Abstract
Tularemia is a rare but potentially serious bacterial zoonosis, which has been reported in the 47 contiguous states of the USA during 2001–2010. This report summarizes the passive surveillance data of tularemia cases reported to the Centers for Disease Control and Prevention from 2011 through 2019. There were 1984 cases reported in the USA during this period. The average national incidence was 0.07 cases per 100,000 person-years (PY), compared to 0.04 cases per 100,000 PY during 2001–2010. The highest statewide reported case 2011–2019 was in Arkansas (374 cases, 20.4% of total), followed by Missouri (13.1%), Oklahoma (11.9%), and Kansas (11.2%). Regarding race, ethnicity, and sex, tularemia cases were reported more frequently among white, non-Hispanic, and male patients. Cases were reported in all age groups; however, individuals 65 years-old and older exhibited the highest incidence. The seasonal distribution of cases generally paralleled the seasonality of tick activity and human outdoor activity, increasing during spring through mid-summer and decreasing through late summer and fall to winter lows. Improved surveillance and education of ticks and tick- and water-borne pathogens should play a key role in efforts to decrease the incidence of tularemia in the USA.
Keywords: Centers for Disease Control and Prevention (CDC), Francisella tularensis, Incidence, National Notifiable Diseases Surveillance System (NNDSS), Tularemia
Graphical abstract
Highlights
-
•
Tularemia is a rare but potentially serious bacterial zoonosis.
-
•
Tularemia cases increase in the USA from January 2011 through December 2019.
-
•
The seasonal distribution of cases increases during spring through mid-summer.
-
•
Improved surveillance and education of ticks and tick- and water-borne pathogens is important.
1. Introduction
The highly infectious causative agent of tularemia is Francisella tularensis (Nelson et al., 2013). Transmission can occur via the bites of arthropods, contact with infected animal tissue, ingestion of contaminated food or water, or inhalation of contaminated aerosols from contaminated soil, water, or plants (Nelson et al., 2013; Carvalho et al., 2014; Frischknecht et al., 2019). In the USA tularemia is primarily associated with two highly infectious bacterial agents Francisella tularensis subspecies tularensis (Type A) or F. tularensis subspecies holarctica (Type B) (Nelson et al., 2013; Mani et al., 2016). Both types differ in their virulence and ecological niches. The majority of tularemia cases in the USA (c.90%) are caused by Type A (Dahlgren et al., 2011). Two genotypes within Type A have been characterized: A1 which occurs in the central states is the most virulent while A2 is found within the western states and is less virulent (Farlow et al., 2005). Subtype A1 has been associated with lagomorphs, cats, and ticks while subtype A2 is associated with deer flies (Chrysops spp.) (Staples et al., 2006). Type B is moderately virulent compared to Type A and is mainly associated with rodents and around water bodies.
Blood-feeding arthropods, particularly ticks (Ixodidae: Dermacentor andersoni, D. occidentalis, D. variabilis and Amblyomma americanum) and deer flies (Chrysops discalis) are the most significant vectors of the agents of tularemia in the USA (Francis & Mayne, 1921; Parker et al., 1924, 1932; Hillman & Morgan, 1937; Calhoun, 1954; Klock et al., 1973; Goethert et al., 2004; Farlow et al., 2005; Mani et al., 2015). Symptoms differ depending on type of exposure and F. tularensis-Type contracted (Tärnvik & Chu, 2007). Generalized symptoms include headaches, coughing, vomiting, body aches, fatigue, and onset fever and chills (Tärnvik & Chu, 2007). Since symptoms are relatively non-specific and flu-like, diagnosis can be delayed or incorrect (Sjöstedt, 2007). However, if the bacteria entered dermatologically or orally, a medical examination depicts tender, enlarged lymph nodes (Nelson et al., 2013). Streptomycin, gentamicin, doxycycline, ciprofloxacin, fluoroquinolones are the choices of antibiotics to treat tularemia (CDC, 2018a; Imbimbo et al., 2020). The prevention methods include avoiding areas with tick infestation, avoiding tick bites by using repellants and wearing long-sleeved shirts and thick pants (Tärnvik & Chu, 2007), avoiding animal contact (Carvalho et al., 2014), and having access to safe drinking water (Hennebique et al., 2019). Moreover, until recently, a vaccine has been available to protect laboratory staff routinely working with F. tularensis (CDC, 2018b). The vaccine is currently under review by the U.S. Food and Drug Administration (FDA) and is not generally available in the USA (CDC, 2018b). Risk from contracting severe forms of tularemia, such as pneumonic or typhoidal tularemia, can result in death or permanent bodily injury (Staples et al., 2006).
