Abstract
We investigated the cellular mechanisms that led to growth inhibition, morphological changes, and lysis of Bacillus cereus WSBC 10030 when it was challenged with a long-chain polyphosphate (polyP). At a concentration of 0.1% or higher, polyP had a bacteriocidal effect on log-phase cells, in which it induced rapid lysis and reductions in viable cell counts of up to 3 log units. The cellular debris consisted of empty cell wall cylinders and polar caps, suggesting that polyP-induced lysis was spatially specific. This activity was strictly dependent on active growth and cell division, since polyP failed to induce lysis in cells treated with chloramphenicol and in stationary-phase cells, which were, however, bacteriostatically inhibited by polyP. Similar observations were made with B. cereus spores; 0.1% polyP inhibited spore germination and outgrowth, and a higher concentration (1.0%) was even sporocidal. Supplemental divalent metal ions (Mg2+ and Ca2+) could almost completely block and reverse the antimicrobial activity of polyP; i.e., they could immediately stop lysis and reinitiate rapid cell division and multiplication. Interestingly, a sublethal polyP concentration (0.05%) led to the formation of elongated cells (average length, 70 μm) after 4 h of incubation. While DNA replication and chromosome segregation were undisturbed, electron microscopy revealed a complete lack of septum formation within the filaments. Exposure to divalent cations resulted in instantaneous formation and growth of ring-shaped edges of invaginating septal walls. After approximately 30 min, septation was complete, and cell division resumed. We frequently observed a minicell-like phenotype and other septation defects, which were probably due to hyperdivision activity after cation supplementation. We propose that polyP may have an effect on the ubiquitous bacterial cell division protein FtsZ, whose GTPase activity is known to be strictly dependent on divalent metal ions. It is tempting to speculate that polyP, because of its metal ion-chelating nature, indirectly blocks the dynamic formation (polymerization) of the Z ring, which would explain the aseptate phenotype.
Inorganic polyphosphate (polyP) is a food additive that is widely used (especially in the meat and dairy industry) to protect flavor and increase yields and because of its water-binding capacity and emulsification properties. polyP is generally recognized as safe. Inhibition of microbial growth by polyP has been the subject of numerous investigations (24, 29). In general, gram-positive bacteria appear to be more sensitive than gram-negative organisms. Still, our knowledge of the basis of the antimicrobial activity of polyP is limited; the ability of polyP to chelate cations is regarded as being responsible for the observed inhibitory effects (11, 13). Lee and coworkers (15, 17) found that certain polyP had a lytic effect on Staphylococcus aureus and concluded that the macromolecules bind to the cell wall, remain bound, and chelate structurally essential metals, which then destabilizes the cell wall and leads to lysis. In contrast, other investigators observed that hexametaphosphate had only a bacteriostatic effect on S. aureus and hypothesized that polyP triggers leakage of magnesium from cells, loss of osmoregulation, and membrane damage (21, 22).
The objective of the present study was to investigate the mechanism of polyP inhibition in more detail. The long-chain polyP used in this study was previously shown to have inhibitory effects on sporeformers and a range of other gram-positive organisms in liquid cultures and processed cheese (18). Here, we used Bacillus cereus as a model organism; this bacterium is an important food-related, toxin-producing, gram-positive sporeformer which was as sensitive to polyP inhibition as various clostridia were (18, 20). We found that higher polyP concentrations are bacteriocidal and cause immediate cell lysis, whereas sublethal concentrations affect septum formation, which results in the formation of filamentous, aseptate, multinucleate cells. Septation and division resume immediately after exposure to divalent cations. Our findings suggest that polyP has an indirect effect on the general division machinery of cells, the Fts protein family.
MATERIALS AND METHODS
Bacterial strains, spores, and culture conditions.
B. cereus WSBC 10030 (Weihenstephan Bacillus Collection) was used in all experiments and was cultivated in plate count media (per liter: 5 g of peptone, 2.5 g of yeast extract, 1 g of glucose) with shaking. Solid medium for agar plates was supplemented with 1.5% agar. The incubation temperature was always 30°C. Spores of B. cereus were produced in liquid culture, in which the organism sporulated very well within 4 days. Spore suspensions were centrifuged, washed five times with ultrapure water (Milli-Q; Millipore, Bedford, Mass.), and stored at 4°C. Before spores were used, they were heat shocked (80°C, 10 min) to inactivate any residual vegetative cells and to induce germination.
Chemicals.
A commercially available, food grade, long-chain sodium polyphosphate glassy (JOHA HBS; 69.0% ± 1% P2O5; white powder; pH in solution, 6.4 ± 0.5; BK Giulini Chemie GmbH, Ladenburg, Germany) was used in this study. A 10% (wt/vol) stock solution was prepared in ultrapure water, adjusted to pH 6.8 with 1 M NaOH, autoclaved for 15 min at 121°C, and stored at 4°C for up to 4 weeks. Metal cations were prepared as 1 M MgCl2 × 6H2O and 1 M CaCl2 × 2H2O stock solutions, which were sterilized by filtration through a 0.2-μm-pore-size polyethersulfone membrane.
Lytic activity of polyP.
A 4-h exponentially growing B. cereus culture (optical density at 600 nm [OD600], approximately 0.2) was harvested by centrifugation (6,000 × g, 10 min), washed twice, and resuspended in 50 mM Tris (pH 7.5). Lysis of the cells after different concentrations of JOHA HBS were added was monitored by recording the OD600 with a dual-beam spectrophotometer (model 550-SE; Perkin-Elmer). The control cell cultures contained no polyP.
