Abstract
Introduction
School readiness includes cognitive, socio-emotional, language and physical growth and development domains which share strong associations with life-course opportunities. Children with cerebral palsy (CP) are at increased risk of poor school readiness compared with their typically developing peers. Recently, earlier diagnosis of CP has allowed interventions to commence sooner, harnessing neuroplasticity. First, we hypothesise that early referral to intervention for children at-risk of CP will lead to improved school readiness at 4–6 years relative to placebo or care as usual. Second, we hypothesise that receipt of early diagnosis and early intervention will lead to cost-savings in the form of reduced healthcare utilisation.
Methods and analysis
Infants identified as at-risk of CP ≤6 months corrected age (n=425) recruited to four randomised trials of neuroprotectants (n=1), early neurorehabilitation (n=2) or early parenting support (n=1) will be re-recruited to one overarching follow-up study at age 4–6 years 3 months. A comprehensive battery of standardised assessments and questionnaires will be administered to assess all domains of school readiness and associated risk factors. Participants will be compared with a historical control group of children (n=245) who were diagnosed with CP in their second year of life. Mixed-effects regression models will be used to compare school readiness outcomes between those referred for early intervention versus placebo/care-as-usual. We will also compare health-resource use associated with early diagnosis and intervention versus later diagnosis and intervention.
Ethics and dissemination
The Children’s Health Queensland Hospital and Health Service, The University of Queensland, University of Sydney, Monash University and Curtin University Human Research Ethics Committees have approved this study. Informed consent will be sought from the parent or legal guardian of every child invited to participate. Results will be disseminated in peer-reviewed journals, scientific conferences and professional organisations, and to people with lived experience of CP and their families.
Trial registration number
ACTRN12621001253897.
Keywords: developmental neurology & neurodisability, health economics, rehabilitation medicine, magnetic resonance imaging
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is a long-term follow-up of all domains of school readiness outcomes of children at risk of cerebral palsy who have participated in early neuroprotection, neurorehabilitation or parent support clinical trials.
Comprehensive school readiness outcomes will be determined for children who participated in randomised controlled trials (RCTs) of neuroprotection (n=1), early neurorehabilitation (n=2) or parent support (n=1) for children at risk of cerebral palsy.
School readiness outcomes will be assessed using standardised measures of cognition, early numeracy and literacy, executive function, socio-emotional skills and behaviour, gross and fine motor skills, functional independence, language and growth.
A cost-effectiveness and cost-consequence analysis will be conducted comparing receipt of early diagnosis and intervention versus later diagnosis of cerebral palsy.
Limitations include the possibility of loss to follow-up due to the ≥2-year time-interval between final follow-up in the RCTs at 24 months corrected age and further follow-up in the present study at 4–6 years 3 months of age, difficulties with performing assessments due to COVID-19 restrictions and possible bias in who is more likely to consent to participate.
Introduction
Cerebral palsy (CP) is the most common childhood physical disability impacting 1 in 700 children in Australia.1 CP is a disorder of movement and posture secondary to an insult or abnormalities to the developing brain, resulting in a lifelong complex disability.2 3 While the early brain injury is static, functional limitations can worsen across the lifespan.4 An international survey of individuals and families with lived experience of CP identified prevention, early intervention and reduction of severity as their highest research priorities.5 Prevention and cures is also one of the key goals in The Australian & New Zealand CP Strategy 2020, as is providing timely intervention and support, improving health and well-being and promoting inclusion in all areas of life, including in education.6
Historically, the most common age of detection of CP was 12–24 months according to the Australian CP Register,1 delaying access to intervention. A 2016 systematic review of early motor interventions showed that only 2 of 15 randomised controlled trials (RCTs) showed any motor gains for children with CP, because (i) traditional interventions have been proven ineffective and contemporary evidence-based neurorehabilitation had not yet been tested in infants; (ii) most interventions were commenced late and were underdosed and (iii) historical studies included and treated many children with typical development because of late diagnosis affecting recruitment accuracy, resulting in dissolved statistical power.7 In line with clinical practice guidelines on the diagnosis of CP, identification of children at high risk of CP now more frequently occurs by 6 months of age in Australia.8 This allows preventative treatments and management practices to commence earlier, and has also allowed early recruitment into large clinical trials of novel contemporary neurorehabilitation, harnessing a critical window of opportunity for neuroplasticity.9 A landmark 2013 systematic review of efficacy of interventions10 which was updated in 202011 identified that intensive motor learning-based rehabilitation and goal-directed therapies have the strongest evidence to improve functional outcomes in CP.12 13 There is a critical need however, for further early intervention research and long-term follow-up of outcomes beyond 2 years of age.11
School readiness is a framework for assessing the strengths and vulnerabilities of a child prior to school.14–16 It is based on their genetic, biological, environmental and maturational status, and it primarily encompasses cognitive, social, attentional, self-regulation, gross and fine motor skills, language, health status and growth domains.17 School readiness is lower in children with disabilities relative to children with typical development,18 19 and on average, children with CP are 0.5–2 SD delayed across all assessed domains of school readiness compared with typically developing children.20 Children with lower school readiness demonstrate persistent academic underachievement and social-emotional risk, and it is an important predictor of later academic performance and employment.16–18 21 Improving school readiness has substantial individual and societal benefits, as demonstrated by the Perry Preschool Project, where the economic investment of a 1-year, high-quality, universal, preschool education estimated a US$7000 net benefit per child, which was equal to a return of US$2.62 for every US$1 invested, with annual return of 10% over 60 years.22 23 A ‘real’ return on investment of 16%, with 75% of benefits identified going to the general public,24 and the ratio of benefit to cost over the child’s lifetime was >8:1,25 with benefits persisting to age 40 years, where more were employed (76% vs 56%) and more earned >US$20 000 per annum (60% vs 40%).24 26 A multitude of factors have been found to influence school readiness, including maternal and perinatal factors such as maternal age, education, employment and socio-economic status, as well as the child’s ethnicity and birth weight27 and accumulation of adverse childhood experiences (ACEs).28 Studies have also demonstrated the critical importance of parenting behaviour on school readiness and academic achievement, including effects of parental engagement and involvement and parenting sense of competence on children’s school readiness outcomes in the areas of language, reading, writing and self-regulatory and socio-emotional skills.29–32 The influence of these factors on the school readiness of children at high risk of CP have however not been explored, although they may be at increased vulnerability due to their early brain injury. Furthermore, brain lesion characteristics have been found to have potential prognostic value for gross motor function33 and communication impairment34 in children with CP, but further research is needed. A better understanding of risk factors which are identifiable in infancy that predict poor school readiness can be used to ensure children at high risk of CP receive early, targeted interventions.
Children with CP frequently have major comorbidities likely to impact long-term outcomes, employment and cost of care.2 Based primarily on CP register data, a systematic review identified that at age 5 years, 3 in 4 children with CP were in pain, 1 in 2 had an intellectual disability, 1 in 3 could not walk, 1 in 3 had hip displacement, 1 in 4 could not talk and 1 in 4 had behaviour disorders and 1 in 15 were tube fed.35 These comorbidities can also impact growth and nutritional status,36–38 habitual physical activity39 and communication,40 however there has been a paucity of interventions to optimise function early, with the aim to increase participation and improve quality of life.41 School readiness is understudied in children with CP, but delays have been identified in children with CP commencing school at 5 years in our Australian CP Child cohort study.20 Children in this study were born in the years of 2006–2009 and were identified and diagnosed with CP at 2.2 years corrected age (CA), and consequently early neuroprotection and/or neurorehabilitation were not received. In the past 6 years, the Australasian Cerebral Palsy Clinical Trials Network has implemented early detection so that at-risk infants have been recruited prior to birth or by <6 months CA into RCTs of neuroprotectants (study 1: Protect-me42), early neurorehabilitation (study 2: Goal-Activity-Motor-Enrichment (GAME),13 study 3: Rehabilitation Early for children at risk of Congenital Hemiplegia (REACH)43) and early parenting support (study 4: Early Parenting Acceptance and Commitment Therapy (Early PACT)44) to determine if these interventions improve motor and cognitive outcomes at 2–2.5 years of age (studies 1–3) or 6 months postintervention (study 4). The present study is a long-term follow-up of these four RCTs of infants at high risk of CP to determine if these interventions improve school readiness outcomes at 4–6 years 3 months of age (from now on shortened to 4–6+3 years). Both contemporary clinical trials (RCT studies 1–4) and a historical prospective cohort (study 5: CP Child45–47) will be used to (i) determine the impact of referral to early intervention versus placebo/care-as-usual (CAU) on school readiness outcomes and (ii) the impact of early versus late diagnosis of CP on health resource use. The historical prospective cohort group consists of children with CP born between 1 September 2005 and 31 September 2009.45–47 These children participated in the Australian CP Child cohort studies, were diagnosed with CP in their second year of life or later and received CAU (ie, no specific clinical trials of early neuroprotectants or neurorehabilitation).45–47 School readiness outcomes for this historical prospective cohort have been published previously.20
Our primary hypothesis is that children who received an early diagnosis of CP and were randomised to an active early intervention will have clinically significant improvements in school readiness (primarily operationalised as general cognition) at 4–6+3 years of age, relative to peers who also received an early diagnosis, but were randomised to receive placebo or CAU. Second, we hypothesise that maternal and prenatal risk factors, and ACEs will impact school readiness at 4–6+3 years. Third, we hypothesise that brain lesion characteristics will predict school readiness at 4–6+3 years. Fourth, we hypothesise maternal mental health and emotional availability will moderate the association between early intervention school readiness at 4–6+3 years. Lastly, we hypothesise that healthcare costs will be higher in the first 2 years of life for children with an early (≤6 months CA) versus late (>6 months CA) diagnosis of CP, however costs will then decrease as earlier diagnosis leads to earlier intervention and better school readiness.
Methods and analysis
Objectives
This long-term follow-up of four RCTs will primarily investigate the effectiveness of early referral to intervention on school readiness at 4–6+3 years of age in children with or at high risk of CP. The comparison groups for the analysis of school readiness will be a contemporary control group of children who received either placebo or CAU. Secondary aims include investigating which maternal and perinatal risk factors and ACEs impact school readiness, identifying which brain structure features from MRI are the most predictive markers of school readiness, and investigating how maternal mental health and emotional availability moderate the association between early intervention and school readiness. Finally, the cost-effectiveness of early versus late diagnosis of CP in terms of differences in healthcare use by the age of 4–6+3 years will be assessed. The comparison groups will be infants identified as high risk of CP <6 months CA versus a historical control group of children with CP who participated in a longitudinal cohort study (CP Child) and were diagnosed with CP in their second year of life or later, and received CAU.45–47
Study design
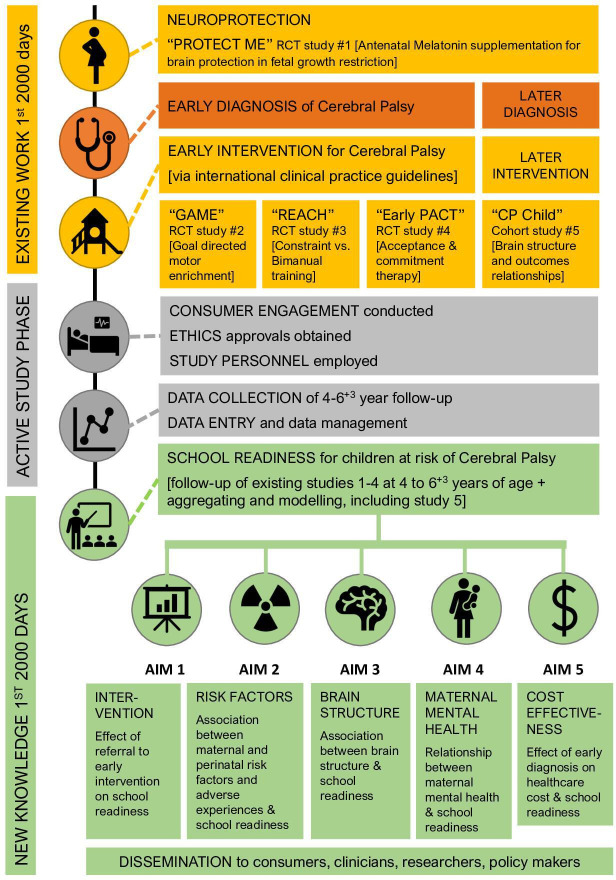
A flow chart of the study design is provided in figure 1. This trial includes sites in the Australian states of Queensland, New South Wales, Victoria and Western Australia. Children at high risk of CP who participated in one of four RCTs as infants (T1, recruited prior to birth or <6 months CA) and completed follow-up at 2 years CA (T2) will be eligible and invited to return to be assessed for school readiness at 4–6+3 years of age (T2). The age range of 4–6+3 years was chosen as children in Australia may enter school as early as 4 years 6 months or as late as 6 years 3 months depending on parental choice and the state in which they reside.
