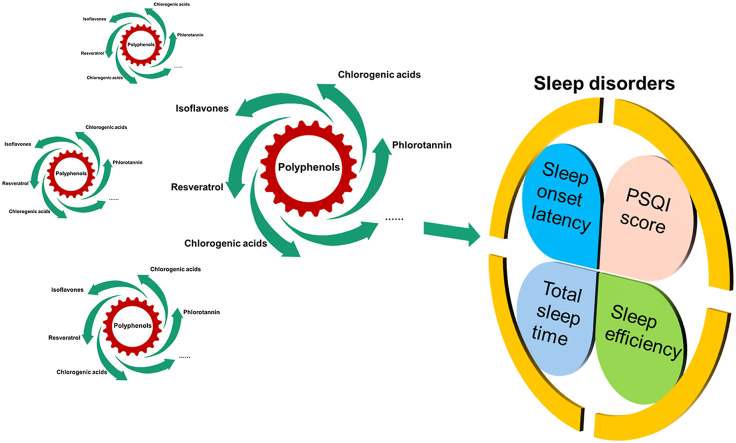
Abstract
Epidemiology studies have indicated that polyphenol consumption was more likely to have higher sleep quality, but some results remain controversial. A general overview of polyphenol-rich interventions on sleep disorders still lacks in the existing literature. Eligible randomized controlled trials (RCT's) literature retrieval was performed in six databases. Sleep efficiency, sleep onset latency, total sleep time, and PSQI were included as objective measures to compare the effects of placebo and polyphenols in patients with sleep disorders. Subgroup-analyses were performed based on treatment duration, geographic location, study design, and sample size. The mean differences (MD) with 95% confidence intervals (CI) were adopted for four continuous variable data of outcomes in pooled analysis. This study is registered on PROSPERO, number CRD42021271775. In total, 10 studies of 334 individuals were included. Pooled data demonstrated that administration of polyphenols decreases sleep onset latency (MD, −4.38 min; 95% CI, −6.66 to −2.11; P = 0.0002) and increases total sleep time (MD, 13.14 min; 95% CI, 7.54 to 18.74; P<0.00001), whereas they have no effect on sleep efficiency (MD, 1.04; 95% CI, −0.32 to 2.41; P = 0.13) and PSQI (MD, −2.17; 95% CI, −5.62 to 1.29; P = 0.22). Subgroup analyses further indicated that treatment duration, study design, and number of participants appeared to be responsible for the largest proportion of accountable heterogeneity. Polyphenols' potential importance is highlighted by these findings in treating sleep disorders. The development of large-scale, randomized, controlled trials is recommended to providing further evidence for therapeutic use of polyphenols in a variety of sleep difficulties.
Keywords: Polyphenol, Sleep disorders outcomes, Sleep onset latency, Total sleep time, Sleep efficiency, Pittsburgh sleep quality index (PSQI)
Graphical abstract
Highlights
-
•
Polyphenols exhibit health-promoting effects on preventing sleep disorders.
-
•
Polyphenols decrease sleep onset latency and increase total sleep time.
-
•
The beneficial effect of polyphenols depend on treatment duration.
1. Introduction
Sleep is an imperative component of health and wellness across the lifespan (Harrington et al., 2022; Svensson et al., 2021). However, at least 10% of western populations are clinically affected by significant sleep disorders, and 1/3 or more of the population suffer daily from excessive daytime sleepiness or a sleep disturbance (Riemann, 2019). More importantly, it is estimated that approximately 50% of older adults have difficulty sleeping (Crowley, 2011; Kim and Duffy, 2018; Saccomano, 2014). Ultimately, as the segment of the elderly population grows, so too will the prevalence of sleep disorders (Stallings et al., 2021). In recent years, the management of sleep disturbances, involving both non-pharmacological and pharmacological interventions (Sewell et al., 2021), continues to be a big issue in sleep medicine. For instance, cognitive behavioral treatment for insomnia is the proposed first-line therapy for insomnia owning to its minimal side effects and efficacy, while there are not sufficient disciplined therapists to treat individuals with chronic insomnia, let alone subclinical sleep troubles (Sweetman et al., 2021). A large number of patients in consequence try to obtain prescription hypnotics or over-the-counter sleep aids, which have more side effects, like cognitive impairments, headaches, nightmares, and daytime fatigue (Chaput et al., 2017). Despite this, older adults with a brand-new sleep disorder diagnosis have the highest rates of being prescribed medications (Pop et al., 2019). A survey about sleep found that almost a third of adults aged 65 and over had been using benzodiazepines every day for an average of 6–7 months (Preville et al., 2012). In fact, the American Geriatrics Society Beer 2015 criteria strongly recommend avoiding prescribed medications in older adults (Patel et al., 2018). It is thus necessary to consider both the effectiveness and safety of these drugs, as well as an unfeigned interest in testing upcoming remedies, that studies have been carried out on screening agents with efficacy, but fewer side effects, for the treatment of sleep disorders derived from natural sources.
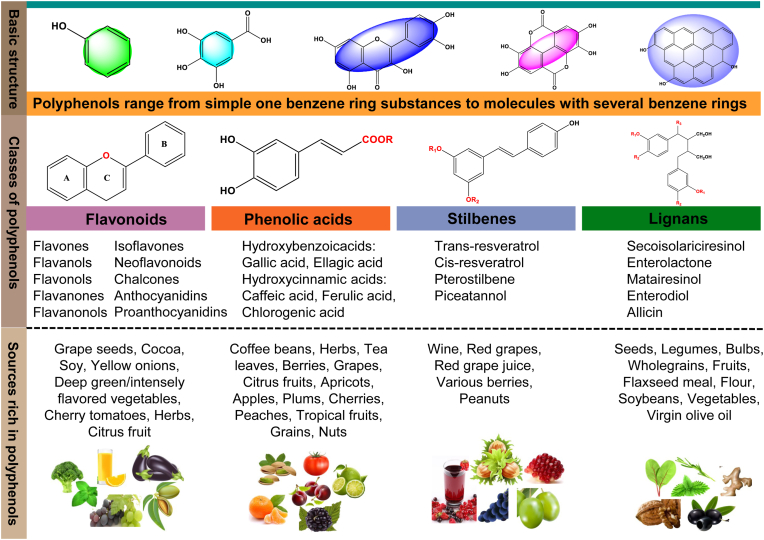
Polyphenols are secondary plant metabolites that are discovered in plant-derived herbal medicine, as well as foods like fruits and vegetables and nuts (Abdel-Moneim et al., 2020; Alara et al., 2021; Gebicki and Nauser, 2021), which are composed of a broad diversity of molecules sharing an analogous elementary polyphenolic structure (e.g. lots of hydroxyl groups of aromatic rings) as well as molecules with a single phenol ring, including phenolic alcohols and phenolic acids. And polyphenols' categorization is based either on the number of phenol rings or the type of structural elements joining the rings to one another (Fig. 1). Some of the primary categories are classified into phenolic acids, flavonoids, stilbenes, and lignans (D'Archivio et al., 2007). Numerous studies have demonstrated that polyphenols possess health-promoting properties for preventing several disturbances, most notably against aging and related neurodegenerative diseases (Ebrahimi and Schluesener, 2012; Rahimifard et al., 2017; Singh and Yadav, 2022). In recent years, the chief interest in polyphenols has been stimulated due to their anti-inflammatory, antioxidant, immunomodulatory and apoptotic properties (Li et al., 2021; Yahfoufi et al., 2018). Furthermore, findings from some clinical studies, such as randomized clinical trials (RCT's), which indicated that individuals who consumed polyphenols were more likely to have higher sleep quality (Hirose et al., 2016). By contrast, other studies in similar populations have reported weak effects on sleep disorders (Liu et al., 2014). Thus, it is crucial to understand whether polyphenols may have an effect on sleep disorders.