Influence of cations.
To determine the influence of divalent metal cations on lysis, 0.1% polyP was added to washed log-phase B. cereus cells. After 30 min, different final concentrations of cations (1, 5, and 10 mM Mg2+; 1 mM Ca2+) were added to samples. It should be noted that addition of higher concentrations of Ca2+ (>3 mM) to polyP-containing solutions resulted in increases in optical density due to a white, insoluble calcium polyP precipitate, which interfered with optical measurements.
Influence of protein synthesis inhibitors on lysis.
To investigate the influence of protein synthesis inhibitors on polyP-induced lysis, a log-phase culture was supplemented with 70 μg of chloramphenicol per ml 20 min before the cells were harvested and washed. The lytic effect of 0.1% polyP on these cells was measured spectrophotometrically as described above. The control contained no chloramphenicol.
Inhibition of spores.
Three final concentrations (0.05, 0.1, and 1%) of JOHA HBS were evaluated to determine their effectiveness in inhibiting the germination and outgrowth of B. cereus spores in liquid culture. Four samples (50 ml of plate count broth) were inoculated with spores (final concentration, 2 × 106 CFU/ml), polyP was added (the control contained no polyP), and the suspensions were incubated at 30°C. Cell counts (CFU per milliliter) were determined at different times (0, 2, 4, 6, 10, and 24 h) by surface plating decimal dilutions and by incubation for 24 h.
Differentiation between bacteriostatic and bacteriocidal effects.
In order to distinguish the bacteriocidal effect of polyP from a bacteriostatic effect of polyP, viable cell counts of B. cereus cultures that were in different growth phases and were treated with 0.1% JOHA HBS were determined. Six 50-ml portions of broth were inoculated with 105 CFU/ml. After 0, 2, 4, 6, and 24 h, 0.1% JOHA HBS was added to each sample, the cultures were incubated, and the viable cells were enumerated by plating at different times. To determine if the bacteriostatic effect could be reversed, 5 mM Ca2+ and 5 mM Mg2+ were added to cultures which had been preincubated with 0.1% JOHA HBS for 24 h, and the increases in viable cell number were monitored by surface plating for the next 8 h.
Resumption of cell division in filaments.
Resumption of cell division was induced after 4 h by adding 5 mM Ca2+. Reinitiated septum formation and the subsequent decay of the filaments were monitored continuously by phase-contrast microscopy. The influence of protein synthesis inhibitors on septation was tested as follows. A 50-ml suspension of filamentous cells in broth was divided into two batches; one batch was supplemented with chloramphenicol (70 μg/ml), and the other was supplemented with calcium (5 mM). After 20 min of incubation, 5 mM calcium were added to the first batch and chloramphenicol was added to the second batch.
DAPI staining.
DNA replication, nucleoid formation, and separation within the filamentous cells were detected by staining preparations with the fluorescent dye DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Boehringer, Mannheim, Germany). Cell samples taken at different times (2 h after 0.05% polyP was added; 1 and 3 h after 5 mM Mg2+ was added) were heat fixed on glass slides and incubated with 30 μl of a DAPI solution (0.5 μg/ml in phosphate-buffered saline [PBS]) for 10 min at 4°C in the dark. The samples were rinsed twice with ultrapure water and observed with an Olympus model BH-2 epifluorescence microscope equipped with a 365-nm excitation filter and a 460-nm emission filter. Fluorescence and phase-contrast micrographs (magnification, ×1,000) of the same field were taken with Kodak Panther ETH P1600 slide film.
Determination of cell length by scanning electron microscopy.
Samples (1 ml) of the elongated Bacillus cells were taken at different times, washed twice in PBS, and prefixed in 5% glutaraldehyde for 12 h at 4°C. Cells were then collected by centrifugation, washed in PBS, and treated for 1 h with 2% osmium tetroxide. Specimens (10 μl) were air dried onto glass coverslips and mounted on aluminum specimen stubs. After sputter coating with a Pd-Au alloy (40:60), cells were examined with a scanning electron microscope (Stereoscan 360; Cambridge Instruments, Cambridge, United Kingdom). About 50 cells per sample were measured, and average cell lengths were calculated.
Transmission electron microscopy.
Samples of filamentous cells and cells exhibiting septum formation after cations were added were fixed for 4 h with 5% glutaraldehyde and subsequently stained with 2% osmium tetroxide for 1 h. A previously described method (34) was used to prepare longitudinally oriented cells. Fixed cells were resuspended in molten 2% agarose and immediately centrifuged in small plastic tubes, each of which contained a solid drop of agarose in the bottom. After solidification, the upper part of the agarose containing the sedimented bacteria was cut into small cubes, which were dehydrated in ethanol and embedded in Spurr resin (30). Thin sections of the polymerized resin blocks were obtained with an ultramicrotome (Ultracut; Reichert & Jung, Vienna, Austria) and placed on Formvar-coated copper grids (200 mesh). A Zeiss model EM 10 electron microscope was used for examination; the acceleration voltage used was 60 kV.
RESULTS
Lytic effect of long-chain polyP.
