Abstract
Introduction
Due to reports of severely reduced insulinotropic effect of the incretin hormone glucose-dependent insulinotropic polypeptide (GIP) in type 2 diabetes (T2D), GIP has not been considered therapeutically viable. Recently, however, tirzepatide, a novel dual incretin receptor agonist (activating the GIP receptor and the glucagon-like peptide 1 (GLP-1) receptor) has demonstrated greater glucose and body weight-lowering properties as compared to GLP-1 receptor agonist therapy. The contribution of GIP receptor activation to effects of tirzepatide remains unknown. We will evaluate the glucose-lowering effect of exogenous GIP in the context of pharmacological GLP-1 receptor activation in patients with T2D.
Methods and analysis
In this randomised, double-blind, four-arm parallel, placebo-controlled trial, 60 patients with T2D will be included (18–74 of age; on diet and exercise and/or metformin therapy only; glycated haemoglobin 6.5–10.5% (48–91 mmol/mol)). Participants will be randomised to an 8-week run-in period with subcutaneous (s.c.) placebo or semaglutide injections once-weekly (0.5 mg). Participants will then be randomised to 6 weeks’ add-on treatment with continuous s.c. placebo or GIP infusion (16 pmol/kg/min). The primary endpoint is change in mean glucose levels (assessed by 14-day continuous glucose monitoring) from the end of the run-in period to end of trial.
Ethics and dissemination
The present study was approved by the Regional Committee on Health Research Ethics in the Capitol Region of Denmark (identification no. H-20070184) and by the Danish Medicines Agency (EudraCT no. 2020-004774-22). All results, positive, negative and inconclusive, will be disseminated at national and/or international scientific meetings and in peer-reviewed scientific journals.
Trial registration numbers
Keywords: diabetes & endocrinology, general diabetes, general endocrinology, lipid disorders
Strengths and limitations of this study.
The contribution of long-term glucose-dependent insulinotropic polypeptide (GIP) receptor activation in the metabolic benefits of dual incretin coagonists such as tirzepatide is not known.
In this study, the effects of 6-week continuous subcutaneous (s.c.) GIP or placebo infusion added to once-weekly s.c. 0.5 mg semaglutide or placebo will be investigated.
This study is designed as a randomised, placebo-controlled, double-blind study and powered to detect clinical relevant changes in the primary outcome: mean glucose levels (assessed by 14-day continuous glucose monitoring).
As native GIP is administered s.c. via an insulin pump device, we have limited the GIP infusion to 6 weeks, which might be too short a period to draw definite conclusions.
Introduction
Due to reports of a severely reduced insulinotropic effect of the incretin hormone glucose-dependent insulinotropic polypeptide (GIP) in type 2 diabetes (T2D), GIP was not considered therapeutically viable in T2D until recently.1–3 In 2013, Finan et al reported metabolic benefits of dual GIP and glucagon-like peptide 1 (GLP-1) receptor agonism4 and in 2021, phase III clinical trial data demonstrated that tirzepatide, a dual GIP/GLP-1 receptor agonist, caused massive improvements in glycaemic control and robust body weight losses in patients with T2D5; greater than observed with the GLP-1 receptor agonist semaglutide dosed subcutaneously (s.c.) once-weekly 1.0 mg.6 However, the contribution of GIP receptor activation to these effects remains unknown.7 The present study will evaluate the glucose-lowering effect of GIP in the context of pharmacological GLP-1 receptor activation in patients with T2D. Secondarily, the study will delineate the metabolic effects of GIP, separately and combined with the GLP-1 receptor agonist semaglutide, beyond glycaemic effects, that is, on body composition, liver status, lipid profile, inflammatory markers and vital signs. Thus, the present study will provide important mechanistic insights into long-term dual GIP/GLP-1 receptor agonism in patients with T2D. We hypothesise that 6 weeks’ continuous s.c. GIP infusion added to semaglutide treatment will reduce continuous glucose monitoring (CGM)-assessed mean glucose levels in patients with T2D and disclose insights into how separate and combined incretin receptor activation affects glucose homeostasis and body weight regulation that may be used therapeutically in the future.
