Abstract
Chimeric antigen receptor (CAR) T cell therapy is an effective salvage therapy for pediatric relapsed B-cell acute lymphoblastic leukemia (B-ALL), yet is challenged by high rates of post-CAR relapse. Literature describing specific relapse patterns and extramedullary (EM) sites of involvement in the post-CAR setting remains limited, and a clinical standard for post-CAR disease surveillance has yet to be established. We highlight the importance of integrating peripheral blood minimal residual disease (MRD) testing and radiologic imaging into surveillance strategies, to effectively characterize and capture post-CAR relapse. Main body: Here, we describe the case of a child with multiply relapsed B-ALL who relapsed in the post-CAR setting with gross non-contiguous medullary and EM disease. Interestingly, her relapse was identified first from peripheral blood flow cytometry MRD surveillance, in context of a negative bone marrow aspirate (MRD <0.01%). Positron emission tomography with 18F-fluorodeoxyglucose revealed diffuse leukemia with innumerable bone and lymph node lesions, interestingly sparing her sacrum, the site of her bone marrow aspirate sampling. Conclusions: We highlight this case as both peripheral blood MRD and 18F-fluorodeoxyglucose positron emission tomography imaging were more sensitive than standard bone marrow aspirate testing in detecting this patient’s post-CAR relapse. Clinical/Biologic Insight: In the multiply relapsed B-ALL setting, where relapse patterns may include patchy medullary and/or EM disease, peripheral blood MRD and/or whole body imaging, may carry increased sensitivity at detecting relapse in patient subsets, as compared with standard bone marrow sampling.
Keywords: Pediatrics; Cell Engineering; Immunotherapy, Adoptive; Receptors, Chimeric Antigen; Hematologic Neoplasms
Background
Therapeutic options for B-cell acute lymphoblastic leukemia (B-ALL) have expanded beyond traditional chemotherapy, to include numerous targeted and cell-based strategies that are capable of achieving high rates of remission.1 This expanded armamentarium of effective therapies has introduced an element of chronicity into acute leukemia, and is permissive of salvage following serial relapses. In specific, chimeric antigen receptor (CAR) T cells targeting the B cell canonical CD19 antigen have achieved unprecedented complete remission (CR) rates of 70%–90% in the B-ALL multiply relapsed setting.2–4 Comparatively, retrospective analysis establishing outcomes across patients with multiply-relapsed B-ALL prior to Food and Drug Administration (FDA) approval of CD19-targeting CAR T cells (tisagenlecleucel), describe CR rates of 51%, 37%, and 31% following second, third, and fourth to eighth respective salvage attempts.5 Despite high CR rates following CAR therapy, 30%–60% of patients will go on to experience post-CAR relapse.6 Literature characterizing properties of sequential relapses in the post-CAR setting remains sparse. Immune privileged sites may be predisposed to relapse and sites of relapse post-CAR may diverge from the historic experience. Many patients undergo clinical surveillance in the post-CAR setting, and it is vital that we effectively characterize relapse patterns, so we can adjust surveillance strategies accordingly.7
Assessment of bone marrow (BM) morphology and cerebrospinal fluid (CSF) status at diagnosis and relapse remains the current gold standard for detecting leukemia. With the development of minimal residual disease (MRD) testing using flow cytometry8 or high-throughput sequencing, leukemia can be detected with increased sensitivity and specificity. Established literature supports the role of peripheral blood MRD testing, as compared with BM, as a minimally invasive, cost-effective, sensitive measure for leukemia surveillance, with next-generation sequencing (NGS) and real-time PCR, when available, having greatest sensitivity.9–11 Here, we describe a case report of a multiply relapse patient with B-cell leukemia who, in the face of negative morphological disease by BM evaluation, had a diffusely remarkable 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET/MR) scan with innumerable bone lesions and lymphoid disease. Despite a preceding BM with no detectable disease by both morphology and flow MRD, the 18F-FDG PET/MR scan was pre-empted by positive peripheral blood flow MRD. This case was distinct in that the disease distribution on 18F-FDG PET/MR scan was patchy and spared the sacrum, likely explaining the negative BM, sampled from the sacrum. The sensitivity of the peripheral blood in detecting this patient’s bulky, yet patchy disease suggests an important role for peripheral blood MRD and 18F-FDG PET scintigraphy in detecting disease in the multiply relapsed post-CAR ALL setting.
