y, Kaya RA, Can SM, Türkmenoǧlu O, Cavusoglu H, Ziyal IM. Minimally invasive anterior contralateral approach for the treatment of cervical disc herniation. Surgical Neurology. 2005;63(3):210-218. doi: 10.1016/j.surneu.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 48.Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. Published online. 1958;166:23. doi: 10.1001/jama.1958.02990010025006 [DOI] [PubMed] [Google Scholar]
- 49.Lunsford LD, Bissonette DJ, Jannetta PJ, Sheptak PE, Zorub DS. Anterior surgery for cervical disc disease. Part 1: Treatment of lateral cervical disc herniation in 253 cases. Journal of Neurosurgery. 1980;53:1-11. Published online. doi: 10.3171/jns.1980.53.1.0001 [DOI] [PubMed] [Google Scholar]