Abstract
Objective
Evaluate relationship between radiographic progression and clinical outcomes in post hoc analyses of patients with psoriatic arthritis (PsA) receiving up to 2 years of guselkumab therapy in the phase 3, placebo-controlled, randomised trial, DISCOVER-2.
Methods
Biologic-naïve adults with active PsA (≥5 swollen joints /≥5 tender joints; C reactive protein ≥0.6 mg/dL) were randomised to guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at week 0, week 4, then every 8 weeks (Q8W); or placebo→guselkumab 100 mg Q4W (week 24). Radiographs (hands/feet) at week 0, week 24, week 52 and week 100 were scored via PsA-modified van der Heijde-Sharp (vdH-S) methodology. In these post hoc analyses, mean changes in vdH-S scores were summarised according to achievement of American College of Rheumatology 20/50/70 response; low disease activity (LDA) defined by Disease Activity in Psoriatic Arthritis (DAPSA) ≤14 or Psoriatic ArthritiS Disease Activity Score (PASDAS) ≤3.2, or minimal/very low disease activity (MDA/VLDA); and normalised physical function (Health Assessment Questionnaire-Disability Index (HAQ-DI) ≤0.5). Response rates for achieving MDA/VLDA and each component were determined among patients with and without radiographic progression (change in total vdH-S score >0.5). No formal hypothesis testing was performed.
Results
664 of 739 treated patients in DISCOVER-2 continued study treatment at week 52 and were included in these analyses. Mean changes in vdH-S scores from weeks 0 to 100 among all patients in the Q4W and Q8W groups were 1.7 and 1.5, respectively. Among all guselkumab-randomised patients, those who achieved ACR20/50/70, DAPSA LDA, PASDAS LDA, MDA, VLDA and HAQ-DI ≤0.5 (normalised physical function) had smaller mean changes in vdH-S scores than did non-responders at week 52 (0.2–1.2 vs 1.7–4.1) and week 100 (0.3–1.2 vs 2.0–4.6). Relative to patients with radiographic progression, those without progression were more likely to achieve the MDA criteria related to swollen and tender joint counts, patient-reported pain and global assessment, and normalised physical function through week 100.
Conclusion
In these post hoc analyses, the achievement of low levels of disease activity, including MDA, was associated with diminished rates of radiographic progression observed in patients receiving up to 2 years of guselkumab. Radiographic non-progressors were more likely to achieve patient-reported MDA criteria of minimal pain and normalised physical function compared with radiographic non-responders.
Trial registration number
Keywords: Arthritis, Psoriatic; Biological Therapy; Autoimmune Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Uncontrolled inflammation of psoriatic arthritis (PsA) can lead to radiographic damage that is associated with impaired physical function and disability, which may be partly irreversible. Patients with PsA treated with guselkumab, a fully human interleukin-23p19 inhibitor, demonstrated low levels of radiographic progression through 2 years in the phase 3 DISCOVER-2 Study.
WHAT THIS STUDY ADDS
In this population of patients at higher risk of future radiographic damage, achieving low/minimal levels of clinical disease activity or normalised physical function, at 1 or 2 years, with guselkumab therapy is associated with less radiographic progression over 2 years.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These results underscore the importance of timely treatment for PsA and suggest that optimising treatment decisions to achieve low levels of disease activity across multiple PsA domains may ultimately improve long-term structural damage outcomes, thus preserving overall physical function.
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic, inflammatory disease characterised by a diverse constellation of signs and symptoms, including peripheral joint damage, psoriatic skin lesions, axial disease, enthesitis and dactylitis. Uncontrolled inflammation resulting from delayed therapy increases the risk of structural joint damage in PsA.1 Structural damage, in turn, is associated with greater impairment of physical function and disability, which may be partly irreversible2 and lead to long-term impairment of health-related quality of life (HRQoL) and work productivity.3 A previous analysis of patients with PsA receiving conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) found that nearly 50% exhibited radiographic progression within 2 years of diagnosis.4 A separate study indicated that a delay in treatment, even as short as 6 months, was associated with greater radiographic damage and impaired physical function.1 Thus, limiting structural damage is an important treatment objective when addressing the potential manifestations of this lifelong disease. In patients with active disease despite traditional therapies, such as csDMARDs and non-steroidal anti-inflammatory drugs (NSAIDs), biological therapies are often recommended to limit progression of structural damage.5–7
Dysregulation of the interleukin (IL)-23/IL-17 axis is known to play a key role in the pathogenesis of psoriasis and PsA and has been the focus of newer biologics developed for patients with psoriatic disease. Findings from animal and human studies have suggested that IL-23 acts through multiple pathways to stimulate osteoclasts, which drive bone loss in inflammatory diseases.8 Guselkumab is a fully human monoclonal antibody that selectively inhibits the IL-23p19 subunit and is approved to treat patients with moderate-to-severe psoriasis and active PsA.9 In the pivotal, phase 3, placebo-controlled studies, DISCOVER-110 and DISCOVER-2,11 guselkumab-treated patients had significantly greater improvements in the signs and symptoms of PsA through week 24 compared with those receiving placebo. Additionally, guselkumab-treated patients exhibited decreases in acute-phase reactants and inflammatory cytokines that are central to the IL-23/IL-17 axis.12 Radiographic progression was assessed in DISCOVER-2, which enrolled a biologic-naïve population enriched for patients at higher risk of radiographic progression. Findings of prespecified analyses demonstrated that patients receiving guselkumab every 4 weeks (Q4W) and every 8 weeks (Q8W) had less radiographic progression through week 24 than did patients receiving placebo, with a statistically significant difference observed with the Q4W regimen.11 Furthermore, low levels of radiographic progression were observed through 2 years of guselkumab treatment.11 13 14 Herein, we report results of post hoc analyses from the 2-year DISCOVER-2 Study, intended to further evaluate the role of selective IL-23 inhibition with guselkumab in slowing radiographic progression and achieving meaningful and durable treatment targets.
