Abstract
Features of amphibian embryos that elucidated the genetics of vertebrate development enable study of the physics that shape morphogenesis and regulate development. Biophysical tools are revealing how genes control mechanical properties of the embryo. The same tools that describe and control mechanical properties are being turned to reveal how dynamic mechanical information and feedback regulate biological programs of development. In this review we outline efforts to explore the various roles of mechanical cues in guiding cilia biology, axonal pathfinding, goblet cell regeneration, epithelial-to-mesenchymal transitions in neural crest and mesenchymal-to-epithelial transitions in heart progenitors. These case studies, reveal the power of Xenopus experimental embryology to expose pathways integrating mechanical cues with programs of development, organogenesis, and regeneration.
Keywords: mechanosensors, mechanotransduction, force, modulus, strain, stress, tension, stiffness, elasticity, viscoelasticity, compliance
Introduction
During development, cells make critical decisions using biochemical and biomechanical cues from their microenvironment Biochemical cues are well documented but many novel roles of biomechanical cues are coming to light. In vitro studies show that biomechanical cues can direct cell differentiation, cell divison, and collective cellular behaviors [1,2]. Resurgent interest in the biomechanics on morphogenesis has resulted in the characterization of mechanical properties of embryoni tissues and the genetic and architectural contributions to those properties (see Box: Glossary for terminology). With these advances, the field of developmental mechanobiology is revealing how the dynamic and diverse microenvironments in the embryo contribute to developing tissues and organs.
Box 1. Glossary.
| Biomechanics | Mechanical processes that directly shape living organisms. Genes and environment are involved in directing material properties. |
| Compliance | The ability to deform under an applied force. |
| Compression | The object is under compression when it experiences a negative strain. |
| Elastic Modulus | Material property that defines the elastic behavior under an applied stress. |
| Force | An interaction with a magnitude and direction that changes the motion of an object or deforms it. |
| Mechanobiology | The feedbacks from mechanical processes that guide biology. |
| Tension | The object is under tension when it experiences a positive strain. |
| Stiffness | The resistance to deformation under an applied force. |
| Strain | A dimensionless term to describe the deformation of an object caused by the force applied. |
| Stress | The amount of force that is applied to a unit area. |
| Viscoelasticity | Viscoelastic is a combination of viscous behaviors and elastic behaviors. Elastic materials completely recover from an applied stress (smaller than yield stress) and viscous materials slowly deform under an applied stress but do not return to original configurations. |
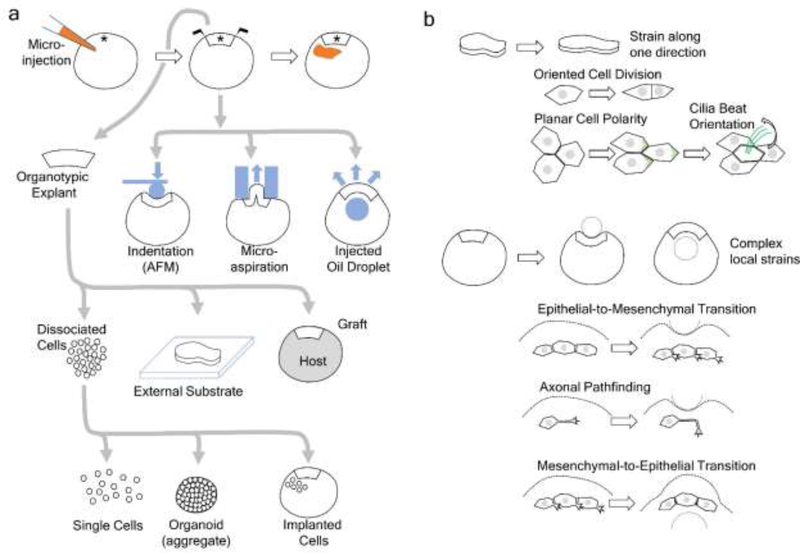
The Xenopus laevis embryo is a unique model system for studying the mechanobiology of development for the following reasons: 1) embryos are available in large numbers, have a well-annotated genome, and can be manipulated by state-of-the-art gain- and loss-of-function technologies (Figure 1a, first row), 2) embryos are ~1.2 mm in diameter and can be easily freed from stiff vitelline membranes, allowing access to pharmaceutical treatments, and 3) embryos and organ primordia can be studied in a range of mechanical contexts. Precisely controlled stress and strain can be applied to intact embryos (Figure 1a; second row), organotypic explants that preserve tissue-tissue interactions (Figure 1a; third row), and even single cells (Figure 1a; fourth row) where the native tissue context is eliminated or reintrodued, as in an aggregate or surrounding cell implants. Challenging cells, tissues, and whole embryos from the same stock material allows detailed multi-scale studies on the contribu ion of molecular, junctional, and tissue level organization to the mechanical properties of the embryo during development. In this review, we will first summarize the types of mechanical cues and how to detect and manipulate them in the tissue, and then introduce several studies using Xenopus embryos to reveal how mechanical cues regulate developmental processes.
