Abstract
Study Design:
Retrospective case-control study.
Objectives:
This study aims to present the clinical and radiographical outcomes of the titanium-polyetheretherketone (Ti/PEEK) composite cage compared to those of the standard PEEK cage in patients receiving minimally invasive transforaminal lumbar interbody fusion (MI-TLIF).
Methods:
Patients receiving 1 level MI-TLIF between October 2015 and October 2017 were included with a minimum of 2-year follow-up. The patients were segregated into 2 groups; Ti/PEEK group and PEEK group. Each patient was propensity-matched using preoperative age, sex, and body mass index. Early fusion rate was evaluated by computed tomography at postoperative 6 months. Clinical outcomes were assessed using the visual analog scale (VAS) and Oswestry Disability Index (ODI) scores.
Results:
After matching, there were 27 patients included in each group. The demographics, diagnosis, and surgical details were not significantly different between the 2 groups. The 6-month rate was 88.9% in Ti/PEEK group. The fusion rate and cage subsidence rate had no difference between the 2 groups. The complication rate in the Ti/PEEK group was comparable to that in the PEEK group. There was no difference in VAS and ODI scores during a 2-year follow-up period.
Conclusions:
The use of Ti/PEEK composite cage was as safe and effective as the use of PEEK cage in MI-TLIF. The 6-month fusion rate was 88.9%. Our finding revealed comparable clinical results for surgeons using Ti/PEEK composite cages in MI-TLIF compared to those using the PEEK cage.
Keywords: minimally invasive transforaminal lumbar interbody fusion (MI-TLIF), titanium-peek composite cage, patient-reported outcomes, fusion rate, cage subsidence, complication
Introduction
Transforaminal lumbar interbody fusion (TLIF) is an established and effective surgical method for treating degenerative lumbar spine diseases. 1 Recently, there has been an increasing trend toward the use of minimally invasive TLIF (MI-TLIF) owing to fewer morbidities, including lesser soft-tissue dissection, shorter hospital stays, and reduced blood loss compared to the traditional open approach.2-5 Both the traditional open approach and MI-TLIF have helped achieve improved patient outcomes and have shown comparable fusion rates and number of complications.6,7
The use of the interbody cage is a key element for the success of TLIF. The cage could help in restoring the disc and foraminal height, maintaining anterior column stability, and promoting solid fusion.8,9 Titanium and polyetheretherketone (PEEK) are the 2 most widely used cage materials with different characteristics. 10 PEEK has similar elastic modulus and mechanical properties to autologous bone, thus minimizing stress shielding compared with metal cages, with the potential for lowering subsidence rates.8,11-13 In addition, the radiolucent property of PEEK allows for better assessment of bony fusion on images. However, the application of PEEK has been limited owing to its bioinert feature, which may limit osteointegration, thereby potentially affecting fusion.11,14 Compared to PEEK, titanium has been found to be biocompatible with higher osteointegration. However, the elastic mismatch of titanium can lead to stress shielding and bone remodeling around the cages, which often results in more incidence of cage subsidence.8,11,13
Recently, titanium-PEEK (Ti/PEEK) cages have been developed that offer the advantages of both the materials. Material modification of PEEK cage with titanium creates an osteoconductive surface that provides short-term stability caused by friction and long-term stability caused by cell adhesion, leading to bony ongrowth or ingrowth.15,16 This cage combines the elastic modulus and radiolucent properties of PEEK with the biocompatibility of titanium surface. 17 Two types of Ti/PEEK cages have been designed and are available on the market: Ti-coated PEEK cage and Ti/PEEK composite cage. Ti-coated PEEK cage involves electron beam coating of titanium onto the surfaces of a PEEK cage. Prior studies have shown that Ti-coated PEEK cage could improve radiographic fusion compared to the standard PEEK cage.17,18 A Ti/PEEK composite cage is made by a combination of PEEK bodies with titanium endplates to augment bone-implants fusion. 10 An animal study demonstrated that the use of Ti/PEEK composite cage could significantly increase biomechanical stiffness and the presence of more bone ingrowth into the titanium endplate. 16
Clinical studies on the outcome and fusion rate achieved after using the Ti/PEEK composite cage are lacking. There are only 2 small case serials reporting the use of a Ti/PEEK composite cage on anterior cervical fusion and anterior lumbar interbody fusion with an average of 1-year follow-up.19,20 To the authors’ knowledge, there are no published studies evaluating their usage for MI-TLIF. This study aims to present the clinical and radiographical outcome of the Ti/PEEK composite cage compared to the standard PEEK cage in patients receiving MI-TLIF with a minimum of 2-year follow-up. The primary outcome is the fusion rate and cage subsidence, and the secondary outcome is the patient-reported outcome scores and complications.