Methods and analysis
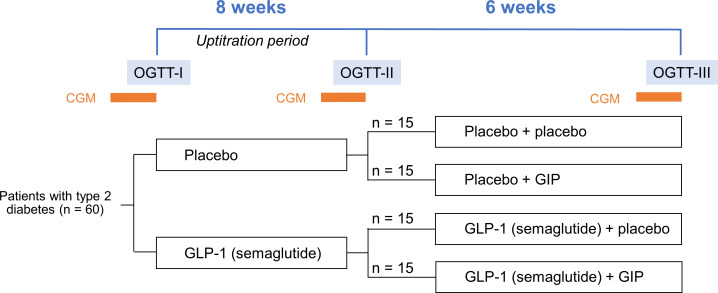
This is a single-centre, randomised, placebo-controlled, four-arm, parallel group (ratio 1:1:1:1), double-blind study (figure 1).
Figure 1.
Study design. CGM periods are 14 days long. CGM, continuous glucose monitoring; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; OGTT, oral glucose tolerance test.
Participants
Sixty patients with T2D will be recruited via diabetes outpatient clinics and general practitioners in the Capital Region of Denmark, the internet and advertisements, for example, in local newspapers.
Inclusion criteria
≥18 and ≤74 years of age.
Diagnosed with T2D for at least 6 months.
Treated with diet and exercise and/or stable metformin and/or stable sodium-glucose co-transporter 2 inhibitor and/or dipeptidyl-peptidase 4 (DPP-4) inhibitor and/or stable sulfonylurea (SU) treatment for at least 3 months (if treated with DPP-4 inhibitor and/or SU, this treatment must be paused for 14 days prior to first CGM period in the trial).
Glycated haemoglobin (HbA1c) ≥6.5 and ≤10.5% (≥48 and ≤91 mmol/mol).
Body mass index (BMI)≥25 and ≤50 kg/m2.
Stable body weight (less than 3 kg self-reported change during the previous 90 days).
Exclusion criteria
Diagnosed with type 1 diabetes.
Known or suspected hypersensitivity to trial product or related products.
Acute decompensation of glycaemic control requiring immediate intensification of treatment to prevent acute complications of diabetes (eg, symptomatic severe hyperglycaemia) which required help from doctor or hospitalisation within 90 days prior to screening.
Previous randomisation in this trial (participation is defined as signed informed consent).
Participation in another clinical trial within 90 days before screening.
Women who are pregnant, breast feeding or intend to become pregnant, or are of childbearing potential and not using adequate contraceptive methods (intrauterine devices or hormonal contraception (oral contraceptive pills, implants, transdermal patches, vaginal rings or long-acting injections)).
If previously treated with GLP-1RA, information about the time and reason for stopping will be collected; based on this, the eligibility will be judged by the investigator.
Participation in an organised weight reduction programme within 3 months before screening.
Anticipated change in lifestyle (eg, smoking, eating or exercise pattern) during the trial.
Any laboratory safety parameter at screening outside the below extended laboratory ranges.
Untreated or uncontrolled hypothyroidism/hyperthyroidism defined as thyroid-stimulating hormone <0.4 mIU/L or >6 mIU/L.
Obesity related to endocrinological disorders (eg, Cushing’s syndrome).
Any blood draw in excess of 25 mL in the past month, or donation of blood or plasma in excess of 400 mL within the 90 days preceding screening.
-
Use of any prescription or non-prescription medication (apart from oral contraceptives, routine vitamins, occasional use of paracetamol, acetylsalicylic acid or ibuprofen) which could interfere with pharmacokinetic or pharmacodynamic results, as judged by the investigator, such as:
Herbal products and non-routine vitamins.
Glucose-lowering medication (except metformin).
Medication that may cause weight gain, including systemic corticosteroids, tricyclic antidepressants and atypical antipsychotics.
Orlistat, zonisamide, topiramate, phenteremine, lorcaserin, bupropion, naltrexone or other weight loss drugs.
Blood pressure and lipid lowering agents (eg, statins) drugs are allowed if treatment has been stable for ≥1 month prior to screening and treatment should preferably be kept unchanged during the trial.
Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2.
History of pancreatitis (acute or chronic).
History of major depressive disorder or other severe psychiatric disorders, for example, schizophrenia or bipolar disorder within the last 2 years or lifetime history of suicide attempt.
Surgery scheduled for the trial duration period, except for minor, non-gastrointestinal surgical procedures at the discretion of the investigator.
Sitting blood pressure (after resting for at least 5 min) ≥160 mm Hg systolic or ≥100 mm Hg diastolic or heart rate of ≥90 beats/min after resting for at least 5 min.