Figure 1. Diverse approaches to characterize and measure biomechanical properties in Xenopus.
a) Gain- or loss-of-function reagents (orange) can be injected into embryos at 1-cell or other early cleavage stages, and target tissues can be identified from 32- or gastrula stage fate maps (*). The whole embryo can be subjected to biomechanical manipulations (blue, device; blue arrows indicate force) to quantify mechanical properties or to apply specific deformations. Organotypic explants that preserve tissues in their native context can be isolated microsurgically. These explants an cultured on external substrates, which allow precise control of the microenvironment, or grafted into a host embryo (grey) where the mechanical microenvironment has been altered. Cell-cell and tissue-tissue interactions can be disrupted by dissociating the explant into single cells, which can be studied as-is by conventional in vitro methods or re-aggregate into an organoid. Cells may also be implanted into whole embryos to observe their reintegration into a native or perturbed microenvironment. b) Tools developed for biomechanical testing can also be used to apply defined strains or deformations to investigate the role of those mechanical cues in guiding cell behaviors. Organotypic explants can be subjected to defined strains to evaluate the role of anisotropic strain on cell division. Tissue strain, anologous to those occurring during epiboly, can specify the planar cell polarity and beat orientation of ciliated cells in both the epidermis and left-right-organizer. Manipulations of whole embryos with indentation or injection of oil droplets can instruct pathfinding in axons and modulate phenotypic transitions between mesenchymal and epithelial cell types. See the main text for more details.
Cues in the mechanical microenvironment
In vitro studies on cultured cells have revealed a vast array of physical features that can be sensed by cells to regular their biology. We refer interested readers to several excellent reviews on this topis [2,3 ]. Physical features and mechanical properties are spatially and temporally patterned during development [1,4] [5]. Some features, such as stiffness, can be measured in vivo while others, such as nanotopology, lie beyond current methods. (Note: we briefly define several terms related to biomechanics and mechanobiology in the Box: Glossary). The case studies that are the focus of this review center on biomechanical material properties that can be measured directly.
How cells sense mechanical cues
Mechanosensors and mechanotransducers are cellular structures that allow cells to identify mechanical cues and to integrate responses to intracellular signaling pathways, respectively. Many types of mechanosensing systems have been identified in cultured cells and bacteria but only a few have been studied in embryos [4]. Studies on cell-matrix adhesion implicate mechanosensitive protein complexes in focal adhesions (FAs) where integrin receptors bind to the extracellular matrix [5]. Studies in Xenopus have identified a novel role for cadherin-11 in mediating adhesion between fibronectin and FAs and aiding in cell migration of neural crest cells [6,7]. In the developing embryo, cell-cell contacts are integral for influencing cell behavior and distributing forces during collective epithelial migration [5]. Epithelial cells are mechanically coupled by several types of junctions: gap, adherens, tight, and desmosomes. These junctions link to the cytoskeleton as well as ion channels and are likely to regulate cell junction remodeling and collective migration through mechanosensing conditions between neighboring cells [3]. Adherens junctions have been well charactered in many different animal models, and their roles in mechanosensing have been verified in Xenopus embryos by an optogenetic tool that induces contact between E-cadherin, and α-catenin [8]. Junctions mediate cell signaling through the actomyosin network and well-characterized mechanosensing proteins such as talin, vinculin, and myosin-II, and recent findings suggest a role for Anillin in mediating tensile forces through vinculin recruitment [9]. Cell-cell signaling can also occur through channel proteins such as Pannexin-1. Under mechanical stress, ATP released through Pannexin-1 activates purinergic receptors in the neighboring cells to regulate cell-cell tension [10].