Methods
Patients and study design
The inclusion and exclusion criteria for DISCOVER-2 have been reported.11 Briefly, biologic-naïve adults with active PsA (≥5 tender joints, ≥5 swollen joints and C reactive protein (CRP) level ≥0.6 mg/dL), current or documented history of psoriasis, and either inadequate response to or intolerance of standard non-biological therapy (eg, csDMARDs, NSAIDs and/or apremilast) were eligible. Patients could continue stable doses of selected csDMARDs, NSAIDs or other analgesics, or oral corticosteroids (≤10 mg/day prednisone or equivalent).
DISCOVER-2 was a randomised, double-blind, placebo-controlled phase 3 study.11 Eligible patients were randomly assigned (1:1:1) to receive subcutaneous injections of guselkumab 100 mg Q4W, guselkumab 100 mg at weeks 0 and 4, and then Q8W, or placebo with crossover to guselkumab Q4W at week 24. The final study agent administration was at week 100.
Assessments
Radiographs of the hands and feet were obtained at weeks 0, 24, 52 and 100 (or at the time of study discontinuation) and scored using the van der Heijde-Sharp (vdH-S) score modified for patients with PsA (inclusion of distal interphalangeal joints in the hands and pencil-in-cup/gross osteolysis deformities)15 in three distinct reading sessions. Reading session 1 included randomised patients who received ≥1 dose of study drug (partial or complete) and had radiographic images obtained at weeks 0 and 24 (or at discontinuation prior to week 24); reading session 2 included patients continuing study treatment at week 24 with images at weeks 0, 24 and 52 (or at discontinuation after week 24); and reading session 3 included patients continuing study treatment at week 52 with images at weeks 0, 24, 52 and 100 (or at discontinuation after week 52). For each reading session, radiographs were independently evaluated by two central primary readers, with a third reader for adjudication, blinded to patient, treatment group and time point. Primary reader scores in each session were averaged in the absence of adjudication.11 13 14 In the presence of adjudication, scores from the adjudicator, if not missing, were used when the difference between primary readers in week 24 change scores was >10 and the difference between the adjudicator and a primary reader was less than the difference between the two primary readers or if the week 24 change score from only one of the two primary readers was missing.
Clinical efficacy assessments and patient-reported outcomes were collected as previously detailed.11 In this post hoc analysis, global PsA disease activity was evaluated using the American College of Rheumatology (ACR) response criteria16 (tender joint count (TJC; 0–68), swollen joint count (SJC; 0–66), patient pain Visual Analogue Scale (VAS; 0–10), physician global assessment of disease activity (0–10 VAS), patient global assessment of disease activity (PtGA; arthritis; VAS 0–10), Health Assessment Questionnaire-Disability Index (HAQ-DI, 0–3) and CRP level (mg/dL)),16 the Psoriatic ArthritiS Disease Activity Score (PASDAS)17 (derived from TJC (0–68), SJC (0–66), physician global assessment (0–100), PtGA (arthritis and psoriasis; VAS 0–100), CRP level (mg/L), enthesitis (Leeds Enthesitis Index; LEI), tender dactylitis count and Physical Component Summary score of the 36-item Short-form Health Survey), and the Disease Activity in Psoriatic Arthritis (DAPSA)18 score (derived from TJC (0–68), SJC (0–66), patient pain VAS (0–10), PtGA (arthritis; VAS 0–10) and CRP level (mg/dL)). Physical function was assessed using the HAQ-DI19; normalised physical function was defined as HAQ-DI score ≤0.5. Minimal disease activity (MDA) and very low disease activity (VLDA) were defined as meeting at least five or all seven, respectively, of the following criteria: TJC ≤1, SJC ≤1, Psoriasis Area and Severity Index (PASI)20 ≤1, patient pain VAS (0-100) ≤15, PtGA (arthritis and psoriasis; VAS 0–100) ≤20, HAQ-DI ≤0.5 and tender entheseal points ≤1.21
DISCOVER-2 (ClinicalTrials.gov: NCT03158285) was conducted in accordance with Declaration of Helsinki and Good Clinical Practice guidelines.
Statistical analysis
Mean changes through week 100 in total PsA-modified vdH-S scores, as well as component erosion and joint space narrowing (JSN) scores, were determined using observed data derived from radiographic reading session 3.14 In post hoc analyses, mean changes in scores were summarised for clinical responders and non-responders, with clinical response defined as ≥20% improvement in ACR criteria (ACR20 response),16 ACR50 response, ACR70 response, PASDAS low disease activity (LDA; score ≤3.2),22 DAPSA LDA (score ≤14),23 MDA, VLDA and normalised HAQ-DI (score ≤0.5; among patients with HAQ-DI >0.5 at baseline). In addition, the proportions of patients achieving MDA and VLDA as well as the individual MDA/VLDA components at weeks 24, 52 and 100 were determined for patients with and without radiographic progression at the same time points, with progression defined as a change from baseline in total vdH-S score >0.5; analyses at weeks 24, 52 and 100 included patients from reading sessions 1, 2 and 3, respectively. Clinical response rates were determined using observed data. DISCOVER-2 was not powered to assess changes in vdH-S scores in the subgroups evaluated in these post hoc analyses; therefore, no formal hypothesis testing was performed.