Cancer (past or present, except basal cell skin cancer or squamous cell skin cancer), which in the investigator’s opinion could interfere with the results of the trial.
Known or suspected alcohol abuse.
Known or suspected drug/chemical substance abuse within 1 year from screening.
Inability or unwillingness to perform self-injection at the screening visit (with a placebo test pen).
Mental incapacity, language barriers or unwillingness to comply with the requirements of the protocol, which may preclude adequate understanding or cooperation during the trial, as judged by the investigator.
Investigators, research assistants, pharmacists, trial coordinators, other staff, sponsor staff or relatives thereof directly or indirectly involved in the conduct of the trial.
Any disorder which in the investigator’s opinion might jeopardise participant’s safety or compliance with the protocol.
Intervention and comparator
Participants will be randomised into four groups of 15. Each group will receive either (1) semaglutide 1.34 mg/mL+GIP, (2) semaglutide 1.34 mg/mL+placebo, (3) placebo+GIP or (4) placebo+placebo. Participants will be randomised to an 8-week run-in treatment period with s.c. placebo or semaglutide injections once-weekly (0.25 mg for 4 weeks and 0.5 mg during the rest of the intervention). Participants will then be randomised to 6 weeks add-on treatment with continuous s.c. placebo or GIP infusion (16 pmol/kg/min). At baseline, in the end of the run-in period and in the end of the add-on treatment period, all participants will undergo a 4-hour oral glucose tolerance test (OGTT) and a 14-day CGM period (see figure 1).
Main outcome
Change from before the run-in period (baseline) to end of trial in 14-day CGM-assessed mean glucose levels.
Key secondary outcomes
Oral glucose tolerance, insulin sensitivity and pancreatic beta cell function assessed by OGTTs. Glycaemic variability (coefficient of variance) and time in range assessed by 14-day CGM-assessed glucose levels. HbA1c, body weight and body composition (total body fat and lean mass percentage) assessed by dual-energy X-ray absorptiometry (DXA).
Randomisation
An unblinded qualified staff member at the study site will generate a randomisation sequence used for allocating participants to the four treatment groups. The unblinded qualified staff member is not otherwise involved in the study. See Section 8 in the full protocol for details. Study drug accountability will be performed by unblinded qualified staff members who will perform an accurate record of all trial products received and dispensed to each participant.
Blinding
Identically appearing vials and injection devices will be provided to all participants by the unblinded study personnel to ensure treatment allocation concealment of participants and blinded study personnel. Likewise, main outcome measurements (CGM-assessed glucose levels) will be registered in a closed database and not visible for participants and study personnel.
Power calculation and statistical analysis plan
The primary comparisons will be between (1) the GIP+semaglutide 1.34 mg/mL group and the placebo+semaglutide 1.34 mg/mL group and (2) the GIP+placebo group and placebo+placebo group. Furthermore, in a secondary explorative comparison, we will test for any synergistic effect of GIP+semaglutide 1.34 mg/mL by comparing the effects of GIP+semaglutide 1.34 mg/mL (as compared with placebo+placebo) with the effects of GIP+placebo (as compared with placebo+placebo) added to the effects of placebo+semaglutide 1.34 mg/mL (as compared with placebo+placebo). The sample size was estimated using the power.t.test function in R statistical software V.4.0.3. We chose a minimal relative difference (MIREDIF) for the two primary comparisons of 1.5 mmol/L, similar to the HbA1c change assumption of −0.74% to −1.44%, corresponding to a change of 1−2 mmol/L in estimated plasma glucose. Furthermore, 1.5 mmol/L corresponds to the end-of-treatment difference in HbA1c between the dulaglutide and intermediate level tirzepatide-treated groups.8 9 Based on our own unpublished CGM data,10 we anticipate that the SD at baseline and follow-up will be approximately 1.9 mmol/L and that the correlation between the baseline and the follow-up measurement will be at least 0.70. Thus, the SD of the change from baseline to follow-up should be at most 1.09 mmol/L. Assuming that this holds true, 13 participants are needed in each treatment group to ensure that the MIREDIF can be detected with a power of 0.85 at a Bonferroni-adjusted significance level of 0.025. To ensure power despite a 5%–10% dropout rate, we plan to include 15 participants in each group.