Methods
Study Design
This retrospective study was conducted based on a prospectively collected database from October 2015 to October 2017 at our institution. Ethical approval and informed consent were obtained from all the participating patients. The database inclusion criteria were as follows: (1) patients aged >18 years; (2) diagnosis of degenerative lumbar spinal diseases, such as lumbar spinal stenosis, or degenerative and isthmic spondylolisthesis; (3) failure of prior conservative treatment for >6 months; (4) received MI-TLIF at 1 or 2 segments of; and (5) a minimum of 2-year follow-up. Additional criteria for this study were patients receiving MI-TLIF at only 1 segment and the usage of 2 specific types of cages, PEEK cage (Rainboo® lumbar cage, A-SPINE, United Orthopedic Corporation, Taiwan) or Ti/PEEK composite cage (Combo® lumbar cage, A-SPINE, United Orthopedic Corporation, Taiwan) (Figure 1). The choices of cage types were by the surgeon’s preference. All surgeries were performed by a single senior surgeon. All the included patients completed follow-up at 6 weeks and 3, 6, 12, and 24 months postoperatively. Patients who had prior spinal surgery or failed to complete the follow-up were excluded.
Figure 1.
(A) Represents PEEK cage (Rainboo® lumbar cage, A-SPINE, United Orthopedic Corporation, Taiwan) and (B) represents Ti/PEEK composite cage (Combo® lumbar cage, A-SPINE, United Orthopedic Corporation, Taiwan)
Surgical Technique
The surgical protocol for MI-TLIF included percutaneous pedicle screw insertion and a paramedian unilateral approach with bilateral decompression. Facetectomy was performed on the same side as the patient’s leg pain. All the decompression, disc preparation, and TLIF procedures were performed under a microscope using a micro-endoscopic retractor. Autologous morselized bone graft from a local bone and 1 cc demineralized bone matrix (OsteoSelect® DBM Putty, Bacterin International, Inc., Belgrade, MT, USA) were used for fusion in all the patients. No bone substitutes were used for increasing the bulk of the bone grafts. No drainage tube was inserted. The patients were asked to wear a brace for 3 to 6 months post-operation.