Results
Baseline demographic and disease characteristics
As previously reported, 739 patients were randomised and received ≥1 study drug administration (guselkumab Q4W, n=245; guselkumab Q8W, n=248; or placebo, n=246).11 Across the three treatment groups, the majority of patients completed the study: 93%–94% through 1 year and 85%–90% did so through week 100.13 14 Patient demographics and disease characteristics were generally well balanced among the treatment groups,11 with baseline assessments indicating active PsA and impaired physical function (mean scores: TJC, 19.8–22.4; SJC, 11.7–12.9; PtGA (arthritis; VAS 0–10), 6.4–6.5; HAQ-DI, 1.2–1.3; PASDAS, 6.6; DAPSA, 46.3–49.7). Additionally, 64%–72% of patients had enthesitis and 40%–49% of patients reported dactylitis at baseline; the mean duration of PsA ranged from 5.1 to 5.8 years (online supplemental table 1). Among patients included in reading session 3, the mean baseline total PsA-modified vdH-S scores were 28.0 in the Q4W group, 23.9 in the Q8W group and 25.6 in the placebo→Q4W group (table 1). Baseline mean erosion and JSN scores from reading session 3 ranged from 12.0 to 14.2 and 11.9 to 13.8, respectively (table 1). Mean baseline vdH-S scores were similar among the three reading sessions (table 1).
Table 1.
Mean PsA-modified vdH-S scores at baseline for DISCOVER-2 patients included in reading sessions 1, 2 and 3
|
|
Guselkumab Q4W | Guselkumab Q8W | Placebo→guselkumab Q4W |
| Baseline vdH-S score | |||
| Reading session 1* | |||
| Patients, N | 245 | 248 | 246 |
| Total | 27.2 (42.3) | 23.0 (37.7) | 23.8 (37.8) |
| Erosion | 13.3 (22.4) | 11.6 (20.3) | 11.0 (19.1) |
| Joint space narrowing | 13.9 (21.5) | 11.5 (18.3) | 12.7 (19.9) |
| Reading session 2† | |||
| Patients, N | 232 | 238 | 231 |
| Total | 25.4 (40.2) | 22.4 (37.9) | 23.0 (39.5) |
| Erosion | 15.1 (22.2) | 13.6 (20.8) | 13.3 (21.4) |
| Joint space narrowing | 10.3 (19.5) | 8.8 (17.9) | 9.7 (19.1) |
| Reading session 3‡ | |||
| Patients, N | 221 | 228 | 215 |
| Total | 28.0 (43.6) | 23.9 (40.4) | 25.6 (42.4) |
| Erosion | 14.2 (23.3) | 12.0 (21.9) | 12.1 (21.9) |
| Joint space narrowing | 13.8 (21.8) | 11.9 (19.5) | 13.5 (21.6) |
Data reported as mean (SD).
*Reading session 1 included randomised patients who received ≥1 administration of study drug (partial or complete) and had radiographic images obtained at weeks 0 and 24 (or at discontinuation prior to week 24).
†Reading session 2 included patients continuing study treatment at week 24 with images at weeks 0, 24 and 52 (or at discontinuation after week 24).
‡Reading session 3 included patients continuing study treatment at week 52 with images at weeks 0, 24, 52 and 100 (or at discontinuation after week 52).
PsA-modified vdH-S score, van der Heijde-Sharp score modified for psoriatic arthritis; Q4W, every 4 weeks; Q8W, every 8 weeks.
rmdopen-2022-002789supp001.pdf (39.4KB, pdf)
Clinical efficacy and radiographic progression
The DISCOVER-2 primary endpoint was achieved; 64% of patients in both guselkumab groups had an ACR20 response at week 24 compared with 33% of placebo patients (p<0.0001).11 In reading session 1 (major secondary endpoint analysis), least squares (LS) mean changes in total vdH-S scores from baseline at week 24 were significantly less in the Q4W group (0.29; p=0.011) and numerically less in the Q8W group (0.52; p=0.072) compared with placebo (0.95).11 Among patients evaluated in reading session 3, mean changes in total vdH-S scores from weeks 0 to 24 (table 2) were consistent with LS mean changes and absolute mean changes derived from reading sessions 111 and 2,13 respectively, and mean changes from weeks 24 to 52 were consistent with those derived from reading session 2 (online supplemental table 2).13
Table 2.
Observed mean changes in PsA-modified vdH-S scores through week 100 in DISCOVER-2 patients evaluated in reading session 3*
| Change in PsA-modified vdH-S score | Guselkumab Q4W | Guselkumab Q8W | Placebo (W0–24)→guselkumab Q4W (W24–100) | |||||||||
| W0–24 N=221 |
W24–52 N=221 |
W52–100 N=211 |
W0–100 N=211 |
W0–24 N=228 |
W24–52 N=228 |
W52–100 N=216 |
W0–100 N=216 |
W0–24 N=215 |
W24–52 N=213 |
W52–100 N=202 |
W0–100 N=204 |
|
| Total | 0.5 (2.7) | 0.6 (2.7) | 0.8 (4.0) | 1.7 (7.0) | 0.7 (2.4) | 0.3 (1.6) | 0.5 (2.4) | 1.5 (4.4) | 1.1 (3.8) | 0.3 (2.8) | 0.1 (3.7) | 1.5 (6.9) |
| Erosion | 0.3 (1.9) | 0.4 (1.8) | 0.4 (2.9) | 1.0 (4.7) | 0.5 (2.0) | 0.2 (1.2) | 0.3 (1.8) | 1.0 (3.4) | 0.7 (2.2) | 0.2 (1.8) | 0.1 (2.0) | 1.0 (4.0) |
| Joint space narrowing | 0.2 (1.2) | 0.2 (1.1) | 0.3 (1.3) | 0.7 (2.7) | 0.2 (0.7) | 0.1 (0.7) | 0.2 (0.9) | 0.5 (1.4) | 0.4 (1.7) | 0.1 (1.1) | 0.04 (1.9) | 0.5 (3.0) |
Data presented as mean (SD).