The ecology and epidemiology of tularemia is complex, and many questions remain as to how the bacteria is maintained and sustained in nature (Telford & Goethert, 2020). Therefore, it is important to continue surveillance to identify geographical and temporal trends to predict and prevent human illness. In this study, we summarize the passive surveillance data associated with tularemia cases reported to the Centers for Disease Control and Prevention (CDC) from January 2011 through December 2019. Our aim is to characterize the epidemiology of reported cases of tularemia with the goal of increasing awareness of recent trends. We focus on recent trends in reported cases of tularemia and demographic profiles, as well as the spatial-temporal patterns of the cases.
2. Materials and methods
2.1. National surveillance systems in the USA
Jurisdictions ranging from local to state and territorial health department levels report surveillance data on roughly 120 diseases to the CDC using the National Notifiable Diseases Surveillance System (NNDSS) (Koo & Wetterhall, 1996). CDC summaries of NNDSS reports contain basic demographic data (age, sex, race, ethnicity, state of residence). Health departments, healthcare providers, hospitals, and laboratories can also report clinical data regarding tularemia infections by manually completing Tularemia Case Report Forms (https://www.cdc.gov/tularemia/resources/TularemiaCaseReportForm.pdf) as supplementary case data (e.g. whether a tick, deerfly, or unknown insect bite was found on the patient’s body). However, not all NNDSS reports have a corresponding Tularemia Case Report Form, and these supplementary data are not publicly available. We accessed the data for the present study via the publicly available Morbidity and Mortality Weekly Report (https://www.cdc.gov/mmwr/mmwr_nd/index.html) at NNDSS.
2.2. Case definition
A confirmed case of tularemia is clinically compatible with confirmatory laboratory results (CDC, 2017). Confirmed cases could also have at least a 4-fold change in serum antibody titer to F. tularensis antigen (CDC, 2017). Probable cases are clinically compatible and have supportive laboratory evidence in which there are elevated serum antibody titers to F. tularensis antigen or detection of F. tularensis in a specimen through fluorescent assay (CDC, 2017). Clinical forms including ulceroglandular, glandular, oculoglandular, oropharyngeal, pneumonic, and typhoidal depend on the different routes of acquisition of infection (CDC, 2018c; Imbimbo et al., 2020).
2.3. Analysis
This analysis includes passive surveillance data of tularemia cases reported to the CDC from January 2011 through December 2019. If the exact date of onset of infection was unknown, the earliest known date associated with the case was used to represent the infection date. Data from the U.S. Census Bureau for the years 2011 through 2019 were used to calculate incidence as infections per 100,000 persons per year (PY) (Nelson et al., 2013). Because cases cannot be generalized to other periods of time or to unreported cases, this report does not contain confidence intervals or results from statistical hypothesis testing (Dahlgren et al., 2011).
3. Results and discussion
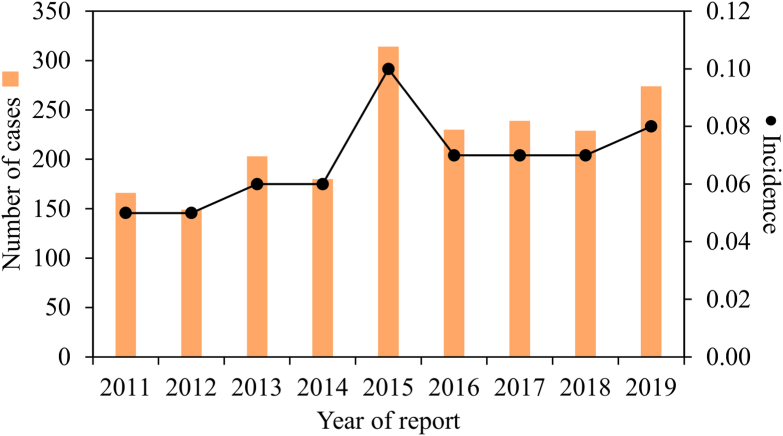
A total of 1984 tularemia cases were reported from 2011 through 2019. The average incidence over this time period was 0.07 cases per 100,000 PY, compared to 0.04 cases per 100,000 PY from 2001 through 2010 (Nelson et al., 2013). There was a steady annual increase in cases from a low of 166 in 2011 to a high of 314 in 2015 (Fig. 1). Overall, tularemia incidence increased by c.60% from 2011 (incidence of c.0.05) to 2019 (incidence of c.0.08).
