Abstract
Background
Acute exacerbations of asthma are common in children, however, treatment decisions for severe exacerbations are challenging due to a lack of robust evidence. In order to create more robust research, a core set of outcome measures needs to be developed. In developing these outcomes, it is important to understand the views of clinicians who care for these children in particular, views that relate to outcome measures and research priorities.
Methods
To determine the views of clinicians, a total of 26 semistructured interviews based on the theoretical domains framework were conducted. These included experienced clinicians from emergency, intensive care and inpatient paediatrics across 17 countries. The interviews were recorded, and later transcribed. All data analyses were conducted in Nvivo by using thematic analysis.
Results
The length of stay in hospital and patient-focused parameters, such as timing to return to school and normal activity, were the most frequently highlighted outcome measures, with clinicians identifying the need to achieve a consensus on key core outcome measure sets. Most research questions focused on understanding the best treatment options, including the role of novel therapies and respiratory support.
Conclusion
Our study provides an insight into what research questions and outcome measures clinicians view as important. In addition, information on how clinicians define asthma severity and measure treatment success will assist with methodological design in future trials. The current findings will be used in parallel with a further Paediatric Emergency Research Network study focusing on the child and family perspectives and will contribute to develop a core outcome set for future research.
Keywords: Asthma, Paediatric asthma
WHAT IS ALREADY KNOWN ON THIS TOPIC
Acute exacerbations of asthma are common in children, however, treatment decisions for severe exacerbations are challenging due to a lack of robust evidence.
WHAT THIS STUDY ADDS
An understanding of clinician perspectives relating to important outcome measures and research questions in acute exacerbations of asthma.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The current findings will be used in parallel with a further Paediatric Emergency Research Network study focusing on child and family perspectives to develop an internationally agreed core outcome set for future research in trials of acute severe asthma in children.
Introduction
An acute exacerbation of asthma, in children, is a common reason for emergency department (ED) presentation and subsequent admission to hospital.1 Hospital admissions for asthma are increasing and are associated with a significant economic burden.2–4 While many children have mild to moderate exacerbations and are discharged home, a recently published study of 14 029 children presenting to Australasian EDs found that 36% of children with acute asthma are admitted to hospital, with 1.1% requiring paediatric intensive care unit admission (PICU).5 In addition, recent studies document increasing PICU admission for severe acute asthma worldwide.4 Despite this concerning scenario, the evidence base informing treatments for these high-risk patients with severe presentations is weak.6 Current knowledge is limited due to a lack of adequate sample size in existing trials, a wide range of treatment options and variation in both clinical management practice and outcome measures use.3 7 8
As such, there is a need to develop a core outcome set for trials in this population to enable collation and systematic review of high-quality and comparable trial results. The process to define core outcomes for a specified study population has been outlined by the Core Outcome Measures in Effectiveness Trials initiative.9 A working group within the Paediatric Emergency Research Network (PERN), an international umbrella collaborative acute care research network, has been established to determine a core outcome set for acute severe asthma in children.10 The primary aim of this paper is to describe the views of clinicians in identifying important outcome measures. The secondary aim is to understand important research priorities and explore the underlying behaviours and knowledge used by clinicians to define, treat and reassess asthma.
Findings from this study will allow the development of a comprehensive list of outcome measures relevant to research in this field. These outcomes will be aligned with those identified by parents and children and will be combined to inform a future Delphi study to generate a core outcome set. This will enable the development of consensus guidelines and standardised protocols for randomised controlled trials in this important condition.10
Methods
Study design
We conducted clinician interviews across multiple hospitals and countries and included multiple specialties. The focus was to understand how and why clinicians treat asthma in the way they do, including identifying what clinicians perceive as important in both research questions and outcome measures.