*Reading session 3 included patients continuing study treatment at week 52 with images at weeks 0, 24, 52 and 100 (or at discontinuation after week 52).
PsA-modified vdH-S score, van der Heijde-Sharp score modified for psoriatic arthritis; Q4W, every 4 weeks; Q8W, every 8 weeks.
Minimal radiographic progression was observed in guselkumab-randomised patients included in reading session 3 during both the first year (mean changes in total vdH-S from week 0 to 52: Q4W, 1.1; Q8W, 1.0), which was consistent with results from reading session 2,13 and also the second year (mean changes in total vdH-S from week 52 to 100: Q4W, 0.8; Q8W, 0.5)14 of guselkumab treatment, regardless of dosing regimen. Through 2 years, mean changes in total vdH-S score from weeks 0 to 100 were 1.7 in the Q4W group, 1.5 in the Q8W group and 1.5 in the placebo→Q4W group. Similar patterns of minimal progression through 2 years of guselkumab therapy were observed for both erosion and JSN scores (table 2).
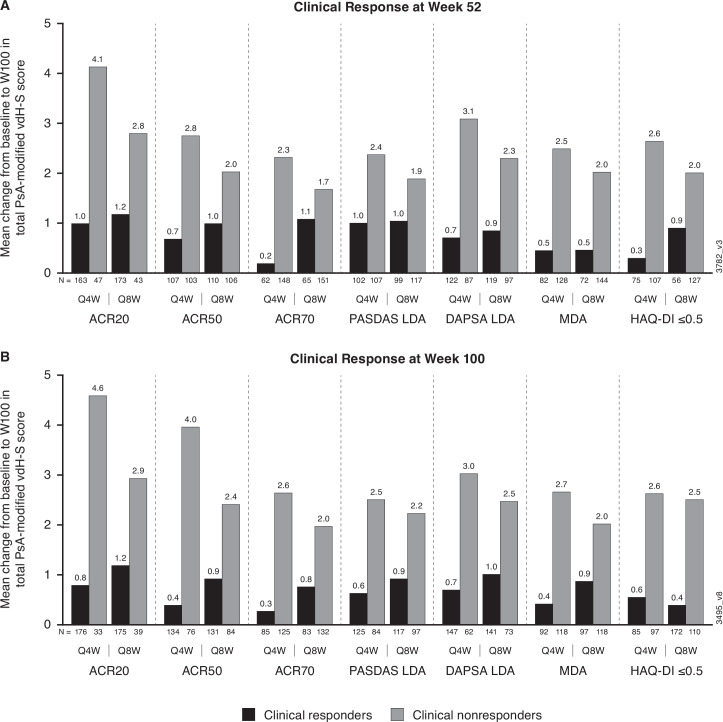
Among patients in the guselkumab groups, mean changes from weeks 0 to 100 in total vdH-S scores were numerically lower in patients who achieved clinical response at week 52 compared with non-responders when assessed by ACR20 (1.0–1.2 vs 2.8–4.1), ACR50 (0.7–1.0 vs 2.0–2.8) or ACR70 (0.2–1.1 vs 1.7–2.3) response (figure 1). Similarly, mean changes from baseline in total vdH-S scores at week 100 were also numerically lower for patients achieving PASDAS LDA (1.0 vs 1.9-2.4), DAPSA LDA (0.7–0.9 vs 2.3–3.1), MDA (0.5 vs 2.0-2.5) or HAQ-DI ≤0.5 (0.3–0.9 vs 2.0–2.6) at week 52 compared with non-responders (figure 1). This effect was also observed for patients who achieved VLDA compared with those who did not (mean change in total vdH-S: −0.4–0.9 vs 1.8–1.9), despite the relatively small sample size. Similar trends were observed for mean changes from weeks 0 to 100 in total vdH-S scores when clinical efficacy was assessed at week 100 (figure 1).
Figure 1.
Mean change from baseline to week 100 in total PsA-modified vdH-S score for patients who achieved clinical response at week 52 (A) or week 100 (B). ACR 20/50/70, ≥20%/50%/70% improvement in American College of Rheumatology criteria; DAPSA, Disease Activity in Psoriatic Arthritis; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; MDA, minimal disease activity; PASDAS, Psoriatic Arthritis Disease Activity Score; PsA-modified vdH-S score, van der Heijde-Sharp score modified for psoriatic arthritis; Q4W, every 4 weeks; Q8W, every 8 weeks.