Figure 1.
Study flow chart. Early PACT, Parenting Acceptance and Commitment Therapy; GAME, Goal-Activity-Motor-Enrichment; RCT, randomised controlled trial; REACH, Rehabilitation Early for children at risk of Congenital Hemiplegia.
Study 1 (Protect-me)42 was an RCT of in utero neuroprotectant treatment with antenatal melatonin supplements versus placebo, while study 2 (GAME)13 was an RCT of early neurorehabilitation comparing goal-directed training in a motor-enriched environment versus CAU, study 3 (REACH)43 was an RCT comparing equal doses of two early neurorehabilitation interventions (modified constraint-induced movement therapy and bimanual training) and study 4 (Early PACT)44 was an RCT of parenting acceptance and commitment therapy versus a wait-list control group. As REACH43 was a comparison of two interventions and Early PACT44 was a wait-list trial, all children in these studies received an intervention. Studies 1, 2 and 4 are still either recruiting or completing final follow-up assessments, therefore not all children participating in these studies will be turning 4 years old in time to be included in the school readiness follow-up as data collection concludes in mid-2025. A total of 425 children are estimated to be eligible to participate in the school readiness study from study 1 (Protect-me, n=55), study 2 (GAME, n=264), study 3 (REACH, n=80) and study 4 (Early PACT, n=26). All RCTs were informed by Consolidated Standards of Reporting Trials (CONSORT) guidelines. This multisite follow-up trial has been designed according to the Standard Protocol Items: Recommendations for Interventional Trials statement,48 will be reported according to the CONSORT statement,49 and is registered on the Australian New Zealand Clinical Trials Registry (ACTRN12621001253897). The WHO Trial Registration Dataset items can be found in online supplemental appendix 1 and this information reflects the study protocol V.1.5 dated 15 September 2022.
bmjopen-2022-068675supp001.pdf (45.3KB, pdf)
Patient and public involvement
Individuals and families with lived experience of CP contributed to a Delphi survey of consumers, researchers and clinicians, resulting in a published consensus of research priorities for CP.5 These research priorities underpinned the development of this study. All participants are encouraged to provide feedback on their experience of participating in the research study either directly or via an anonymous online form, and receive a flyer with a QR code on the conclusion of the assessment session to encourage feedback. Study updates and findings will be communicated to participant in institution newsletters and at the conclusion of the study after the primary analyses, a summary flyer of the main outcomes of the study will be emailed and/or mailed to participants.
Eligibility criteria
Children recruited into the Protect-me, GAME, REACH and Early PACT trials who (i) consented to receive information on clinical trials, (ii) who are aged between 4 years and 6+3 years at the time of assessment and (iii) can attend a study visit, will be eligible for participation in this follow-up of RCTs. All eligible children (n=425) will be offered the 4–6+3-year follow-up regardless of their original group allocation in studies 1–4.
Interventions
The interventions delivered in studies 1–4 have been described in detail in their respective protocol papers.13 42–44 Similarly, assessments performed in the historical prospective cohort study has also been reported in protocol papers.45–47 Short summaries are provided below.
Study 1: Protect-me: RCT of antenatal melatonin supplementation in fetal growth restriction (FGR) for fetal neuroprotection (ACTRN12617001515381).42 FGR is a risk factor for CP, and this is the first triple-blinded, parallel-group RCT which aims to determine if antenatal maternal melatonin supplementation improves neurodevelopment at 2 years compared with placebo for survivors of FGR. Women (n=336) with a singleton pregnancy complicated by FGR were recruited between 23+0 and 31+6 weeks’ gestation.50 The intervention group received 10 mg melatonin versus placebo. Antenatal assessments of maternal and fetal well-being were performed, as well as a term-corrected neonatal MRI and general movements assessment. This trial has Monash Health Human Research and Ethics committee approval (17-0000-583A) and is recruiting across Australia and New Zealand.
Study 2: GAME harnessing neuroplasticity: RCT goal-directed motor enrichment for infant CP (ACTRN12617000006347).13 Worldwide, GAME is the largest RCT (n=300, 150 per group) of early intervention, and aims to identify if goal-directed training in a motor-enriched environment (ie, novel neuroplasticity neurorehabilitation) produces superior motor and cognitive outcomes to CAU in infants at high risk of CP. Ethics approval was obtained through the Sydney Children’s Hospital Network Human Research Ethics Committee (HREC/17/SCHN/37), with site-specific approvals across four states of Australia.
Study 3: REACH RCT (ACTRN12615000180516).43 This was the first RCT to compare the effect of an equal dose of Baby modified Constraint Induced Movement Therapy to bimanual training on early reaching and grasping in infants at high risk of unilateral CP (n=98). Full ethics approval for this study was obtained from the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/14/QRCH/376) with ethical review provided by another 20 sites across 4 states of Australia.
Study 4: Early PACT: RCT of web-based intervention for parents of children with CP (ACTRN12616000351415).44 This is an RCT recruiting parents of children with CP (n=66) allocated to Early PACT or a wait-list control. PACT is an online parenting course grounded in ACT, using environmental enrichment through enhanced mother-infant emotional availability. Outcomes include the parent-child relationship, parental adjustment and child’s quality of life. Ethics approvals were obtained through the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/15/QRCH/115) and The University of Queensland (2015001743).
Study 5: CP Child studies and PREDICT-CP: comprehensive surveillance to predict outcomes for school-aged children with CP (ACTRN12616001488493).45–47 These three studies provide a historical control comparison group of children who did not receive a diagnosis of CP until their second year of life or later and received CAU. The CP Child study45 and its concurrent substudy CP Child: Growth, Nutrition and Physical Activity (GNPA)47 formed a prospective longitudinal cohort study of children from Queensland from the birth years 2006–2009 who were assessed at regular intervals between the ages of 18 months and 5 years. PREDICT-CP46 was a follow-up study of these children at one time point between 8 and 12 years of age. The aim was to investigate the associations between brain structure, neurodevelopment, growth,36 body composition,37 dietary intake,51 52 oropharyngeal function,53 54 habitual physical activity,39 musculoskeletal development (hip status, bone health) and muscle performance during the preschool period (2–5 years) on motor attainment and performance,55 cognition,20 56 executive function,57 communication,58 participation, quality of life and related health resource use costs at school age (8–12 years). Ethics approvals were obtained for the Australian CP Child and GNPA studies through the University of Queensland Human Research Ethics Committee (2007001784 and 2008002260), the Children’s Health Services District Ethics Committee (HREC/07/QRCH/107 and HREC08/QRCH/112/AM01) and eight other sites across Australia.45 47 Subsequent ethics approvals were obtained for the PREDICT study from The University of Queensland Human Research Ethics Committee (2014001487) and the Children’s Health Services District Ethics Committee (HREC/14/QRCH/329).46
Characterisation of the sample
Two measurement time points will be included: baseline/infant assessment and the present study follow-up at 4–6+3 years for school readiness. To characterise the sample at baseline, the age of identification of high risk of CP and age at formal diagnosis of CP (if any) will be identified from studies 1–4. Age of diagnosis will also be identified for study 5. Furthermore, any data on the General Movements Assessment59 at writhing and fidgety ages, GM Motor Optimality Score60 or Hammersmith Infant Neurological Exam61 score completed between birth and 24 months CA will also be collected.
Demographic characteristics will be collected to characterise the sample at the at 4–6+3 years follow-up. The child’s age, sex, ethnic identity of mother and father (Aboriginal, Torres Strait Islander, Māori, Caucasian, Asian, Africa, Hispanic or Latin, Pacific Islander, Indian, Arabic and/or specify other), language(s) spoken at home and whether they have attended an early learning programme (preschool/kindergarten) will be collected. The mother’s age at time of child’s birth, the family’s postcode (to identify Socio-Economic Indexes for Areas Index of relative Socio-Economic Advantage and Disadvantage rank),62 and the parent’s marital status, ethnicity and highest completed level of education will also be collected.
Classification measures
Clinical signs of CP. Motor type will be classified as spastic, dystonic, ataxic, hypotonic, choreoathetosis or no motor type detected according to the Australian Cerebral Palsy Register (ACPR) guidelines.63 The ACPR Description Form will be used to record the motor type and distribution of clinical signs of atypical muscle tone.63 For spastic CP, distribution will be classified by number of limbs impaired, unilateral (hemiplegia) and bilateral distribution (diplegia, triplegia, quadriplegia) on the Australian Spasticity Assessment Scale.64 If the child does not have a diagnosis of CP and does not present with clinical signs of CP as per the ACPR, the CP-specific classifications and measures outlined below will not be completed.
Functional severity. Participants will be classified using the Gross Motor Function Classification System (GMFCS). The GMFCS has internationally established validity, reliability and stability for the classification and prediction of motor function of children with CP aged 2–12 years.65 66 It has an acceptable inter-rater and intra-rater reliability.67
Manual ability will be classified as level I–V using the Manual Abilities Classification System (MACS).68 The MACS classifies a child’s ability to hand objects in daily life on a five-level ordinal scale, and has established construct validity and excellent inter-rater reliability.68
Communication and speech. Based on a naturalistic 5 min language sample with familiar and unfamiliar communication partners, a speech therapist will rate participants using the Communication Function Classification System,69 the Functional Communication Classification System70 and the Viking Speech Scale.71
Eating and drinking abilities. The Eating and Drinking Ability Classification System (EDACS) for children with CP will be used to rate children’s safety and efficacy when eating and drinking via (i) parent report and (ii) speech therapist observation of a standardised feeding assessment.72 73 The EDACS has excellent inter-rater reliability and good agreement between speech therapist and parent rating.72
Study aims
Aim 1: early intervention versus CAU/placebo
Records of children in studies 1–4 will be assessed to identify (i) age at intervention study commencement, (ii) allocation to treatment versus CAU/placebo, (iii) total dose of intervention in hours and (iv) content of intervention.