Theoretical framework
Methodological orientation and theory
In order to change behaviour, there is a need to explore the underlying drivers of current practice. The theoretical domains framework (TDF), underpinned by behavioural change theories, provides a structured and comprehensive framework to understand the different elements of behavioural change, thus allowing for more effective implementation research.11 Broadly speaking, the TDF covers the following domains: knowledge, skills, social/professional role and identity, belief about capabilities, belief about consequences, motivation and goals, memory, attention and decision process, environmental context, social influences, emotion and behavioural regulation.11 Based on this framework, interview questions were formulated according to each domain to ensure a breadth of understanding of why clinicians assess and manage asthma in the way they do. The questions were formulated initially by the research working group to cover what was identified as the key questions for understanding asthma and research and outcome priorities. This was then compared with the outline of the TDF to ensure all components were covered. This was then refined by the initial research working group; including researchers (CG, SC, FB and SD) with final interview questions based on consensus. For each interview, to allow for comprehensive data collection at the end of each interview an open-ended question was asked ‘is there anything else on this topic or thoughts around this topic you would like to add or ask?’. This component was also transcribed and used in analysis of the data as per the rest of the data set.
The semistructured interview allowed flexibility within the components of the interview and included collection of demographic features of participants. The questions (online supplemental appendix 1) explored how clinicians define an acute severe exacerbation of asthma in children, the relative clinician-perceived importance of different outcomes and aspects of clinical care, and opinions on priorities for future research. The interview format was not modified during the interview process.
bmjresp-2022-001502supp001.pdf (61.9KB, pdf)
Participant selection and sampling
Experienced clinicians in paediatric emergency medicine, intensive care and inpatient paediatrics across 17 countries were recruited through convenience sampling from the PERN asthma group by email. The total number of recipients of the initial email was 27. Each member from the group was asked to either participate or invite another clinician (such as intensive care paediatric physicians, emergency physicians, general and respiratory paediatricians) within their local health network to participate. In order to have a diverse view, a maximum of five clinicians in one country and no more than two clinicians from the same hospital were included. This recruitment process commenced to ensure adequate diversity both geographically across the globe and to ensure representation, where possible, across both high-income and low-middle income counties (table 1). Once this was ensured we continued with interviews, until thematic saturation was reached. Interview data were analysed after the first 10 interviews, and after then every 5 interviews thereafter.
Table 1.
Characteristics and demographics of interview participants in alphabetical order‡
| Country | Specialty | Practice setting | Years of experience |
| America | Emergency physician | Tertiary | >15 |
| Argentina | Paediatrician | Tertiary | 5–10 |
| Australia | Paediatric emergency physician* | Tertiary | >10 |
| Australia | General paediatrician* | Tertiary | 5–10 |
| Australia | Intensive care physician | Tertiary | >10 |
| Australia | Emergency physician | Tertiary | 0–5 |
| Canada | Paediatric emergency physician | Tertiary | >10 |
| China | Paediatrician† | Tertiary | >10 |
| Costa Rica | Paediatrician | Tertiary | 5–10 |
| Costa Rica | Paediatric respiratory physician | Tertiary | 5–10 |
| India | Paediatric emergency physician | Tertiary | 5–10 |
| India | Paediatric emergency physician | Tertiary | 5–10 |
| New Zealand | Paediatric emergency physician | Tertiary | >10 |
| New Zealand | General paediatrician | Tertiary | >10 |
| Paraguay | Paediatric emergency physician | Tertiary | 0–5 |
| Qatar | Paediatric emergency physician | Tertiary | 5–10 |
| Romania | Paediatric emergency physician | Secondary | 5–10 |
| Singapore | Paediatric emergency physician | Tertiary | >10 |
| South Korea | Paediatric allergist† | Tertiary | >10 |
| Spain | Paediatric emergency physician | Secondary | 5–10 |
| Switzerland | Paediatric emergency physician | Tertiary | >10 |
| South Africa | Paediatric emergency physician | Tertiary | 5–10 |
| Tanzania | Paediatrician† | Tertiary | 0–5 |
| United Kingdom | Emergency physician | Secondary | >10 |
| United Kingdom | Paediatric intensivist | Tertiary | 5–10 |
| Uruguay | Paediatric intensivist | Tertiary | 5–10 |
All other interviews these were conducted via ZOOM conference software.
*These interviews occurred in person at researcher’s primary place of work.
†These occurred via written correspondence.