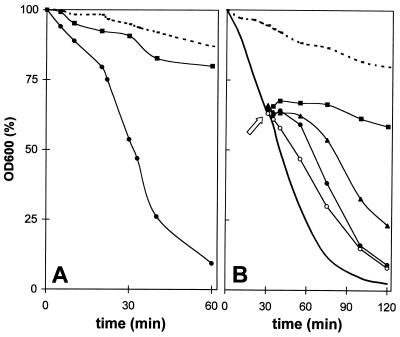
Figure 1A shows the lysis of log-phase B. cereus WSBC 10030 cells under the influence of JOHA HBS. Cells lysed rapidly in a 0.1% polyP solution, whereas no lysis was observed in the control. In contrast, stationary-phase cells challenged with polyP did not lyse (data not shown). Increased concentrations of polyP led to accelerated lysis; the initial OD600 was reduced approximately 20% by 0.01% polyP in 1 h, whereas 0.05 and 0.1% polyP resulted in reductions of 50% and more than 90%, respectively. Examination of the cultures after lysis by phase-contrast microscopy revealed that the cellular debris consisted of empty cell sacculi with almost complete cell wall cylinders, most of which were open in the polar regions or the septum (data not shown).
FIG. 1.
Lytic effect of the polyP JOHA HBS on exponential-phase cultures of B. cereus WSBC 10030 and influence of divalent metal cations. (A) Lytic effect of 0.1% polyP. Addition of 70 μg of chloramphenicol per ml to an exponential-phase culture for 20 min decreased the lytic effect of polyP. Dashed line, control cells in water. Symbols: ●, polyP; ■, polyP and chloramphenicol. (B) Influence of cations on polyP-induced lysis. Thirty minutes after 0.1% polyP was added to cell suspensions, 10 mM (■), 5 mM (▴), or 1 mM (●) Mg2+ or 1 mM (○) Ca2+ was added. Dashed line, control cells in water without polyP but with 10 mM Mg2+; solid line, control cells with polyP and no cations.
Influence of cations and protein synthesis inhibitors on lysis.
Lysis could be effectively stopped by adding divalent cations (Fig. 1B). When 10 mM Mg2+ was added to a cell suspension in which lysis had been induced, the decrease in OD600 (cell wall destruction) stopped immediately. Lower cation concentrations only delayed the lysis effect, which was most probably a concentration-dependent titration effect and, therefore, directly proportional to the polyP concentration used. Similar results were obtained when calcium cations were used. Preincubation of exponentially growing cells with chloramphenicol completely prevented polyP-induced cell lysis (Fig. 1A), which indicated that protein synthesis is required for polyP-triggered lysis.
polyP inhibits spore germination.
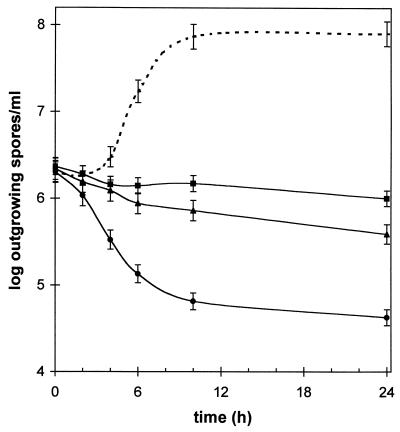
Figure 2 shows that not only was polyP effective against vegetative cells, but spores were also affected. Lower concentrations of JOHA HBS (0.05 and 0.1%) completely inhibited outgrowth of Bacillus spores. A higher concentration (1%) even had a sporocidal effect; the spore concentration (initial inoculum, 2 × 106 CFU/ml) decreased to less than 105 CFU/ml within 8 h. In polyP-containing samples, no vegetative cells were detected by phase-contrast microscopy. We also observed that compared to the control, a significant portion (approximately 10%) of the phase-bright spores remained phase bright during incubation, indicating that the influx of water associated with germination events was inhibited.
FIG. 2.
Effect of polyP on the outgrowth of B. cereus spores. Liquid media were inoculated with 2 × 106 heat-induced spores per ml, and different amounts of JOHA HBS polyP (0.05% [■], 0.1% [▴], and 1% [●]) were added at zero time. Dashed line, control culture without polyP. Samples were taken at different times, and outgrowing spores were enumerated (expressed as CFU per milliliter) by plating them onto solid media. The error bars indicate the approximate variability in CFU determinations.
polyP has a bacteriocidal effect and a bacteriostatic effect.
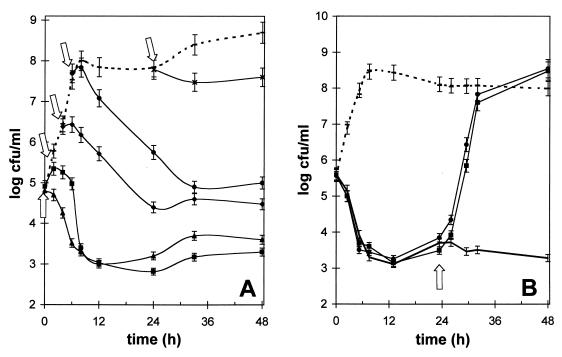
The qualitative effects of polyP on viable cells in liquid broth cultures were determined in order to distinguish between a bacteriocidal (lethal) effect and a bacteriostatic (transient inhibition) effect. Our results indicate that a polyP concentration of 0.1% or higher had a bacteriocidal effect, which was dependent on the growth phase (Fig. 3A). The most rapid decreases in number of viable cells were observed in log-phase cultures; the cell counts decreased by approximately 3 log units within 6 to 8 h. In contrast, stationary-phase cells (24-h culture) were not significantly affected. It was interesting that approximately 0.1 to 1% of the cells that were initially present were not killed by polyP. Although these cells exhibited no growth in liquid cultures containing otherwise lethal concentrations of polyP, they could be enumerated by colony formation after they were plated onto noninhibitory solid media, which indicated that polyP had a bacteriostatic effect on nondividing cells. Addition of divalent cations to the surviving CFU in a polyP-treated culture could reverse this bacteriostatic effect, and this was followed by a very rapid increase in the viable cell count (Fig. 3B). The generation time in the normally growing Bacillus cultures was estimated to be approximately 28 min, whereas the sudden increase in CFU after calcium was added revealed that the apparent doubling time was only 20 min.