Fig. 1.
Number of reported cases and associated incidence of tularemia in the USA from 2011 to 2019. Bars represent total number of reported cases. Dots represent incidence (number of infections per 100,000 persons per year). Based on data from the Nationally Notifiable Diseases Surveillance System (NNDSS).
Demographic patterns of tularemia cases regarding sex, age, race, and ethnicity indicated there was higher incidence among males (64.6%) compared to females (34.6%), among the ≥ 65 years of age (20.9%), among whites (67.1%), and among non-Hispanics (72.6%) (Table 1). The highest incidence by race, which remained the same as Nelson et al. (2013) reported during 2001–2010, was among American Indians or Native Americans (0.33 cases per 100,000 PY) (Table 1). It is also important to note there was a high percentage of instances that were “unspecified/unknown” with regard to both ethnicity (23.3%) and race (21.3%). As expected, over half of the total cases (59.9%) occurred in adults 40 years and older, and children (1–14 years-old) also showed a high tularemia incidence (0.07 cases per 100,000 PY). The patterns likely reflect behavioral differences among demographic groups affecting ways of transmission (e.g. outdoor activity patterns, portal of entry) (Bayles et al., 2013; Imbimbo et al., 2020), as well as age-specific physiological differences (Mogg et al., 2020). Generally, the demographic patterns during 2011–2019 were similar to those reported during 2001–2010 (Nelson et al., 2013). Of course, the inherent bias in reported data must be considered, as not all demographic groups have equal access to healthcare in the USA (Jones et al., 2012; Bishop et al., 2021) while increasing testing may have resulted in more cases being reported (Ly et al., 2017). Moreover, missing data and small numbers can limit statistical analyses, reduce statistical power, and produce biased estimates and interpretation (Nelson et al., 2013).
Table 1.
Demographic profiles of tularemia cases in the USA reported to the Centers for Disease Control and Prevention from January 2011 through December 2019.
| Variable | Category | No. of cases | Incidencea |
|---|---|---|---|
| Sex | Male | 1282 | 0.09 |
| Female | 687 | 0.05 | |
| Unknown | 15 | – | |
| Race | American Indian or Native American | 132 | 0.33 |
| Asia/Pacific Islander | 16 | 0.01 | |
| Black or African American | 34 | 0.01 | |
| White | 1332 | 0.06 | |
| Other | 48 | – | |
| Unspecified/Unknown | 422 | – | |
| Ethnicity | Hispanic | 82 | 0.02 |
| Non-Hispanic | 1440 | 0.06 | |
| Unspecified/Unknown | 462 | – | |
| Age group | < 1 year | 6 | 0.02 |
| 1–4 years | 107 | 0.07 | |
| 5–14 years | 258 | 0.07 | |
| 15–24 years | 136 | 0.04 | |
| 25–39 years | 262 | 0.04 | |
| 40–64 years | 774 | 0.08 | |
| ≥ 65 years | 414 | 0.10 | |
| Unknown/Not specified | 27 | – |
Incidence defined as the number of reported cases per 100,000 persons per year.