Fig. 1.
Polyphenols' basic skeletal structure, polyphenols classes, and sources.
While a number of reviews have demonstrated the beneficial effects of polyphenol-rich foods such as fruit and vegetables on sleep (Binks et al., 2020; Noorwali et al., 2019), a systematic review and meta-analysis have yet to be conducted to quantitatively synthesize data from RCT's that assess the direct effects of polyphenols. The purpose of this study was to use meta-analyses to evaluate the prevailing evidence-base for the function of administering pure polyphenols and quantitative polyphenol extracts in the treatment of sleep disorders.
2. Methods
2.1. Search strategy
This meta-analysis was guided accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). A systematical search was performed on PubMed, Embase, Cochrane Library, Science direct, MEDLINE, and Web of Science with no time limitations. Search terms included “polyphenols”, or “phenolic acid”, or “polyphenolic compounds”, or “flavonoids”, or “resveratrol”, or “EGCC”, or “catechin”, or “quercetin”, or “curcumin”, or “anthocyanin”, or “phloridzin” in combination with “sleep disorders”, or “sleep dysfunction”, or “sleep disturbance”, or “insomnia”. For instance, the search strategy in PubMed was listed in Table 1. Furthermore, the references of related researches, reviews, editorials, letters, and conference summaries were likewise searched. The research work was done independently and in duplicate.
Table 1.
The search strategy in PubMed of the relationship between polyphenols and sleep disorders.
| Search Terms | |
|---|---|
| 1 | Polyphenols [MeSH Terms] |
| 2 | Polyphenols OR polyphenol OR polyphenolic compounds OR phenolic acid OR flavonoids OR isoflavone OR flavanol OR phloridzin OR resveratrol OR EGCC OR catechin OR quercetin OR curcumin OR anthocyanin |
| 3 | 1 OR 2 |
| 4 | Sleep disorders [MeSH Terms] |
| 5 | Sleep disorder OR insomnia OR sleep dysfunction OR sleep disturbance OR sleep OR sleep duration OR DSPS OR ASPS OR parasomnia |
| 6 | 4 OR 5 |
| 7 | Randomized controlled trial [Publication Type] |
| 8 | Clinical trial OR phase III clinical trial OR controlled clinical trial OR randomized controlled trial OR randomized clinical trial |
| 9 | 7 OR 8 |
| 10 | 3 AND 6 AND 9 |
2.2. Inclusion and exclusion criteria
Study selection was based on the inclusion and exclusion criteria. The inclusion criteria included: (1) study design is double-blind RCT's (crossover or parallel); (2) populations were adult patients (>18 years old) with sleep disorders; (3) intervention comparisons of polyphenols versus placebo; (4) outcomes of nocturnal sleep (instead of daytime) include any or all of sleep parameters, including sleep onset latency, sleep efficiency, total sleep time, and Pittsburgh sleep quality index (PSQI), as measured by either subjective or objective approaches, such as sleep diary, actigraphy, polysomnogram or electroencephalogram. When the same outcome variables were measured using two different methods (namely sleep questionnaires and polysomnography), separate effect sizes for subjective and objective measures were calculated and included in the analyses. Sleep onset latency was the primary outcome. PSQI, total sleep time and sleep efficiency were secondary outcomes. There were no restrictions on sample size, trial duration, participant sex or comorbidity, although full text publications had to be published in the English language. And the exclusion criteria were: (1) non- RCT's, such as cases, letters, editorials, systemic reviews and expert opinions; (2) trials articles not peer-reviewed or published; (3) populations that included children (<18 years old); (4) studies that were repeatedly published or had no qualitative outcomes; (5) outcomes that analyzed daytime without the night sleep; (6) studies that included confounding factors (like the potential pharmacological confounding effects and others).
2.3. Data extraction and quality assessment
Both authors independently assessed the articles and extracted data based on participants, randomization, interventions (drug used, dose, and formulation), outcomes, duration, reasons for discontinuation and author information, while disagreements were resolved through discussion with a third author. In the case of duplicate publications and companion papers of a primary study, the authors maximized the yield of information by simultaneous evaluation of all available data. Clinical outcomes included continuous variables of sleep onset latency, total sleep time, PSQI, and sleep efficiency, expressed as mean and standard deviation (SD). Only if errors were performed as standard error (SE), they are converted to SD using the formula provided in the Cochrane Handbook (Cumpston et al., 2019). Meanwhile, the risk of bias of each trial was also assessed by both authors, independently, using random-effects models and Review Manager (RevMan) 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark), with any disagreement resolved by consensus.
2.4. Statistical analysis
The efficacy of polyphenols versus placebo was evaluated on the continuous outcomes of sleep onset latency (mean [SD]), total sleep time (mean [SD]), sleep efficiency (mean [SD]), and PSQI score (mean [SD]). Based on the knowledge of evidence-based medicine and the Cochrane Handbook for Systematic Review of Interventions (version 5.1) weight is determined by the mean [SD] and the size of all enrolled studies (Cumpston et al., 2019; Elbourne et al., 2002). Results were measured and reported in the same way across all included studies, so a weighted mean difference was used. The mean differences (MD) with 95% confidence intervals (CI) were adopted for these four continuous variable data of outcomes in pooled analysis. The Z-test determined an overall significance of treatment effect of polyphenols versus placebo with values of P < 0.05.