Clinical response in patients with and without radiographic progression
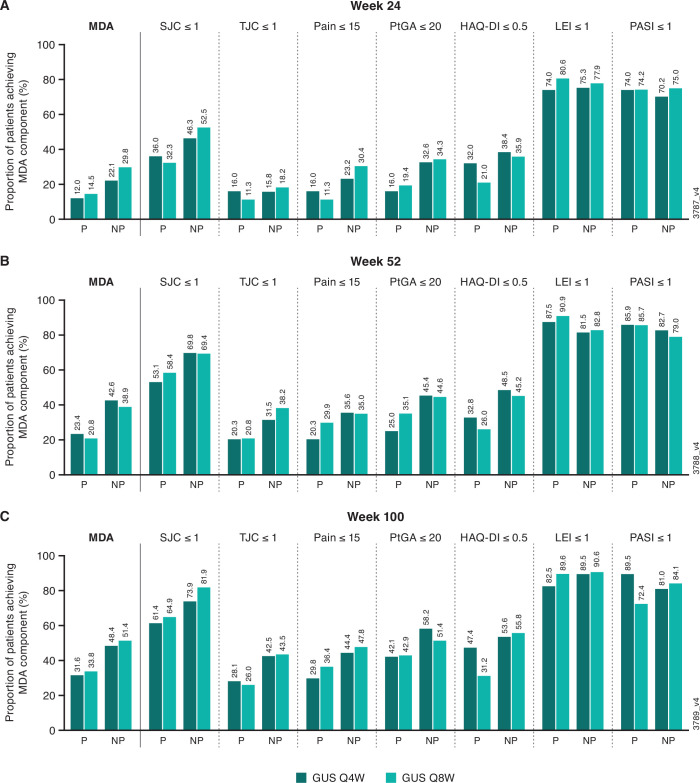
At week 24 (reading session 1), guselkumab-randomised patients classified as radiographic non-progressors had numerically greater response rates than progressors for achieving SJC ≤1 (46%–52% vs 32%–36%), patient pain VAS ≤15 (23%–30% vs 11%–16%), PtGA (arthritis and psoriasis) ≤20 (33%–34% vs 16%–19%) and HAQ-DI ≤0.5 (36%–38% vs 21%–32%) as well as overall MDA (22%–30% vs 12%–14%) (figure 2). While clinical response rates increased or were maintained at weeks 52 (reading session 2) and 100 (reading session 3) in both cohorts of patients, numerically greater proportions of radiographic non-progressors than progressors achieved SJC ≤1, TJC ≤1, patient pain VAS ≤15, PtGA (arthritis and psoriasis) ≤20, HAQ-DI ≤0.5 and MDA (figure 2). At week 24, 5%–6% of radiographic non-progressors and 2%–3% of radiographic progressors achieved VLDA; response rates continued to separate between these cohorts at week 100 (18%–25% vs 8%–9%, respectively; data not shown). The vast majority (>70%) of both radiographic non-progressors and progressors achieved LEI ≤1 and PASI ≤1 at all three time points (figure 2).
Figure 2.
Proportions of patients achieving MDA components at weeks 24 (A; reading session 1), 52 (B; reading session 2) and 100 (C; reading session 3) summarised by radiographic progression status at the same time points, with progression defined as change from baseline in total PsA-modified vdH-S score >0.5. Progressors (P)—week 24: Q4W, N=50; Q8W, N=62; week 52: Q4W, N=64; Q8W, N=77; week 100: Q4W, N=57, Q8W, N=77. Non-progressors (NP)—week 24: Q4W, N=190; Q8W, N=181; week 52: Q4W, N=162; Q8W, N=157; week 100: Q4W, N=153, Q8W, N=138. GUS, guselkumab; HAQ-DI, Health Assessment Questionnaire-Disability Index; LEI, Leeds Enthesitis Index; MDA, minimal disease activity; PASI, Psoriasis Area and Severity Index; PsA-modified vdH-S score, van der Heijde-Sharp score modified for psoriatic arthritis; PtGA, patient global assessment; Q4W, every 4 weeks; Q8W, every 8 weeks; SJC, swollen joint count; TJC, tender joint count.
Discussion
Structural damage in patients with PsA can lead to functional disability that may be irreversible.2 Previous studies have identified risk factors for radiographic progression in PsA, including elevated CRP,24–26 higher SJC,24 27 28 and the presence of dactylitis29 and bone erosions.26 The DISCOVER-2 population was enriched for patients at higher risk of radiographic progression with the inclusion criteria of ≥5 tender joints, ≥5 swollen joints and CRP ≥0.6 mg/dL. In addition, although the presence of dactylitis was not required for study enrolment, 45% of patients in this study were affected at baseline. CRP and SJC, respectively, are indicative of systemic and local inflammation, and these variables, as well as the presence of dactylitis, are included in the composite indices used to assess disease activity in the current analyses. Achieving meaningful improvements or low levels of disease activity across several disease domains, as assessed by these composite measures, at 1 year of treatment with guselkumab was associated with less radiographic progression over 2 years.
Low rates of radiographic progression were observed through 2 years in patients receiving guselkumab in DISCOVER-2, regardless of the dosing regimen. In the guselkumab groups, achievement of clinical response and treatment targets at 1 year (ie, ACR20/50/70, PASDAS LDA, DAPSA LDA, MDA and normalised physical function (HAQ-DI ≤0.5)) was associated with smaller mean changes in total vdH-S scores from weeks 0 to 100. In addition, patients classified as non-progressors (change in total vdH-S ≤0.5) were more likely to achieve MDA. Specifically, radiographic non-progressors were more likely to achieve the MDA criteria for swollen and tender joints (0 or 1) and, importantly, patient-reported outcomes indicative of minimal pain (VAS ≤15 (0–100) and overall disease activity (PtGA of arthritis and psoriasis; VAS ≤20 (0–100)), as well as normalised physical function (HAQ-DI ≤0.5). Additionally, previous research has demonstrated that among patients with PsA treated with secukinumab, those who achieved either MDA or DAPSA LDA had significantly greater improvements in patient-reported measures of HRQoL and fatigue and less overall work impairment compared with MDA and DAPSA LDA non-responders through 2 years.30
The IL-23/IL-17 axis has been implicated in the pathogenesis of psoriasis and PsA. IL-23 maintains the differentiation of naïve T cells into Th17 cells, which are the primary source of the proinflammatory cytokine IL-17A and have been linked to several autoimmune diseases including PsA.31 In a murine model of psoriasis, mice with increased levels of IL-23 in the skin also developed joint swelling,32 and in patients with PsA, IL-23 expression has been correlated with SJC and CRP.33 IL-23 has been identified as a ‘master regulator’ in psoriasis,34 and selective inhibition of IL-23 in PsA provides efficacy across several aspects of disease as demonstrated by results of the current analyses.