Measurement and manipulation of mechanical cues in vivo
There have been many methods available, including sessile drop [11], laser ablation, strain mapping, and 3D stress mapping [12], but in this review we will focus on those used in the case studies discussed below, including: cantilever-based systems, microaspiration, and approaches to stretched or compressed organotypic explants.
Cantilever-based systems are some of the most common methods in measuring tissue mechanical properties. Both off-the-shelf and custom devices use cantilevers as force-transducers which are key for indenting or deforming small regions of exposed embryonic tissues (Figure 1a, second row). Contact forces and depth of indentation or strain with the tissue are recorded and can be used to calculate a modulus (see Box: Glossary). Commercial atomic force microscopy (AFM) systems are cantilever-based systems that were first adopted for in vitro studies of cell monolayers [13]. AFM can report mechanical properties from 1 to 5 μm of the surface. Additionally, custom cantilever-based systems can be used to measure forces produced by spreading explants, blastopore closure, and force convergent extension [14–16].
Microaspiration involves applying pressure to pull a tissue into a narrow channel (Figure 1a, second row). The modulus or compliance of a patch of cells on an embryo or aggregate can be calculated from the geometry of the channel, the pressure applied, and the distance the tissue moves into the channel [15]. In addition to being used to measure tissue stiffness non-destructively in different stages of embryos, aspiration can also apply defined strains to test the putative role of mechanical cues [15,17,18**,19**,20].
Compressive or tensile forces can be applied across a tissue, either within the intact embryo (e.g. Figure 1a, second row) or to explants. A uniaxial stress-relaxation has been extensively used to describe the elastic modulus of organotypic explants (first described in [21], most recently used in [22**]) (Figure 1b). Tensile testing, using various designs of tissue stretchers can also exert tension across explants ([23–25]). Explants can be either fully bonded to an elastic membrane (Figure 1a, third row) or suspended between two adherent mounts, and then responses are recorded as the tissue is stretched. The elastic modulus of the tissue can also be calculated combined with a cantilever system. Such devices have been used in studying the role of mechanical stress in cell division, convergent extension, and numerous other studies.
Strain can be introduced in a purely mechanical form through inert mineral oil injections (see [35]). A moderate level of strain (between 10 and 20 %) can be achieved in one tissue by injecting a droplet of inert immiscible fluid into an adjacent location (Figure 1a, second row). Additionally, droplet volume can be precisely controlled with standard nano-injetion sstems and can be tracked with fluorescent dye. Stress and compliance can be calculated exactly from image analysis techniques such as strain mapping [54] and biophysical measurements obtained through micro-aspiration or AFM.
Developmental processes regulated by mechanical cues: examples in Xenopus embryos
There have been rapid advances in the field of mechanobiology of development, thanks to the ability to microsurgically expose tissues to biophysical testing and manipulation. Below, we discuss several recent studies using Xenopus embryos to show how embryos and organotypic tissues are regulated by mechanical cues from the microenvironment (summarized in Figure 1b).
Axon guidance
Pathfinding of dendrites and axons is well known to be instructed by biochemical cues [26]; however, in vitro studies have shown that neurite growth can be affected by tension and mechanical properties of the substrate [27,28]. The observation that the central nervous system is mechanically heterogeneous [29,30] suggests that mechanical cues also play a role in neuronal guidance in vivo.