FIG. 3.
Lytic and bacteriostatic effects of long-chain polyP on B. cereus cells. (A) Influence of the growth phase on polyP lytic activity. Cultures were inoculated at zero time, and 0.1% polyP was added at different times (0, 2, 4, 6, and 24 h; indicated by arrows). polyP clearly had a bacteriocidal (lytic) effect on growing cells, but there also was a bacteriostatic effect. (B) Addition of 5 mM Ca2+ (●) or 5 mM Mg2+ (■) to polyP-treated cells after 24 h (arrow) reversed the bacteriostatic effect and reinitiated rapid cell division and growth. Dashed lines, control cultures without polyP; thick solid line, control with no cations added. The error bars indicate the approximate variability in enumeration of CFU on solid media.
polyP can induce filamentation.
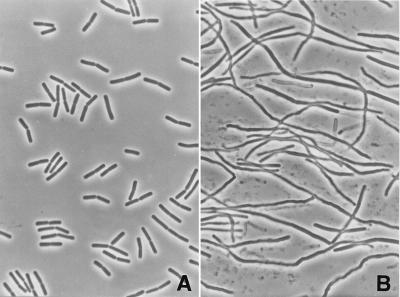
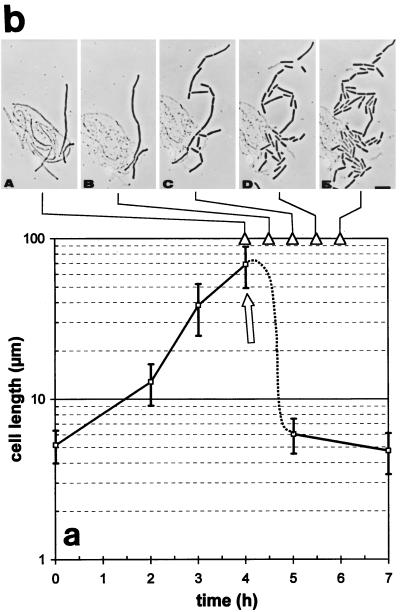
Addition of a sublethal concentration of polyP (0.05%) to log-phase cultures of B. cereus led to macroscopically visible aggregates of long, filamentous cells within 2 to 3 h (Fig. 4). This effect was strictly dependent on the polyP concentration. As stated above, higher concentrations (concentrations higher than 0.1%) completely inhibited cell growth and led to cell lysis, whereas very low concentrations (concentrations less than 0.03%) had little effect on cell growth or cell elongation (i.e., normal growth occurred). The cell elongation events were quantified by measuring individual cell lengths by scanning electron microscopy, and Fig. 5A shows the increases in the average length of Bacillus cells at different times following a polyP challenge. The mean length of normal log-phase cells was about 5 μm, but we could distinguish short, single cells (length, 2.5 to 4 μm) and longer, dividing cells (length, 4 to 6 μm), usually consisting of two individual cell bodies. Addition of 0.05% polyP resulted in a more than 10-fold increase in the average cell length within 3 h (average length, up to 70 μm). If the cells were maintained under these conditions, however, the filaments eventually started to lyse.
FIG. 4.
Effects of sublethal concentrations of long-chain polyP on the morphology of B. cereus WSBC 10030. (A) Phase-contrast micrograph showing normal-size, log-phase B. cereus cells. (B) Phase-contrast micrograph showing filamentous cells after addition of 0.05% polyP and subsequent incubation for 3 h.
FIG. 5.
Increase in cell length (filamentation) of B. cereus WSBC 10030 after treatment with sublethal polyP concentrations and reinitiation of cell division by divalent metal cations. (a) Addition of 0.05% polyP to a log-phase culture led to cell elongation (i.e., a dramatic increase in individual cell length). Fifty cells were measured at each time point by using scanning electron microscopy; the error bars indicate the standard deviations of the means. (b) Resumption of cell division and subsequent breakdown of the filaments following addition of 5 mM Ca2+ or 5 mM Mg2+ (at 4 h; indicated by an arrow in panel a). Panels A to E are phase-contrast micrographs of the same field taken at 0, 30, 60, 90, and 120 min after cations were added, respectively (indicated by arrowheads).
Metal cations induce rapid septation and division in filamentous cells.
When divalent cations (5 mM Ca2+ or 5 mM Mg2+) were added, rapid and dramatic morphological changes in the filamentous cells were observed. Phase-contrast microscopy revealed that cell division resumed almost instantaneously, which led to separation of each filament into three or more individual cells of different lengths within 30 to 60 min (Fig. 5B). The daughter cells then divided normally, and after 3 h only normal-length viable cells were present in the samples.