Pooled analyses of serum biomarkers from patients in the DISCOVER-1 and DISCOVER-2 Studies found decreases in acute-phase reactants and inflammatory cytokines following guselkumab treatment, with post-treatment serum levels of IL-17A and IL-17F consistent with those in healthy controls.12 In a separate analysis of DISCOVER-2 patients, guselkumab-treated patients had greater decreases in serum levels of several collagen degradation biomarkers as early as week 4 than did patients receiving placebo.35 When evaluating clinical efficacy by various composite indices of disease activity, greater proportions of guselkumab-treated patients achieved meaningful improvements at week 24 compared with placebo, with separation observed as early as week 8.36
A treat-to-target approach has been widely implemented in managing patients with rheumatoid arthritis (RA) and is associated with improved outcomes, including radiographic progression and physical function.37 This approach is currently considered the standard of care for patients with RA.38 39 The Tight Control of PsA (TICOPA) Study evaluated this concept using MDA as the treatment target and demonstrated that greater proportions of patients with PsA in the tight control group achieved ACR and PASI responses compared with patients in the standard care group.40 Although no difference in radiographic progression between the two treatment groups was apparent at week 48, it should be noted that TICOPA participants received only non-biological therapies,40 which are known to be inferior to biologics in slowing radiographic progression.41–49 Current recommendations from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis support using MDA as a treatment target, although no consensus has been reached on a preferred continuous measure of PsA disease activity. Actual real-world use of a treat-to-target approach has remained relatively limited, with fewer than half of clinicians surveyed reporting regular use of a composite measure for PsA in their practice.50 The results of these post hoc analyses from DISCOVER-2 demonstrate the association between less radiographic progression and achieving low levels of disease activity as assessed by several composite indices, including MDA. The findings reported here suggest that using a treat-to-target approach in patients with PsA may result in long-term benefits in terms of both radiographic damage and physical function.
Uncontrolled inflammation in patients with PsA can lead to progressive radiographic damage and ultimately disability.4 In a qualitative study of treatment outcomes in patients with PsA, prevention of joint damage was an important factor to many patients when considering their treatment options.51 Thus, therapies that are effective in diminishing progression of structural damage and maintaining function may lead to improved treatment persistence. An observational analysis of patients with PsA in Ireland found that those who had a delay >6 months between symptom onset and their first visit with a rheumatologist were more likely to have peripheral erosions and greater impairments in physical function and HRQoL compared with those who were evaluated by a rheumatologist earlier in their disease course.1 Previous results from DISCOVER-2 have demonstrated that among biologic-naïve patients, those treated with guselkumab had lower levels of radiographic progression and less impairment of overall HRQoL and work productivity at week 24 than did patients receiving placebo.11 52 53 Of note, DISCOVER-2 patients who crossed over to guselkumab after 6 months of placebo had mean changes in total vdH-S scores at week 100 that were similar to those in patients who had been receiving guselkumab from baseline.
Response rates for criteria related to enthesitis and skin disease were high overall, with no apparent differences between progressors and non-progressors in achieving responses defined by minimal symptoms (LEI ≤1 and PASI ≤1). Dysregulation of IL-23 is known to play a central role in both the keratinocyte proliferation leading to psoriatic skin lesions32 as well as entheseal inflammation.54 Inhibiting the IL-23p19 subunit with guselkumab therapy demonstrated robust efficacy in treating both of these aspects of PsA with over half of patients in DISCOVER-2 achieving complete skin clearance and over 65% of patients achieving resolution of enthesitis at week 100.14
These analyses were conducted post hoc, and DISCOVER-2 was not powered to assess radiographic progression in the various subgroups evaluated, some of which comprised relatively small numbers of patients and, thus, may have been susceptible to a high degree of variability in radiographic scores. Owing to these limitations, formal hypothesis testing was not performed. Of note, the retention rate in this study was high, with nearly all patients enrolled and treated in DISCOVER-2 completing study treatment through 2 years, providing a robust dataset. All patients in DISCOVER-2 were biologic-naïve and were selected using inclusion criteria designed to enrich the population for patients at higher risk of radiographic progression. These results may not be generalisable to all patients with PsA; however, it should be noted that the treatment effect with guselkumab has been observed across subgroups from DISCOVER-1 and DISCOVER-2 defined by various baseline demographic and disease characteristics.55 In addition, 2 years may be a relatively short follow-up time for observing radiographic progression in PsA. To that end, a phase 3b study (APEX; NCT04882098) is being conducted to further evaluate the effects of guselkumab on radiographic progression in at-risk biologic-naïve patients with PsA. In APEX, an elevated risk of radiographic progression is defined by the presence of ≥2 joint erosions of the hands and feet and CRP ≥0.3 mg/dL at baseline, and patients will be followed for up to 3 years.
As noted, previous findings from DISCOVER-1 and DISCOVER-2 have demonstrated the efficacy of guselkumab in improving signs and symptoms across several PsA disease domains across diverse patient subgroups.55 The patients with an elevated risk of future structural damage who were enrolled in DISCOVER-2 demonstrated low rates of radiographic progression through 2 years, with 85%–90% completing the study through week 100. Results of the current post hoc analyses from DISCOVER-2 indicate that achieving low levels of clinical disease activity, following 1 or 2 years of treatment with guselkumab, is associated with less radiographic progression over 2 years. Our results suggest that the association of achieving low levels of disease activity across disease domains and less radiographic progression over time may be an important consideration in the shared decision-making process when evaluating PsA treatment options.
Taken together, these data provide a robust analysis of radiographic progression through 2 years in a phase 3 study of guselkumab in patients with PsA11 13 14 and also highlight the importance of addressing structural damage in a timely manner to optimise long-term patient outcomes, including preservation of function.