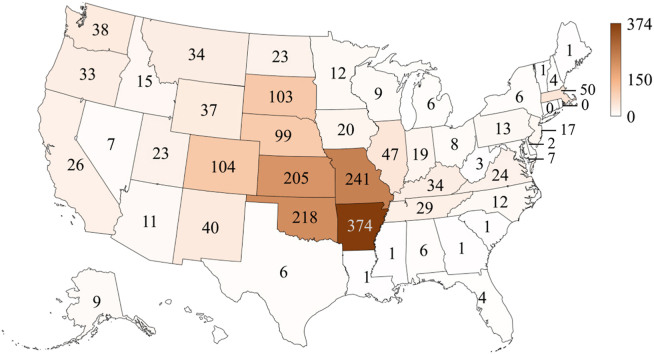
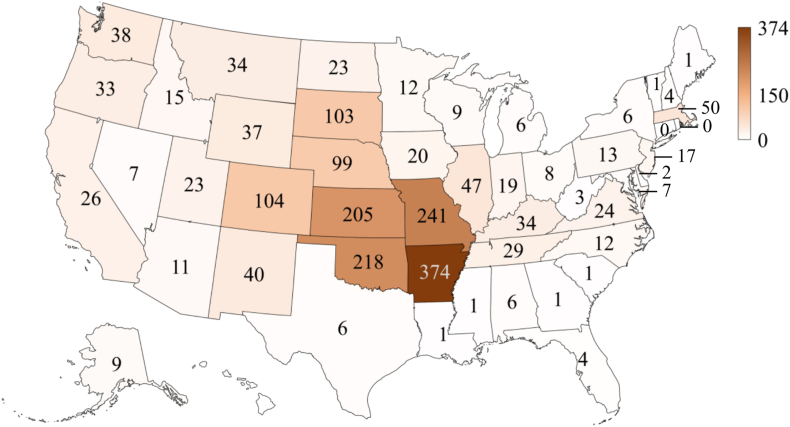
As for the reported cases, Arkansas had the highest number of cases (n = 374, 20.4% of the total), followed by Missouri (n = 241, 13.1%), Oklahoma (n = 218, 11.9%), and Kansas (n = 205, 11.2%) while Connecticut, the District of Columbia, Georgia, Hawaii, Maine, Mississippi, New York City, and Rhode Island did not have any case reported (Fig. 2). Tularemia-associated tick species include A. americanum (lone star tick), D. andersoni (Rocky Mountain wood tick), D. occidentalis (Pacific coast tick), and D. variabilis (American dog tick) in the USA (Zellner & Huntley, 2019). Among the four tick species, D. variabilis and A. americanum are the two most important tick vectors of human tularemia found within the central USA occurring in Arkansas, Kansas, Missouri and Oklahoma (Petersen & Schriefer, 2005; Zellner & Huntley, 2019) where the states had the highest number of reported tularemia cases. Dermacentor andersoni along with C. discalis are often associated with human tularemia in the western USA (Farlow et al., 2005). Dermacentor occidentalis is one of the most common tick species found in California and has been shown as a vector of human tularemia within the state (Gurfield et al., 2017). Moreover, the high number of tularemia cases in the central USA could also result from animal contact (e.g. a rabbit enzootic cycle) (Mani et al., 2016).
Fig. 2.
Spatial patterns of reported cases of tularemia in the USA from 2011 to 2019 (n = 1984) based on data from the Nationally Notifiable Diseases Surveillance System (NNDSS).
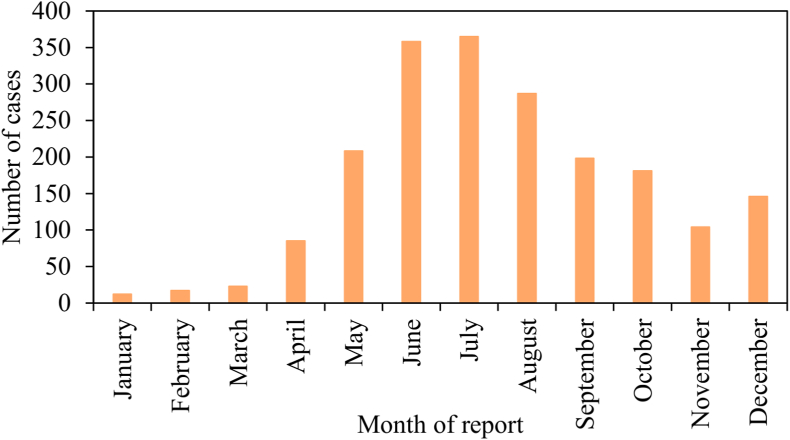
The seasonal distribution of cases generally paralleled the seasonality of tick host-seeking activity, vegetation development, host availability, and human outdoor activity (Randolph, 2011; Baker et al., 2020; Bishop et al., 2022), increasing markedly during April and May, peaking during June and July, and decreasing through late summer and fall to winter lows (Fig. 3). Cases were relatively uncommon between January and March. The survival and activity levels of host-seeking ticks depend on microclimate conditions, and abundances fluctuate seasonally depending on the availability of hosts (Wang et al., 2012, 2015, 2016). Thus, a causal chain of ecological factors likely contributing to the seasonal fluctuations in tularemia cases consists of seasonally varying rainfall, which drives vegetation growth, affecting host (rodent and rabbit) populations and pathogen survival (Pedati et al., 2015).