This hypothesis is later verified using Xenopus retinal ganglion cells (RGC) [31]. Neurite outgrowth of cultured RGCs was found to respond to substrate stiffness, which depends on the stretch-activated ion channel Piezo1. After microsurgically exposing the RGCs in the brain, substrate stiffness along the track of RGC axon growth was measured by an AFM. By altering brain stiffness and Piezo1 activity, the study showed that RGC axons sense a stiffness gradient in the brain and grow towards softer tissue [31]. In a follow-up study, to better understand changes of brain tissue stiffness over time, the authors mapped stiffness and correlated axon growth in vivo [32**] and discovered that the stiffness gradient in the brain is established just prior to axon turning and requires a round of cell proliferation. These two studies provided in vivo evidence of axon guidance mediated by dynamically regulated mechanical cues, highlighting the effectiveness of the Xenopus embryo as a model system to study mechanobiology during neuronal development.
Neural crest cell migration
Xenopus neural crest (NC) has been used to investigate the role of biomechanical cues in the initiation of epithelial-to-mesenchymal transition (EMT) and collective migration. While the molecular signals that pattern and guide NC cell migration are relatively well understood, the observation that these cells pause before initiating migration [33] suggests that they are waiting for additional cues from the microenvironment.
A recent stuy tested the substrate requirements for NC cell migration in vitro and then moved to identify mechanical changes in substrate stiffness in vivo [34**]. NC cells were found to wait for their mesoderm substrates to age and stiffen before they would initiate migration. Analysis of fibronectin ruled out cues from cell-ECM interactions; however, increasing mesoderm cell density and stiffness triggered NC migration and increased junctional protein expression. Mechanical perturbations of the embryos through ablation, myosin II inhibitors, and morpholinos were able to rule out the contribution of mesoderm contractility. Since mesoderm compaction relies on convergent extension of dorsal axial tissues, the authors perturbed the planar cell polarity (PCP) pathway to inhibit mesoderm stiffening, which could be restored through indentation via AFM. Furthermore, manipulation of the mechanosensors integrin, Vinculin, and Talin inhibited NC migration, suggesting essential roles of focal adhesions in sensing the mechanical properties of the adjacent mesoderm [34**].
Mesenchymal-to-epithelial transition (MET)
Heart progenitor cells (HPCs) begin collective migration as mesenchymal cells but end as epithelial cells. By tracking Xenopus mesenchymal HPCs and mapping the heart forming region using traction force microscopy, our recent work revealed a correlation between increased strain rates and the initiation of MET [35]. The ability of HPCs to sense stiffness of the adjacent endoderm was confirmed through fate-map targeted modulation of actomyosin contractility using constitutively active Rho or myosin II modulators. MET could be driven precociously or inhibited by modulating endoderm substrate stiffening. As with the previous cases, physically modulating local stiffness by microinjecting an oil droplet could also accelerate MET [35].
Organoids of stem cells are well known for their ability to initiate complex programs of morphogenesis and differentiation [36]. Our group recently discovered a role of biomechanical cues in driving MET and inducing goblet cell fates on the surface of Xenopus deep ectoderm organoids [19**]. Due to the rapid onset of MET in less than 5 hours, the Xenopus model allows quantitation of both tissue mechanics measured by microaspiration and cell behaviors recorded by live cell confocal microscopy. The nuclear localization of Yes-associated protein 1(YAP), a transcription factor correlated with mechanical changes, was found to coincide with cell contractility and apicobasal polarity. Like the study of HPCs, actomyosin contractility in ectoderm cells could be manipulated through both small molecules and gain-of-function mutant proteins to inhibit or drive precocious MET. Following MET, newly epithelialized cells undergo goblet cell differentiation, a step that was also sensitive to mechanical perturbation [19**]. In summary, our work established a role of mechanical cues in cardiogenesis and regeneration, but the identity of mechanosensors as well as the signaling pathways involved in triggering MET remains unresolved.