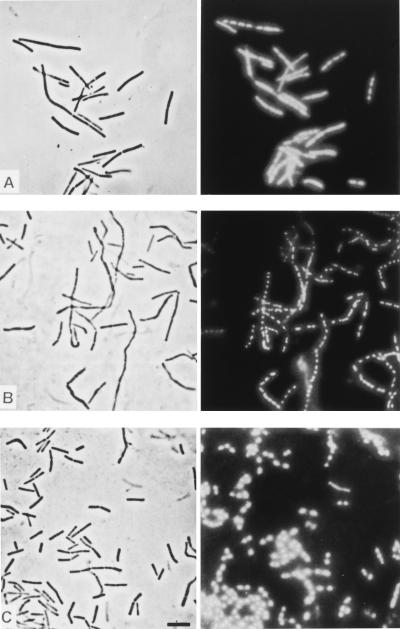
To further confirm that polyP specifically inhibited cell division events, DAPI-treated samples were examined by epifluorescence microscopy (Fig. 6). DAPI interacts with double-stranded DNA, making separated nucleoids visible as brightly stained objects within dark cell bodies. In normally growing cultures one or two discrete nucleoids were present in each cell, depending on the cell cycle, whereas the filament-forming cells in polyP-treated samples contained multiple discrete nucleoids (Fig. 6A). Apparently, DNA replication and chromosome segregation were not disturbed; the daughter nucleoids were distributed over the whole lengths of the filaments, with equal distances between them. After cations were added, septation and cell division resumed (Fig. 6B), which eventually resulted in single, short cells with one or two nucleoids (Fig. 6C).
FIG. 6.
Same-field micrographs of B. cereus cells obtained by phase-contrast microscopy (left panels) and epifluorescence microscopy after DAPI staining (right panels). (A) Shortly after 0.05% polyP was added (1 h), beginning filamentation was apparent, but this process did not affect chromosome replication and coordinated nucleoid segregation in each individual elongated cell body. (B) Addition of 5 mM Ca2+ (after 3 h) reinitiated normal division events (septation) in the multinucleoid filaments. (C) Almost normal cell growth, division, and nucleoid distribution in the cells 3 h after Ca2+ was added. Bar = 10 μm.
Fine structure of septation-blocked filamentous cells.
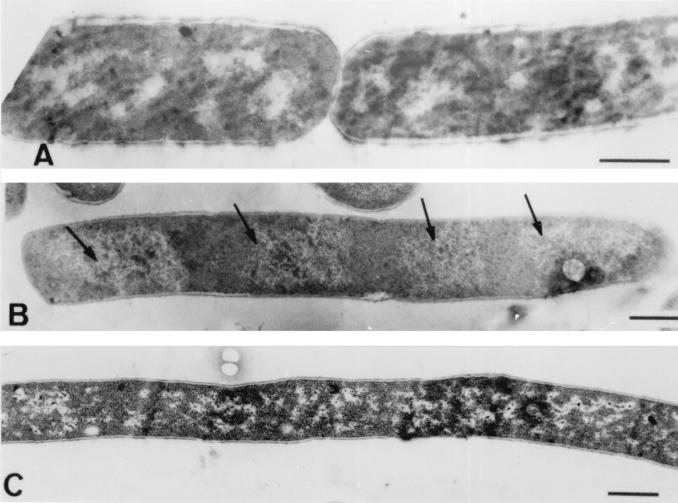
Convincing evidence that polyP causes a defect in septum formation was obtained by transmission electron microscopy, which was carried out by using samples of polyP-treated cells and cation-supplemented filaments collected at different times. Figure 7 shows that septation was completely arrested after polyP was added; there was a total lack of invaginating septum formation at either the midcell region or the polar region, and no cell wall indentations or other signs of division initiation were observed. The nucleoids in the cells were visible and clearly separated. There were no other obvious differences in the appearance of the cell wall, the membrane, or other cellular structures between normal and polyP-treated cells.
FIG. 7.
Transmission electron micrographs of thin sections of B. cereus WSBC 10030 cells following treatment with polyP. (A) Control cells before polyP addition. (B) Cells treated with 0.05% polyP after 30 min. The arrows indicate segregated nucleoids. (C) Cells treated with 0.05% polyP after 2 h. A complete lack of septum formation and the resulting filamentation is clearly evident. Bars = 0.5 μm.
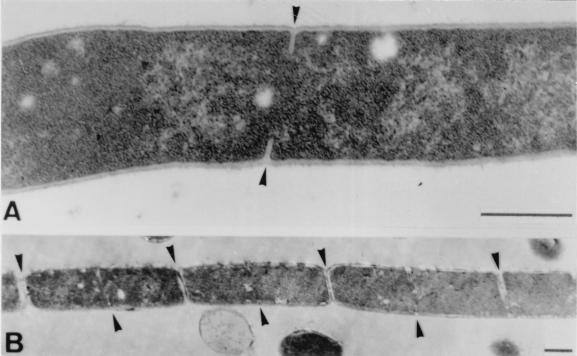
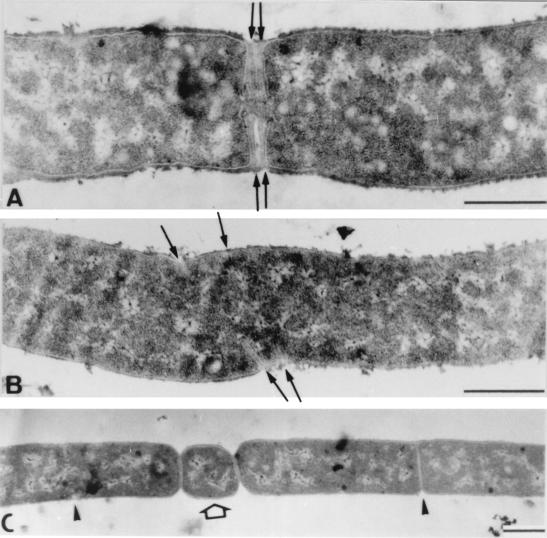
Figure 8A shows the events that occurred almost immediately (10 min) after metal ions added to filamentous cells. Septation resumed, which was clearly visible as ring-shaped invaginating edges of septal walls within the cell bodies. After 30 to 45 min (Fig. 8B), septation was complete, cells were beginning to divide, and the next round of ring formation and septum building had already started. While most septa were correctly initiated with normal distances between them, we quite frequently observed aberrant septum formation in filaments to which cations were added (Fig. 9). Occasionally, two cross walls were initiated simultaneously very close to each other. Other malformations included forked Y-shaped septa, incomplete septa without opposite edge formation, multiple septa, and very short, minicell-like segments, which were formed at various positions within the long filaments.