Heterogeneity was assessed using chi-squared test and I2 test in accordance with Cochrane collaboration's guidance for assessing heterogeneity in meta-analyses. P value < 0.1 for the chi-square was defined as indicating the presence of heterogeneity. The level of heterogeneity was classified as low heterogeneity (I2 = 0–25%), moderate heterogeneity (I2 = 25–50%), substantial heterogeneity (I2 = 50–75%), and considerable heterogeneity (I2 = 75–100%) (Higgins and Thompson, 2002). If the results have low heterogeneity or moderate heterogeneity (P < 0.1 and I2 < 50%), a fixed-effect model was used; otherwise, a random effect model was applied. To investigate the robustness of results, publication bias and sensitivity analysis were evaluated. All statistical analyses were performed using Review Manager (RevMan) 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark).
3. Results
3.1. Literature search and study characteristics
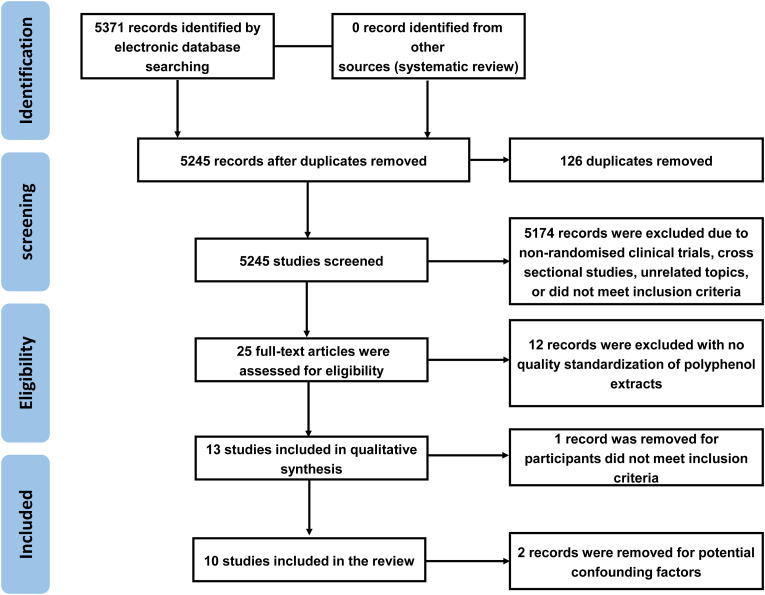
The initial search by two reviewers that are identified, 5371 records, of which 126 duplicate records were removed, and 5245 studies remained. Afterwards, the abstract and title of remained literature were scrutinized, and 5174 records were excluded because of non-randomized clinical trials, cross-sectional studies, unrelated topics, or did not meet inclusion criteria. Thereafter, 25 full-text papers were evaluated for eligibility and 12 records were eliminated with polyphenol extracts' no quality standardization of polyphenol extracts. One study limited the age range of participants to 18–40 years to reduce the likelihood of age-related sleep disorders that have been reported in older adults, so the study was dropped because the cohort did not meet the inclusion criteria. In addition, two studies were removed because of confounding factors (like the potential pharmacological confounding effects and others). Ultimately, 10 RCT's were involved in this meta-analysis (Hachul et al., 2011; Kuratsune et al., 2010; Losso et al., 2018; Park et al., 2017; Pennisi et al., 2017; Pigeon et al., 2010; Taherzadeh et al., 2020; Tubbs et al., 2021; Um et al., 2018; Umigai et al., 2018). The detailed research selection is showed in Fig. 2.
Fig. 2.
PRISMAS flow diagram of included/excluded studies for systematic review regarding the effect of polyphenol ingestion on sleep disorders.
A specific depiction of involved studies' characteristics was introduced in Table 2. Among the nine studies of 334 participants were conducted in Iran (1) (Taherzadeh et al., 2020), Japan (3) (Kuratsune et al., 2010; Park et al., 2017; Umigai et al., 2018), USA (3) (Losso et al., 2018; Pigeon et al., 2010; Tubbs et al., 2021), Brazil (1) (Hachul et al., 2011), Italy (1) (Pennisi et al., 2017), South Korea (1) (Um et al., 2018). All studies were randomized, double-blind. The five trials applied a parallel-group design, with five studies following a cross-over design. Almost all of studies were mixed gender, with exception of two studies (One trial included only postmenopausal women, the other included only men) (Hachul et al., 2011; Kuratsune et al., 2010). All the subjects were healthy except one study involving hepatitis C (Pennisi et al., 2017). The average age of participants in the involved trials ranged from 26.6 to 71.6 years, at the same time, subjects with either mild sleep complaint, primary insomnia or sleep disorders. The duration of researches ranged from five days to twelve months, with four of the studies lasting more than four weeks (Hachul et al., 2011; Pennisi et al., 2017; Taherzadeh et al., 2020; Tubbs et al., 2021).
Table 2.
Characteristic of included studies.
| Study | Setting [Country] | Study design | Study population | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|---|
| Kuratsune et al. (2010) | Kansai University of Welfare Sciences, Japan | A randomized, double-blind, placebo-controlled, crossover trial | 21 men with a mild sleep complaint, 25–59 years old | Received 7.5 mg crocetin daily for 2 weeks | Placebo | Sleep onset latency (min), Sleep efficiency |
| Pigeon et al. (2010) | University of Rochester Medical Center, USA | A randomized, double-blind, placebo-controlled, crossover trial | 15 older adults with chronic insomnia, men (8) and women (7), mean age 71.6 years old | Received 8 ounce tart cherry juice beverage (60 mg anthocyanins) for 2 weeks (2 times/day) | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
| Hachul et al. (2011) | Universidade Federal de São Paulo, São Paulo, Brazil | A randomized, double-blinded, placebo-controlled trial | 38 postmenopausal women with insomnia, 50–65 years old | Received 80 mg isoflavones daily for 4 months | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
| Pennisi et al. (2017) | University of Catania, Italy | A randomized, double-blinded, placebo-controlled trial | 60 participants with sleep disorders, men (35) and women (25), mean age 46.4–46.8 years old | Received 19.8 mg resveratrol daily for 12 months | Placebo | Pittsburgh Sleep Quality Index (PSQI) |