‡Tertiary and secondary refers to the capacity of the hospital to either be a specialist paediatric hospital, with access to a wide range of medical and surgical specialties and most commonly a paediatric intensive care unit or secondary where a paediatric patient would be seen in ED but if unwell or requiring complex care would need to be referred. For this study, the interviewees denoted themselves as working at a tertiary or secondary hospital.
ED, emergency department.
Each participant received an invitation letter with a participant information sheet from the principal investigator (CG). A total of twenty-six interviews were conducted; twenty one interviews were conducted using Zoom videoconferencing12 and recorded, two interviews which were conducted face to face at the primary researcher’s own hospital. Three interviews were completed with the exchange of written questions and answers: two participants preferred written documentation due to English as a second language, and one due to unreliable internet service. All interviews were recorded and transcribed by a professional transcription service. The interviewees with each type of interview method are denoted in Table 1. Verbal consent was obtained at the commencement of each interview. No potential interviewees refused consent and no interviewees withdrew from the study.
Table 2.
Overview of themes and subthemes characterised by theoretical domain framework and presented in hierachy of the number of interviews in which dominant theme/subtheme was mentioned
| Theoretical domains framework | Themes | Subthemes |
| Knowledge | Definition of asthma | History Clinical examination Age Excluding differential diagno Response to treatment Reference to guidelines |
| Defining severity of asthma | Level of consciousness Oxygen saturations Observations Whole picture Scoring system Reference to guidelines Need to put IV in Parental concern |
|
| Treatment decisions for escalated treatment | Clinical features Experience Knowledge of research |
|
| Environmental context and resources | Access to resources Access to Intensive care |
|
| Skills/beliefs about capabilities | Belief in clinical practice guidelines Belief in ability of treatment to work |
|
| Motivation and goals | Measurement of treatment success | Improving observations Avoiding of consequences Patient centred goals |
| Emotion | Perceived unwellness of child Deteriorating child |
|
| Social influences | Similar treatments to colleagues Legacy of treatments |
|
| Behavioural regulation | Practice change | |
| Beliefs about consequences | Avoiding consequences Best treatment regime |
|
| Social/professional role and identity | Similar treatments to colleagues Perception of staff skill set |
Two of the interviewees were colleagues of the principal investigator working in the same healthcare system. To mitigate interview bias a general email was sent to all emergency physicians, intensive care physicians and general paediatric clinicians. The two interviewed were those that expressed interest and availability to participate. The same set of questions was asked as was done for all other interviewees and the interview recorded and transcribed verbatim. All other interviewees were unknown to the investigator prior to this study.
Researcher characteristics and reflexivity
The research team has reflected on our sociodemographic, cultural and educational backgrounds and the influence on our data collection and interpretation. The lead author (CG) is an Australian medical specialist having completed both her medical degree and specialist qualifications in paediatrics. This study comprised part of her PhD and she is experienced in contributing to multicentre research in the field of acute, severe asthma. During the data collection and analysis for this study, the lead author worked as a paediatric emergency physician at a major hospital in Adelaide Australia and was a PhD candidate at University of Adelaide. The second author is a bicultural (Chinese/Australian), PhD qualified researcher with 10 years’ experience in triangulation research combining quantitative and qualitative methods. The rest of the authors represent a geographically diverse group of clinicians across the globe, all working clinically within paediatric emergency medicine and collectively with extensive experience in both qualitative and quantitative research. Interviews were only conducted by the lead author and data analysis was conducted by the first and second authors.
Data analyses
The recordings of interviews were deidentified and transcribed by a central secure academic transcription service (www.gotranscript.com). The transcripts were then sent to the interviewer for review. Once checked and approved, transcripts were uploaded into NVivo V.12 to assist with data analysis (18 March 2020, QSR International, London, UK).
Detailed inductive analysis occurred on raw data. Data were then categorised into themes that were then mapped to the TDF.13 The two researchers (CG/YX) independently reviewed the transcribed interviews. Text relevant to more than one theme were cross-indexed. Coding was discussed after the first five interviews to ensure consistency. Discrepancies were identified, discussed and consensus was reached.