FIG. 8.
Transmission electron micrographs of thin sections of polyP-treated B. cereus cells (i.e., filaments) following addition of 5 mM Ca2+. (A) After 10 min, ring-shaped septum formation and growth resumed (arrowheads). (B) After 45 min, newly formed septa were completed, and cell division (i.e., separation of daughter cells) began (upper arrowheads), while a second cycle of septation is already evident (lower arrowheads). Bars = 0.5 μm.
FIG. 9.
Transmission electron micrographs of polyP treated cells taken 1 h after Ca2+ was added. The arrows indicate various types of septum malformations, probably due to the hyperdivisionlike phenotype. (A) Invagination of two growing septa very close to each other. (B) Apparently uncoordinated multiple ring formation and septum growth. (C) Formation and separation of a minicell-like short rod (arrow) and normal septation (arrowheads) Bars = 0.5 μm.
DISCUSSION
The results of the present study clearly show that the long-chain polyP JOHA HBS has a concentration-dependent antimicrobial effect on cells of B. cereus. Higher concentrations lead to cell lysis and have bacteriocidal and bacteriostatic effects, and sublethal concentrations inhibit cell septum formation, resulting in filamentous cell growth. These effects are dependent on the growth phase of the cells.
Lethal concentrations of polyP resulted in direct lysis of growing cells, whereas stationary-phase (resting) cells were not affected. The remaining cellular debris consisted of empty cell wall cylinders and polar caps, which was the first indication that polyP-induced lysis is spatially specific. Our observation that lysis could be effectively prevented by divalent cations confirmed the previous finding that addition of free Mg2+ can overcome the inhibitory effect of polyP on B. cereus (13). Lysis could also be hindered by the translation inhibitor chloramphenicol. These results showed that the lytic action associated with polyP involves interactions with cations and clearly suggested that cation-dependent enzymatic reactions, which should be cell wall associated and should occur only in growing cells, are involved.
The lethal effect of higher doses of polyP was confirmed by the results of the plating experiments, which indicated that polyP has a bacteriocidal effect. Although the viable cell counts in growing Bacillus cultures could be reduced by about 3 log units, complete killing of the culture was not possible. Presumably, the surviving cells were not actively growing and, therefore, were only bacteriostatically inhibited and remained viable. We also found that higher concentrations of polyP resulted in injury and inactivation of Bacillus spores; outgrowth of germinated spores was completely blocked. A similar effect due to the action of a chelating compound has been reported for EDTA, which had an inhibitory effect on the outgrowth of B. cereus spores (7). Growth inhibition could also be reversed by adding cations. These previous findings primarily implicated nutritional deficiencies created by chelation of essential metal cations, which then affected outgrowth.
In addition to the lytic, bacteriocidal effects of higher JOHA HBS concentrations, sublethal (i.e., nonlytic) concentrations had a surprising effect on growing cells of B. cereus. In the presence of 0.05% polyP, normal rod-shaped cells (length, 3 to 5 μm) transformed into long, filamentous cells (which were 4- to 10-fold longer). Addition of divalent cations led to rapid decay of the filaments into three or more viable cells, which explains the very short apparent doubling time observed (Fig. 3B).
The supposed mechanism of polyP-mediated growth inhibition and cell lysis certainly involves the sequestering of cations by polyP (9, 29, 36). polyP molecules are polyanionic macromolecules and are fairly strong chelating agents (10). It has been were proposed that they interact with the divalent cations localized on the cell surface and make them unavailable to the cell (15). Cations can overcome this effect and have been shown to be able to stop polyP-induced lysis (2, 16). One previous hypothesis was that other essential, sequestered cations were released during the process; i.e., cations with high stability constants (such as Ca2+ and Mg2+) may displace other polyP-chelated cations and make them “reavailable” for bacterial utilization (11, 13). An important finding (obtained by using S. aureus as a model organism) was that polyP molecules bind to the bacterial cell wall, chelate metals, and remain bound without a significant release of metal cations into the surrounding medium (17). None of the previous studies, however, was able to determine the identity of the actual effector in the bacterial cell. Since the phenotypic effects of polyP challenge are very similar in various microorganisms, we suggest that at least filamentous cell growth and lysis of growing cells should be considered the results of very similar mechanisms and events in different bacteria.