| Park et al. (2017) | University of Tsukuba, Japan | A randomized, double-blind, placebo-controlled, crossover trial | 9 participants with sleep disorders, men (4) and women (5), mean age 25.7 years old | Received 600 mg chlorogenic acids daily for 5 days | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
| Um et al., 2018 | Korea Food Research Institute, Seongnam, South, Korea | A randomized, double-blinded, placebo-controlled trial | 20 subjects (man and women) with difficulty falling asleep, mean age 28.6–32.4 years old | Received 500 mg phlorotannin daily for 1 week | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency, Pittsburgh Sleep Quality Index (PSQI) |
| Losso et al. (2018) | Louisiana State University Agricultural Center, USA | A randomized, double-blind, placebo-controlled, crossover trial | 15 participants with insomnia, men (3) and women (5), mean age 68 years old | Received 240 ml tart cherry juice beverage (451.56 μg/ml procyanidin B-2, 123.33 μg/ml cyanidin-3-O-glucosylrutinoside, 20.26 μg/ml cyanidin-3-O-rutinoside, and 3.51 μg/ml cyanidin-3-O-glucoside) for 2 weeks (2 times/day) | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
| Umigai et al. (2018) | Riken Vitamin Co., Ltd., Japan | A randomized, double-blind, placebo-controlled, crossover trial | 24 men (14) and postmenopausal women (10) with mild sleep complaints, 35–60 years old | Received 7.5 mg crocetin daily for 14 days | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
| Taherzadeh et al. (2020) | Mashhad University of Medical Sciences, Iran | A randomized, double-blinded, placebo-controlled trial | 50 participants (man and women) with primary chronic insomnia, 18–40 years old | Received polyphenol extracts (0.02 mg/ml crocin and 4 μg/ml isoquercitrin) for 8 weeks | Placebo | Pittsburgh Sleep Quality Index (PSQI) |
| Tubbs et al. (2021) | University of Arizona College of Medicine, USA | A randomized, double-blinded, placebo-controlled trial | 89 participants with sleep disorders, men (35) and women (54), 22–50 years old | Received 485 mg polyphenol botanical blend daily for 30 days | Placebo | Sleep onset latency (min), Total sleep time (min), Sleep efficiency |
All studies, with the exception of one research (Taherzadeh et al., 2020), used an orally administered containing polyphenols or polyphenol extracts, including crocetin, isoflavones, tart cherry juice beverage (containing a measured level of procyanidin), resveratrol, chlorogenic acids, phlorotannin, and polyphenol botanical blend for at any rate one of the active interventions assessed. Both studies were based on tart cherry juice beverages containing a measured level of procyanidin at doses of 240 ml (2 times/day) and 8 ounces (2 times/day), respectively (Losso et al., 2018; Pigeon et al., 2010). In addition, the dosage of crocetin was reported in two studies (Kuratsune et al., 2010; Umigai et al., 2018), with a day-to-day dose of 7.5 mg. Other active interventions were assessed in one study each, which the daily dose ranging from 19.8 mg to 600 mg.
The 10 included trials tested distinct outcomes. Eight studies reported the efficacy of polyphenols in sleep efficiency, total sleep time, and sleep onset latency (Hachul et al., 2011; Park et al., 2017; Pigeon et al., 2010; Tubbs et al., 2021; Um et al., 2018; Umigai et al., 2018), except for one study that did not involve the total sleep time (Kuratsune et al., 2010). Pittsburgh sleep quality index (PSQI) was evaluated in three trials (Pennisi et al., 2017; Taherzadeh et al., 2020; Um et al., 2018).
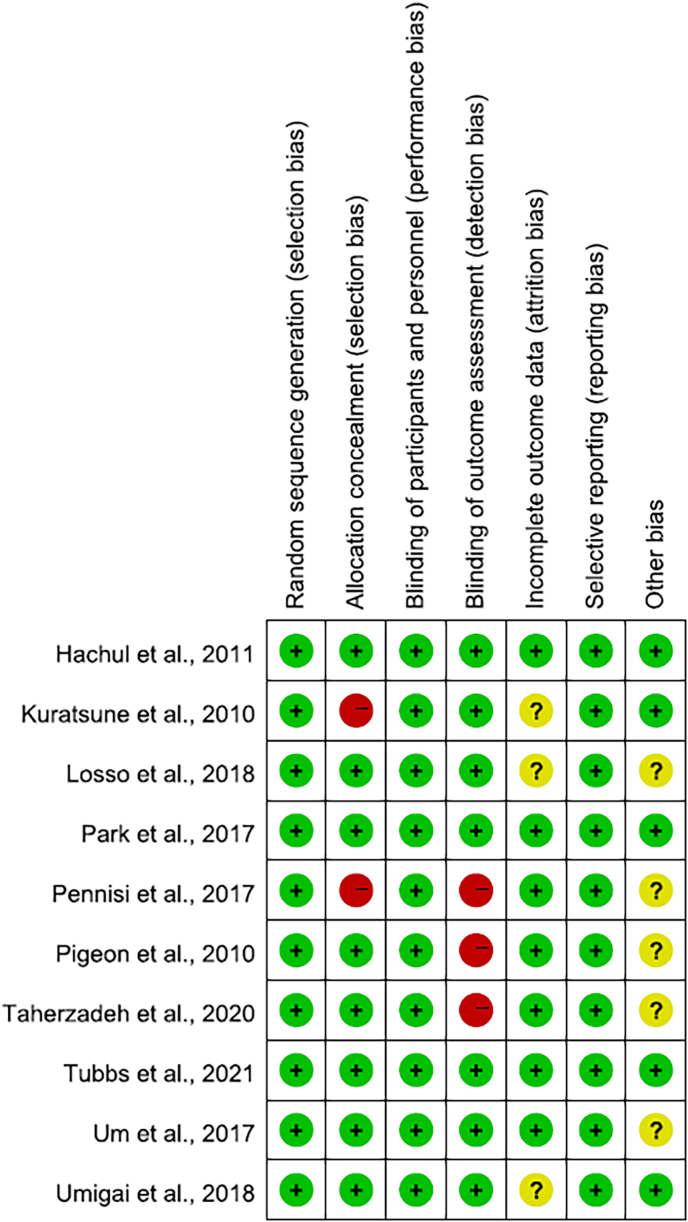
3.2. Risk of bias
With exception of two studies (Kuratsune et al., 2010; Pennisi et al., 2017), the majority of the seven bias parameters were described in sufficient detail in the methodology so that most of the involved studies possessed a low risk of bias (Fig. 3). In only three trials, several participants did not complete the final outcomes data (briefly explained in the studies), so the risk of bias in these three studies was generally unclear (Kuratsune et al., 2010; Losso et al., 2018; Umigai et al., 2018). In the three studies, questionnaires or sleep diaries were only used as screening tools or results, and there was no objective measurement of sleep such as polysomnography, resulting in a high risk of detection bias (Pennisi et al., 2017; Pigeon et al., 2010; Taherzadeh et al., 2020). Additionally, five researches were dictated to get unclear risk of other biases, including potential sponsorship bias, sampling bias, or bias due to problems not covered elsewhere exists (Losso et al., 2018; Pennisi et al., 2017; Pigeon et al., 2010; Taherzadeh et al., 2020; Um et al., 2018).
Fig. 3.
Risk of bias summary. The figure showed review authors' judgements about each risk of bias item for each included study.