Data Collection and Radiographical Assessment
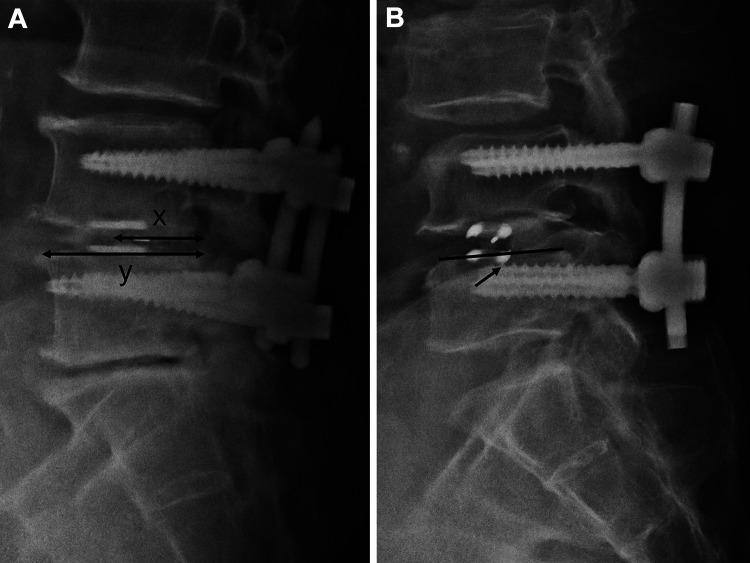
Patient demographic and surgical details were collected, including age, sex, body mass index (BMI), comorbidities, bone mineral density, surgical level, surgical time, estimated blood loss, and length of hospital stay. Radiographical parameters were obtained using plain static radiographs, including supine anteroposterior and lateral images taken at the preoperative baseline and each follow-up visit. Two radiographical parameters were recorded: disc height and segmental disc angle at the cage inserted level. Cage-related parameters included cage size, cage position, and cage subsidence. Cage position was recorded using central point ratio (CPR), which is the ratio of the distance between the midpoint of the cage and the posterior extent of the superior endplate of the inferior vertebra divided by the length of the superior endplate of the inferior vertebra (x/y × 100%) 21 (Figure 2). A CPR value >0.5 indicated a more anterior displaced cage. Cage subsidence was evaluated using lateral radiographs and was defined as >2 mm migration of the cage into the adjacent vertebral body. 22
Figure 2.
(A) Represents the central point ratio = x/y × 100% and (B) represents the cage subsidence.
Early interbody fusion was assessed using standard computed tomography (CT) scanning with 3 mm slices at the 6-month follow-up using the Brantigan-Steffee-Fraser (BSF) classification. 23 A classification of BSF-3 was considered as radiographical union, BSF-2 as locked pseudarthrosis, and BSF-1 as non-union. All complications or reoperations were also recorded. Patient-reported outcomes (PRO) were analyzed based on the Oswestry Disability Index (ODI) questionnaire and visual analog scale (VAS) for back pain during preoperative and every postoperative follow-up visit.
Study Endpoints
The primary endpoint were the 6-month fusion rates assessed using CT scans and cage subsidence evaluated using lateral radiographs. The secondary endpoint were the patient-reported outcome using VAS and ODI scores and all perioperative or implant-related complications.
Matched Method and Statistical Analysis
The patients were segregated into 2 groups: Ti/PEEK group and PEEK group based on the types of cages used. Each of the patients was then propensity-matched using preoperative parameters, including age, sex, and BMI. Statistical analysis was performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA). Categorical data was compared using the chi-squared or Fisher’s exact tests. Continuous data was compared using an independent t-test or Wilcoxon rank-sum tests where appropriate. A 2-tailed significance level was set at P < .05.
Results
A total of 125 patients who met the inclusion criteria and completed the follow-ups were included in the study. The average age of the included patients was 66.9 years, with a mean follow-up of 23.7 months. Eighty-two of the included patients were women (65.6%). There were 27 patients receiving the Ti/PEEK composite cage and 98 patients receiving the PEEK cage. After having the propensity-matched one by one using age, sex, and BMI, there were 27 patients in the Ti/PEEK group and 27 patients in the PEEK group. As shown in Table 1, the comparison analysis demonstrated that the patients’ demographics, diagnosis, and surgical details were not significantly different between the 2 groups.
Table 1.