Ciliated cell differentiation
Directional flow driven by ciliated cells regulates diverse functions during embryonic development. As cilia are cell protrusions and generally considered a sensory organ of the cell, it comes as no surprise that cilia are suspected of sensing fluid flow, although the detailed mechanism remains to be determined [37]. Similar to mammals, Xenopus embryos have motile monocilia in the left-right organizer (LRO) and multiciliated cells in the skin [38,39]. To drive unidirectional fluid flow, the orientation of these cilia is based toward the posterior end of the cells [38,40]. The cilia polarity of multiciliated cell is established during gastrulation and refined by aligning themselves with the flow direction, forming a positive feedback loop [40]. The planar cell polarity (PCP) pathway is required for cilia polarization during gastrulation, but the mechanism remained obscure [41].
Following observation of strain patterns during early movements of gastrulation, a recent study hypothesized that cilia reorient in response to directional mechanical strains along the anteroposterior axis [17]. To test this hypothesis, the authors showed that microaspiration of embryos and organotypic explants could re-align cilia along the new direction of strain. In a follow-up study, the same group examined the role of early strain in the alignment of cilia in the LRO [18**]. The LRO is the organ that specifies left-right asymmetry and features long motile cilia at the center and short immotile cilia at the lateral region of the tissue [42]. Under directional mechanical strains, LRO cells responded similarly to ectodermal cells by polarizing the microtubule orientation and PCP protein distribution. Interestingly, mechanical strains not only instruct the direction of cilia beat but also regulate cilia length during differentiation in coordination with the transcription factor Foxjl [18**]. These studies highlight the significance of morphogenesis-induced mechanical strains as global cues to instruct cilia differentiation and polarity in ciliated tissue of different origin.
Orientation of cell division
Cell division can play a critical role in the early developmental processes, and mechanical cues are highly correlated with the orientation and timing of cell division [43–45] However the molecular pathways that integrate cell division with external mechanical cues are unclear. A recent study suggested that mechanical forces deform cell shapes, but are not directly sensed by the mitotic spindle to orient cell division, rather acting as a cue for mitosis [46**]. Organotypic animal cap explants were cultured in a custom-designed stretch apparatus and subjected to uniaxial 19.67 ± 1.91% strain. Cell shapes elongated and cell division was significantly aligned along the axis of stretch, and the authors found that the position of tricellular junctions (TCJs) predicted cell division orientation better than other morphometric features. Simulation with a vertex-based model [47] showed that local cell-level stress aligns more with the orientation of TCJs than with global tissue-level stress. This phenomenon was disturbed by manipulating C-cadherin activity, implying that C-cadherin may play a role in recruiting the spindle orientation protein LGN to the TCJs Moreover, cell division rate was reduced by Myosin II knockdown but partially rescued by stretch, suggesting that mechanical cues are required to trigger mitosis [46**]. Computational modeling side-by-side with biomechanical manipulations suggest a new perspective on the integration of mechanical cues with cadherins and TCJs during cell division; yet, the molecular pathways integrating mechanical cues with mitosis remain to be identified.
Conclusions and Future Perspectives
Rigorous mechanobiology studies in Xenopus test hypotheses suggested in mammalian systems regarding the regulation of stem cell specification [2], stem cell migration [2], early cell fate decision [36,48], and tissue patterning [49–51]. It is increasingly clear that mechanical cues in the microenvironment play pivotal roles in multiple morphogenetic and patterning events during embryogenesis.