Aim 2: risk factors for adverse neurodevelopmental outcome
a. Maternal and perinatal risk factors will be retrieved from clinical trial and neonatal hospital discharge summaries and maternal interview including the child’s gestational age at birth, method of delivery, maternal risk factors (family genetic history, multiple gestation, infection, substance use, smoking, alcohol intake using the Alcohol Use Disorders Identification Test- Consumption74 and perinatal variables (eg, days of neonatal intensive care unit admission, oxygenation, ventilation)). Medical data will be collected using standardised Australian & New Zealand Neonatal Network (ANZNN) data definitions (eg, GA, birth weight, disease severity, structural MRI, periventricular haemorrhage, periventricular leukomalacia, hypoxic ischaemic encephalopathy).
b. Adverse childhood experiences: Centre for Youth Wellness Adverse Childhood Experiences Questionnaire for Children (ACE-Q) is a 17-item instrument completed by the parent or caregiver for children aged 0–12 years. Items in section 1 assess the exposure to the original 10 ACEs and the section 2 items assess for exposure to additional early life stressors relevant to children. Adverse events include: emotional or physical abuse or neglect, sexual abuse, separation from caregiver, domestic violence, caregiver’s substance abuse, mental illness or physical illness, community violence, discrimination, housing instability, food insecurity or low family cohesio.75 76 A cumulative number of exposures is collected to deidentify specific events to protect privacy.
Aim 3: brain structure
Brain lesion severity and qualitative type: neonatal MRIs will be retrieved, and early brain structure will be classified according to Kidokoro et al75 76 (studies 1–4, n=425 and study 5, n=150 (collected)). Structural MRIs from age ≥2 years will also be retrieved and brain structure will be classified using the Fiori semi-quantitative brain lesion severity scale77 and qualitative type will be classified according to Krägeloh-Mann and Cans.78 Where possible both neonatal and later MRIs will be analysed using automated analysis pipelines to measure brain structure.79 Measures of brain structure include anatomical volumes, cortical shape like cortical thickness and sulcal depth, qualitative measures from Kidokoro et al and microstructure (including fractional anisotropy, mean diffusivity and fixel-based measures)80–82 as well as clinical information (gestational age, gender).
Aim 4: maternal mental health and family measures
a. Maternal mental health: Depression Anxiety Stress Scale (DASS-21) is a 21-item self-reported measure that assesses symptoms of depression, anxiety, stress in adults and will be used to assess parental mental health. The DASS-21 has good test-retest reliability and its three subscales have good internal consistency ranging from 0.87 to 0.94.83 The DASS also has good discriminant and concurrent validity for identifying diagnostic presence of depression and anxiety.83 Cut-off scores for depression, anxiety and stress classify symptoms as normal, mild, moderate, severe or extremely severe.83
b. Parent-child relationship: Emotional Availability-Self-Report (EA-SR) is a 32-item parent-report measure of emotional availability within parent-child relationship. The EA-SR has five subscales: mutual attunement, affect quality, capacity to involve parent, intrusiveness and hostility, validated against the EAS.84 A parental emotional availability scale can be calculated by reverse coding the intrusiveness and hostility subscales, such that higher scores reflected higher parental emotional availability.85
c. Life stressors: life events list is a self-reported 24-item instrument to assess the number and types of stressful life events along with the degree of stress experienced across 21 specific life events and 3 optional events during the past 12 months. Items include events that normatively are considered negative (eg, deaths, crime) as well as events that are more ambiguous (eg, change in finances, relocating).86
Aim 5: early versus late diagnosis of CP
Age of diagnosis/identified as ‘high risk’ of CP: children participating in studies 1–4 (‘early diagnosis/identification’) will be compared with children in study 5 (‘late diagnosis’).
Outcome measures
The primary outcome measures for aim 1 (effect of early intervention), aim 2 (risk factors) and aim 4 (maternal mental health) on school readiness is general cognition (Wechsler Preschool & Primary Scale of Intelligence (WPPSI-IV A&NZ)). The two secondary outcome measures for these aims are motor capacity (Gross Motor Function Measure (GMFM-66)) and a novel School Readiness Index. The GMFM-66 is specific to the CP population, whereas all the measures included in the index have normative scores/values available.
The School Readiness Index comprises nine measures of general cognition, early literacy and numeracy, executive function, behaviour and socio-emotional skills, gross and fine motor skills, functional independence, language and growth (table 1). For the purposes of the index score, children with a standardised score below the average range as specified for each measure will be considered in the ‘low range’ for school readiness, and receive a score of zero for this specific measure.87 For growth measures, children with height-for-age, weight-for-age or body mass index-for-age ≥2 SD below the mean of age-matched and sex-matched peers were considered to have poor nutritional status as per WHO growth standards, and receive a score of zero for the growth domain.88 For domains where participants are within or above the normative range, they receive a score of 1. The maximum score for the School Readiness Index is 9, and a greater score indicates a greater level of school readiness. School readiness is a broad conceptual framework, and the School Readiness Index has been constructed to quantify the number of school readiness domains where the participants are within or above the average normative range. The index allows comparison of a broader measure of school readiness between groups by using the continuous index score as the outcome measure. The use of an index to assess the number of affected school readiness domains has been used in children born very preterm.18 89 The number of readiness domains affected at age 4 in this population strongly predicted later educational risk, and delays in ≥two domains was found to be an effective criterion for educational surveillance and/or additional support during the transition to school.89
Table 1.
School Readiness Index domains
| School readiness domains | Measure | Domain/Score | ‘School ready’ cut-off | School Readiness Index |
| General cognition | WPPSI-IV | Full Scale IQ | >90 | 1 point |
| Literacy and numeracy | WIAT-III | Early reading skills | >90 | 1 point if above cut-off for both |
| Maths problem solving | >90 | |||
| Executive function | BRIEF-P | Global executive composite | <65 | 1 point |
| Intellectual and developmental disability | Vineland-3 | Adaptive behaviour composite | >85 | 1 point |
| Behaviour and socio-emotional skills | BASC-3 | Behavioural symptoms index | <60 | 1 point if both below/above cut-off |
| Adaptive skills | >40 | |||
| Gross and fine motor skills | PDMS-2 | Total motor quotient | >90 | 1 point |
| Functional independence | PEDI-CAT | Daily activities | >40 | 1 point if all above cut-off or all |
| Mobility | >40 | |||
| Social/Cognitive | >40 | |||
| Responsibility | >40 | |||
| Language | PLS-5 | Total language score | >85 | 1 point |
| Growth/Nutritional status | Anthropometry (z-scores) | Height, weight and BMI-for-age | >−2 SD | 1 point if all above cut-off |
BASC-3, Behaviour Assessment System for Children, Third Edition; BMI, body mass index; BRIEF-P, Behaviour Rating Inventory of Executive Function-Preschool; PDMS-2, Peabody Developmental Motor Scales; PEDI-CAT, Paediatric Evaluation of Disability Inventory-Computerised Assessment Test; PLS-5, Preschool Language Scale, Fifth Edition; Vineland-3, Vineland Adaptive Behaviour Scale; WIAT-III, Wechsler Individual Achievement Test; WPPSI-IV, Wechsler Preschool & Primary Scale of Intelligence, Fourth Edition A&NZ.
The primary outcomes for aim 3 (early biomarkers) are general cognition (WPPSI-IV A&NZ), motor capacity (GMFM-66) and fine and gross motor skills (Peabody Developmental Motor Scales (PDMS-2)). The primary outcome measure for aim 5 (early vs late diagnosis) is health resource use, and secondary measures include motor capacity (GMFM-66), functional independence (Paediatric Evaluation of Disability Inventory-Computerised Assessment Test (PEDI-CAT)) and growth status (height, weight and body mass index z-scores) as these were also collected in the CP Child studies.45 47 Apart from the primary and secondary school readiness outcomes, additional subdomains have been considered for a more comprehensive profiling of the nature of impairments, including manual ability, feeding skills, diet quality, body composition, habitual physical activity and quality of life. Table 2 presents the school readiness variables and data pooling plan.
Table 2.
School readiness variables and data pooling plan
| Studies 1–4 (aims 1–4) | Study 5 (aim 5) | |
| Exposure | ||
| Aim 1: intervention—active treatment | Yes/No | n/a |
| Predictors/Moderators | ||
| Aim 2: maternal/perinatal risk | ANZNN Assess | Partial |
| Aim 2: adverse childhood experiences | ACE-Q | n/a |
| Aim 3: brain structure via MRI | Kidokoro et al, Fiori et al scale, Krägeloh-Mann and Cans | |
| Aim 4: mental health (maternal) | DASS-21 | n/a |
| Aim 4: emotional availability (maternal) | EA-SR | n/a |
| Aim 4: life stressors (maternal) | LEL | n/a |
| School readiness outcome measures | ||
| Cognition and behaviour | ||
| General cognition (primary outcome) | WPPSI-IV | n/a |
| Literacy and numeracy | WIAT-III | n/a |
| Executive function | BRIEF-P | n/a |
| Intellectual and developmental disability | Vineland-3 | n/a |
| Behaviour and socio-emotional skills | BASC-3 | n/a |
| Motor capacity and performance | ||
| Motor capacity | GMFM-66 | |
| Gross and fine motor skills | PDMS-2 | n/a |
| Manual abilities | AHA/BoHA | n/a |
| Functional independence | PEDI-CAT | |
| Communication | ||
| Language | PLS-5 | n/a |
| Nutritional status, dietary intake and feeding skills | ||
| Growth/Nutritional status | Height, weight, BMI, BIA | |
| Dietary intake/Quality | AES-FFQ | n/a |
| Feeding skills | DDS, 3-oz water test | DDS |
| Physical activity performance | ||
| Habitual physical activity | Accelerometer | |
| Quality of life | ||
| Quality of life | ITQOL | n/a |
| Healthcare costs | ||
| Health resource use | Health resource use | |
Study 1=Protect-me; study 2=GAME; study 3=REACH; study 4=Early PACT; study 5=CP Child.
ANZNN; ACE-Q139; LEL86; MRI; Kidokoro et al76; Fiori et al scale77; Krägeloh-Mann and Cans78; EA-SR84; DASS-2183; GMFM-6695; WPPSI-IV90; WIAT-III93; Vineland-391; BRIEF-P92; BASC-394; AHA103; BoHA106; PEDI-CAT109; PLS-5114; BIA115; AES-FFQ121; DDS53; 3-oz water drinking test125; ITQOL133; HRU.134
ACE-Q, Centre for Youth Welfare Adverse Childhood Experiences Questionnaire for Children; AES-FFQ, Australian Eating Survey Food Frequency Questionnaire; AHA, Assisting Hand Assessment; ANZNN, Australian & New Zealand Neonatal Network; BASC-3, Behaviour Assessment System for Children, Third Edition; BIA, bioelectrical impedance analysis; BMI, body mass index; BoHA, Both Hands Assessment; BRIEF-P, Behaviour Rating Inventory of Executive Function-Preschool; CP, cerebral palsy; DASS-21, Depression Anxiety Stress Scale; DDS, Dysphagia Disorder Scale; Early PACT, Early Parenting Acceptance and Commitment Therapy; EA-SR, Emotional Availability-Self Report; GAME, Goal-Activity-Motor-Enrichment; GMFM-66, Gross Motor Function Measure; HRU, Health Resource Use Questionnaire; ITQOL, Infant Toddler Quality of Life Questionnaire; LEL, life events list; n/a, not available; PEDI-CAT, Paediatric Evaluation of Disability Inventory-Computerised Assessment Test; PLS-5, Preschool Language Scale, Fifth Edition; REACH, Rehabilitation Early for children at risk of Congenital Hemiplegia; Vineland-3, Vineland Adaptive Behaviour Scale; WIAT-III, Wechsler Individual Achievement Test; WPPSI-IV, Wechsler Preschool & Primary Scale of Intelligence, Fourth Edition A&NZ.