Patient Demographics and Surgical Details in the 2 Groups.
| Variables | Ti-PEEK group (n = 27) |
PEEK group (n = 27) |
P-value |
|---|---|---|---|
| Age (years) | 67.9 ± 13.4 | 68.6 ± 10.3 | .83 |
| Sex, female | 21 (77.8%) | 21 (77.8%) | 1 |
| BMI (kg/m2) | 25.5 ± 2.4 | 25.9 ± 3.7 | .671 |
| Diabetes | 7 (25.9%) | 9 (33.3%) | .551 |
| Smoking | 3 (11.1%) | 1 (3.7%) | .61 |
| T-score for BMD | −1.0 ± 1.3 | −1.3 ± 1.2 | .497 |
| Follow-up duration (months) | 24.6 ± 1.5 | 24.4 ± 1.2 | .621 |
| Diagnosis | .547 | ||
| Spinal stenosis | 17 (63%) | 13 (48.1%) | |
| Degenerative spondylolisthesis | 7 (25.9%) | 10 (37%) | |
| Isthmic spondylolisthesis | 3 (11.1%) | 4 (14.8%) | |
| Surgical level | .34 | ||
| L3-4 | 4 (14.8%) | 2 (7.4%) | |
| L4-5 | 16 (59.3%) | 21 (77.8%) | |
| L5-S1 | 7 (25.9%) | 4 (14.8%) | |
| Surgical details | |||
| EBL (mL) | 448.0 ± 231.6 | 350.0 ± 131.6 | .064 |
| Surgical times (min) | 230.2 ± 61.8 | 231.7 ± 72.5 | .936 |
| Length of hospital stays (days) | 8.2 ± 2.2 | 8.0 ± 2.4 | .812 |
BMI, body mass index; BMD, bone mineral density; MIS, minimally invasive surgery; EBL, estimated blood loss. Note: Results are shown as mean ± standard deviation or the number of patients (%).
The radiographic, cage-related variables, and fusion status between the 2 groups are presented in Table 2. The disc height and segmental disc angle were comparable between the 2 groups during preoperative evaluations and 2-year follow-up. The cage size was not different between the 2 groups. The CPR was 64.4% ± 10.8% and 66.3% ± 11.7% in the Ti/PEEK and PEEK group (P = .541), respectively, which indicated that the cage position is in the anterior two-thirds of the disc space for the 2 groups. The use of Ti/PEEK composite cage showed lower cage subsidence rate than that for the PEEK cage (18.5% vs. 37%, P = .129), but with no statistical significance. The early fusion rate evaluated by CT scan at 6-month follow-up was higher in the Ti/PEEK group (88.9%) than in the PEEK group (74.1%, P = .293), but with no statistical significance.
Table 2.
Radiographic, Cage-Related Variables, and Fusion Status in the Groups.
| Variables | Ti-PEEK group (n = 27) |
PEEK group (n = 27) |
P-value |
|---|---|---|---|
| Radiographic parameters | |||
| Segmental disc angle (°) | |||
| at preoperative | 5.0 ± 3.9 | 5.4 ± 4.5 | .71 |
| at 2-year follow-up | 5.8 ± 4.6 | 5.7 ± 3.6 | .864 |
| Disc height (mm) | |||
| at preoperative | 8.0 ± 2.4 | 8.4 ± 2.6 | .513 |
| at 2-year follow-up | 7.7 ± 1.5 | 8.4 ± 2.0 | .145 |
| Cage-related parameters | |||
| Cage size | .932 | ||
| 8 mm | 3 (13.6%) | 2 (10%) | |
| 9 mm | 9 (40.9%) | 9 (45%) | |
| 10 mm | 8 (36.4%) | 8 (40%) | |
| 11 mm | 2 (9.1%) | 1 (5%) | |
| Central point ratio (%) | 64.4 ± 10.8 | 66.3 ± 11.7 | .541 |
| Cage subsidence (%) | 18.5% | 37.0% | .129 |
| Fusion rate at 6 months (%) | 88.9% | 74.1% | .293 |
Note: Results are shown as mean ± standard deviation or the number of patients (%).
Table 3 summarizes the perioperative complications and implant-related complications identified during the follow-up. There were 2 complications in the Ti/PEEK group and 4 complications in the PEEK group. The total number of complications was not statistically different between the 2 groups (P = .669). There were no complications that needed reoperation for the 2 groups during the follow-up.