Just as with genetics, mechanobiology can be investigated using the forward and reverse approaches: the former involves an unbiased screen to identify mechanical cues contributing to a certain process, while the latter is carried out by manipulating a mechanical property and looking for processes being affected. Xenopus embryos are highly suitable for both of the approaches. Organotypic explants from Xenopus embryos can be easily prepared and exposed to chemical and mechanical manipulations, allowing straightforward and detailed analyses similar to those obtained from stem cell cultures. Moreover, Xenopus embryos are more accessible to observation and manipulation compared to mammalian embryos, and therefore it is feasible and often very effective to verify hypotheses derived from in vitro studies on Xenopus embryos. In addition to the robust methods discussed above, new techniques allowing improved detection and finer spatiotemporal control of mechanical cues, such as the ferrofluid microdroplets and optogenetic tools [8,52,53] are being developed or adapted to use with Xenopus embryos, explants, and cells. The robust quantitative analysis of biomechanics and mechanobiology at multiple different scales during embryogenesis is unique to Xenopus and provides novel insights into the role of mechanical cues in guiding cell behaviors necessary for development and responsible for disease progression.
Highlights.
Mechanical cues can be manipulated indentation, microaspiration, and stretch.
Mechanical cues can pattern tissues and drive differentiation in many cell types.
Accessibility and ease of manipulation make Xenopus embryos ideal for mechanobiology.
Acknowledgements
We regret that we could not cite the full range of biomechanical and mechanobiological studies that leverage the many advantages of the Xenopus embryo. We acknowledge the many helpful discussions with current and former lab members - these debates have shaped our way of thinking and guide our future work. This work was supported by grants to LAD from the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (R01 HD044750). We dedicate this paper in the memory of Lev Beloussov, for his lifelong contributions to Xenopus mechanobiology.
Footnotes
The authors do not have any conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
** of outstanding interest
- 1.Petridou NI, Spiro Z, Heisenberg CP: Multiscale force sensing in development. Nat Cell Biol 2017, 19:581–588. [DOI] [PubMed] [Google Scholar]
- 2.Argentati C, Morena F, Tortorella I, Bazzucchi M, Porcellati S, Emiliani C, Martino S: Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinheiro D, Bellaiche Y: Mechanical Force-Driven Adherens Junction Remodeling and Epithelial Dynamics. Dev Cell 2018, 47:3–19. [DOI] [PubMed] [Google Scholar]
- 4.Miller CJ, Davidson LA: The interplay between cell signalling and mechanics in developmental processes. Nat Rev Genet 2013, 14:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barriga EH, Mayor R: Adjustable viscoelasticity allows for efficient collective cell migration. Semin Cell Dev Biol 2019, 93:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlici D: Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev 2009, 23:1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langhe RP, Gudzenko T, Bachmann M, Becker SF, Gonnermann C, Winter C, Abbruzzese G, Alfandari D, Kratzer MC, Franz CM, et al.: Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat Commun 2016, 7:10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ollech D, Pflasterer T, Shellard A, Zambarda C, Spatz JP, Marcq P, Mayor R, Wombacher R, Cavalcanti-Adam EA: An optochemic l tool for light-induced dissociation of adherens junctions to control mechanical coupling between cells. Nat Commun 2020, 11:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold TR, Shawky JH, Stephenson RE, Dinshaw KM, Higashi T, Huq F, Davidson LA, Miller AL: Anillin regulates epithelial cell mechanics by structuring the medial-apical actomyosin network.Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao L, Locovei S, Dahl G: Pannexin membrane channels are mechanosensitive conduits for ATP FEBS Lett 2004, 572:65–68. [DOI] [PubMed] [Google Scholar]
- 11.Kalantarian A, Ninomiya H, Saad SM, David R, Winklbauer R, Neumann AW: Axisymmetric drop shape analysis for estimating the surface tension of cell aggregets by centrifugation. Biophys J 2009, 96:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stooke – Vaughan GA, Davidson LA, Woolner S: Xenopus as a model for studies in mechanical stress and cell division. Genesis 2017, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoh JH, Schoenenberger CA: Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J Cell Sci 1994, 107 (Pt 5): 1105–1114. [DOI] [PubMed] [Google Scholar]
- 14.Hara Y, Nagayama K, Yamamoto TS, Matsumoto T, Suzuki M, Ueno N: Directional migration of leading-edge mesoderm generates physical forces: Implication in Xenopus notochord formation during gastrulation. Dev Biol 2013, 382:482–495. [DOI] [PubMed] [Google Scholar]
- 15.von Dassow M, Strother JA, Davidson LA: Surprisingly simple mechanical behavior of a complex embryonic tissue. PLoS One 2010, 5:e15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfister K, Shook DR, Chang C, Keller R, Skoglund P: Molecular model for force production and transmission during vertebrate gastrulation. Development 2016, 143:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien YH, Keller R, Kintner C, Shook DR: Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr Biol 2015, 25:2774–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chien YH, Srinivasan S, Keller R, Kintner C: Mechanical Strain Determines Cilia Length, Motility, and Planar Position in the Left-Right Organizer. Dev Cell 2018, 45:316–330 e314. ** This paper reports that directional mechanical strain during gastrulatioN coordinates with Foxj1 to regulate cilia differentiation in the left-right organizer, exemplifying a developmental process that integrates both the genetic and mechanical inputs.