Case presentation
We report the case of a school-age female with a history of multiply relapsed B-ALL. The patient additionally had a history of autism with baseline developmental speech delay. She was diagnosed at age 3 with low-risk B-ALL with cytogenetics significant for ETV6:RUNX1. She tolerated upfront chemotherapy on the COG AALL0932 protocol without complication. She achieved a CR following induction and completed 2 years of chemotherapy at age 6. Unfortunately, she developed late isolated BM relapse 3 years following completion of chemotherapy. She was started on reinduction chemotherapy with vincristine, peg-asparaginase and dexamethasone and unfortunately developed catastrophic infectious complications with Streptococcus viridans and Escherichia coli bacteremia, followed by Bacillus cereus meningitis, rendering her non-ambulatory and non-verbal. Despite toxicities, a CR was achieved, however, the patient was unable to tolerate further consolidative chemotherapy or allogeneic hematopoietic stem cell transplantation (HSCT) and underwent leukemia surveillance. She developed a second relapse with combined BM and extramedullary disease, with extraocular involvement 9 months following her initial relapse and was referred to our center for CD19-specific CAR T cell therapy (tisagenlecleucel).
On extraocular relapse, she received reinduction chemotherapy with high-dose methotrexate (MTX; 5 g/m2 ×4 doses). Focal radiation was declined by family at that time. Leukapheresis product was then collected and sent to novartis for tisagenlecleucel production. During the production, the patient was bridged with oral 6-mercaptopurine and oral MTX. A baseline BM and lumbar puncture prior to CAR T cell did not demonstrate detectable disease, with exception of one atypical cell in her CSF of unclear significance. She did not have established NGS diagnostic clones, so MRD using NGS was not an available tool for pre or post-CAR disease surveillance. Baseline pre-CAR MRI of the orbit showed resolution of measurable lesions. She received standard lymphodepletion doses with fludarabine 30 mg/m2 × 4 days and cyclophosphamide 500 mg/m2 × 2 days, followed by infusion with tisagenlecleucel. She developed grade 1 cytokine release syndrome (CRS), managed with supportive care, and did not experience neurotoxicity. Tocilizumab was not required and CAR was overall well tolerated.
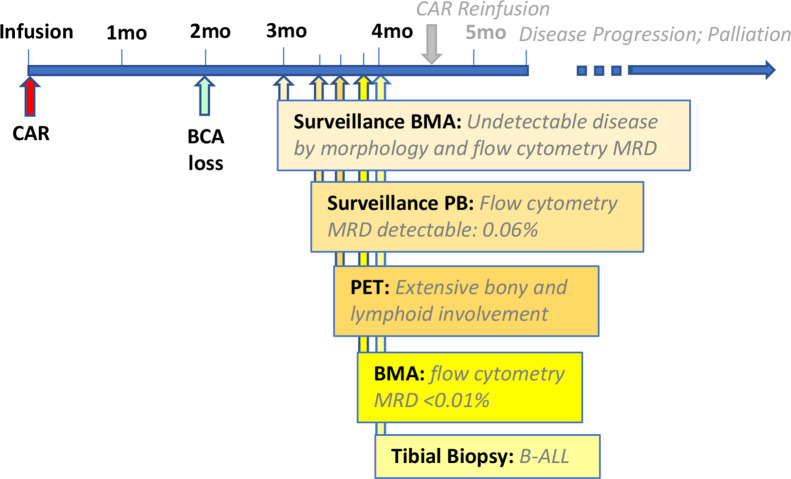
Her post-tisagenlecleucel course (figure 1) was significant for loss of B cell aplasia at 2 months post-CAR, with lymphocyte subsets demonstrating 25% CD19+ cells. Surveillance BM aspirate remained negative for morphological disease and flow cytometry at her 3-month evaluation. Screening MRD from the peripheral blood 2 weeks following her negative BM was unexpectedly positive for an 0.06% abnormal immature B cell population consistent with her prior B-ALL. Follow-up BM aspirate 12 days later continued to show negative morphological disease and MRD by flow cytometry of <0.01%. An 18F-FDG PET/MR scan was pursued to further evaluate the positive peripheral blood MRD in context of BM MRD of <0.01%. 18F-FDG PET/MR scan revealed innumerable osseous hypermetabolic leukemic lesions and disseminated hypermetabolic lymph nodes (figure 2). Interestingly, the 18F-FDG PET/MR scan demonstrated sparing of the iliac crest, site of the prior BM aspirate. The MR component of the study for attenuation correction and anatomic localization of the 18F-FDG PET imaging confirmed sites of normal and abnormal anatomy corresponding to the 18F-FDG metabolic imaging. A bone biopsy of an involved hypermetabolic tibial lesion was pursued for tissue confirmation of disease and was consistent by morphology and immunophenotype with prior disease, with 100% CD19 with slightly dim expression and 95% dim CD22 expression.
Figure 1.
Timeline of post-CAR clinical course: schema highlights events leading up to and establishing post-CAR relapse. CAR, chimeric antigen receptor.
Figure 2.