Our study showed that synthesis of the lateral cell wall, DNA replication and segregation, and other essential physiological processes in polyP-treated cells did not seem to be affected by polyP. Instead, the data presented here clearly indicate that polyP blocks septation, probably via cellular proteins involved in an early stage of cell division. One early event in bacterial cell division is the formation of the so-called Z ring. The major division protein responsible, FtsZ, is highly conserved, has been found in all eubacteria investigated so far, and seems to be essential for cell division in all procaryotes. It forms a dynamic ring structure at the future division site. The Z ring “contracts” during cell division while it maintains a position at the leading edge of the invaginating septum (5, 19, 31). Most importantly, FtsZ exhibits a strictly Mg2+-dependent GTPase activity (28), and Mg2+ and Ca2+ are required for the GTP-dependent dynamic behavior of FtsZ polymers (25, 35). If the data described above are considered along with our observations, it seems quite reasonable to speculate that these strictly metal ion-dependent proteins involved in septation and cell division might be the (indirect) key targets for polyP. In other words, polyP binds to the bacterial cell wall and, by means of its sequestering nature, probably creates an acute metal cation deficiency. This could then block the GTPase activity of FtsZ and prevent growth and extension of the leading edge polymers in the Z ring. Our finding that exogeneous addition of an excess of divalent metal cations resulted in instantaneous septum formation, which then caused hyperdivision activity (displayed as a minicell-like phenotype), is identical to what was observed in ftsZ-overexpressing Escherichia coli (32). This suggests that FtsZ is present in polyP-treated aseptate cells but its dynamic assembly is temporarily blocked due to inhibition of the GTPase activity.
Our hypothesis that inhibition of Fts proteins may be responsible for the polyP-induced filamentous phenotype is also supported by other, related findings. FtsZ-minus mutants of E. coli form multinucleate, filamentous cells (1), and it is known that Fts-defective, temperature-sensitive mutants of Bacillus subtilis also display a filamentous phenotype at restrictive temperatures (8, 23). Septum formation in multinucleate filaments can be reinitiated when filaments are returned to permissive temperatures, but recovery can be blocked by inhibitors of protein synthesis (6). This is in excellent agreement with our observations.
polyP has yet another property that could support the theory outlined here. Due to its polyanionic nature, polyP readily interacts with basic proteins and with basic domains of proteins, such as those in polymerases or nonhistone nuclear proteins (14, 26). Inspection of the B. subtilis FtsZ amino acid sequence (4) revealed that is has a predicted acidic pI of 4.7. However, the C-terminal 80 amino acids consist of a highly charged, very basic domain with a predicted pI of 10.1, which could possibly interact with and bind to polyP molecules. With respect to the GTP-hydrolyzing activity of FtsZ, it is known that the guanosine pentaphosphate hydrolase of E. coli has an affinity for polyP via its polyphosphatase domain (12).
It should be noted, however, that the absence of other proteins of the Fts family also resulted in filamentous bacterial cell morphology (e.g., in the case of FtsA [3] or FtsI [27]). FtsI is also known as penicillin-binding protein 3 (PBP-3), and filamentous cell growth can be induced with FtsI-specific β-lactam antibiotics. However, unlike FtsZ, these other Fts proteins are not known to be dependent on divalent cations. Moreover, our micrographs of B. cereus filaments did not reveal any indentations along the cell wall, indicating that the Z ring was not yet formed. FtsA localizes at a future division site after the ring has been established (33). Therefore, we concluded that the divalent cation-dependent GTPase activity of the bacterial septum-directing division protein FtsZ is the most likely candidate target for polyP and is responsible for the observed aseptate phenotype. A recently described in vitro assay used to analyze the polymerization and assembly of FtsZ by light scattering (25) in the presence of different concentrations of polyP may provide a way to directly test our hypothesis in an inhibition assay.
A similar mechanism might be postulated for other gram-positive organisms (e.g., Clostridium, Staphylococcus, and Listeria species) which exhibit polyP-mediated growth inhibition accompanied by cell elongation and changes in cellular morphology (15, 18, 20, 36).
ACKNOWLEDGMENTS
We thank Waltraud Knapp and Hans-Christian Bartscherer (Freising, Germany) for use of the electron microscopy facility, and we are grateful to Patrick Schiwek for technical assistance.