Table 3.
Complications and Reoperations in the 2 Groups.
| Complications or reoperations | Ti-PEEK group (n = 27) |
PEEK group (n = 27) |
P-value |
|---|---|---|---|
| Peri-operative | |||
| Dural tear | 1 | 1 | - |
| Wound problem/infection | 0 | 1 | - |
| Nerve root injury | 0 | 0 | - |
| Epidural hematoma | 0 | 0 | - |
| Implant-related | |||
| Screw loosening | 1 | 1 | - |
| Screw broken | 0 | 1 | - |
| Cage retropulsion/migration | 0 | 0 | - |
| Total | 2 | 4 | .669 |
| Reoperation due to complication | 0 | 0 | - |
Note: data was expressed as number of patients.
Table 4 summarizes the VAS and ODI scores for the 2 groups. The PRO scores based on VAS and ODI scores were not different during the preoperative and postoperative follow-ups. Both the VAS and ODI scores improved significantly after the index surgery at 2-year follow-up (P < .001). The reduction in the VAS score was found to be significantly higher in the Ti/PEEK group than in the PEEK group at 2-year follow-up (5.2 ± 2.2 vs. 3.7 ± 2.3, P = .026). The reduction in the ODI scores was not different in the 2 groups at 2-year follow-up (Ti/PEEK group, 35.8 ± 24.1; PEEK group, 33.8 ± 18.2, P = .732).
Table 4.
VAS and ODI Scores in the 2 Groups.
| Variables | Ti-PEEK group (n = 27) |
PEEK group (n = 27) |
P-value |
|---|---|---|---|
| VAS | |||
| preop | 6.9 ± 2.0 | 6.2 ± 2.1 | .194 |
| 3 months | 2.0 ± 1.8 | 2.3 ± 1.7 | .288 |
| 6 months | 1.6 ± 1.3 | 2.2 ± 2.0 | .149 |
| 1 year | 1.4 ± 1.3 | 1.8 ± 1.4 | .313 |
| 2 year | 1.7 ± 1.4 | 2.4 ± 1.7 | .124 |
| Change* | 5.2 ± 2.2 | 3.7 ± 2.3 | .026 |
| ODI | |||
| preop | 49.6 ± 18.1 | 48.7 ± 12.5 | .847 |
| 3 months | 16.1 ± 12.4 | 22.0 ± 14.1 | .11 |
| 6 months | 11.9 ± 11.4 | 13.9 ± 16.1 | .601 |
| 1 year | 13.8 ± 12.5 | 12.6 ± 14.3 | .735 |
| 2 year | 13.7 ± 12.8 | 14.9 ± 12.7 | .735 |
| Change* | 35.8 ± 24.1 | 33.8 ± 18.2 | .732 |
VAS, visual analogue scale; ODI, Oswestry disability index. Results are shown as mean ± standard deviation.
* Change was compared between preoperative and 2-year follow-up.
Discussion
The results of our study revealed that the Ti/PEEK composite cage could be safely used in MI-TLIF, and the complication rate achieved with the use of the Ti/PEEK composite cage was comparable to that of the PEEK cage. The 6-month fusion rate was high in the Ti/PEEK group (88.9%). However, the fusion rate and cage subsidence rate were not significantly different between the 2 groups. Both groups showed significantly improved PRO scores after undergoing the index surgery at 2-year follow-up. Interestingly, the reduction in the VAS scores was higher in the Ti/PEEK group than that noted in the PEEK group. One possible reason could be the relatively higher fusion rate and lower cage subsidence in the Ti/PEEK group than in the PEEK group, even though a significance was not reached.