General cognition: WPPSI-IV A&NZ
The WPPSI-IV A&NZ estimated Full Scale IQ subscales (information, similarities, block design, matrix reasoning, picture memory, bug search) will be used to determine children’s general cognitive development at 4–6+3 years will be assessed.90 The WPPSI-IV is the gold standard for identifying general cognitive delays/deficits and has been standardised on Australian children with the normative sample representative of the Australian census for age, gender, parental education, geographic location and Indigenous status. The WPPSI-IV has strong internal consistency (0.85–0.95) and test-retest reliability (0.70–0.88), along with strong correlations with the Wechsler Intelligence Scale for Children.90 To minimise misclassification of children’s general cognitive abilities because of their underlying motor impairments (GMFCS IV/V), only the motor free subtests of the WPPSI-IV will be used to estimate Full Scale IQ as per current recommendations and our ongoing validation project. Furthermore, if deemed appropriate by the assessor, the five subtests of the WPPSI-IV that do not require verbal responses will be administered to estimate the non-verbal index.
Intellectual and developmental disability
The Vineland Adaptive Behaviour Scales, Third Edition (Vineland-3)91 will supplement the WPPSI-IV assessment for identifying children with intellectual and developmental disabilities. The Vineland-3 produces a global score (adaptive behaviour composite) and standard scores in three domains (communication, daily living skills, socialisation). It has good internal consistency (0.94–0.98) and its domains and overall score has moderate to high correlations (r=0.67–0.81) with other measures of adaptive behaviour (ie, Bayley Scales of Infant and Toddler Development Third Edition).91
Executive function: Behaviour Rating Inventory of Executive Function-Preschool
Behaviour Rating Inventory of Executive Function-Preschool (BRIEF-P) is a parent rated, comprehensive measure of children’s emerging executive function abilities across three broad indexes (inhibitory self-control, flexibility and emergent metacognition), and one composite score.92 BRIEF-P has strong internal consistency (0.80–0.90), test-retest reliability (0.80–0.90), with strong correlations (>0.70) with other standardised behaviour rating scales.92
Early academic skills: Wechsler Individual Achievement Test-Australian and New Zealand Standardised, Third Edition
Wechsler Individual Achievement Test-Australian and New Zealand Standardised, Third Edition is a standardised measure of academic achievement across domains of oral language, reading, written language and mathematics for individuals from 4:0 to 5:11 years of age.93 The early reading skills and maths problem solving subtests will be used. The internal consistency coefficients are strong (r=0.83–0.95).93
Behaviour Assessment System for Children
Behaviour Assessment System for Children, Third Edition (BASC-3) is a parent-rated questionnaire of child socio--emotional development across the domains of internalising and externalising problems including aggression, anxiety, inattention, conduct problems and withdrawal.94 The BASC-3 has strong internal consistency (0.76–0.96) and test-retest reliability (0.80–0.93), and strong correlations with other standardised rating scales.94
Gross Motor Function Measure
GMFM-66 is a criterion-referenced observation measure designed to assess changes in gross motor function in children with CP.95 The original GMFM-88 is an 88-item measure with items spanning the spectrum of gross motor activities in five dimensions (A: lying and rolling, B: sitting, C: crawling and kneeling, D: standing and E: walking, running and jumping).96 The GMFM-66 is a 66-item subset of the original 88 items, developed using Rasch modelling to represent gross motor function on a unidimensional interval scale (score 0–100).97 The GMFM-66 will be administered and provide an overall measure of gross motor function capacity (ie, what they are able to do in a standardised environment)98 in the current study for children with CP. It has established construct validity, high test-retest reliability (intraclass correlation coefficient (ICC)=0.99), responsive to change (minimal clinically important difference (MCID)=3.7) and has concurrent validity with Bayley-III (r=0.42 fine motor, 0.64 gross motor).97 99 100
Peabody Developmental Motor Scales
PDMS-2 will be used to evaluate gross and fine motor skill performance, that is, what skills they demonstrate in their daily environment.98 101 This standardised, norm-referenced measure for children from birth to 5 years 11 months of age has been validated as a discriminative measure for motor skill delays, and demonstrated responsiveness to change for children with CP aged 2–5 years.102
Manual ability
The Assisting Hand Assessment (AHA) measures the effectiveness with which a child with unilateral CP uses their more affected hand during bimanual activity performance.103 Test-retest reliability is high (ICC=0.98) and the AHA is responsive to change following upper limb intervention with a smallest detectable difference of 3.89 raw score points.104 105 The Both Hands Assessment (BoHA) has been adapted from the AHA, and measures how children with bilateral CP use their hands in bimanual activities.106 Rasch measurement modelling showed strong evidence of internal construct validity106 and scores on the BoHA are highly correlated with measures of upper limb capacity (Melbourne Assessment 2 subscales=0.48–0.83, Box and Blocks Test=0.85, n=39)107 and measures of self-care performance (PEDI-CAT r=0.73, n=44).108
Paediatric Evaluation of Disability Inventory-Computerised Assessment Test
PEDI-CAT is a standardised, norm-referenced assessment of independence in self-care, mobility, social cognitive and responsibility domains.109 110 The test is valid, reliable and responsive in children with developmental delay and/or CP.111 112 Convergent validity has been established between domains of the PEDI and the Functional Mobility Scale, the Daily Activities and Speech and Communication domains of the Paediatric Quality of Life CP and the Personal Care and Communications domains of the Caregiver Priorities and Child Health Index of Life with Disabilities questionnaire (r=0.42–0.85, p<0.001).111 Test-retest reliability has been found to be excellent (ICC=0.96–0.99). The PEDI-CAT is completed by parents, and the content balanced version will be used.113
Preschool Language Scale, Fifth Edition
Preschool Language Scale, Fifth Edition is a comprehensive developmental language assessment (receptive and expressive subtests) for children up to 7 years 11 months.114 It is an interactive, play-based assessment featuring items to address a wider variety of early play behaviours. Split half reliabilities range from 0.80 to 0.97. The total language score has a sensitivity of 0.83 and specificity of 0.80 for detecting language delay disorder in children from birth through age 7 years 11 months.114
Growth and nutritional status
Growth and nutritional status will be measured by body weight, height, length or knee height and body composition using bioelectrical impedance analysis validated for use in the CP Child study (study 5).115 116 Anthropometric data will be converted to z-scores using Centers for Disease Control growth charts for children.88 117 Nutritional status will be determined using height, weight and body mass index z-score cut-offs.118 119
Dietary quality: the Australian Eating Survey Food Frequency Questionnaire
The Australian Eating Survey Food Frequency Questionnaire will be completed via parent report and the Australian Recommended Food Score-Preschool (ARFS-P) version will be calculated.120 The ARFS-P has demonstrated validity in characterising nutrient intakes of children aged 2–5 years.120 121 Diet quality is an important determinant of child growth and development, and poor preschool dietary quality has been associated with parental concerns of poorer school readiness.122 123
The Dysphagia Disorder Survey Part 2
The Dysphagia Disorder Survey (DDS) Part 2 raw score will be used to evaluate oral, pharyngeal and oesophageal phase dysphagia, scored from video by a DDS-certified speech therapist from a standardised feeding assessment (including puree, lumpy/mash, chewable, tough chewable and fluid textures).53 124 The DDS had excellent reliability (intra-rater agreement >90% and inter-rater agreement >85%) and acceptable convergent validity (sensitivity=100% and specificity=47.1%) for identifying oropharyngeal dysphagia in children with CP.53 124 Children will also complete the 3 oz (90 mL) water swallow test, which has acceptable psychometric properties for detecting oropharyngeal dysphagia in children (sensitivity=100%, specificity=51.2%).125 Clinical signs suggestive of pharyngeal phase impairment will be assessed premeal and postmeal as the presence or absence of (i) wet/gurgly voice, (ii) wet/gurgly breathing, (iii) rattly chest and (iv) cough.38 A parent-report feeding questionnaire used in study 5 will also be completed.54 Feeding difficulties and dysphagia can negatively impact nutritional status and present as a challenge and point of stress for children and parents when attending school.126
Habitual physical activity
Triaxial accelerometers will be used to evaluate the frequency, intensity and duration of physical activity.127 While the GMFM-66 measures activity capacity (what children are capable of in terms of walking, running, etc), habitual physical activity is a measure of physical activity performance (what children do in their daily life).128 Accelerometry is valid, reliable and feasible in children with CP.129 130 A combined thigh/wrist model has been validated for GMFCS III and IV.131 Accelerometers will be fitted on the less affected wrist and thigh during assessment and worn during waking hours for 7 days.127 Raw accelerometer data will be processed using machine learning algorithms specifically trained and validated for assessing habitual physical activity in CP.131 132 The machine learning algorithms identify activity type and quantify time spent in sedentary activities (sitting or lying down), standing utilitarian movements (light intensity), comfortable walking and brisk walking (moderate-to-vigorous intensity activity).131 132
Quality of life: the Infant Toddler Quality of Life Questionnaire
The Infant Toddler Quality of Life Questionnaire (ITQOL) is a 103-item parent-reported questionnaire of quality of life for infants and toddlers aged 2 months to 5 years, and measures quality of life across physical, mental and social well-being. For each of the 10 concepts (physical functioning, growth and development, bodily pain, temperament and moods, general behaviour, getting along, general health perceptions, change in health and parental impact: emotional/time/family activities/family cohesion) item responses are scored, summed and transformed to a scale from 0 (worst health) to 100 (best health).133 The ITQOL has demonstrated acceptable internal consistency (Cronbach’s alpha >0.70) and test-retest reliability (ICC ≥0.50, p<0.01) for all 10 scales.133
Economic evaluation
Resource use and direct costs of treatment will be measured using our previously developed Health Resource Use Questionnaire (HRU)134 supplemented by data linkage from the Medical Benefits Scheme (MBS) and Pharmaceutical Benefits Scheme (PBS) from the Department of Human Services. Health resource use data include screening assessments, therapy frequency and duration, hospital admissions, general practitioner and other medical visits, medications, equipment and parent time taken for appointments. Costs of the early interventions will be based on actual staff and equipment costs in the trials. Standard cost sources including MBS, PBS, hospital and diagnostic-related grouping for inpatient and outpatient services will be applied to the resource use and a total cost of care will be calculated for each child up to age of assessment of school readiness (4–6+3 years).
Participant timeline
The follow-up time-point for the School Readiness study consists of a one-off assessment that takes approximately 6 hours including at least two scheduled breaks. To improve feasibility, the assessments may be completed over two or more visits depending on parent preference and child tolerance. Assessments may be completed at research centres or as home visits, and parent questionnaires may be completed online after the assessment or as interviews over the phone or videoconference. Primary caretakers may opt to only do online/phone questionnaires if an in-person assessment is not feasible. Child participants are also requested to wear a wrist and thigh accelerometer during waking hours for 7 days, starting the day following the assessments. Parents or caregivers will be required to fill out a wear log for the accelerometer. After the study visit, participants will receive a summary report of their child’s developmental progress using findings from key assessments. Parents and caregivers will be encouraged to contact the study team for further information about assessments performed on the day should they wish to receive this.