3.3. Sleep onset latency
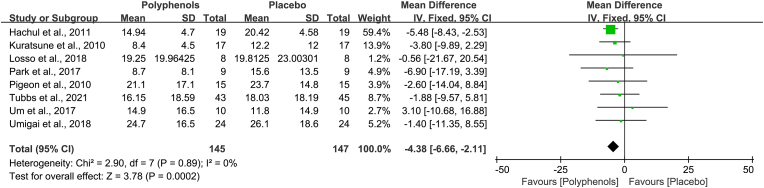
Sleep onset latency is the prime result in this meta-analysis. Eight researches with available data were encompassed in this meta-analysis as the other studies did not report quantitative data of sleep onset latency in polyphenols and placebo groups. There was low heterogeneity throughout the involved studies (Chi2 = 2.90, I2 = 0%, P = 0.89), and the fixed-effects model was applied. Pooled analysis of eight studies showed that administration of polyphenols significantly decreased sleep onset latency, versus placebo (MD, −4.38 min; 95% CI, −6.66 to −2.11). The overall estimated effect of polyphenols was significant (Z = 3.78, P = 0.0002) (Fig. 4).
Fig. 4.
Effects of polyphenols on sleep onset latency. This forest plot demonstrated that administration of polyphenols decreases sleep onset latency.
3.4. Total sleep time
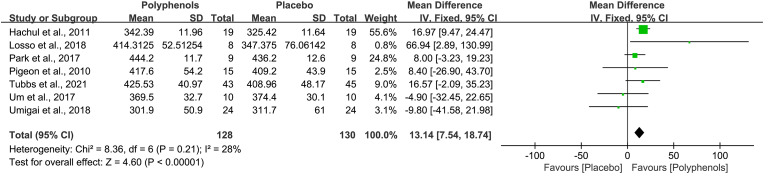
Total sleep time is the secondary result in this meta-analysis. Seven studies presented data suitable for the efficacy of polyphenols in total sleep time. Analysis of these studies suggested that administration of polyphenols increased total sleep time (MD, 13.14 min; 95% CI, 7.54 to 18.74), and the substantial heterogeneity was present (Chi2 = 8.36, I2 = 28%, P = 0.21). The overall calculated effect of polyphenols was significant (Z = 4.60, P<0.00001) (Fig. 5).
Fig. 5.
Effects of polyphenols on total sleep time. This forest plot demonstrated that administration of polyphenols increases total sleep time.
3.5. Sleep efficiency
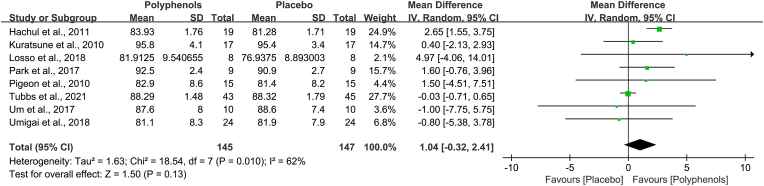
Sleep efficiency is also the secondary outcome in this meta-analysis. Adequate data on sleep efficiency were recorded in eight trials. Nevertheless, there were no significant differences between polyphenols and placebo in sleep efficiency (MD, 1.04; 95% CI, −0.32 to 2.41), and the Z value of polyphenols was 1.50 (P = 0.13). Meanwhile, the substantial heterogeneity was present across the seven studies (Chi2 = 18.54, I2 = 62%, P = 0.01, Fig. 6).
Fig. 6.
Effects of polyphenols on sleep efficiency. This forest plot revealed that administration of polyphenols has no significant effects on sleep efficiency.
3.6. PSQI score
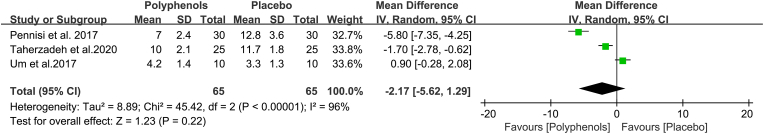
Likewise, PSQI score is the secondary outcome in this meta-analysis. Three studies were assessed by meta-analysis to assess the action of polyphenols on sleep quality, and results revealed marginal evidence for effects of polyphenols compared with the placebo group in improving sleep quality using the PSQI score (MD, −2.17; 95% CI, −5.62 to 1.29). Simultaneously, the overall calculated effect of polyphenols was not statistically significant (Z = 1.23, P = 0.22) (Fig. 7). Nevertheless, at the same time, there was considerable heterogeneity among studies (Chi2 = 45.42, I2 = 96%, P < 0.00001). Therefore, subgroup analyses were conducted to further examine the determinants of the heterogeneity.
Fig. 7.
Effects of polyphenols on sleep quality. This forest plot revealed that administration of polyphenols has no significant effects on PSQI score.
3.7. Subgroup analysis
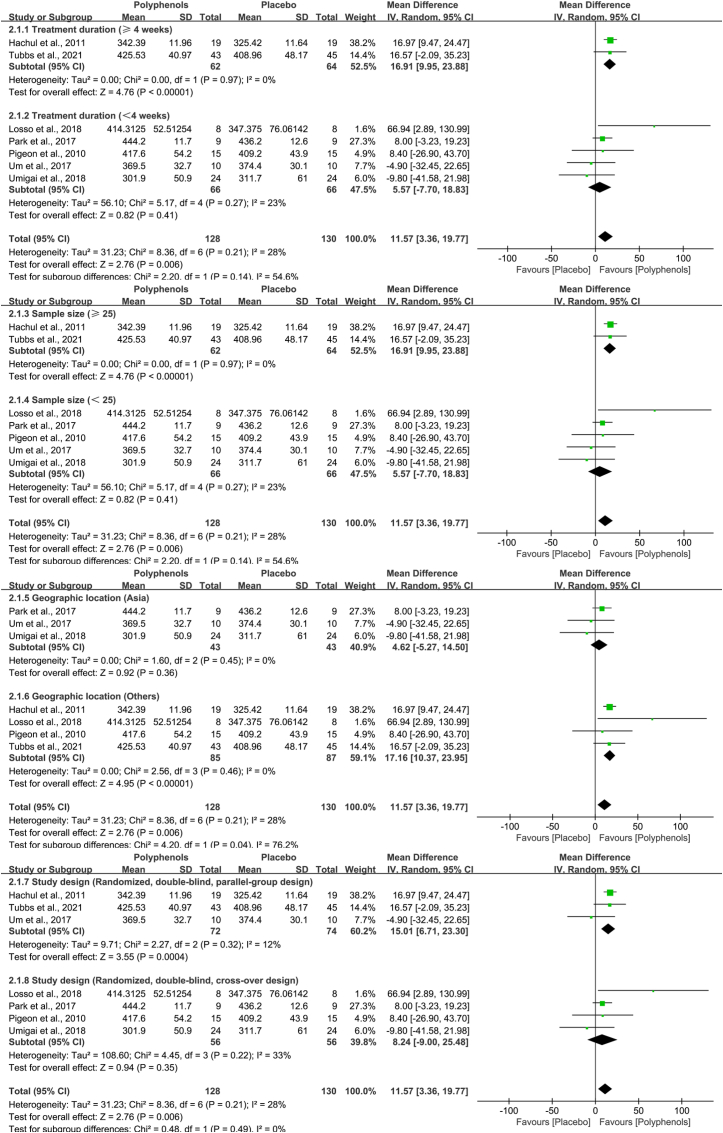
Subgroup analysis was performed to inquire into heterogeneity's sources and to handle our review's secondary objectives. The category of participants in the subgroup, which contributed the most weight in the random-effects model, involved man, menopausal women, and patients with chronic hepatitis, produced data too sparse to be able to statistically infer heterogeneity. Thus, it was stratified the included studies based on treatment duration, location of study (settings), the type of study design, and number of participants (Fig. 8, Table 3). A subgroup of treatment duration analyses provided results that participants received polyphenols treatment for less than four weeks (Chi2 = 5.17, I2 = 23%, P = 0.27) compared with those who did not (Chi2 = 0.00, I2 = 0.0%, P = 0.97), yielded substantial