Fig. 3.
Month of report of tularemia cases in the USA from 2011 to 2019 (n = 1984) based on data from the Nationally Notifiable Diseases Surveillance System (NNDSS).
4. Conclusions
Tularemia incidence in the USA increased by c.60% from 2011 to 2019, which is particularly alarming given the high infectivity and high morbidity of this disease. Prevention and diagnosis of tularemia are directly linked with knowledge of medically important tick vectors, such as D. variabilis and A. americanum (Eisen & Paddock, 2021), animal contact (Carvalho et al., 2014), and contaminated water (Hennebique et al., 2019). To better combat the disease, efficiency of tick and tick-borne pathogen surveillance should be improved at both state and local levels (Eisen & Paddock, 2021), and access to safe drinking water via treatment of municipal and private sources should be provided (Hennebique et al., 2019). Water reservoirs should be monitored in the overall tularemia surveillance, in addition to a range of wild and domestic animals which may function as the reservoir for tularemia (Carvalho et al., 2014; Hennebique et al., 2019). At the individual level, residents and visitors to areas where tick vectors are common should use insect repellent frequently, wear gloves when handling animals, avoid mowing in areas where sick or dead animals have been reported, and do not drink untreated surface water (Pedati et al., 2015; Hennebique et al., 2019).
Funding
The authors received no specific funding for this work.
Ethical approval
Not applicable.
CRediT author statement
Conceptualization: Alexandra Bishop and Hsiao-Hsuan Wang. Methodology: Alexandra Bishop and Hsiao-Hsuan Wang. Data curation: Alexandra Bishop, Emily Brockinton, Esha Kothapalli, Scott Clark, Tanvi Vishwanath, Tatyana Canales and Krishnendu Sreekumar. Validation: Hsiao-Hsuan Wang. Formal analysis: Alexandra Bishop, Hsiao-Hsuan Wang, Emily Brockinton, Esha Kothapalli, Scott Clark, Tanvi Vishwanath, Tatyana Canales and Krishnendu Sreekumar. Writing - original draft: Alexandra Bishop and Hsiao-Hsuan Wang. Writing - review and editing: Taylor G. Donaldson, William E. Grant, Pete D. Teel. Visualization: Alexandra Bishop and Hsiao-Hsuan Wang. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the Editor-in-Chief, Dr. Aneta Kostadinova, and two anonymous reviewers for their time and effort. The manuscript is greatly improved as a result of their comments.