Within themes, all subthemes were defined, and reported as the number of times the thematic concept was coded. Verbatim quotation for themes are highlighted in results and online supplemental appendix 2.
bmjresp-2022-001502supp002.pdf (195.3KB, pdf)
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
This paper is reported according to the Standards for Reporting Qualitative Research.14
Results
Interviews were conducted between December 2018 and August 2020. Demographic characteristics, location of clinical practice and method of interviewing for each participant are outlined in table 1. The average interview duration was 33 min (range from 15 to 60 min). Interviews conducted via written communication were excluded from the timing range reported. Overall themes are outlined in table 2 and are reported with reference to the TDF which they are mapped. Several dominant themes were identified and the subthemes within each have been outlined. The results outlined below report the dominant themes with regard to the primary aim of this study, to explore outcome priorities and the secondary aims of both identifying research questions and how clinicians define asthma and severity and response to treatment.
Dominant theme: defining asthma and severity of asthma (knowledge)
The subthemes identified in defining an asthma exacerbation in descending order of frequency were history, clinical examination, age, excluding differential diagnosis, use of local guidelines and response to treatment. A peak flow meter was only mentioned in one interview as being in routine use. There was variation in the age range from when an asthma exacerbation was considered, with age ranging from 2 to 5 years old. No interviewee considered asthma exacerbation as a diagnosis in children younger than 2 years old. While asthma severity was viewed as a combination of features, the dominant subtheme associated with severe or life-threatening asthma exacerbation was the level of consciousness.
A child that’s agitated, distressed or drowsy makes me more concerned that I'm dealing with severe asthma. Uruguay, Paediatric Intensivist.
Other subthemes included oxygen saturations, observations and the number of an asthma score. The value of oxygen saturations considered as a sign of severe exacerbation ranged from 90% to 94%. Three asthma scoring systems were identified, the Pulmonary Respiratory Assessment Measure (PRAM)15 the Pulmonary Index score16 and the Wood and Downe’s score.17 The use of the scores was identified as both guiding treatment choices and measuring success and clinical response to treatments.
It’s throughout our whole department and it’s part of our EMR. It has a direct order entry. It’s all based on PRAM scoring and then automatic order-filling based on that. Switzerland, Emergency Physician.
Dominant theme: drivers of treatment decisions (knowledge)
There were similarities among providers in the first-line treatments for children with non-severe asthma, with all clinicians mentioning supplemental oxygen and short-acting beta agonists. There was some variability regarding the indication for steroids in mild-moderate asthma. There was considerable variation in the management of severe asthma: when discussing the use of a particular intravenous agent, the drivers of using escalated therapy and how the outcomes of treatment were measured (Knowledge, Motivation and Goals). Reasons for variation were attributed to personal clinical experience and knowledge of the existing literature.
My experience with magnesium is [that] it seems to be [a] safe and effective therapy. Australia, Paediatric Emergency Physician
I'm probably using aminophylline more in the last one to two years for the severe critical asthma that’s going to ICU. Australia, Emergency Physician
Changed threshold of using IV/Oral corticosteroids. In the past reserved them only for patients who did not respond initially to therapy. Nowadays prescribe them to any patient with an exacerbation needing a hospital visit. Based on some research I had read at some point. Tanzania, Emergency Physician
The complexity of treatment decisions was highlighted as outlined below.
I would say that the decision is probably influenced by the time of the shift, whether it’s day or night, whether there’s a space where … [in] the ward or they're going to be needing ED for a long time, what the feeling of the nurses is. Costa Rica, Respiratory physician
There were different opinions on when respiratory support therapies such as the use of high flow nasal cannulae and non-invasive ventilation were used. (Skills/belief about capabilities)
We give high flows or at least more flows of oxygen, that might improve their oxygenation. Helping them with some PEEP, might help them decrease, at least, their work of breathing. Costa Rica, Paediatrician
Then as far as non-invasive ventilation like BiPAP or CPAP, those are usually applied if we continue to have a child that shows signs of poor ventilation. South Korea, Paediatric allergist
A dominant theme was the need for admission and the likelihood of Intensive Care admission if the children required IV therapy. (Emotion/motivation, goals and social influences)
If you are on an infusion of whatever your choice of the day is, in our hospital, that means you go to intensive care. New Zealand, General Paediatrician
It’s very [very] hard to discharge a child who I've given intravenous treatment to because fear of judgment by my peers. Australia, Emergency Physician
The theme of limited access to resources, particularly in availability of high flow or access to intensive care, was more apparent in low-income and middle-income countries (Environmental context and resources).