Prior laboratory-based studies have reported promising results for the use of Ti/PEEK composite cages. An in vitro study reported that titanium promoted an inflammatory cellular response in its environment that favored bone formation, whereas PEEK implants promoted fibrosis. 24 Olivares-Navarrete et al. 25 found that titanium alloy substrates increase osteoblast maturation and produce an osteogenic environment that contained bone morphogenic proteins (BMP). The same study group also found that the microscopic rough surface of titanium alloy stimulated the osteogenic-angiogenic microenvironment. The osteogenic-angiogenic responses to titanium alloy were greater than those to PEEK. These factors may increase bone formation, enhance integration, and improve implant stability in interbody spinal fusions. 26 McGilvray et al. 16 conducted an animal study and found that the Ti/PEEK composite cage significantly increased the biomechanical stiffness and potentially led to a more robust intervertebral fusion than observed with the standard PEEK cage.
Only 2 clinical studies have reported the clinical outcome of Ti/PEEK composite cages, and both studies were case series without a control group. To the best of our knowledge, the present study is the first case-control study with a propensity-matched PEEK control group for MI-TLIF. Chong et al. reported a series of 25 patients using the Ti/PEEK composite cage for anterior cervical fusion. 19 The fusion rate was 96% at 6 months, and a good to excellent outcome in 92% of patients was reported with a mean follow-up of 14.6 months. Mobbs et al. reported using Ti/PEEK composite cages for anterior lumbar interbody fusion in 15 patients. 20 The fusion rate was 95% at 9-12 months, with a mean follow-up of 15 months. Both studies reported no cage related complications during the follow-up. The fusion rate observed in our study was lower than that observed in these 2 cohorts. One major reason is the difference in surgical approaches and anatomic locations between these studies. Our study focused on the outcome in MI-TLIF. We reported a 6-month fusion rate only, and the prior study reported a 2-year fusion rate of 91-97%. 27 Prior studies also reported that the fusion rate may increase over time.28,29 Furthermore, the definition of solid fusion and timing of CT scan were also different between these studies. One previous review had addressed the heterogeneity regarding a radiographic criterion for bony fusion assessment 17 ; therefore, we used CT scans instead of plain film radiography to evaluate fusion status. The CT scans provide better resolution of the bridging callus than plain films, which may overestimate the fusion rate. 30 We did not use BMP for enhancing fusion. Several studies have reported that the use of BMP exhibit a higher fusion success rate in MI-TLIF.31,32
The success of interbody fusion is assessed based on not only osteointegration over the vertebral endplate and cage surface but also on bridging bone formation within the cage and disc space. Our prior study reported that the bone graft area ratio of the whole disc space was 39.2%, including the cage surface area in solid union TLIF. 30 This meant that most of the bridging bone grew within the disc space rather than the cage surface. Moreover, the biologic properties of the microporous titanium endplate of the cage could not only enhance osteointegration on the cage but also produce an osteogenic-angiogenic microenvironment that increased the bone formation in the whole disc space. 16
The elastic modulus of the PEEK cage did not change after adding the titanium endplate.10,12,13 Theoretically, the stress shielding effect did not change in the Ti/PEEK composite cage, and the subsidence rate was comparable to that of the PEEK cages. Assem et al. reviewed the use of Ti/PEEK composite cages, which exhibited encouraging radiological results and validated their potential for increasing fusion rates and reducing cage subsidence (P > .05). 17 Our study reported similar findings with comparable fusion rates and cage subsidence with the use of the Ti/PEEK composite cage than with the use of the PEEK cage.
There were no prior studies that compared the PRO scores between the Ti/PEEK composite cage and the PEEK cage. We reported that using both the types of cages in MI-TLIF led no differences in the VAS and ODI scores in a 2-year follow-up period. However, the reduction in the VAS score was greater in the Ti/PEEK group than that in the PEEK group. Part et al. found that in MI-TLIF patients, a better reduction of back pain was noted in patients who achieved radiographic solid fusion. 33 This explains our results that the significantly higher VAS score reduction observed in the Ti/PEEK group than in the PEEK may be due to more solid fusion observed in the Ti/PEEK group.