- 19. Kim HY, Jackson TR, Stuckenholz C, Davidson LA: Tissue mechanics drives regeneration of a mucociliated epidermis on the surface or Xenopus embryonic aggregates. Nat Commun 2020, 11:665. ** By manipulating the compliance of Xenopus aggregates with microaspiration and loss-of function techniques, this study demonstrates that mesenchymal cells transition into mucociliated epithelium under stiff conditions, emphasizing the importance of mechanical cues on cell fate decision.
- 20.von Dassow M, Davidson LA: Natural variation in embryo mechanics: gastrulation in Xenopus laevis is highly robust to variation in tissue stiffness. Dev Dyn 2009, 238:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore SW: A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Transaction on Biomedical Engineering 1994, 41:45–50. [DOI] [PubMed] [Google Scholar]
- 22. Shawky J, Balakrishnan UL, Stuckenholz C, Davidson LA: Multiscale analysis of architecture, cell size and the cell cortex reveals cortical F-actin density and composition are major contributors to mechanical properties during convergent extension. Development 2018, 145. ** This study exemplifies the flexibility of Xenopus in biomechanics by investigating tissue architecture, cell cortex, and cell size in a multiscale level, using multiple techniques involving mechanical testing and measuring.
- 23.Nestor-Bergmann A, Johns E, Woolner S, Jensen OE: Mechanical characterization of disordered and anisotropic cellular monolayers. Physical Review E 2018, 97:052409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shook DR, Davidson L, Kasprowicz EM, Keller R: Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation. Elife 2018:126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiebe C, Brodland GW: Tensile properties of embryonic epithelia measured using a novel instrument. J Biomech 2005, 38:2087–2094. [DOI] [PubMed] [Google Scholar]
- 26.Stoeckli ET: Understanding axon guidance: are we nearly there yet? Development 2018, 145. [DOI] [PubMed] [Google Scholar]
- 27.Balgude AP, Yu X, Szymanski A, Bellamkonda RV: Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 2001, 22:1077–1084. [DOI] [PubMed] [Google Scholar]
- 28.Athamneh AI, Suter DM: Quantifying mechanical force in axonal growth and guidance Front Cell Neurosci 2015, 9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chighizola M, Dini T, Lenardi C, Milani P, Podesta A, Schulte C: Mechanotransduction in neuronal cell development and functioning. Biophys Rev 2019, 11:701–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkin BS, Azeloglu EU, Costa KD, Morrison B 3rd: Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma 2007, 24:812–822. [DOI] [PubMed] [Google Scholar]
- 31.Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, et al.: Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 2016, 19:1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson AJ, Pillai EK, Dimov IB, Foster SK, Holt CE, Franze K: Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. Elife 2019, 8. ** Using time-lapse in vivo atomic force microescopy, the authors demonstrate that axon growth in the Xenopus embryonic brain follw a sbstrate stiffness gradient generated by local cell proliferation. This is a great example of how mechanical cues are produced and used for axon pathfinding in vivo.