The circuit we use is a single-use circuit. That is the only problem with high-flow nasal cannula, but being a third-world country, the problem in certain patients, it won't be affordable. India, Paediatric Emergency Physician
Dominant theme: measurements of successful treatment (motivation and goals)
There were three subthemes that encapsulated the way clinicians identified treatment success by: Improvement in the clinical status during an observational period, minimisation of the need for invasive ventilation and meeting patient-centred goals. With respect to clinical improvement, the most common goal was the work of breathing, although this measure was often combined with general improvement in mental status and hypoxia.
The work of breathing in terms of respiratory rate, use of accessory muscles is lessening. Then I think the treatment is successful. Australia, Emergency Physician
In contrast, physicians in less-well resources regions expressed an opinion that escalation of therapy was usually instituted to avoid respiratory failure requiring mechanical ventilation, respiratory arrest and death.
All we are trying to prevent is the cardiac arrest ultimately. India, Paediatric Emergency Physician
Patient-centred goals emphasised the importance of making the child feel more comfortable and diminishing the concern of staff and parents.
Makes the child feel more comfortable. They feel calmer, and their look and their symptoms settle down. Australia, General Paediatrician
Dominant theme: research goals and outcome measures (goals and skills)
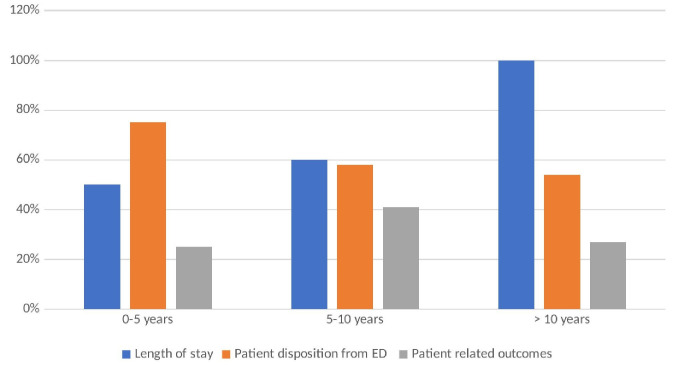
Participants outlined several research themes and outcome measures outline in Table 3. In addition, figure 1 reflects the dominant themes of outcome measures by years of experience. The more experienced clinicians all highlighted length of stay as an outcome measure, with the less experienced clinicians focusing more on disposition from ED. All clinicians across the years of experience highlighted the importance of including patient-related outcome measures. Table 4 provides suggestions for future research questions and important trial outcome measures are listed in Table 5. Verbatim examples of each research question are provided in online supplemental appendix 2.
Table 3.
Outcome measures and research questions mapped to the domain of goals and skills from the TDF and ordered by number of interviews in which theme was mentioned
| Outcome priorities | Length of stay parameters Patient disposition from ED Patient-related outcomes Rate of complications Using a validated score Length of non-invasive ventilation (NIV) and invasive ventilation (IV) How much treatment was needed Death and respiratory arrest |
| Entrance into studies | Understanding different phenotypes Defining severity for an acute exacerbation How asthma is defined |
| Research questions | |
| Diagnosis and classification | Peak flow usefulness Aligning guideline Identifying those with secondary pneumonia Point of care US |
| Treatment goals | Respiratory function Target oxygen level Prevention of exacerbations |
| Treatment options | Best IV therapy Nebulised magnesium/heliox/intramuscular epinephrine/ketamine Steroids NIV and high flow Use of IV therapy on the ward Role of Ipratoprium Bromide Efficacy of asthma care plan |
ED, emergency department; TDF, theoretical domains framework.
Figure 1.
Dominant outcome measures identififed by years of experience of clinician. ED, emergency department.
Table 4.