There are some limitations to this study. First, the choice of cage type was not randomized; however, we used a propensity-matched method for reducing the effect of confounding bias between the 2 groups. Second, there was a lack of longer radiographic fusion rate evaluation and the postoperative CT scan was restricted to patients with refractory symptoms in the author’s country. Third, the sample size was small. Further large, prospective, randomized studies will be needed for studying the effects of the 2 cage types in MI-TLIF.
Conclusion
Our study reported that the Ti/PEEK composite cage could be safely used in patients receiving MI-TLIF, without causing a higher number of complications than those observed with the PEEK cage. There was no difference between fusion rate and the cage subsidence rate in patients receiving these cage types. There were significantly improved PRO scores with both the Ti/PEEK composite cage and PEEK cage during 2-year follow-up. Our finding revealed comparable clinical results for surgeons using Ti/PEEK composite cages in MI-TLIF compared to those using the PEEK cage.
Footnotes
Authors’ Note: Ethical approval was obtained from the Institutional Review Board (IRB-TPEVGH No.:2015-08-006ACF).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yu-Cheng Yao, MD  https://orcid.org/0000-0001-7555-4321
https://orcid.org/0000-0001-7555-4321
Po-Hsin Chou, MD, PhD  https://orcid.org/0000-0001-5899-1124
https://orcid.org/0000-0001-5899-1124
Ming-Chau Chang, MD  https://orcid.org/0000-0002-4799-9339
https://orcid.org/0000-0002-4799-9339
References
- 1.Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14(6):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LE, , Bhattacharyya S, , Pracyk J. Minimally invasive versus open transforaminal lumbar interbody fusion for single-level degenerative disease: a systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 2020;133:358–365.e4. [DOI] [PubMed] [Google Scholar]
- 3.Ge DH, Stekas ND, Varlotta CG, et al. Comparative analysis of two transforaminal lumbar interbody fusion techniques: open TLIF versus Wiltse MIS TLIF. Spine (Phila Pa 1976). 2019;44(9): E555–E560. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Mok J, Patel P. Transforaminal lumbar interbody fusion: traditional open versus minimally invasive techniques. J Am Acad Orthop Surg. 2018;26(4):124–131. [DOI] [PubMed] [Google Scholar]
- 5.Price JP, Dawson JM, Schwender JD, Schellhas KP. Clinical and radiologic comparison of minimally invasive surgery with traditional open transforaminal lumbar interbody fusion: a review of 452 patients from a single center. Clin Spine Surg. 2018;31(2): E121–E126. [DOI] [PubMed] [Google Scholar]
- 6.Weiss H, Garcia RM, Hopkins B, Shlobin N, Dahdaleh NS. A systematic review of complications following minimally invasive spine surgery including transforaminal lumbar interbody fusion. Curr Rev Musculoskelet Med. 2019;12(3):328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makanji H, Schoenfeld AJ, Bhalla A, Bono CM. Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J. 2018;27(8):1868–1876. [DOI] [PubMed] [Google Scholar]
- 8.Patel DV, Yoo JS, Karmarkar SS, Lamoutte EH, Singh K. Interbody options in lumbar fusion. J Spine Surg. 2019;5(Suppl 1): S19–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Eltorai AE, Ruttiman R, Daniels AH. Advances in spinal interbody cages. Orthop Surg. 2016;8(3):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan K, Mobbs RJ. Evolution of design of interbody cages for anterior lumbar interbody fusion. Orthop Surg. 2016;8(3):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong E, Pelletier MH, Mobbs RJ, Walsh WR. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review orthopedics and biomechanics. BMC Musculoskelet Disord. 2015;16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23–29. [DOI] [PubMed] [Google Scholar]