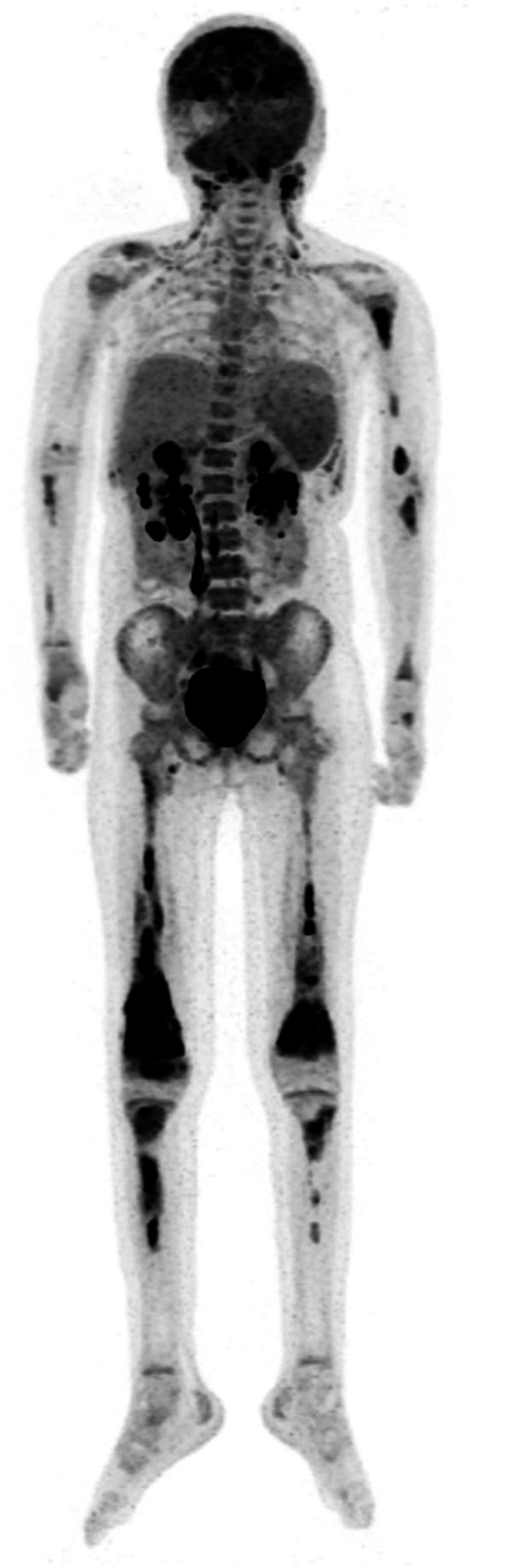
18F-FDG PET scan on post-tisagenlecleucel relapse. 18F-FDG PET illustrates sacral sparing, yet, grossly patchy enumerable osseous and lymphoid lesions (in context of flow cytometry MRD from bone marrow aspirate of <0.01%, with detectable flow cytometry MRD in peripheral blood of 0.06%). 18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; MRD, minimal residual disease.
In context of confirmed relapse with ongoing CD19 expression, the patient proceeded with reinfusion of available tisagenlecleucel product. She received higher lymphodepleting doses of chemotherapy with cyclophosphamide of 900 mg/m2 × 2 and unchanged fludarabine dosing and was infused with her second tisagenlecleucel infusion 4.5 months after her initial CAR infusion. She tolerated her second CAR-infusion without CRS or neurotoxicity. Unfortunately, at day 28 following CAR reinfusion, the patient established herself as tisagenlecleucel-resistant and was found to have 27% B-ALL lymphoblasts in her peripheral blood. Lymphoblasts continued to express both CD19 and CD22 with unchanged phenotype. X-ray at that time revealed leukemic lesions in the proximal tibia and distal femur. Family opted to limit further workup at that time and palliative care was consulted. The patient did receive multiple cycles of inotuzumab, with good quality of life and transient disease control, yet ultimately continued to have combined BM and extramedullary disease progression. The patient received palliative focal radiation and chemotherapy (mini-hyper-CVAD), which was well tolerated, until expiring from B-ALL, 12 months following her initial CAR infusion.
Conclusions
This current report describes a patient rendered non-verbal and non-ambulatory from chemotherapy-related morbidity. She was not a candidate for potentially curative HSCT or chemotherapy, due to prior morbidity. Serial CAR infusions were well tolerated, highlighting the paramount role of CAR therapy as a tolerable option for patients with high toxicity profiles. Relapse, as illustrated, remains a major challenge in the post-CAR setting. This case report of post-CAR relapse illustrates that characterizing relapse patterns and adapting surveillance strategies to accurately measure disease burden and detect subclinical sites of relapse is of vital importance.