heterogeneity. The results of total sample sizes in subgroups revealed that there was low heterogeneity when the number of participants was greater than 25 (Chi2 = 0.00, I2 = 0.0%, P = 0.97), while there was considerable heterogeneity when the number of participants was less than 25 (Chi2 = 5.17, I2 = 23%, P = 0.27). Analogously, the type of study design is that crossover trial produced substantial heterogeneity (Chi2 = 4.45, I2 = 33%, P = 0.22), whereas parallel controlled trial produced low heterogeneity (Chi2 = 2.27, I2 = 12%, P = 0.32). However, the location of study (settings) in those subgroups generated low heterogeneity (Chi2 = 1.60, I2 = 0.0%, P = 0.45; and Chi2 = 2.56, I2 = 0.0%, P = 0.46, respectively). Therefore, treatment duration, study design, and number of participants appeared to be responsible for the largest proportion of accountable heterogeneity.
Fig. 8.
Subgroup analysis of the association between the source of heterogeneity and four factors: treatment duration, number of participants, geographic location, and study design.
Table 3.
Pooled estimates for polyphenols on total sleep time in subgroups of trials.
| Group | Subgroups | Number | Chi2 | P-Value | I2 (%) |
|---|---|---|---|---|---|
| All | 7 | 10.92 | 0.09 | 45% | |
| Treatment duration | ≥4 weeks | 2 | 0.00 | 0.97 | 0.0% |
| <4 weeks | 5 | 5.17 | 0.27 | 23% | |
| Sample size | ≥25 | 2 | 0.00 | 0.97 | 0.0% |
| <25 | 5 | 5.17 | 0.27 | 23% | |
| Geographic location | Asia | 3 | 1.60 | 0.45 | 0.0% |
| Others | 4 | 2.56 | 0.46 | 0.0% | |
| Study design | Parallel trial | 3 | 2.27 | 0.32 | 12% |
| Crossover trial | 4 | 4.45 | 0.22 | 33% |
3.8. Sensitivity analysis
To establish the robustness of the outcomes, the sensitivity analysis was performed by omitting a class of study at a time. The estimate effects of 95% CI ranged from −2.78 (−6.35, −0.79) to −4.62 (−7.00, −2.24), and P < 0.05 was conferred by all values. The sensitivity analyses revealed that dismissing any single research from the whole sample had little considerably affect in the estimate effect, implying that our outcomes were robust.
3.9. Publication bias
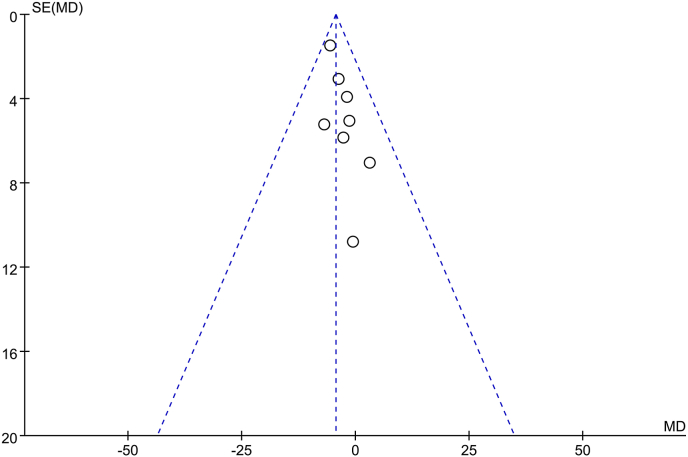
In order to investigate whether the studies involved in our meta-analysis is a representative sample of all the research in this field, we examined the publication bias. Since symmetrical distributions of studies were on both sides of the average, visual inspection of the funnel plot (Fig. 9) and the performance of the Egger's linear regression test demonstrated that there was no publication bias.
Fig. 9.
Publication bias. Symmetrical funnel plot indicated minimal publication bias.
4. Discussion
Of late, with numerous countries reporting an unusual incidence of sleep dysfunction in the elderly, sleep disorders appear to be an important public health affair that requires suitable management and precise diagnosis (Folmer et al., 2020; Siegel, 2022). So far, there is no efficacious therapy for sleep disturbances, which is due to the hangover impact and poor half-life of the existing pharmacotherapies (Wilkerson and McRae-Clark, 2021), and the complex non-pharmacotherapies like relaxation treatment and cognitive therapy, leading to poor compliance of patients (Sweetman et al., 2021). The development of dietary supplements for sleep disorders has get a precedence for scientific study in clinical psychiatry and sleep medicine (Zhao et al., 2020), but few dietary supplements have won as much scientific attention as polyphenols. A plethora of animal studies have illustrated considerable reductions in sleep disorders-induced oxidative stress injury and inflammation with polyphenols-rich administration in recent years (Wang et al., 2020), and in fact several RCT's have been attempting to inquire into polyphenols' action on sleep disorders (Tubbs et al., 2021). In general, the level of evidence that may make polyphenols easier as a potential therapeutic candidate for sleep disorders was considered to be low.