Research questions in each of the dominant themes with examples
| 1. Treatment options | What is the best IV bronchodilator therapy and in what order should the IV options be given? What is the utility of NIV and high flow? Which steroid is ‘best’ for exacerbations of asthma, what dosing? What are the risks of giving IV therapy on the ward? What is the role of nebulised magnesium? What is the role of ketamine? How does ipratropium help prevent admission or escalation of care in an acute exacerbation of asthma? What is the role of IM epinephrine? What is the role of Heliox? |
| 2. Diagnosis and classification | What is the usefulness of peak flow in diagnosis and classification of severity? How do you identify those with secondary pneumonia? What is the utility of point of care ultrasound? What should an international clinical practice guideline look like and what are the research goals? |
| 3. What are reasonable treatment goals? | For clinical parameters including respiratory function? What should be the target oxygen level? |
| 4. Questions related to families and children | What are ways in which you can prevent asthma exacerbations? How do you ensure knowledge translation of asthma care plans? |
IV, invasive ventilation; NIV, non-invasive ventilation.
Table 5.
Outcome measures in each of the dominant themes
| 1. Length of stay | Hospital length of stay PICU length of stay |
| 2. Patient flow/disposition | Disposition from ED Rate of repeated attendance in ED or primary care |
| 3. Duration of treatment | Length of non-invasive and invasive treatment |
| 4. Patient-related outcomes | Time to return to normal activity Time to return to school Financial impact on families for example, time off work Patient feeling better Resolution of symptoms |
| 5. Rate of complications | Complications from IV treatment Toxicity from salbutamol—how much is too much? Long-term outcomes |
| 6. Using a validated score | In trials, as an outcome measure to compare different treatments For predicting outcomes |
| 7. How much treatment was needed | Frequency of interventions Need to escalate therapy |
| 8. Death and respiratory arrest | Rate of occurrence |
ED, emergency department; IV, invasive ventilation; PICU, paediatric intensive care unit.
In addition, clinicians have emphasised the importance of a consensus for key core outcome measures, including factors perceived as important by children and families. This also included the need to consider how core outcomes would differ in different economic and social settings. It was commented on that higher mortality rates and severity at presentation in lower socioeconomic countries would affect the outcome and research goals identified.
Well, they die before we can take care of them. They die. They just simply die… If you make a trial in that country or in that country, outcomes are different than I guess in Australia or in Uruguay. Uruguay, Paediatric Intensivist
Dominant themes around future research from clinician’s perspective were categorised into three main areas: defining entrance into studies, identifying important outcome measures for clinical trials and determining important research questions.
With regard to entrance into studies, dominant themes included an understanding of different asthma phenotypes, the clinical diagnosis of asthma and defining severity of an acute exacerbation.
You have the severe obese asthmatics that have a completely different phenotype to significant severe eosinophilic asthmatic. I guess you need to rule out which group of asthmatic[s] you want to study and what is your aim of the study. Costa Rica, Paediatrician
Is getting a clear definition of what asthma is and that’s probably the biggest challenge in these studies is like what are we calling asthma and making sure that, that’s defined, then the people running the trials are getting the same groups of kids in. Australia, General Paediatrician
Discussion
This study explored the priorities in research questions and outcome measures for children with acute exacerbations of asthma. This was examined by using the TDF to gain an understanding of how clinicians diagnosed asthma, classified severity management decisions and measurement of success (or otherwise) of the treatment administered. Findings from this study can now be used to help us prioritise specific research areas in paediatric acute asthma and inform investigators about the use of relevant patient populations and how to best assess treatment response.
Diagnostic criteria for acute exacerbation of asthma in children varied between clinicians. It is common that individuals have different understanding or interpretation of a concept however, without internationally recognised criteria to diagnose the severity of acute asthma in the ED setting, it is challenging to systematically examine the quality of practice including treatments, outcomes and costs.
Respondents have suggested some common features useful in defining entry criteria for future asthma studies. These include a minimum age of 2 years, and the need for definitions of asthma to include components from medical history (such as a previous salbutamol responsive wheeze episode) and examination findings such as respiratory distress or work of breathing.