REFERENCES
- 1.Addinall S, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres H M, Furr J R, Russell A D. Effect of divalent cations on permeabilizer-induced lysozyme lysis of Pseudomonas aeruginosa. Lett Appl Microbiol. 1998;27:372–374. doi: 10.1046/j.1472-765x.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 3.Beall B, Lutkenhaus J. Impaired cell division and sporulation of a Bacillus subtilis strain with the ftsA gene deleted. J Bacteriol. 1992;174:2398–2403. doi: 10.1128/jb.174.7.2398-2403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall B, Lowe M, Lutkenhaus J. Cloning and characterization of Bacillus subtilis homologs of Escherichia coli cell division genes ftsZ and ftsA. J Bacteriol. 1988;170:4855–4864. doi: 10.1128/jb.170.10.4855-4864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramhil D. Bacterial cell division. Annu Rev Cell Dev Biol. 1997;13:395–424. doi: 10.1146/annurev.cellbio.13.1.395. [DOI] [PubMed] [Google Scholar]
- 6.Breakefield X O, Landman O E. Temperature-sensitive divisionless mutant of Bacillus subtilis defective in the initiation of septation. J Bacteriol. 1973;113:985–998. doi: 10.1128/jb.113.2.985-998.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulgarelli M A, Shelef L A. Effect of ethylenediaminetetraacetic acid (EDTA) on growth from spores of Bacillus cereus. J Food Sci. 1985;50:661–664. [Google Scholar]
- 8.Callister H, Wake R G. Characterization and mapping of temperature-sensitive division initiation mutations of Bacillus subtilis. J Bacteriol. 1981;145:1042–1051. doi: 10.1128/jb.145.2.1042-1051.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott R P, Straka, Garibaldi J A. Polyphosphate inhibition of growth of pseudomonads from poultry meat. Appl Microbiol. 1964;12:517–522. doi: 10.1128/am.12.6.517-522.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irani R R, Callis C F. Calcium and magnesium sequestration by sodium and potassium polyphosphates. J Am Oil Chem Soc. 1962;39:156–159. [Google Scholar]
- 11.Jen C M C, Shelef L A. Factors affecting sensitivity of Staphylococcus aureus 196E to polyphosphate. Appl Environ Microbiol. 1986;52:842–846. doi: 10.1128/aem.52.4.842-846.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keasling J D, Bertsch L, Kornberg A. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. Proc Natl Acad Sci USA. 1993;90:7029–7033. doi: 10.1073/pnas.90.15.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knabel S J, Walker H W, Hartman P A. Inhibition of Aspergillus flavus and selected gram-positive bacteria by chelation of essential metal cations by polyphosphates. J Food Prot. 1991;54:360–365. doi: 10.4315/0362-028X-54.5.360. [DOI] [PubMed] [Google Scholar]
- 14.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee R M, Hartmann P A, Olson D G, Williams F D. Bactericidal and bacteriolytic effects of selected food-grade polyphosphates, using Staphylococcus aureus as a model system. J Food Prot. 1994;57:276–283. doi: 10.4315/0362-028X-57.4.276. [DOI] [PubMed] [Google Scholar]
- 16.Lee R M, Hartmann P A, Olson D G, Williams F D. Metal ions reverse the inhibitory effects of selected food-grade polyphosphates in Staphylococcus aureus. J Food Prot. 1994;57:284–288. doi: 10.4315/0362-028X-57.4.284. [DOI] [PubMed] [Google Scholar]
- 17.Lee R M, Hartmann P A, Stahr H M, Olson D G, Williams F D. Antibacterial mechanism of long-chain polyphosphates in Staphylococcus aureus. J Food Prot. 1994;57:289–294. doi: 10.4315/0362-028X-57.4.289. [DOI] [PubMed] [Google Scholar]
- 18.Loessner M J, Maier S K, Schiwek P, Scherer S. Long-chain polyphosphates inhibit growth of Clostridium tyrobutyricum in processed cheese spreads. J Food Prot. 1997;60:493–498. doi: 10.4315/0362-028X-60.5.493. [DOI] [PubMed] [Google Scholar]
- 19.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Maier S K. Die antimikrobielle Wirkung von Polyphosphaten. Ph.D. thesis. Freising, Germany: Technical University of Munich; 1999. [Google Scholar]
- 21.Matsuoka A, Tsutsumi M, Watanabe T. Inhibitory effect of hexametaphosphate on the growth of Staphylococcus aureus. J Food Hyg Soc Jpn. 1995;36:588–594. [Google Scholar]
- 22.Matsuoka A, Tsutsumi M, Watanabe T. Influence of hexametaphosphate on Staphylococcus aureus cytoplasmic membrane. J Food Hyg Soc Jpn. 1997;38:441–445. [Google Scholar]
- 23.Mendelson N H, Cole R M. Genetic regulation of cell division initiation in Bacillus subtilis. J Bacteriol. 1972;112:994–1003. doi: 10.1128/jb.112.2.994-1003.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molins R A. Phosphates in foods. Boca Raton, Fla: CRC Press; 1991. [Google Scholar]
- 25.Mukherjee A, Lutkenhaus J. Analysis of FtsZ assembly by light scattering and determination of the role of divalent metal cations. J Bacteriol. 1999;181:823–832. doi: 10.1128/jb.181.3.823-832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offenbacher S, Kline E S. Evidence for polyphosphate in phosphorylated nonhistone nuclear proteins. Arch Biochem Biophys. 1984;231:114–123. doi: 10.1016/0003-9861(84)90368-0. [DOI] [PubMed] [Google Scholar]
- 27.Pogliano J, Pogliano K, Weiss D S, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 29.Shelef L A, Seiter J A. Indirect antimicrobials. In: Davidson P M, editor. Antimicrobials in foods. 2nd ed. New York, N. Y.: Marcel Dekker, Inc.; 1993. pp. 539–569. [Google Scholar]
- 30.Spurr A R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 32.Ward J E, Lutkenhaus J F. Overproduction of FtsZ induces minicells in E. coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 33.Weiss D S, Chen J C, Ghigo J-M, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehouse R L S, Bénichou J-C, Ryter A. Procedure for the longitudinal orientation of rod-shaped bacteria and the production of a high cell density of procaryotic and eucaryotic cells in thin sections for electron microscopy. Biol Cell. 1977;30:155–158. [Google Scholar]
- 35.Yu X-C, Margolin W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997;16:5455–5463. doi: 10.1093/emboj/16.17.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaika L L, Scullen O J, Fanelli J S. Growth inhibition of Listeria monocytogenes by sodium polyphosphate as affected by polyvalent metal ions. J Food Sci. 1997;62:867–869. [Google Scholar]