Recruitment
Children recruited into the Protect-me, GAME, REACH and Early PACT trials will be approached by study personnel for reconsent for this follow-up at 4–6+3 years of age. Families with a child meeting eligibility will be invited to join this follow-up at the four collaborating sites (Queensland, New South Wales, Victoria, Western Australia) and associated clinical services (Queensland Children’s Hospital, Cerebral Palsy Alliance, Monash Children’s Hospital, Perth Children’s Hospital). Recruitment from the four major centres will enable the target sample size to be achieved. Recruitment at each site will begin following receipt of ethical and governance approvals. Recruitment will draw on current databases from the previous studies.
Allocation and blinding
Mothers recruited in the Protect-me trial (study 1) were randomly allocated to receive melatonin or placebo via an online computerised randomisation service.42 Children recruited for studies 2–4 were randomised according to each study protocol to receive intervention or CAU (GAME13), equal dose of one of two different early interventions (REACH43) and intervention or wait-list control (Early PACT44) using an electronic allocation system determined by non-study personnel via Research Electronic Data Capture (REDCap). Where possible assessors on the school readiness outcomes will be masked to group allocation in studies 1–4 but in some instances, they may have knowledge of the participants from previous interactions in other studies.
Data management
Data types and collection
Objective data will be collected using standardised assessment during face-to-face interaction with the child, which may be videotaped, and 7-day accelerometry will be collected. Primary caregivers will complete questionnaires about the child and themselves via online surveys/scoring software and/or paper questionnaires. Medical chart reviews will be used to confirm or clarify medical information, and MRIs will be retrieved for scoring. All data are re-identifiable. Data quality monitoring of the REDCap database will be undertaken quarterly by the centre data manager to ensure data are accurate and complete.
Data transfer and storage
Data management will comply with relevant privacy protocols, such as the Australian Standard on personal privacy protection. All data collected will be coded with a participant ID number. Consent forms and identification codes will be stored in a different place from the data records to which they are linked. Data recorded on paper will be stored at the trial sites in locked filing cabinets during the data collection phase and within an archive box located at the Centre for Child Health Research at the conclusion of the data collection phase. Electronic data will be stored on secure Australian servers using REDCap (database) and UQ research data manager. Data will be retained until HREC-approved retention period following closure of study (15 years).
Statistical methods
Sample size and power calculations
Up to 425 children at high risk of or with CP, aged between 4 years and 6+3 years are eligible for inclusion based on study 1–4 participation numbers. The total number of participants with data for the primary outcome (WPPSI-IV) and secondary outcome (GMFM-66) is expected to be n=373 (conservatively assume 4–6+3-year-old data from 90% of 425 potential participants from studies 1 to 4). This gives 90% power (alpha=0.05) to detect between intervention versus placebo/CAU differences of 0.26 SD or greater on the WPPSI-IV. This is equivalent to a difference of >3.9 units on WPPSI-IV standardised norms. When examined by intervention type, we have 90% power (alpha=0.05) to detect a between-group difference of >6.4 units for neuroprotectants and >4.8 units for neurorehabilitation. For ambulant children, we can detect between-group differences of >5.2 units and >5.5 units for non-ambulant children. For the secondary outcome of GMFM-66, we assume an SD of 8.6 units.55 Consequently, we have >95% power to detect an MCID of at least 5 units overall (and >95% power for ambulant (MCID=7), >80% power non-ambulant (MCID=5). For the novel secondary outcome of the School Readiness Index, if we assume an SD of 2 units, we have 80% power to detect differences between groups of >0.52 units.
Statistical analyses
Summary statistics will be displayed for continuous variables as either mean and SD or median and IQR as appropriate, while categorical variables will be displayed as frequency (percentage). To investigate the primary outcomes for aim 1, the association between group (intervention vs placebo/CAU) and general cognition (WPPSI-IV) we will build a mixed-effects linear regression model. Group and time will be included as fixed effects, with a group-by-time interaction. Participant will be included as a random effect to account for the non-independence of observations from the same child. Balance between groups will be investigated and potentially confounding variables will be included in model as co-variables as appropriate. Models for secondary outcomes will be constructed using the same principles. For secondary outcomes assessed using continuous data we will construct linear models, for outcomes using binary data we will construct logistic models and for outcomes using count data we will construct Poisson models. The same analysis principles will also be used to investigate the secondary outcomes for aim 5 (gross motor capacity (GMFM-66), functional independence (PEDI-CAT) and growth status (height, weight and body mass index z-scores)), with diagnosis time (early vs late) included as the main effect in mixed-effects linear regression models.
To investigate aim 2, which involves identifying maternal and perinatal risk factors for adverse neurodevelopmental outcomes, we will build a series of regression models. First, we will run univariable models, with explanatory variables investigated chosen based on knowledge from literature and from the dataset. The best multivariable models will be identified using the Bayesian Information Criterion. Model calibration will be tested graphically, and internal validation will use bootstrap resampling. To investigate aim 4, which involves identifying the moderating effects of maternal mental health and emotional availability on primary and secondary outcomes, moderated regression models will be used.
For aim 3, brain structure data and other relevant variables (gestational age, gender) will be input into machine learning models (multivariable regression and random forests).135 Outcomes of these machine learning models will be general cognition (WPPSI-IV), gross motor capacity (GMFM-66) and gross and fine motor skills (PDMS-2). A stepwise feature selection procedure will identify the most predictive markers of clinical outcome and will be validated with a model cross-validation strategy.
For primary outcome of health resource use in aim 5, a cost-effectiveness analysis and a cost-consequence analysis (CCA) to compare the cohort that had early diagnosis and early intervention (studies 1–4: Protect-me, GAME, REACH and Early PACT)13 42–44 versus later diagnosis of CP and later commencement of interventions (study 5: CP Child).45 47 136 For the cost-effectiveness, we will calculate the costs per additional child meeting the school readiness outcome. The base case will be performed at 4–6+3 years and a limited societal perspective will be taken, with carer costs included. Patterns of HRU and costs will be described for children aged 4–6+3 years. The CCA presents options in the form of a table of disaggregated costs and a range of outcomes, allowing decision makers to form their own opinion on the relative importance of the outcomes presented.137 CCAs are recommended for complex interventions with multiple effects and will enable the incorporation of wider benefits to the child and family into the economic evaluation.138 The benefits include broader health and non-health effects.
For all analysis, missing data will be treated case-by-case depending on patterns of data missingness, that is, multiple imputation methods if ‘missing at random’ and pattern-mixture models if ‘not missing at random’. Adjustment for multiple comparisons will be made, bearing in mind type I and II error rates.
Participant safety and withdrawal
Risk management and safety
Participant risks
While the accelerometers (ActiGraph GT3X+, Axivity AX3) pose no health and safety risks themselves, discomfort could be caused by tight application or contact allergic reactions to cleaning fluid or the adhesive used in the hypoallergenic patches used to fix the thigh accelerometer. This will be monitored closely with verbal prompting, and visual checking of devices and skin contact twice a day by parents/carers.
Study risks
The main risks to this follow-up study are recruitment shortfalls and participant attrition. High recruitment is anticipated as all families will receive a comprehensive report about their child’s school readiness. Should further lockdowns occur due to COVID-19, these are likely to be isolated to specific areas/states, therefore having four sites enables recruitment to continue in other sites should one be restricted due to COVID-19. Assessments may be rescheduled if the child is under 6 years 3 months of age at the time of assessment. All staff involved in delivering the assessments will be trained and supervised by senior experienced personnel. Regular monitoring will occur, and any discomfort reported by the child or their caregiver will be immediately responded to by staff members.
Adverse events reporting
Any adverse events associated with assessments will be screened using open-ended questions. Adverse events will be documented and reported to the site Chief Investigators by the assessor. Major adverse events will be reported to HREC within 24 hours. Major adverse events include (i) injuries that require medical treatment (such as severe strains or broken bones), (ii) referral for assessment and intervention in context of significant anxious or depressive symptoms. After reporting to the site Chief Investigator, local site processes will be followed as necessary. Minor adverse events include (i) near miss accidents (such as falling during overground walking), (ii) sore muscles, bruises, other minor injuries not requiring medical treatment, (iii) feeling upset, guiltyor sad.
Handling withdrawals
Participants can withdraw at any time. Participants who choose to withdraw from the study will not be penalised in any way. Participants are informed of their right to withdraw at any time without consequences at the time of reading participant information forms and signing of consent forms. Data will be analysed on an intention-to-treat basis based on their group allocation in studies 1–4.
Ethics and dissemination
Informed consent process
Informed consent will be sought from the parent or legal guardian of every child invited to participate. A copy of a consent form can be found in online supplemental appendix 2. Potential participants will be provided with a copy of the participant information statement after agreeing to enrol in the study via phone or email contact. Potential participants will have at least 24 hours and typically >1 week to read information about the study and decide whether they would like to participate. Families will be invited to ask questions and discuss any aspect of the study with the site contact, Chief Principal Investigator and/or Study Coordinator should they require more information to decide. Prior to booking an appointment or collecting pre-appointment data, verbal or written informed consent will be required. A copy of the consent form will be signed again at the first face-to-face meeting and countersigned by the assessing/treating assessor and a witness. Families will be given the option to only complete online questionnaires if they do not wish to attend a face-to-face appointment.
bmjopen-2022-068675supp002.pdf (166.2KB, pdf)
Results of the study will be disseminated via (i) conference abstracts and presentations, (ii) peer-reviewed articles in scientific journals, (iii) participant, organisation and institution newsletters and (iv) media releases. At the conclusion of the study after the primary analyses, a summary flyer of the main outcomes of the study will be emailed and/or mailed to participants.
Data access and confidentiality
In accordance with the National Health and Medical Research Council/Medical Research Future Fund (NHMRC/MRFF) statement on data sharing: ‘NHMRC/MRFF encourages data sharing and providing access to data and other research outputs (metadata, analysis code, study protocols, study materials and other collected data) arising from NHMRC supported research’, data will be made available to other researchers or funding bodies including the NHMRC as necessary for the purposes of meta-analysis/systematic review and/or confirmation of statistical results. These data will be made available at group-level. If individual-level data are required, a limited, codified dataset will be made available to reduce or eliminate the possibility of re-identification of the data. A description of the dataset (metadata) will be published so that it can be discovered and/or cited. Data will be shared directly with individuals or institutions that approach the custodians. Future use and sharing of data are addressed on the Parent Information Sheet. Identifiable data will not be available for future use unless by separate ethics application.
Ethics approval
This project has received ethical approval from the following committees: Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/21/QCHQ/77124); The University of Queensland Human Research Ethics Committee (2021/HE002003), The University of Sydney Human Research Ethics Committee (2021/835); The Cerebral Palsy Alliance (2022_01_01); Commonwealth Scientific and Industrial Research Organisation Health and Medical Human Research Ethics Committee (CSIRO, 2021_081_RR). Site-specific approval has been granted for Perth Children’s Hospital and Monash University. Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the participant or may affect participant safety will require a formal amendment to the protocol. Such amendments will be agreed on by the chief investigators and approved by the relevant ethics committees prior to implementation.
Dissemination
Results will be disseminated in peer-reviewed journals, scientific conferences and professional organisations, and to people with lived experience of CP and their families.
Study status
Assessments commenced in February 2021 and will continue until July 2025.