References
- Baker A., Wang H.-H., Mogg M., Derouen Z., Borski J., Grant W.E. Increasing incidence of anaplasmosis in the United States, 2012 through 2016. Vector Borne Zoonotic Dis. 2020;20:855–859. doi: 10.1089/vbz.2019.2598. [DOI] [PubMed] [Google Scholar]
- Bayles B.R., Evans G., Allan B.F. Knowledge and prevention of tick-borne diseases vary across an urban-to-rural human land-use gradient. Ticks Tick Borne Dis. 2013;4:352–358. doi: 10.1016/j.ttbdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Bishop A., Borski J., Wang H.-H., Donaldson T.G., Michalk A., Montgomery A., et al. Vector-Borne Zoonotic Dis; 2022. Increasing incidence of spotted fever group rickettsioses in the United States, 2010–2018. [DOI] [PubMed] [Google Scholar]
- Bishop A., Wang H.-H., Grant W.E. Using data surveillance to understand the rising incidence of babesiosis in the United States, 2011–2018. Vector Borne Zoonotic Dis. 2021;21:391–395. doi: 10.1089/vbz.2020.2754. [DOI] [PubMed] [Google Scholar]
- Calhoun E.L. Natural occurrence of tularemia in the lone star tick, Amblyomma americanum (Linn.), and in dogs in Arkansas. Am. J. Trop. Med. Hyg. 1954;3:360–366. doi: 10.4269/ajtmh.1954.3.360. [DOI] [PubMed] [Google Scholar]
- Carvalho C.L., Lopes de Carvalho I., Zé-Zé L., Núncio M.S., Duarte E.L. Tularaemia: a challenging zoonosis. Comp. Immunol. Microbiol. Infect. Dis. 2014;37:85–96. doi: 10.1016/j.cimid.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, USA: 2017. Case Definition: Tularemia.https://ndc.services.cdc.gov/case-definitions/tularemia-2017/ [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, USA: 2018. Diagnosis & Treatment: Tularemia.https://www.cdc.gov/tularemia/diagnosistreatment/ [Google Scholar]
- CDC . Tularemia. Centers for Disease Control and Prevention; Atlanta, USA: 2018. Prevention.https://www.cdc.gov/tularemia/prevention/index.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, USA: 2018. Signs & Symptoms: Tularemia.https://www.cdc.gov/tularemia/signssymptoms/index.html [Google Scholar]
- Dahlgren F.S., Mandel E.J., Krebs J.W., Massung R.F., McQuiston J.H. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R.J., Paddock C.D. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 2021;58:1490–1502. doi: 10.1093/jme/tjaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow J., Wagner D.M., Dukerich M., Stanley M., Chu M., Kubota K., et al. Francisella tularensis in the United States. Emerg. Infect. Dis. 2005;11:1835–1841. doi: 10.3201/eid1112.050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis E., Mayne B. Experimental transmission of tularaemia by flies of the species Chrysops discalis. US Public Health Serv. Hyg. Lab. Bul. 1921;138:8–16. [Google Scholar]
- Frischknecht M., Meier A., Mani B., Joerg L., Kim O.C.-H., Boggian K., Strahm C. Tularemia: An experience of 13 cases including a rare myocarditis in a referral center in Eastern Switzerland (Central Europe) and a review of the literature. Infection. 2019;47:683–695. doi: 10.1007/s15010-019-01269-7. [DOI] [PubMed] [Google Scholar]
- Goethert H.K., Shani I., Telford S.R. Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Marthaʼs Vineyard, Massachusetts. J. Clin. Microbiol. 2004;42:4968–4973. doi: 10.1128/JCM.42.11.4968-4973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfield N., Grewal S., Cua L.S., Torres P.J., Kelley S.T. Endosymbiont interference and microbial diversity of the Pacific coast tick, Dermacentor occidentalis, in San Diego County, California. PeerJ. 2017;5 doi: 10.7717/peerj.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebique A., Boisset S., Maurin M. Tularemia as a waterborne disease: A review. Emerg. Microb. Infect. 2019;8:1027–1042. doi: 10.1080/22221751.2019.1638734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.C., Morgan M.T. Tularemia. Report of a fulminant epidemic transmitted by the deer fly. JAMA. 1937;108:538–540. [Google Scholar]
- Imbimbo C., Karrer U., Wittwer M., Buettcher M. Tularemia in children and adolescents. Pediatr. Infect. Dis. J. 2020;39:e435–e438. doi: 10.1097/INF.0000000000002932. [DOI] [PubMed] [Google Scholar]