To the best of the authors’ knowledge, this is the first systematic review and set of meta-analyses to research the action of polyphenols ingestion versus placebo on sleep disorders based on sleep quality parameters. These meta-analyses' results proved that there is a substantial favorable effect of polyphenols administration on sleep quality parameters such as total sleep time and sleep onset latency, but no significant effect on sleep efficiency and PSQI score. Though the resulting data showed large amounts of variability, subgroup analysis suggested that which was partially occupied for by known reasons for heterogeneity, including the treatment duration, study design, and number of participants. Nevertheless, the sensitivity analyses revealed that getting rid of any single research from the intact sample had little considerably influence in the estimate effect, indicating that our outcomes were robust. Plenty of data has reported a more eminent incidence of sleep disorders in older adults (Miner et al., 2022), however, our study included not only older adults but also younger adults. In addition, previous systematic reviews on herbal medicines for sleep disorders all suggested that witness was not adequate to gain a determined conclusion (Leach and Page, 2015; Ni et al., 2015), which means that the exact role of the active ingredients in these herbs on sleep disorders remains to be studied. Moreover, systematic reviews provided promising findings regarding the usefulness of polyphenols intervention as an inexpensive approach for enhancing brain health (Ammar et al., 2020). Thus, the inclusion criteria were narrowed by our work to studies in which polyphenols was the exclusive intervention on sleep disorders.
This meta-analysis was guided under the guidance of PRISMA (Page et al., 2021). The search strategy that was utilized in our review was rigorous and the inclusion standards were spacious so all related publications from a broad variety of sources were included from 1950 to 2022. Entered researches were analyzed by two authors independently applying unitive criteria and disagreement was resolved by means of consultation with a third author. 10 RCT's that investigated the evidence for polyphenols' efficacy for sleep disorders' management was identified by the literature's systematic search. To the best of our knowledge, this is the maiden review, that has attempted directly to compare the outcomes of total sleep time, sleep onset latency, sleep efficiency, and PSQI score interventions with pure polyphenols, all of which are internationally accepted indexes of sleep evaluation and especially the previous three are non-subjective sleep parameters. Indeed, these four characteristic sleep quality parameters were the most common measures of sleep disorders in the included trials (Li et al., 2019). In the analyses for the separate categories of outcomes, polyphenols simplify reaching more estimable sleep for these patients through reducing the sleep onset latency, or by increasing total sleep time, while effect of polyphenols on sleep efficiency and PSQI score of patients with sleep dysfunctions was insignificant. Furthermore, all polyphenols were administered as oral mono-preparations in all but one of the studies using intranasal dosage form (Taherzadeh et al., 2020), and included studies used different types of polyphenols (pure polyphenols and standard quantified extracts) to evaluate the therapeutic effects on sleep disorders. Particularly, the majority of trails (n = 6) evaluated the efficacy of isoflavones, resveratrol, chlorogenic acids, phlorotannin, polyphenol extracts (0.02 mg/ml crocin and 4 μg/ml isoquercitrin), and polyphenol botanical blend in one study each. Both studies were based on tart cherry juice beverages containing a measured level of procyanidin. Additionally, two of the included studies suggested that crocetin supplementation (7.5 mg/day for two weeks) conduces to sleep maintenance, causing ameliorated subjective sleep quality (Kuratsune et al., 2010; Umigai et al., 2018). The pooled analysis of the acute and chronic administrations (treatment duration more than four weeks) of polyphenols supplementation suggests a beneficial effect on the majority of assessed sleep quality compared to placebo condition. On the whole, it could be seen from these data that polyphenols improve sleep quality with regard to sleep onset latency and total sleep time, which lends support to polyphenols as an expected manner to sleep disorders.
Although meta-analyses emphasized the positive aspect, the large level of heterogeneousness that was noted across the individual studies was not unforeseen, especially while regarding the data as whole, ever since multiple sorts of interventions (flavonoids, phenolic acids, stilbenes or lignans containing polyphenol) were included, as well as participants with different types of sleep disorders. Results of subgroup analysis showed that treatment duration, study design, and number of participants seem to take responsibility for the largest portion of accountable heterogeneity, in addition to location of study (settings), which have anteriorly been referred to dominating sources of heterogeneity (Thompson, 1994). Of the 10 trials included in this review, the intervention doses of polyphenols ranged from 7.5 mg to 600 mg daily and the period of the intervention extended from 5 days to 12 months. Interestingly, significant effects were observed for the administration 485 mg/day polyphenol botanical blend for 30 days in improving sleep quality (Tubbs et al., 2021). A period of five-day chlorogenic acids consumption (600 mg/day) significantly shortened sleep latency and did not adversely affect sleep quality (Park et al., 2017). However, no significant changes in sleep efficiency, sleep onset latency, and total sleep time after two weeks of 7.5 mg/day crocetin treatment, except subjective sleep quality (Umigai et al., 2018).
Definitely, it ought to be noticed that there are possible matters with a doses of polyphenol supplementation. Generally, clinical studies have reported that several polyphenols are safe and effective at doses of 250 mg (Panahande et al., 2019; Shi et al., 2017). In fact, there are explicit advantages to supplementation with pure polyphenols and more complex extracts in isolation, since it is purged and yielded at an exact concentration per dose (Blumberg et al., 2015). Whole extracts can diverge in individual components' relative content and optimum dosage will alter rely on types of polyphenols and extraction process (Bohn et al., 2015). Meanwhile, the dosage form is one of the key factors leading to the dose size, like that intranasal dosage form can avoid hepatic first-pass metabolism and gastrointestinal tract and utilize the advantages of low doses (Taherzadeh et al., 2020). In addition, to identify an efficacious polyphenol dosage, a more explicit comprehension of the accurate mechanisms of action must also be garnered (Carey et al., 2021). Polyphenols have conventionally been considered as antioxidants with poor bioavailability, and in high doses they can scavenge excess free radicals produced in the body or cells (Di Meo et al., 2020). However, it is worth mentioning that the understanding of the polyphenols' operation has changed markedly in recent years (Gardener et al., 2021). Recent study has ascribed the capability of polyphenols to have an impact on sleep disturbances to alterations in cell that signals tension response pathways, which induces upregulation of the yield of endogenous antioxidant enzymes like superoxide dismutase (Scuto et al., 2021). Importantly, polyphenols' ability to up-regulate the nuclear factor erythroid 2-related factor 2/electrophiles via the reaction element pathway that interacts with Keap1 when exposed to excess free radicals (Saha et al., 2020). In fact, the potential mechanisms of polyphenols on sleep disorders remain unclear and there is no consensus, such as possibly modulating the histaminergic arousal system, or improving the influence of the neurotransmitter gamma-aminobutyric acid (GABA) at the GABAA receptor (Yoon and Cho, 2018). Therefore, polyphenol dosages' aforementioned studies made specific investigations into optimum dosage and polyphenol composition inconceivable in the current review.