Supplementary Material
Acknowledgments
The authors would like to thank the QCPRRC Research Governance Officer Jacquie Robinson for her work, and Dr Morgan Carlton for her administrative assistance in setting up the study. The authors would like to thank the staff at Children’s Physical Activity Group, Denise Brookes and Kate West who handle the logistics of the accelerometer data collection. The authors would also like to thank the study assessors, including Christine Finn, Bernadette Shannon, Sarah Gibson, Jemima Walker, Emma Sanders, Sarah Goodman, Candice Stevenson, Naomi Hodder, Monica Toohey, Anna te Velde, Emma Stanton and Eliza Walker.
Footnotes
Twitter: @DrCathyMorgan, @hss_1
Correction notice: This article has been corrected since it was first published. Co-author 'Jane Wotherspoon' has been added to the list of authors.
Contributors: RNB, IN, CM, LS, MCF, RSW, TC, KW, ST, KP and SB conceived the trial. RNB, IN, CM, LS, MCF, RSW, TC, KW, ST and KP achieved study funding as chief investigators, and SB, SM, AP, NB, HSS, KRP and KB as associate investigators. RNB and SO completed the initial draft of the manuscript. RNB, CM, AH, TC, MCF and AH undertook clinical trials registration and completion of ethics and site-specific applications. SO developed the study manual and assessment programme with input from the investigators (RNB, IN, CM, LS, MCF, RSW, TC, KW, ST, KP, SB, SB, SM, AP, NB, HSS, KRP and KB), postdoctoral researchers working on the study (AB, AK, AH, KB) and the clinical trials coordinator (LG-P). SL reviewed the study and manuscript, and provided feedback as a consumer living with cerebral palsy. All authors designed the study, have read, edited and approved the final manuscript.
Funding: This work is supported by a Medical Research Future Fund Preventive and Public Health Research Initiative—Maternal First 2000 Days and Childhood Health grant (MRFF 2007292). RNB is supported by a NHMRC Research Fellowship (NHMRC 1037220). LS is supported by a NHMRC Career Development Fellowship (NHMRC 1160694). KRP is supported by an NHMRC Investigator Grant (GNT2009765). AB is supported by a UQ Research Stimulus (Allocation 2, Stream 3).
Disclaimer: This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. In accordance with the National Health and Medical Research Council/Medical Research Future Fund (NHMRC/MRFF) statement on data sharing: 'NHMRC/MRFF encourages data sharing and providing access to data and other research outputs (metadata, analysis code, study protocols, study materials and other collected data) arising from NHMRC supported research', data will be made available to other researchers or funding bodies including the NHMRC as necessary for the purposes of meta-analysis/systematic review and/or confirmation of statistical results. These data will be made available at group-level. If individual-level data are required, a limited, codified dataset will be made available to reduce or eliminate the possibility of re-identification of the data. A description of the dataset (metadata) will be published so that it can be discovered and/or cited. Data will be shared directly with individuals or institutions that approach the custodians. Future use and sharing of data are addressed on the Parent Information Sheet. Identifiable data will not be available for future use unless by separate ethics application.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Australian Cerebral Palsy Register . Report of the Australian cerebral palsy register birth years 2009-2018. 2018. Available: https://cpregister.com/wp-content/uploads/2019/02/Report-of-the-Australian-Cerebral-Palsy-Register-Birth-Years-1995-2012.pdf
- 2.Access Economics . The economic impact of cerebral palsy in Australia in 2007. 2008. Available: https://cpaustralia.com.au/media/20379/access_economics_report.pdf
- 3.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007;109(Supp):8–14. [PubMed] [Google Scholar]
- 4.Hanna SE, Rosenbaum PL, Bartlett DJ, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol 2009;51:295–302. 10.1111/j.1469-8749.2008.03196.x [DOI] [PubMed] [Google Scholar]
- 5.McIntyre S, Novak I, Cusick A. Consensus research priorities for cerebral palsy: a Delphi survey of consumers, researchers, and clinicians. Dev Med Child Neurol 2010;52:270–5. 10.1111/j.1469-8749.2009.03358.x [DOI] [PubMed] [Google Scholar]
- 6.Australian and New Zealand Cerebral Palsy Strategy Collaboration . Australian and new zealand cerebral palsy strategy. 2020. Available: www.cerebralpalsystrategy.com.au [DOI] [PubMed]
- 7.Morgan C, Darrah J, Gordon AM, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol 2016;58:900–9. 10.1111/dmcn.13105 [DOI] [PubMed] [Google Scholar]
- 8.Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897–907. 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston MV. Clinical disorders of brain plasticity. Brain Dev 2004;26:73–80. 10.1016/S0387-7604(03)00102-5 [DOI] [PubMed] [Google Scholar]
- 10.Novak I, McIntyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 2013;55:885–910. 10.1111/dmcn.12246 [DOI] [PubMed] [Google Scholar]
- 11.Novak I, Morgan C, Fahey M, et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep 2020;20:3. 10.1007/s11910-020-1022-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan C, Novak I, Dale RC, et al. Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatr 2015;15:30. 10.1186/s12887-015-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan C, Novak I, Dale RC, et al. Game (goals-activity-motor enrichment): protocol of a single blind randomised controlled trial of motor training, parent education and environmental enrichment for infants at high risk of cerebral palsy. BMC Neurol 2014;14:203. 10.1186/s12883-014-0203-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuckerman B, Halfon N. School readiness: an idea whose time has arrived. Pediatrics 2003;111(6 Pt 1):1433–6. 10.1542/peds.111.6.1433 [DOI] [PubMed] [Google Scholar]
- 15.Prior M, Bavin E, Ong B. Predictors of school readiness in five‐ to six‐year‐old children from an Australian longitudinal community sample. Educational Psychology 2011;31:3–16. 10.1080/01443410.2010.541048 [DOI] [Google Scholar]
- 16.Pagani LS, Fitzpatrick C, Archambault I, et al. School readiness and later achievement: a French Canadian replication and extension. Dev Psychol 2010;46:984–94. 10.1037/a0018881 [DOI] [PubMed] [Google Scholar]
- 17.Williams PG, Lerner MA, COUNCIL ON EARLY CHILDHOOD, et al. School readiness. Pediatrics 2019;144:2019–1766. 10.1542/peds.2019-1766 [DOI] [PubMed] [Google Scholar]
- 18.Roberts G, Lim J, Doyle LW, et al. High rates of school readiness difficulties at 5 years of age in very preterm infants compared with term controls. J Dev Behav Pediatr 2011;32:117–24. 10.1097/DBP.0b013e318206d5c9 [DOI] [PubMed] [Google Scholar]
- 19.Woodward LJ, Moor S, Hood KM, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed 2009;94:F339–44. 10.1136/adc.2008.146282 [DOI] [PubMed] [Google Scholar]
- 20.Gehrmann FE, Coleman A, Weir KA, et al. School readiness of children with cerebral palsy. Dev Med Child Neurol 2014;56:786–93. 10.1111/dmcn.12377 [DOI] [PubMed] [Google Scholar]
- 21.Duncan GJ, Dowsett CJ, Claessens A, et al. School readiness and later achievement. Dev Psychol 2007;43:1428–46. 10.1037/0012-1649.43.6.1428 [DOI] [PubMed] [Google Scholar]
- 22.Rolnick A, Grunewald R. Early childhood development: economic development with a high public return. The Region 2003;17:6–12. [Google Scholar]
- 23.Friedman-Krauss A, Barnett WS. Early childhood education: pathways to better health. preschool policy brief issue 25. National Institute for Early Education Research, 2013. [Google Scholar]
- 24.Schweinhart LJ, Montie J, Xiang Z, et al. Lifetime effects: the high/scope perry preschool study through age 40. Ypsilanti, Minnesota: High/Scope Press, 2005. [Google Scholar]
- 25.Karoly LA, Bigelow JH. The economics of investing in universal preschool education in california. RAND Corporation, 2005. 10.7249/MG349 [DOI] [Google Scholar]
- 26.Campbell FA, Ramey CT, Pungello E, et al. Early childhood education: young adult outcomes from the abecedarian project. Applied Developmental Science 2002;6:42–57. 10.1207/S1532480XADS0601_05 [DOI] [Google Scholar]
- 27.Camacho C, Straatmann VS, Day JC, et al. Development of a predictive risk model for school readiness at age 3 years using the UK millennium cohort study. BMJ Open 2019;9:e024851. 10.1136/bmjopen-2018-024851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson DB, Testa A, Vaughn MG. Adverse childhood experiences and school readiness among preschool-aged children. J Pediatr 2021;230:191–7. 10.1016/j.jpeds.2020.11.023 [DOI] [PubMed] [Google Scholar]
- 29.Sheridan SM, Knoche LL, Edwards CP, et al. Parent engagement and school readiness: effects of the getting ready intervention on preschool children’s social-emotional competencies. Early Educ Dev 2010;21:125–56. 10.1080/10409280902783517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marti M, Merz EC, Repka KR, et al. Parent involvement in the getting ready for school intervention is associated with changes in school readiness skills. Front Psychol 2018;9:759. 10.3389/fpsyg.2018.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan SM, Knoche LL, Kupzyk KA, et al. A randomized trial examining the effects of parent engagement on early language and literacy: the getting ready intervention. J Sch Psychol 2011;49:361–83. 10.1016/j.jsp.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucsea O, Kosmerly S, Rogers MA. Effects of mothers’ parenting sense of competence and child gender on academic readiness in preschool children with symptoms of ADHD. Journal of Applied School Psychology 2023;39:40–70. 10.1080/15377903.2021.2012862 [DOI] [Google Scholar]
- 33.Himmelmann K, Horber V, Sellier E, et al. Neuroimaging patterns and function in cerebral palsy-application of an MRI classification. Front Neurol 2020;11:617740. 10.3389/fneur.2020.617740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman A, Fiori S, Weir KA, et al. Relationship between brain lesion characteristics and communication in preschool children with cerebral palsy. Res Dev Disabil 2016;58:55–64. 10.1016/j.ridd.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 35.Novak I, Hines M, Goldsmith S, et al. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics 2012;130:e1285–312. 10.1542/peds.2012-0924 [DOI] [PubMed] [Google Scholar]
- 36.Oftedal S, Davies PSW, Boyd RN, et al. Longitudinal growth, diet, and physical activity in young children with cerebral palsy. Pediatrics 2016;138:e20161321. 10.1542/peds.2016-1321 [DOI] [PubMed] [Google Scholar]
- 37.Oftedal S, Davies PS, Boyd RN, et al. Body composition, diet, and physical activity: a longitudinal cohort study in preschoolers with cerebral palsy. Am J Clin Nutr 2017;105:369–78. 10.3945/ajcn.116.137810 [DOI] [PubMed] [Google Scholar]
- 38.Benfer KA, Weir KA, Bell KL, et al. Longitudinal cohort protocol study of oropharyngeal dysphagia: relationships to gross motor attainment, growth and nutritional status in preschool children with cerebral palsy. BMJ Open 2012;2:e001460. 10.1136/bmjopen-2012-001460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oftedal S, Bell KL, Davies PSW, et al. Sedentary and active time in toddlers with and without cerebral palsy. Med Sci Sports Exerc 2015;47:2076–83. 10.1249/MSS.0000000000000653 [DOI] [PubMed] [Google Scholar]