- 33.Kerosuo L, Bronner-Fraser M: What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin Cell Dev Biol 2012, 23:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barriga EH, Franze K, Charras G, Mayor R: Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 5’4:523–527. ** The authors show that Xenopus neural crest cell migration is initiated by mesoderm stiffening caused by convergent extension, providing another example of morphogenetic movement that produces mechanical cues to guide collective cell migration.
- 35.Jackson TR, Kim HY, Balakrishnan UL, Stuckenholz C, Davidson LA: Spatiotemporally Controlled Mechanical Cues Drive Progenitor Mesenchymal-to-Epithelial Transition Enabling Proper Heart Formation and Function. Curr Biol 2017, 27:1326-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim EJY, Korotkevich E, Hiiragi T: Coordination of Cell Polarity, Mechanics and Fate in Tissue Self-organization. Trends Cell Biol 2018, 28:541–550. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira RR, Fukui H, Chow R, Vilfan A, Vermot J: The cilium as a force sensor-myth versus reality. J Cell Sci 2019, 132. [DOI] [PubMed] [Google Scholar]
- 38.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M: Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol 2007, 17:60–66. [DOI] [PubMed] [Google Scholar]
- 39.Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C: A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development 1999, 126:4715–4728. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell B, Jacobs R, Li J, Chien S, Kintner C: A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 2007, 447:97–101. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C: The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol 2009, 19:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum M, Ott T: Animal left-right asymmetry. Curr Biol 2018, 28: R301–R304. [DOI] [PubMed] [Google Scholar]
- 43.Benham-Pyle BW, Pruitt BL, Nelson WJ: Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry.Science 2015, 348:1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fink J, Carpi N, Betz T, Betard A, Chebah M, Azioune A, Bornens M, Sykes C, Fetler L, Cuvelier D, et al.: External forces control mitotic spindle positioning. Nat Cell Biol 2011, 13:771–778. [DOI] [PubMed] [Google Scholar]
- 45.Gloerich M, Bianchini JM, Siemers KA, Cohen DJ, Nelson WJ: Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat Commun 2017, 8:13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nestor-Bergmann A, Stooke-Vaughan GA, Goddard GK, Starborg T, Jensen OE, Woolner S: Decoupling the Roles of Cell Shape and Mechanical Stress in Orienting and Cueing Epithelial Mitosis. Cell Rep 2019, 26:2088–2100 e2084. ** Combining tissue-stretch experiments with computational modeling, this paper demonstrates that cell shape and mechanical stress instruct cell division orientation and mitotic entry respectively exemplifying the complexity of mechanical cues in regulating cell division.
- 47.Chiou KK, Hufnagel L, Shraiman BI: Mechanical stress inference for two dimensional cell arrays. PLoS Comput Biol 2012, 8:e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maitre JL: Mechanics of blastocyst morphogenesis. Biol Cell 2017, 109:323–338. [DOI] [PubMed] [Google Scholar]
- 49.Aw WY, Heck BW, Joyce B, Devenport D: Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr Biol 2016, 26:2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM: Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 2017, 357:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller E, Fuchs E: Tissue patterning and cellular mechanics. J Cell Biol 2015, 211:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serwane F, Mongera A, Rowghanian P, Kealhofer DA, Lucio AA, Hockenbery ZM, Campas O: In vivo quantification of spatially varying mechanical properties in developing tissues. Nat Methods 2017, 14:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krueger D, Izquierdo E, Viswanathan R, Hartmann J, Pallares Cartes C, De Renzis S: Principles and applications of optogenetics in developmental biology. Development 2019, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y, Hazar M, Vijayraghavan DS, Song J, Jackson TR, Joshi SD, Messner WC, Davidson LA, LeDuc PR: Mechanochemical actuators of embryonic epithelial contractility. Proc Natl Acad Sci U S A 2014, 111:14366–14371. [DOI] [PMC free article] [PubMed] [Google Scholar]