- Jones S.G., Conner W., Song B., Gordon D., Jayakaran A. Comparing spatio-temporal clusters of arthropod-borne infections using administrative medical claims and state reported surveillance data. Spat. Spatiotemporal Epidemiol. 2012;3:205–213. doi: 10.1016/j.sste.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Klock L.E., Olsen P.F., Fukushima T. Tularemia epidemic associated with the deerfly. JAMA. 1973;226:149–152. [PubMed] [Google Scholar]
- Koo D., Wetterhall S.F. History and current status of the National Notifiable Diseases Surveillance System. J. Publ. Health Manag. Pract. 1996;2:4–10. doi: 10.1097/00124784-199623000-00004. [DOI] [PubMed] [Google Scholar]
- Ly K.N., Jiles R.B., Teshale E.H., Foster M.A., Pesano R.L., Holmberg S.D. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann. Intern. Med. 2017;166:775–782. doi: 10.7326/M16-2350. [DOI] [PubMed] [Google Scholar]
- Mani R.J., Metcalf J.A., Clinkenbeard K.D. Amblyomma americanum as a bridging vector for human infection with Francisella tularensis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani R.J., Morton R.J., Clinkenbeard K.D. Ecology of tularemia in central US endemic region. Curr. Trop. Med. Rep. 2016;3:75–79. doi: 10.1007/s40475-016-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg M., Wang H.H., Baker A., Derouen Z., Borski J., Grant W.E. Increased incidence of Ehrlichia chaffeensis infections in the United States, 2012 through 2016. Vector Borne Zoonotic Dis. 2020;20:547–550. doi: 10.1089/vbz.2019.2595. [DOI] [PubMed] [Google Scholar]
- Nelson C., Kugeler K., Petersen J., Mead P. Tularemia - United States, 2001–2010. MMWR Morb. Mortal. Wkly. Rep. 2013;62:963–966. [PMC free article] [PubMed] [Google Scholar]
- Parker R., Spencer R., Francis E. Tularæmia: XI. Tularæmia infection in ticks of the species Dermacentor andersoni stiles in the bitterroot valley, mont. Publ. Health Rep. 1924;39:1057–1073. https://www.jstor.org/stable/pdf/4577151.pdf 1896–1970. [Google Scholar]
- Parker R.R., Phillips C.B., Davis G.E. Tularaemia: Occurrence in the sage hen, Centrocercus urophasianus. Publ. Health Rep. 1932;47:479–487. [Google Scholar]
- Pedati C., House J., Hancock-Allen J., Colton L., Bryan K., Ortbahn D., et al. Increase in human cases of tularemia - Colorado, Nebraska, South Dakota, and Wyoming, January-September 2015. MMWR Morb. Mortal. Wkly. Rep. 2015;64:1317–1318. doi: 10.15585/mmwr.mm6447a4. [DOI] [PubMed] [Google Scholar]
- Petersen J.M., Schriefer M.E. Tularemia: Emergence/re-emergence. Vet. Res. 2005;36:455–467. doi: 10.1051/vetres:2005006. [DOI] [PubMed] [Google Scholar]
- Randolph S.E. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labudaʼs enduring paradigm. Ticks Tick Borne Dis. 2011;2:179–182. doi: 10.1016/j.ttbdis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Sjöstedt A. Tularemia: History, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- Staples J.E., Kubota K.A., Chalcraft L.G., Mead P.S., Petersen J.M. Epidemiologic and molecular analysis of human tularemia, United States, 1964–2004. Emerg. Infect. Dis. 2006;12:1113–1118. doi: 10.3201/eid1207.051504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tärnvik A., Chu M.C. New approaches to diagnosis and therapy of tularemia. Ann. N. Y. Acad. Sci. 2007;1105:378–404. doi: 10.1196/annals.1409.017. [DOI] [PubMed] [Google Scholar]
- Telford S.R., III, Goethert H.K. Ecology of Francisella tularensis. Annu. Rev. Entomol. 2020;65:351–372. doi: 10.1146/annurev-ento-011019-025134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-H., Grant W.E., Teel P.D. Simulation of climate-host-parasite-landscape interactions: A spatially explicit model for ticks (Acari: Ixodidae) Ecol. Model. 2012;243:42–62. [Google Scholar]
- Wang H.-H., Grant W.E., Teel P.D., Hamer S.A. Simulation of climate-tick-host-landscape interactions: Effects of shifts in the seasonality of host population fluctuations on tick densities. J. Vector Ecol. 2015;40:247–255. doi: 10.1111/jvec.12161. [DOI] [PubMed] [Google Scholar]
- Wang H.-H., Grant W.E., Teel P.D., Hamer S.A. Tick-borne infectious agents in nature: Simulated effects of changes in host density on spatial-temporal prevalence of infected ticks. Ecol. Model. 2016;323:77–86. [Google Scholar]
- Zellner B., Huntley J.F. Ticks and tularemia: Do we know what we donʼt know? Front. Cell. Infect. Microbiol. 2019;9:146. doi: 10.3389/fcimb.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]