- 40.Coleman A, Weir K, Ware RS, et al. Predicting functional communication ability in children with cerebral palsy at school entry. Dev Med Child Neurol 2015;57:279–85. 10.1111/dmcn.12631 [DOI] [PubMed] [Google Scholar]
- 41.Sakzewski L, Carlon S, Shields N, et al. Impact of intensive upper limb rehabilitation on quality of life: a randomized trial in children with unilateral cerebral palsy. Dev Med Child Neurol 2012;54:415–23. 10.1111/j.1469-8749.2012.04272.x [DOI] [PubMed] [Google Scholar]
- 42.Palmer KR, Mockler JC, Davies-Tuck ML, et al. Protect-me: a parallel-group, triple blinded, placebo-controlled randomised clinical trial protocol assessing antenatal maternal melatonin supplementation for fetal neuroprotection in early-onset fetal growth restriction. BMJ Open 2019;9:e028243. 10.1136/bmjopen-2018-028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyd RN, Ziviani J, Sakzewski L, et al. Reach: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open 2017;7:e017204. 10.1136/bmjopen-2017-017204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whittingham K, Sheffield J, Mak C, et al. Early parenting acceptance and commitment therapy “ early PACT ” for parents of infants with cerebral palsy: a study protocol of a randomised controlled trial. BMJ Open 2020;10:e037033. 10.1136/bmjopen-2020-037033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd RN, Jordan R, Pareezer L, et al. Australian cerebral palsy child study: protocol of prospective population based study of motor & brain dev. of preschool CP. BMC Neurol 2013;13:13–57. 10.1186/1471-2377-13-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyd RN, Davies PS, Ziviani J, et al. PREDICT-CP: study protocol of implementation of comprehensive surveillance to predict outcomes for school-aged children with cerebral palsy. BMJ Open 2017;7:e014950. 10.1136/bmjopen-2016-014950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell KL, Boyd RN, Tweedy SM, et al. A prospective, longitudinal study of growth, nutrition and sedentary behaviour in young children with cerebral palsy. BMC Public Health 2010;10:179. 10.1186/1471-2458-10-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller SL, Yawno T, Alers NO, et al. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res 2014;56:283–94. 10.1111/jpi.12121 [DOI] [PubMed] [Google Scholar]
- 51.Walker JL, Bell KL, Stevenson RD, et al. Relationships between dietary intake and body composition according to gross motor functional ability in preschool-aged children with cerebral palsy. Ann Nutr Metab 2012;61:349–57. 10.1159/000342557 [DOI] [PubMed] [Google Scholar]
- 52.Walker JL, Bell KL, Boyd RN, et al. Energy requirements in preschool-age children with cerebral palsy. Am J Clin Nutr 2012;96:1309–15. 10.3945/ajcn.112.043430 [DOI] [PubMed] [Google Scholar]
- 53.Benfer KA, Weir KA, Bell KL, et al. Oropharyngeal dysphagia and gross motor skills in children with cerebral palsy. Pediatrics 2013;131:e1553–62. 10.1542/peds.2012-3093 [DOI] [PubMed] [Google Scholar]
- 54.Benfer KA, Weir KA, Ware RS, et al. Parent-reported indicators for detecting feeding and swallowing difficulties and undernutrition in preschool-aged children with cerebral palsy. Dev Med Child Neurol 2017;59:1181–7. 10.1111/dmcn.13498 [DOI] [PubMed] [Google Scholar]
- 55.Burgess A, Reedman S, Chatfield MD, et al. Development of gross motor capacity and mobility performance in children with cerebral palsy: a longitudinal study. Dev Med Child Neurol 2022;64:578–85. 10.1111/dmcn.15112 Available: 10.1111/dmcn.15112 [DOI] [PubMed] [Google Scholar]
- 56.Laporta-Hoyos O, Pannek K, Pagnozzi AM, et al. Cognitive, academic, executive and psychological functioning in children with spastic motor type cerebral palsy: influence of extent, location, and laterality of brain lesions. Eur J Paediatr Neurol 2022;38:33–46. 10.1016/j.ejpn.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 57.Bodimeade HL, Whittingham K, Lloyd O, et al. Executive function in children and adolescents with unilateral cerebral palsy. Dev Med Child Neurol 2013;55:926–33. 10.1111/dmcn.12195 [DOI] [PubMed] [Google Scholar]
- 58.Coleman A, Weir KA, Ware RS, et al. Relationship between communication skills and gross motor function in preschool-aged children with cerebral palsy. Arch Phys Med Rehabil 2013;94:2210–7. 10.1016/j.apmr.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 59.Einspieler C, Prechtl HFR. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev 2005;11:61–7. 10.1002/mrdd.20051 [DOI] [PubMed] [Google Scholar]
- 60.Einspieler C, Marschik PB, Pansy J, et al. The general movement optimality score: a detailed assessment of general movements during preterm and term age. Dev Med Child Neurol 2016;58:361–8. 10.1111/dmcn.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romeo DM, Ricci D, Brogna C, et al. Use of the Hammersmith infant neurological examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol 2016;58:240–5. 10.1111/dmcn.12876 [DOI] [PubMed] [Google Scholar]
- 62.Statistics. ABo . Socio-economic indexes for areas (SEIFA 2016). In: Statistics. ABo. Canberra, Australia, 2016. [Google Scholar]
- 63.Love S, Gibson N, Blair E, et al. Cerebral palsy description form for the Australian cerebral palsy register. In: Kids Rehab: Children’s Hospital Westmead and Cerebral Palsy Alliance. 2020. [Google Scholar]
- 64.Love S, Gibson N, Smith N, et al. Interobserver reliability of the Australian spasticity assessment scale (ASAS). Dev Med Child Neurol 2016;58 Suppl 2:18–24. 10.1111/dmcn.13000 [DOI] [PubMed] [Google Scholar]
- 65.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23. 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 66.Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 2000;80:974–85. 10.1093/ptj/80.10.974 [DOI] [PubMed] [Google Scholar]
- 67.Wood EC, Rosenbaum P. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Dev Med Child Neurol 2000;42:292–6. 10.1017/s0012162200000529 [DOI] [PubMed] [Google Scholar]
- 68.Eliasson A-C, Krumlinde-Sundholm L, Rösblad B, et al. The manual ability classification system (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 2006;48:549–54. 10.1017/S0012162206001162 [DOI] [PubMed] [Google Scholar]
- 69.Hidecker MJC, Paneth N, Rosenbaum PL, et al. Developing and validating the communication function classification system for individuals with cerebral palsy. Dev Med Child Neurol 2011;53:704–10. 10.1111/j.1469-8749.2011.03996.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barty E, Caynes K, Johnston LM. Development and reliability of the functional communication classification system for children with cerebral palsy. Dev Med Child Neurol 2016;58:1036–41. 10.1111/dmcn.13124 [DOI] [PubMed] [Google Scholar]
- 71.Pennington L, Virella D, Mjøen T, et al. Development of the viking speech scale to classify the speech of children with cerebral palsy. Res Dev Disabil 2013;34:3202–10. 10.1016/j.ridd.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 72.Sellers D, Mandy A, Pennington L, et al. Development and reliability of a system to classify the eating and drinking ability of people with cerebral palsy. Dev Med Child Neurol 2014;56:245–51. 10.1111/dmcn.12352 [DOI] [PubMed] [Google Scholar]
- 73.Benfer KA, Weir KA, Bell KL, et al. The eating and drinking ability classification system in a population-based sample of preschool children with cerebral palsy. Dev Med Child Neurol 2017;59:647–54. 10.1111/dmcn.13403 [DOI] [PubMed] [Google Scholar]
- 74.O’Leary CM, Bower C, Zubrick SR, et al. A new method of prenatal alcohol classification accounting for dose, pattern and timing of exposure: improving our ability to examine fetal effects from low to moderate alcohol. J Epidemiol Community Health 2010;64:956–62. 10.1136/jech.2009.091785 [DOI] [PubMed] [Google Scholar]
- 75.George JM, Fiori S, Fripp J, et al. Validation of an MRI brain injury and growth scoring system in very preterm infants scanned at 29- to 35-week postmenstrual age. AJNR Am J Neuroradiol 2017;38:1435–42. 10.3174/ajnr.A5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–14. 10.3174/ajnr.A3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiori S, Cioni G, Klingels K, et al. Reliability of a novel, semi-quantitative scale for classification of structural brain magnetic resonance imaging in children with cerebral palsy. Dev Med Child Neurol 2014;56:839–45. 10.1111/dmcn.12457 [DOI] [PubMed] [Google Scholar]
- 78.Krägeloh-Mann I, Cans C. Cerebral palsy update. Brain Dev 2009;31:537–44. 10.1016/j.braindev.2009.03.009 Available: 10.1016/j.braindev.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 79.Makropoulos A, Robinson EC, Schuh A, et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 2018;173:88–112. 10.1016/j.neuroimage.2018.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pannek K, Fripp J, George JM, et al. Fixel-based analysis reveals alterations is brain microstructure and macrostructure of preterm-born infants at term equivalent age. Neuroimage Clin 2018;18:51–9.:S2213-1582(18)30003-2. 10.1016/j.nicl.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pannek K, Guzzetta A, Colditz PB, et al. Diffusion MRI of the neonate brain: acquisition, processing and analysis techniques. Pediatr Radiol 2012;42:1169–82. 10.1007/s00247-012-2427-x [DOI] [PubMed] [Google Scholar]
- 82.Pannek K, Hatzigeorgiou X, Colditz PB, et al. Assessment of structural connectivity in the preterm brain at term equivalent age using diffusion MRI and T2 relaxometry: a network-based analysis. PLoS One 2013;8:e68593. 10.1371/journal.pone.0068593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther 1995;33:335–43. 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- 84.Vliegen N, Luyten P, Biringen Z. A multimethod perspective on emotional availability in the postpartum period. Parenting 2009;9:228–43. 10.1080/15295190902844514 [DOI] [Google Scholar]
- 85.Luyten P, Mayes LC, Nijssens L, et al. The parental reflective functioning questionnaire: development and preliminary validation. PLoS One 2017;12:e0176218. 10.1371/journal.pone.0176218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med 1991;325:606–12. 10.1056/NEJM199108293250903 [DOI] [PubMed] [Google Scholar]
- 87.Halle T, Hair E, Buchinal M, et al. The running for successful outcomes: exploring the evidence for thresholds of school readiness. Washington DC: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, 2012. [Google Scholar]
- 88.WHO Multicentre Growth Reference Study Group . Who child growth standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76–85. 10.1111/j.1651-2227.2006.tb02378.x [DOI] [PubMed] [Google Scholar]
- 89.Pritchard VE, Bora S, Austin NC, et al. Identifying very preterm children at educational risk using a school readiness framework. Pediatrics 2014;134:e825–32. 10.1542/peds.2013-3865 [DOI] [PubMed] [Google Scholar]
- 90.Wechsler DW. Preschool & primary scale of intelligence, Australia and new zealand. 4th edn. Sydney, Australia: Pearson Clinical and Talent Assessment, 2014. [Google Scholar]
- 91.Sparrow SS, Cicchetti DV, Saulnier C. Vineland adaptive behavior scales. 3rd edn. Bloomington, MN: Pearson, 2016. [Google Scholar]
- 92.Sherman EMS, Brooks BL. Behavior rating inventory of executive function – preschool version (BRIEF-P): test review and clinical guidelines for use. Child Neuropsychology 2010;16:503–19. 10.1080/09297041003679344 [DOI] [Google Scholar]
- 93.Joshua N, Wilson C, Bendrups N. WIAT-III A&NZ examiner’s manual. Syndey, Australia: NCS Pearson, 2016. [Google Scholar]
- 94.Reynolds C, Kamphaus R. Behaviour assessment system for children – third edition manual. Circle Pines, MN: American Guidance Service, 2015. [Google Scholar]