Footnotes
Contributors: Study conception and design or acquisition of data—ABG, IBM, PR, APK, XLX, YJ, SS, MS, SDC, FL and PJM. Data analysis—YJ and SS. Data interpretation—ABG, IBM, PR, APK, XLX, YJ, SS, MS, SDC, FL and PJM. Drafting the article or revising it critically for important intellectual content—ABG, IBM, PR, APK, XLX, YJ, SS, MS, SDC, FL and PJM. Final approval of the version to be published—ABG, IBM, PR, APK, XLX, YJ, SS, MS, SDC, FL and PJM. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved:—ABG, IBM, PR, APK, XLX, YJ, SS, MS, SDC, FL and PJM. SDC is the guarantor for this work.
Funding: This study was funded by Janssen Research & Development, LLC. Medical writing support was provided by Rebecca Clemente, PhD, of Janssen Scientific Affairs, LLC, under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2022;175:1298-1304).
Competing interests: ABG has received honoraria as an advisory board member, non-promotional speaker or consultant for: Amgen, AnaptysBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dice Therapeutics, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharma, UCB Pharma and Xbiotech (stock options for an RA project); research/educational grants from: AnaptysBio, Bristol Myers Squibb, Janssen, Novartis, Ortho Dermatologics, Sun Pharma and UCB Pharma; all funds go to the Icahn School of Medicine at Mount Sinai. IBM has received consultant fees from AstraZeneca, AbbVie, Bristol Myers Squibb, Amgen, Eli Lilly and Company, Cabaletta, Compugen, GSK, Gilead, Janssen, Novartis, Pfizer, Sanofi, Roche and UCB; grant/research support from AstraZeneca, Bristol Myers Squibb, Amgen, Eli Lilly and Company, GSK, Janssen, Novartis, Roche and UCB; and is a shareholder for Causeway Therapeutics, and Evelo Compugen (non-executive roles), NHS GGC Board Member, Evelo Board of Directors and Versus Arthritis Trustee Status. PR has received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer and UCB; and research grants from Janssen and Novartis. APK, XLX and SS are employees of Janssen Research & Development, and own stocks in Johnson & Johnson. YJ is a consultant employed by Cytel and funded by Janssen to provide statistical support. MS is an employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson and owns a stock in Johnson & Johnson. SDC is an employee of Janssen Scientific Affairs, and owns a stock in Johnson & Johnson. FL is an employee of Janssen Cilag Global Medical Affairs and owns a stock in Johnson & Johnson. PJM has received research support from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun Pharma and UCB; consultant fees from AbbVie, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma and UCB; and speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by each site’s governing ethical body (in the USA: Stirling institutional review board approval number: 5910C), and all patients provided written informed consent.
References
- 1.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015;74:1045–50. 10.1136/annrheumdis-2013-204858 [DOI] [PubMed] [Google Scholar]
- 2.Kerschbaumer A, Baker D, Smolen JS, et al. The effects of structural damage on functional disability in psoriatic arthritis. Ann Rheum Dis 2017;76:2038–45. 10.1136/annrheumdis-2017-211433 [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T 2010;35:680–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Kane D, Stafford L, Bresnihan B, et al. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. 10.1093/rheumatology/keg384 [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Guyatt G, Ogdie A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79. 10.1038/s41584-022-00798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razawy W, van Driel M, Lubberts E. The role of IL-23 receptor signaling in inflammation-mediated erosive autoimmune arthritis and bone remodeling. Eur J Immunol 2018;48:220–9. 10.1002/eji.201646787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremfya: package insert. Horsham, PA: Janssen Biotech, Inc, 2022. [Google Scholar]
- 10.Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 11.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- 12.Sweet K, Song Q, Loza MJ, et al. Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: results from phase 3 trials. RMD Open 2021;7:e001679. 10.1136/rmdopen-2021-001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol 2021;73:604–16. 10.1002/art.41553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McInnes IB, Rahman P, Gottlieb AB, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol 2022;74:475–85. 10.1002/art.42010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64 Suppl 2:ii61–4. 10.1136/ard.2004.030809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 17.Helliwell PS, FitzGerald O, Fransen J, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. 10.1136/annrheumdis-2012-201341 [DOI] [PubMed] [Google Scholar]
- 18.Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. 10.1136/ard.2009.122259 [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson T, Pettersson U. Severe psoriasis -- oral therapy with a new retinoid. Dermatologica 1978;157:238–44. 10.1159/000250839 [DOI] [PubMed] [Google Scholar]
- 21.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 22.Helliwell PS, FitzGerald O, Fransen J. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. J Rheumatol 2014;41:1212–7. 10.3899/jrheum.140172 [DOI] [PubMed] [Google Scholar]
- 23.Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PSA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. 10.1136/annrheumdis-2015-207507 [DOI] [PubMed] [Google Scholar]
- 24.Borst C, Alasti F, Smolen JS, et al. Role of clinical and biochemical inflammation in structural progression of patients with psoriatic arthritis. RMD Open 2021;7:e002038. 10.1136/rmdopen-2021-002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Heijde D, Gladman DD, FitzGerald O, et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J Rheumatol 2019;46:1089–96. 10.3899/jrheum.180971 [DOI] [PubMed] [Google Scholar]
- 26.Gladman DD, Mease PJ, Choy EHS, et al. Risk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPT. Arthritis Res Ther 2010;12:R113. 10.1186/ar3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bond SJ, Farewell VT, Schentag CT, et al. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6. 10.1136/ard.2006.056457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon P, Pfoehler C, Bergner R, et al. Swollen joint count in psoriatic arthritis is associated with progressive radiological damage in hands and feet. Clin Exp Rheumatol 2012;30:45–50. [PubMed] [Google Scholar]