Further, for trials in severe asthma exacerbations, it is important to standardise the assessment of severity. Asthma scores were a popular choice, as they allow for both measurement as study entry criteria and a way to measure change. The use of asthma scores is widespread in some regions (particularly North America and Europe) however, robust validation is lacking.3 18 19 Experienced clinicians described the utility of altered conscious state as a marker for critical illness; however, this is not routinely captured in most asthma scores. In addition, the measurement properties of this symptoms/sign are not well explored.
It was more common that clinicians from low-income and middle-income countries (LMIC) reported need to manage more severe or life-threatening disease and how treatment decisions may be influenced by cost. This is likely multifactorial and reflects accessibility and affordability of high-quality timely healthcare. It is clear that there needs to be better understanding of how cost impacts families or clinicians’ choices of treatment and that treatment choices that are more readily used in low-income and middle-income countries, for example, subcutaneous or intramuscular epinephrine need to feature in trial design.
This study has reiterated the variability of treatment options outlined in previous research6 7 and provided some depth of understanding for it. It has been found that the clinicians often relied on their clinical guidelines as their knowledge basis, along with their colleagues’ opinions to guide their treatment decisions. A presumption was made by the clinicians that these guidelines were based on evidence although this was not verified and there continues to exist significant disparity in guidelines both within countries and internationally.
Hospital length of stay was the most commonly suggested outcome measure, and has been regularly used in paediatric asthma studies.8 As an outcome parameter, the length of stay is easy to measure, impacts families and hospitals, and has clear fiscal implications. However, length of stay may not necessarily reflect the outcome from treatment only, but also may be influenced by historical factors, time of day, or local hospital policies (eg, requiring hospital admission and/or PICU admission after IV therapy) and affordability in countries without easily accessible free healthcare for children. Important considerations relevant to patients and families include the impact of treatment and hospitalisation on a child and their family unit.
Limitations
It is recognised that there is a small number of respondents per region and the representation from low-income and middle-income countries and non-English-speaking participants was small. It would be important when defining research questions to consider how these would be useful or transferable to low-income and middle-income families. However, data saturation was reached adding confidence to these findings. This study is also limited to the view of professionals and further work is required to refine findings and include the view of families and children.
Conclusion
This study has gathered opinions and priorities of experienced clinicians from different geographical regions on which children with acute asthma should participate in future trials, how we should measure response to treatments and what research areas should be prioritised. This study also highlights the importance of tailoring the use of research outcomes to local clinical and socioeconomic circumstances and geographic context of the patient.
The current findings will be used in parallel with a further PERN study focusing on the child and family perspectives. These will contribute to develop a core outcome set for future research.
Acknowledgments
We would like to acknowledge the PERN network and its clinicians who identified colleagues to be interviewed for this study. In addition, to all the clinicians who participated in these interviews and freely gave their time.
Footnotes
Twitter: @DrCharmaineGray, @DrSimonCraig
Contributors: This paper in its formulation, conduction and reporting was as part of Paediatric Research Network (PERN). The primary author conducted all interview and secondary author analysed all data. All authors reviewed and contributed to writing of this paper. CSG accepts full responsibility for the work, access to data and controlled the decision to publish
Funding: This work is supported by the NHMRC Centre of Research Excellence in Paediatric Emergency Medicine (GNT1171228). SC’s contribution was funded by the Thoracic Society of Australia and New Zealand / National Asthma Council Fellowship, 2020: Generating new knowledge and seeking global consensus to inform future RCTs in acute severe paediatric asthma and the Australasian College for Emergecny Medicine Foundation Al Spilman Early Career Research Grant 2017. SRDs time was in part funded by Cure Kinds in New Zealand. FEB's time was funded by an NHMRC Investigator Leadership grant and the Royal Children's Hospital Foundation, PArkville, Australia
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Central ethics approval was obtained from the Women’s and Children’s Hospital and University of Adelaide, Human Research Ethics Committees, Australia HREC Ref:18/WCHN/120. Participants gave informed consent to participate in the study before taking part.