No statistical analyses of unblinded data will be performed until the database is locked. Statistical analyses will be performed using SAS software (V.9.4; SAS Institute) or similar software. All analyses will be performed on randomised patients who received at least one dose of the trial compound (semaglutide 1.34 mg/mL or placebo). Withdrawals will be replaced to ensure that at least 52 participants complete the trial—though a maximum of 8 participants can be replaced.
Primary endpoint
The primary endpoint (change in mean glucose assessed by CGM) will be analysed using a constrained linear mixed model with inherent baseline adjustment and with an unstructured covariance pattern to account for correlation between repeated measurements on each study participant. Differences in mean changes from baseline to follow-up will be reported for treatment with GIP+semaglutide 1.34 mg/mL versus placebo+semaglutide 1.34 mg/mL and treatment with GIP+placebo versus placebo+placebo with 97.5% CIs.
Patient and public involvement
We engaged two persons with T2D to (1) gain insights on disease progression and management, (2) identify unmet needs and most relevant outcomes and (3) identify opportunities to reduce disease burden and provide support for trial participants. Based on the feedback received from the interviews the following considerations are implemented. (1) An overview of visits and pictures of the CGM and insulin pump for GIP have been added to the participant information material and (2) the material was made more appropriate for the layperson.
Ethics and dissemination
Ethical considerations
This trial population consists of participants with T2D. The use of placebo semaglutide and GIP is needed in this study to separate any possible placebo effects from effects from the study drugs. Titration algorithms are used to ensure participants receive optimal treatment with minimal side effects. Participants will receive thorough information on correct administration of the trial product. They will, besides the planned study visits, receive intra-visit follow-up phone calls, if needed. If participants do not feel comfortable injecting themselves, additional study visits will be planned to administer injections at the clinical unit, Gentofte Hospital. The participants will receive trial products from free of charge from the clinical unit, Gentofte Hospital.
During the trial, participants will receive GIP or placebo (saline). There are no known side effects of GIP. Furthermore, participants will receive semaglutide 1.34 mg/mL or placebo. Known side effects of semaglutide are mild to moderate and are listed in the summary of product characteristics.11
At baseline, visit 4, and during visit 7 (end of study) CGM initiation, DXA scan and liver FibroScan will be performed. When collecting blood, some patients may experience minor discomfort when the needle penetrates the skin and rarely a small bleeding occurs. Complication to skin penetration could be a superficial phlebitis that will be treated with antibiotics should any sign of infection be present.
A total amount of 760 mL of blood will be drawn during the trial (~20 weeks), which include blood samples for safety assessments, efficacy assessments and efficacy assessments during the three OGTTs. A normal blood donation is 450 mL of blood and this can be done four times a year in Denmark.12 The amount of blood drawn in this study corresponds to approximately 1.5 blood donation. Persons with anaemia will not be included.
The CGM is minimally invasive and requires insertion of a sensor probe into the subcutaneous adipose tissue to measure glucose in the interstitial fluid. Potential complication to the CGM are rare and minimal and include local infection, pain and irritation of the skin. Any sign of infection the CGM will be removed and attached elsewhere. During the DXA scan and liver FibroScan the participant will be lying down during the procedure that lasts for estimated 15 min. A DXA scan is associated with a modest radiation dosage of approximately 0.1 mSv (corresponding to 2–3 times the dosage of a dental X-ray), it is painless, and no side effects are expected. FibroScan is not associated with radiation, is safe and not associated with any known side effects.
When the participants have given their informed written consent to participate in the trial, we will perform a review of their records to make sure that there is no contraindication towards the treatment with semaglutide 1.34 mg/mL or GIP(1-42). Overall, the potential benefits and scientific significance are expected to outweigh any potential risks or disadvantages in this clinical trial.
Ethics approval
The present study was approved by the Regional Committee on Health Research Ethics in the Capitol Region of Denmark (identification no. H-20070184) and by the Danish Medicines Agency (EudraCT no. 2020-004774-22). The investigators will ensure that this study is conducted in full conformance with the Edinburgh, Scotland (2000), amendment to the Declaration of Helsinki 1964 and with national laws and regulations for clinical research. The principal investigator is responsible for informing the ethics committees and regulatory authorities of any SAE and/or major amendments to the protocol as per national requirements.