Patients with multiply relapsed B-ALL may be more susceptible to having non-contiguous, patchy medullary disease or extramedullary disease, and peripheral blood may in fact be more sensitive than BM in a subset of patients. To date, as per commercial indication, tisagenlecleucel is by definition reserved for patients with refractory B-ALL or ≥2 relapses, skewing patient selection toward the multiply-relapsed setting. Specifically in the post-CAR setting, there is an additional rationale that immune privileged extramedullary sites may carry an increased risk of relapse, yet data characterizing sites of relapse in the post-CAR setting is just starting to emerge.7
It is vital that as we introduce new mechanistic classes of therapies, our monitoring strategies should be optimized to capture all possible patterns of disease relapse, including combined or extramedullary only disease. Additional to peripheral blood and BM evaluations, 18F-FDG PET scintigraphy is an emerging complementary functional molecular imaging technique that, combined with anatomic imaging as either PET/CT or PET/MR study, broadly captures both medullary and extramedullary sites of disease.12 Whole body imaging is not embedded into the standard disease evaluation or surveillance of patients with B-ALL. We likely underestimate the extent of disease, as we traditionally limit our assessment of disease burden to sites that are evaluated as per the clinical standard, including BM, peripheral blood, CSF or sites of clinical signs or symptoms. Subthreshold extramedullary disease that has yet to manifest with clinical symptoms likely often goes unrecognized. 18F-FDG PET scans have a clinical role in monitoring B-ALL in the pre-CAR and post-CAR setting in patients with known extramedullary disease. However, formal studies are warranted to establish the sensitivity and role of 18F-FDG PET scans in detecting disease in relapsed B-ALL, when patients otherwise do not exhibit symptoms of extramedullary disease. Here, we describe a case of post-CAR relapse where disease relapse was detected first by peripheral blood MRD surveillance in an asymptomatic patient. Disease was quite disseminated, as identified by 18F-FDG PET/MR scan, despite a morphologically negative BM, with flow cytometry MRD of <0.01. We; therefore, suggest that current standard for leukemia evaluation may need to adapt as relapse patterns evolve and treatment strategies mechanistically depart from standard chemotherapy, and we highlight the role for peripheral blood MRD and imaging in post-CAR disease assessments.
Acknowledgments
We acknowledge the clinical cell therapy (CCT) team, the pediatric oncology leukemia team and the Bass Center staff, nurses and fellows for their role in patient care and adherence to best patient clinical standards.
Footnotes
Twitter: @SRamakrishna_MD
Contributors: LS directed patient clinical care and was primary author of the manuscript. AW, CE, CB, KLD, SR, CA, NL, HRN, JO, and CM were involved in patient clinical care. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CM is an inventor on multiple patents for CAR T cells. CM is a cofounder and holds equity in Lyell Immunopharma and Syncopation Life Sciences, which are developing CAR-based therapies, and consults for Lyell, NeoImmune Tech, Apricity, Nektar and Immatics, Ensome and Mammoth.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Davis KL, Mackall CL. Immunotherapy for acute lymphoblastic leukemia: from famine to feast. Blood Adv 2016;1:265–9. 10.1182/bloodadvances.2016000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129:3322–31. 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 2019;134:2361–8. 10.1182/blood.2019001641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun W, Malvar J, Sposto R, et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia 2018;32:2316–25. 10.1038/s41375-018-0094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov 2018;8:1219–26. 10.1158/2159-8290.CD-18-0442 [DOI] [PubMed] [Google Scholar]
- 7. Holland EM, Yates B, Ling A, et al. Characterization of extramedullary disease in B-ALL and response to CAR T-cell therapy. Blood Adv 2022;6:2167–82. 10.1182/bloodadvances.2021006035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a children’s oncology group study. Blood 2008;111:5477–85. 10.1182/blood-2008-01-132837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartram J, Wright G, Adams S, et al. High-throughput sequencing of peripheral blood for minimal residual disease monitoring in childhood precursor B-cell acute lymphoblastic leukemia: A prospective feasibility study. Pediatr Blood Cancer 2022;69:e29513. 10.1002/pbc.29513 [DOI] [PubMed] [Google Scholar]
- 10. Brisco MJ, Sykes PJ, Hughes E, et al. Monitoring minimal residual disease in peripheral blood in B-lineage acute lymphoblastic leukaemia. Br J Haematol 1997;99:314–9. 10.1046/j.1365-2141.1997.3723186.x [DOI] [PubMed] [Google Scholar]
- 11. Pulsipher MA, Han X, Maude SL, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov 2022;3:66–81. 10.1158/2643-3230.BCD-21-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Z, Hu Y, Li J, et al. Applications of PET in diagnosis and prognosis of leukemia. Technol Cancer Res Treat 2020;19:1533033820956993. 10.1177/1533033820956993 [DOI] [PMC free article] [PubMed] [Google Scholar]