References
- 1. Acworth J, Babl F, Borland M, et al. Patterns of presentation to the Australian and New Zealand paediatric emergency research network. Emerg Med Australas 2009;21:59–66. 10.1111/j.1742-6723.2009.01154.x [DOI] [PubMed] [Google Scholar]
- 2. Gill PJ, Goldacre MJ, Mant D, et al. Increase in emergency admissions to hospital for children aged under 15 in England, 1999-2010: national database analysis. Arch Dis Child 2013;98:328–34. 10.1136/archdischild-2012-302383 [DOI] [PubMed] [Google Scholar]
- 3. Boeschoten S, de Hoog M, Kneyber M, et al. Current practices in children with severe acute asthma across european picus: an ESPNIC survey. Eur J Pediatr 2020;179:455–61. 10.1007/s00431-019-03502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boeschoten SA, Boehmer AL, Merkus PJ, et al. Risk factors for intensive care admission in children with severe acute asthma in the netherlands: a prospective multicentre study. ERJ Open Res 2020;6:00126-2020. 10.1183/23120541.00126-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig S, Powell CVE, Nixon GM, et al. Treatment patterns and frequency of key outcomes in acute severe asthma in children: a paediatric research in emergency departments international collaborative (predict) multicentre cohort study. BMJ Open Respir Res 2022;9:e001137. 10.1136/bmjresp-2021-001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Craig SS, Dalziel SR, Powell CV, et al. Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane reviews. Cochrane Database Syst Rev 2020;8:CD012977. 10.1002/14651858.CD012977.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyttle MD, O’Sullivan R, Doull I, et al. Variation in treatment of acute childhood wheeze in emergency departments of the United Kingdom and ireland: an international survey of clinician practice. Arch Dis Child 2015;100:121–5. 10.1136/archdischild-2014-306591 [DOI] [PubMed] [Google Scholar]
- 8. Gray CS, Powell CVE, Babl FE, et al. Variability of outcome measures in trials of intravenous therapy in acute severe paediatric asthma: a systematic review. Emerg Med J 2019;36:225–30. 10.1136/emermed-2018-207929 [DOI] [PubMed] [Google Scholar]
- 9. Williamson PR, Altman DG, Bagley H, et al. The comet handbook: version 1.0. Trials 2017;18(Suppl 3):280. 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Craig S, Babl FE, Dalziel SR, et al. Acute severe paediatric asthma: study protocol for the development of a core outcome set, a pediatric emergency reserarch networks (PERN) study. Trials 2020;21:72. 10.1186/s13063-019-3785-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkins L, Francis J, Islam R, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77. 10.1186/s13012-017-0605-9 Available: 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbu CM. ZOOM: A spatial data visualization tool (version 2.0.4). 2013. Available: https://githubcom/cbarbu/R-package-zoom
- 13. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7:37. 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: A synthesis of recommendations. Acad Med 2014;89:1245–51. 10.1097/ACM.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 15. Chalut DS, Ducharme FM, Davis GM. The preschool respiratory assessment measure (PRAM): a responsive index of acute asthma severity. J Pediatr 2000;137:762–8. 10.1067/mpd.2000.110121 [DOI] [PubMed] [Google Scholar]
- 16. Hsu P, Lam LT, Browne G. The pulmonary index score as a clinical assessment tool for acute childhood asthma. Ann Allergy Asthma Immunol 2010;105:425–9. 10.1016/j.anai.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 17. Wood DW. A clinical scoring system for the diagnosis of respiratory failure. Am J Dis Child 1972;123:227. 10.1001/archpedi.1972.02110090097011 [DOI] [PubMed] [Google Scholar]
- 18. Bekhof J, Reimink R, Brand PLP. Systematic review: insufficient validation of clinical scores for the assessment of acute dyspnoea in wheezing children. Paediatr Respir Rev 2014;15:98–112. 10.1016/j.prrv.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 19. Chacko J, King C, Harkness D, et al. Pediatric acute asthma scoring systems: a systematic review and survey of UK practice. J Am Coll Emerg Physicians Open 2020;1:1000–8. 10.1002/emp2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001502supp001.pdf (61.9KB, pdf)
bmjresp-2022-001502supp002.pdf (195.3KB, pdf)
Data Availability Statement
Data are available on reasonable request.