- 14.Warburton A, Girdler SJ, Mikhail CM, Ahn A, Cho SK. Biomaterials in spinal implants: a review. Neurospine. 2020;17(1):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon BJ, Xavier F, Walker BR, Grinberg S, Cammisa FP, Abjornson C. Optimizing surface characteristics for cell adhesion and proliferation on titanium plasma spray coatings on polyetheretherketone. Spine J. 2016;16(10):1238–1243. doi:10.1016/j.spinee.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 16.McGilvray KC, Waldorff EI, Easley J, et al. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses. Spine J. 2017;17(12):1907–1916. [DOI] [PubMed] [Google Scholar]
- 17.Assem Y, Mobbs RJ, Pelletier MH, Phan K, Walsh WR. Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: a systematic review and preclinical evaluation. Eur Spine J. 2017;26(3):593–605. [DOI] [PubMed] [Google Scholar]
- 18.Massaad E, Fatima N, Kiapour A, Hadzipasic M, Shankar GM, Shin JH. Polyetheretherketone versus titanium cages for posterior lumbar interbody fusion: meta-analysis and review of the literature. Neurospine. 2020;17(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong E, Mobbs RJ, Pelletier MH, Walsh WR. Titanium/polyetheretherketone cages for cervical arthrodesis with degenerative and traumatic pathologies: early clinical outcomes and fusion rates. Orthop Surg. 2016;8(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobbs RJ, Phan K, Assem Y, Pelletier M, Walsh WR. Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: early clinical and radiological results. J Clin Neurosci. 2016;34:94–99. [DOI] [PubMed] [Google Scholar]
- 21.Landham PR, Don AS, Robertson PA. Do position and size matter? An analysis of cage and placement variables for optimum lordosis in PLIF reconstruction. Eur Spine J. 2017;26(11):2843–2850. [DOI] [PubMed] [Google Scholar]
- 22.Kim MC, Chung HT, Cho JL, Kim DJ, Chung NS. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(2):87–92. [DOI] [PubMed] [Google Scholar]
- 23.Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8(4):570–577. [DOI] [PubMed] [Google Scholar]
- 24.Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine (Phila Pa 1976). 2015;40(6):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater. 2014;10(8):3363–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Formica M, Vallerga D, Zanirato A, et al. Fusion rate and influence of surgery-related factors in lumbar interbody arthrodesis for degenerative spine diseases: a meta-analysis and systematic review. Musculoskelet Surg. 2020;104(1):1–15. doi:10.1007/s12306-019-00634-x. [DOI] [PubMed] [Google Scholar]
- 28.Ito Z, Imagama S, Kanemura T, et al. Volumetric change in interbody bone graft after posterior lumbar interbody fusion (PLIF): a prospective study. Eur Spine J. 2014;23(10):2144–2149. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Lee JH, Park JW, Lee HS. Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three-dimensional computed tomography scans. Spine J. 2011;11(7):647–653. [DOI] [PubMed] [Google Scholar]
- 30.Yao YC, Lin HH, Chou PH, Wang ST, Chang MC. Differences in the interbody bone graft area and fusion rate between minimally invasive and traditional open transforaminal lumbar interbody fusion: a retrospective short-term image analysis. Eur Spine J. 2019;28(9):2095–2102. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Wang Y, Liang Z, Zhou M, Chen C.Comparative clinical effectiveness and safety of bone morphogenetic protein versus autologous iliac crest bone graft in lumbar fusion: a meta-analysis and systematic review. Spine (Phila Pa 1976). 2020;45(12): E729–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parajón A, Alimi M, Navarro-Ramirez R, et al. Minimally invasive transforaminal lumbar interbody fusion: meta-analysis of the fusion rates. What is the optimal graft material? Neurosurgery. 2017;81(6):958–971. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Ha JW, Lee YT, Sung NY. The effect of a radiographic solid fusion on clinical outcomes after minimally invasive transforaminal lumbar interbody fusion. Spine J. 2011;11(3):205–212. [DOI] [PubMed] [Google Scholar]