- 95.Russell D, Rosenbaum P, Avery L, et al. Gross motor function measure (GMFM-66 and GMFM-88) user’s manual. Cambridge, UK: MacKeith press, 2002. [Google Scholar]
- 96.Ko J, Kim M. Reliability and responsiveness of the gross motor function measure-88 in children with cerebral palsy. Phys Ther 2013;93:393–400. 10.2522/ptj.20110374 [DOI] [PubMed] [Google Scholar]
- 97.Russell DJ, Avery LM, Rosenbaum PL, et al. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther 2000;80:873–85. [PubMed] [Google Scholar]
- 98.World Health Organisation . International classification of functioning, disability, and health. Geneva: World Health Organisation, 2001. [Google Scholar]
- 99.Wang HY, Yang YH. Evaluating the responsiveness of 2 versions of the gross motor function measure for children with cerebral palsy. Arch Phys Med Rehabil 2006;87:51–6. 10.1016/j.apmr.2005.08.117 [DOI] [PubMed] [Google Scholar]
- 100.Wright FV, Boschen K, Jutai J. Exploring the comparative responsiveness of a core set of outcome measures in a school-based conductive education programme. Child Care Health Dev 2005;31:291–302. 10.1111/j.1365-2214.2005.00511.x [DOI] [PubMed] [Google Scholar]
- 101.Folio M, Fewell R. Peabody developmental motor scales: examiner’s manual. 2nd edn. Austin, Texas: PRO-ED Inc, 2000. [Google Scholar]
- 102.Wang HH, Liao HF, Hsieh CL. Reliability, sensitivity to change, and responsiveness of the peabody developmental motor scales-second edition for children with cerebral palsy. Phys Ther 2006;86:1351–9. 10.2522/ptj.20050259 [DOI] [PubMed] [Google Scholar]
- 103.Krumlinde-sundholm L, Eliasson A. Development of the assisting hand assessment: a rasch-built measure intended for children with unilateral upper limb impairments. Scandinavian Journal of Occupational Therapy 2003;10:16–26. 10.1080/11038120310004529 [DOI] [Google Scholar]
- 104.Krumlinde-Sundholm L, Holmefur M, Kottorp A, et al. The assisting hand assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol 2007;49:259–64. 10.1111/j.1469-8749.2007.00259.x [DOI] [PubMed] [Google Scholar]
- 105.Holmefur M, Aarts P, Hoare B, et al. Test-Retest and alternate forms reliability of the assisting hand assessment. J Rehabil Med 2009;41:886–91. 10.2340/16501977-0448 [DOI] [PubMed] [Google Scholar]
- 106.Elvrum A-KG, Zethræus B-M, Vik T, et al. Development and validation of the both hands assessment for children with bilateral cerebral palsy. Phys Occup Ther Pediatr 2018;38:113–26. 10.1080/01942638.2017.1318431 [DOI] [PubMed] [Google Scholar]
- 107.Elvrum A-KG, Johansen GO, Vik T, et al. External validity of the both hands assessment for evaluating bimanual performance in children with bilateral cerebral palsy. Dev Med Child Neurol 2022;64:586–92. 10.1111/dmcn.15127 [DOI] [PubMed] [Google Scholar]
- 108.Burgess A, Boyd RN, Chatfield MD, et al. Hand function and self-care in children with cerebral palsy. Dev Med Child Neurol 2021;63:576–83. 10.1111/dmcn.14783 [DOI] [PubMed] [Google Scholar]
- 109.Haley SM. Pediatric evaluation of disability inventory (PEDI): development, standardization and administration manual. Boston, Massachusetts: Therapy Skill Builders, 1992. [Google Scholar]
- 110.Haley SM, Coster WJ, Dumas HM, et al. Accuracy and precision of the pediatric evaluation of disability inventory computer-adaptive tests (PEDI-CAT). Dev Med Child Neurol 2011;53:1100–6. 10.1111/j.1469-8749.2011.04107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shore BJ, Allar BG, Miller PE, et al. Measuring the reliability and construct validity of the pediatric evaluation of disability inventory-computer adaptive test (PEDI-CAT) in children with cerebral palsy. Arch Phys Med Rehabil 2019;100:45–51. 10.1016/j.apmr.2018.07.427 [DOI] [PubMed] [Google Scholar]
- 112.Berg M, Jahnsen R, Frøslie KF, et al. Reliability of the pediatric evaluation of disability inventory (PEDI). Phys Occup Ther Pediatr 2004;24:61–77. 10.1300/j006v24n03_05 [DOI] [PubMed] [Google Scholar]
- 113.Fragala-Pinkham MA, Miller PE, M Dumas H, et al. Development and validation of equations to link pediatric evaluation of disability inventory (PEDI) functional skills scores to PEDI-computer adaptive test scores for youth with cerebral palsy. Phys Occup Ther Pediatr 2020;40:106–20. 10.1080/01942638.2019.1628160 [DOI] [PubMed] [Google Scholar]
- 114.Zimmerman I, Steiner V, Pond E. Preschool language scales- fifth edition (PLS-5). San Antonio, Texas: Pearson, 2011. [Google Scholar]
- 115.Bell KL, Boyd RN, Walker JL, et al. The use of bioelectrical impedance analysis to estimate total body water in young children with cerebral palsy. Clin Nutr 2013;32:579–84. 10.1016/j.clnu.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 116.Bell KL, Davies PSW. Prediction of height from knee height in children with cerebral palsy and non-disabled children. Ann Hum Biol 2006;33:493–9. 10.1080/03014460600814028 [DOI] [PubMed] [Google Scholar]
- 117.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the united states: methods and development. Vital Health Stat 2000;2002:1–190. [PubMed] [Google Scholar]
- 118.Becker P, Carney LN, Corkins MR, et al. Consensus statement of the Academy of nutrition and dietetics/american Society for parenteral and enteral nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract 2015;30:147–61. 10.1177/0884533614557642 [DOI] [PubMed] [Google Scholar]
- 119.de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes 2010;5:458–60. 10.3109/17477161003615583 [DOI] [PubMed] [Google Scholar]
- 120.Burrows TL, Collins K, Watson J, et al. Validity of the Australian recommended food score as a diet quality index for pre-schoolers. Nutr J 2014;13:87. 10.1186/1475-2891-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collins CE, Burrows TL, Truby H, et al. Comparison of energy intake in toddlers assessed by food frequency questionnaire and total energy expenditure measured by the doubly labeled water method. J Acad Nutr Diet 2013;113:459–63. 10.1016/j.jand.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 122.Omand JA, Janus M, Maguire JL, et al. Nutritional risk in early childhood and parent-reported school concerns. Public Health Nutr 2021;24:6169–77. 10.1017/S1368980021001725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwarzenberg SJ, Georgieff MK, Daniels S, et al. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018;141:e20173716. 10.1542/peds.2017-3716 [DOI] [PubMed] [Google Scholar]
- 124.Benfer KA, Weir KA, Bell KL, et al. Validity and reproducibility of measures of oropharyngeal dysphagia in preschool children with cerebral palsy. Dev Med Child Neurol 2015;57:358–65. 10.1111/dmcn.12616 [DOI] [PubMed] [Google Scholar]
- 125.Suiter DM, Leder SB, Karas DE. The 3-ounce (90-cc) water swallow challenge: a screening test for children with suspected oropharyngeal dysphagia. Otolaryngol Head Neck Surg 2009;140:187–90. 10.1016/j.otohns.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 126.Angell ME, Bailey RL, Stoner JB. Family perceptions of facilitators and inhibitors of effective school-based dysphagia management. Lang Speech Hear Serv Sch 2008;39:214–26. 10.1044/0161-1461(2008/021 [DOI] [PubMed] [Google Scholar]
- 127.Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:S531–43. 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 128.World Health Organisation . International classification of functioning, disability, and health: children & youth version: ICF-CY. Geneva: World Health Organisation, 2007. [Google Scholar]
- 129.Trost SG, Fragala-Pinkham M, Lennon N, et al. Decision trees for detection of activity intensity in youth with cerebral palsy. Med Sci Sports Exerc 2016;48:958–66. 10.1249/MSS.0000000000000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keawutan P, Bell KL, Oftedal S, et al. Validation of accelerometer cut-points in children with cerebral palsy aged 4 to 5 years. Pediatr Phys Ther 2016;28:427–34. 10.1097/PEP.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 131.Goodlich BI, Armstrong EL, Horan SA, et al. Machine learning to quantify habitual physical activity in children with cerebral palsy. Dev Med Child Neurol 2020;62:1054–60. 10.1111/dmcn.14560 [DOI] [PubMed] [Google Scholar]
- 132.Ahmadi MN, O’Neil ME, Baque E, et al. Machine learning to quantify physical activity in children with cerebral palsy: comparison of group, group-personalized, and fully-personalized activity classification models. Sensors (Basel) 2020;20:3976. 10.3390/s20143976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raat H, Landgraf JM, Oostenbrink R, et al. Reliability and validity of the infant and toddler quality of life questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res 2007;16:445–60. 10.1007/s11136-006-9134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benfer KA, Weir KA, Bell KL, et al. Oropharyngeal dysphagia and cerebral palsy. Pediatrics 2017;140:e20170731. 10.1542/peds.2017-0731 [DOI] [PubMed] [Google Scholar]
- 135.Pagnozzi AM, Dowson N, Doecke J, et al. Identifying relevant biomarkers of brain injury from structural MRI: validation using automated approaches in children with unilateral cerebral palsy. PLoS One 2017;12:e0181605. 10.1371/journal.pone.0181605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boyd RN, Davies PS, Ziviani J, et al. PREDICT-CP: study protocol of implementation of comprehensive surveillance to PREDICT outcomes for school-aged children with cerebral palsy. BMJ Open 2017;7:e014950. 10.1136/bmjopen-2016-014950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kirch W. Cost-consequence analysiscost-consequence analysis. In: Kirch W, ed. Encyclopedia of Public Health. Dordrecht: Springer Netherlands, 2008: 168. [Google Scholar]
- 138.Mauskopf JA, Paul JE, Grant DM, et al. The role of cost-consequence analysis in healthcare decision-making. Pharmacoeconomics 1998;13:277–88. 10.2165/00019053-199813030-00002 [DOI] [PubMed] [Google Scholar]
- 139.Koita K, Long D, Hessler D, et al. Development and implementation of a pediatric adverse childhood experiences (aces) and other determinants of health questionnaire in the pediatric medical home: a pilot study. PLoS One 2018;13:e0208088. 10.1371/journal.pone.0208088 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068675supp001.pdf (45.3KB, pdf)
bmjopen-2022-068675supp002.pdf (166.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. In accordance with the National Health and Medical Research Council/Medical Research Future Fund (NHMRC/MRFF) statement on data sharing: 'NHMRC/MRFF encourages data sharing and providing access to data and other research outputs (metadata, analysis code, study protocols, study materials and other collected data) arising from NHMRC supported research', data will be made available to other researchers or funding bodies including the NHMRC as necessary for the purposes of meta-analysis/systematic review and/or confirmation of statistical results. These data will be made available at group-level. If individual-level data are required, a limited, codified dataset will be made available to reduce or eliminate the possibility of re-identification of the data. A description of the dataset (metadata) will be published so that it can be discovered and/or cited. Data will be shared directly with individuals or institutions that approach the custodians. Future use and sharing of data are addressed on the Parent Information Sheet. Identifiable data will not be available for future use unless by separate ethics application.