Data dissemination
All results, positive, negative and inconclusive, will be disseminated at national and/or international scientific meetings and in peer-reviewed scientific journals. All investigators will be given access to the cleaned data sets and will be given 14 days to review and comment on any manuscript/abstract or other means intended for publication or presentation of the data. The datasets generated and analysed during the study will not be publicly available but will be available from the corresponding author on motivated request and in full compliance with the EU General Data Protection Regulation and the Danish Data Protection Act.
Study status
Protocol version 0.5.2, from September 10, 2021. Recruitment began in January 2022, and the final patient is expected to be recruited in January 2024.
Full protocol
The full protocol is attached as an additional file, accessible from the BMJ Open website (online supplemental file 1). The study protocol has been reported in accordance with the Standard Protocol Items: Recommendations for Clinical Interventional Trials guidelines (online supplemental file 2).
bmjopen-2022-065736supp001.pdf (880.4KB, pdf)
bmjopen-2022-065736supp002.pdf (72.1KB, pdf)
Discussion
We hypothesise that 6 weeks’ continuous s.c. GIP infusion added to semaglutide treatment will reduce CGM-assessed mean glucose levels and in patients with T2D and provide insights into other gluco-metabolic effects of separate and combined activation of the GIP and GLP-1 receptors.
Supplementary Material
Acknowledgments
We would like to thank laboratory technician Dorthe B Nielsen for her help with describing the laboratory procedures and study coordinator Anne-Marie Ellegaard, MSc PhD, for proof reading of the manuscript. We acknowledge all the study personnel involved in this study.
Footnotes
Twitter: @GasbjergL, @Filip_Knop
Contributors: MMH, LSG, TV, CKN, MBC and FKK all contributed substantially to the study design and methodology. JLF was responsible for the statistical analysis plan. FKK is the sponsor of the study and has overall responsibility of the study design, data collection, data management, data analysis, interpretation of data, writing of the report, and the decision to submit the report for publication, and have ultimate authority over these activities. All authors wrote, edited and approved the present manuscript.
Funding: This work was supported by the investigator-sponsored studies programme of Novo Nordisk under universal trial number U1111-1259-1491. The grant is received as pure support without any obligation with Novo Nordisk and covers the expenses of the study. The investigators are all employed at University Hospital, University of Copenhagen and/or University of Copenhagen.
Competing interests: LSG and MBC are minority shareholders of Antag Therapeutics. TV has served on scientific advisory panels, been part of speaker's bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GSK, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharmaceuticals. FKK has served on scientific advisory panels, been part of speaker's bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Norgine, Novo Nordisk, Sanofi, ShouTi, SNIPR Biome, Zealand Pharma and Zucara; and is a minority shareholder of Antag Therapeutics. Other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Nauck M, Stöckmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986;29:46–52. 10.1007/BF02427280 [DOI] [PubMed] [Google Scholar]
- 2.Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007;56:1951–9. 10.2337/db07-0100 [DOI] [PubMed] [Google Scholar]
- 3.Gasbjerg LS, Bergmann NC, Stensen S, et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 2020;125:170183. 10.1016/j.peptides.2019.170183 [DOI] [PubMed] [Google Scholar]
- 4.Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med 2013;5:209ra151. 10.1126/scitranslmed.3007218 [DOI] [PubMed] [Google Scholar]
- 5.Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–55. 10.1016/S0140-6736(21)01324-6 [DOI] [PubMed] [Google Scholar]
- 6.Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once Weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–15. 10.1056/NEJMoa2107519 [DOI] [PubMed] [Google Scholar]
- 7.Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020;5:e140532. 10.1172/jci.insight.140532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. The Lancet 2018;392:2180–93. 10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- 9.Frías JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab 2020;15:379–94. 10.1080/17446651.2020.1830759 [DOI] [PubMed] [Google Scholar]
- 10.Bomholt T. Glycaemic markers in persons with type 2 diabetes on haemodialysis - full text view - clinicaltrials.gov. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT03909269
- 11.Ozempic product information . SUMMARY OF PRODUCT CHARACTERISTICS 2017. 2017. Available: https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf
- 12.Danish Blood Donation Organisation . Givblod.dk. n.d. Available: https://givblod.dk/tapning-hvor-og-hvordan/sadan-foregar-en-tapning/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065736supp001.pdf (880.4KB, pdf)
bmjopen-2022-065736supp002.pdf (72.1KB, pdf)