- 29.Geijer M, Lindqvist U, Husmark T, et al. The Swedish Early Psoriatic Arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of dactylitis. J Rheumatol 2015;42:2110–7. 10.3899/jrheum.150165 [DOI] [PubMed] [Google Scholar]
- 30.Coates LC, Nash P, Kvien TK, et al. Comparison of remission and low disease activity states with DAPSA, MDA and VLDA in a clinical trial setting in psoriatic arthritis patients: 2-year results from the FUTURE 2 study. Semin Arthritis Rheum 2020;50:709–18. 10.1016/j.semarthrit.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Ghilardi N, Xie M-H, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003;278:1910–4. 10.1074/jbc.M207577200 [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Deshpande M, Grisotto M, et al. Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci Rep 2020;10:8259. 10.1038/s41598-020-65269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celis R, Planell N, Fernández-Sueiro JL, et al. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res Ther 2012;14:R93. 10.1186/ar3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gooderham MJ, Papp KA, Lynde CW. Shifting the focus-the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Dermatol Venereol 2018;32:1111–9. 10.1111/jdv.14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schett G, Loza MJ, Palanichamy A, et al. Collagen turnover biomarkers associate with active psoriatic arthritis and decrease with guselkumab treatment in a phase 3 clinical trial (DISCOVER-2). Rheumatol Ther 2022;9:1017–30. 10.1007/s40744-022-00444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coates LC, Ritchlin CT, Gossec L, et al. Guselkumab provides sustained domain-specific and comprehensive efficacy using composite indices in patients with active psoriatic arthritis. Rheumatology (Oxford) 2023;62:606–16. 10.1093/rheumatology/keac375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. 10.1016/S0140-6736(04)16676-2 [DOI] [PubMed] [Google Scholar]
- 38.Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 39.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 40.Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allard A, Antony A, Shaddick G, et al. Trajectory of radiographic change over a decade: the effect of transition from conventional synthetic disease-modifying antirheumatic drugs to anti-tumour necrosis factor in patients with psoriatic arthritis. Rheumatology (Oxford) 2019;58:269–73. 10.1093/rheumatology/key297 [DOI] [PubMed] [Google Scholar]
- 42.D’Angelo S, Palazzi C, Olivieri I. Psoriatic arthritis: treatment strategies using biologic agents. Reumatismo 2012;64:113–21. 10.4081/reumatismo.2012.113 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Leal M, Reyes-Soto MA, Hernandez-Galarza I, et al. Does current evidence on disease-modifying antirheumatic drugs for psoriatic arthritis reinforce an effect on radiographic progression? Results from a systematic review and meta-analysis. Clin Rheumatol 2021;40:3499–510. 10.1007/s10067-021-05622-w [DOI] [PubMed] [Google Scholar]
- 44.Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford) 2015;54:20–8. 10.1093/rheumatology/keu237 [DOI] [PubMed] [Google Scholar]
- 45.Kang EJ, Kavanaugh A. Psoriatic arthritis: latest treatments and their place in therapy. Ther Adv Chronic Dis 2015;6:194–203. 10.1177/2040622315582354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kavanaugh A, Husni ME, Harrison DD, et al. Radiographic progression inhibition with intravenous golimumab in psoriatic arthritis: week 24 results of a phase III, randomized, double-blind, placebo-controlled trial. J Rheumatol 2019;46:595–602. 10.3899/jrheum.180681 [DOI] [PubMed] [Google Scholar]
- 47.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis 2014;73:1000–6. 10.1136/annrheumdis-2013-204741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landewé R, Ritchlin CT, Aletaha D, et al. Inhibition of radiographic progression in psoriatic arthritis by adalimumab independent of the control of clinical disease activity. Rheumatology (Oxford) 2019;58:1025–33. 10.1093/rheumatology/key417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. 10.1002/art.40851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coates LC, FitzGerald O, Merola JF, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/Outcome Measures in Rheumatology consensus-based recommendations and research agenda for use of composite measures and treatment targets in psoriatic arthritis. Arthritis Rheumatol 2018;70:345–55. 10.1002/art.40391 [DOI] [PubMed] [Google Scholar]
- 51.Dures E, Hewlett S, Lord J, et al. Important treatment outcomes for patients with psoriatic arthritis: a multisite qualitative study. Patient 2017;10:455–62. 10.1007/s40271-017-0221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtis JR, McInnes IB, Rahman P, et al. The effect of guselkumab on work productivity in biologic-naïve patients with active psoriatic arthritis through week 52 of the phase 3, randomized, placebo-controlled DISCOVER-2 trial. Adv Ther 2022;39:4613–31. 10.1007/s12325-022-02270-7 [DOI] [PubMed] [Google Scholar]
- 53.Curtis JR, McInnes IB, Rahman P, et al. The effect of guselkumab on general health state in biologic-naïve patients with active psoriatic arthritis through week 52 of the phase 3, randomized, placebo-controlled DISCOVER-2 trial. Adv Ther 2022;39:4632–44. 10.1007/s12325-022-02269-0 [DOI] [PubMed] [Google Scholar]
- 54.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γT+ CD3+CD4-CD8- entheseal resident T cells. Nat Med 2012;18:1069–76. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 55.Ritchlin CT, Mease PJ, Boehncke W-H, et al. Sustained and improved guselkumab response in patients with active psoriatic arthritis regardless of baseline demographic and disease characteristics: pooled results through week 52 of two phase III, randomised, placebo-controlled studies. RMD Open 2022;8:e002195. 10.1136/rmdopen-2022-002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002789supp001.pdf (39.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.