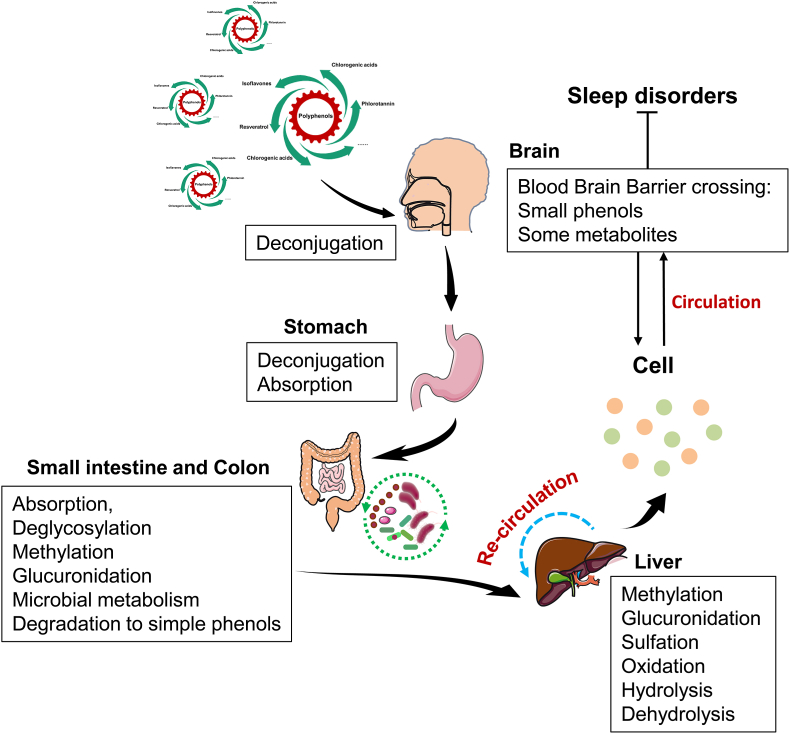
One of the crucial reasons for this heterogeneity is the variable treatment duration. The duration of studies ranged from five days to twelve months, with four of the studies lasting more than four weeks, and no side effects were reported. On the one hand, it is considered that polyphenols like conventional vitamin antioxidants, for example, vitamin C and E (Lalkovicova and Danielisova, 2016), can be used in the long term. On account of the antioxidant action, nonetheless, associated with polyphenol ingestion being lately ascribed to upregulation of the human body's endogenous antioxidant response system in preference to the straightforward basal scavenging capability as is the case with traditional nutritional antioxidants (Yahfoufi et al., 2018), it appears to be believable that protracted ingestion of polyphenols in sleep disorders may produce varying results. On the other hand, studies have shown that the bioavailability of some polyphenols is low, with less than 5% of ingested phenolic compounds reaching the bloodstream, while the majority of the remaining 95% is fermented by the gut microflora (Clifford, 2004). Furthermore, polyphenol pharmacokinetic researches have revealed that peak plasma polyphenols in the initial 1–2 h post ingestion and also have reported elevated concentrations for up to 12 h post-acute ingestion (Mertens-Talcott et al., 2008; Zhong et al., 2017). Among them, compounds containing stilbene structures as one of the polyphenols, such as study has emphasized that though resveratrol's oral absorption is comparatively high, the bioavailability in the blood stream was low (Chimento et al., 2019). Thus, it appears entirely appropriate to extend the polyphenol ingestion cycle in studies on improving sleep quality (Fig. 10). On the contrary, the same few polyphenols, like pterostilbene, that show similar effects to resveratrol while showing better bioavailability (Peng et al., 2018). Moreover, the polyphenol's intranasal administration can result in bioavailability and nicer absorption owing to bypassing blood-brain barrier and the delivery of the drug straightly to the brain (Laffleur and Bauer, 2021), so such polyphenol supplements may be taken for a shorter period.
Fig. 10.
Digestion and absorption of polyphenols in the human body.
Other factors contributing to this heterogeneity is the lack of an adequate samples, and almost half of the included study design are not parallel controlled trials, which are the potential limitations of this work. And this analysis shares the general limitations of any meta-analysis. Though so many databases were searched, the number of embraced references in this systematic review and meta-analysis was limited. Thus, the relatively few studies limited further subgroup analysis to explore the potential confounders. Meanwhile, the selection of trials with polyphenol postscripts restrains considerations on the health benefits that are derived from a polyphenol-enriched naturally diet, where dissimilar components' interactive impacts may become visible. Similarly, the ingestion of individual polyphenols may not yield the equivalent advantages that are detected in epidemiological or experimental researches. Besides, data that deriving from studies on consumption of polyphenolic compounds, where polyphenol ingestion is higher than general, could not be naturalistic in terms of interaction with drugs or other dietary components. While polyphenols are used in drug formulations, they ought to be viewed as nutraceuticals, whose safety must likewise be handled, peculiarly suppose their biological activity and bioavailability are increased.
5. Conclusion
In conclusion, the results of the meta-analysis and systematic review indicated that polyphenols might be an efficacious adjuvant therapy to decrease odds of sleep disorders. Furthermore, the beneficial effect of polyphenols was dependent on the treatment duration. Nevertheless, the contemporary study process for the development of bioactive polyphenols for application within the sleep medicine part is for the most part in its infancy. There is an unambiguous necessity for further experimental data to identify whether prolonged polyphenol ingestion may have adverse health effects in humans like first-line medications for sleep disorders.
CRediT authorship contribution statement
Wenjun Wang: Investigation, Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft, Visualization, and, Writing – review & editing. Tianlong Liu: Investigation, Data curation, Formal analysis, Writing – original draft. Yi Ding: Writing – review & editing, Software, Data curation, Visualization. Yi Zhang: Conceptualization, Methodology, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 82204761and 82274313), and the National Key Research and Development Program of China (No. 2017YFC1703904).
Handling Editor: Dr. Quancai Sun
Contributor Information
Yi Ding, Email: dingyi1324@163.com.
Yi Zhang, Email: zhangyi0238@163.com.
Data availability
Data will be made available on request.
References
- Abdel-Moneim A.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020;104:1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Alara O.R., Abdurahman N.H., Ukaegbu C.I. Extraction of phenolic compounds: a review. Curr Res Food Sci. 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A., Trabelsi K., Boukhris O., Bouaziz B., Muller P., J M.G., Bott N.T., Muller N., Chtourou H., Driss T., Hokelmann A. Effects of polyphenol-rich interventions on cognition and brain health in healthy young and middle-aged adults: systematic review and meta-analysis. J. Clin. Med. 2020;9 doi: 10.3390/jcm9051598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binks H., G E.V., Gupta C., Irwin C., Khalesi S. Effects of diet on sleep: a narrative review. Nutrients. 2020;12 doi: 10.3390/nu12040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg J.B., Vita J.A., Chen C.Y. Concord grape juice polyphenols and cardiovascular risk factors: dose-response relationships. Nutrients. 2015;7:10032–10052. doi: 10.3390/nu7125519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T., McDougall G.J., Alegria A., Alminger M., Arrigoni E., Aura A.M., Brito C., Cilla A., El S.N., Karakaya S., Martinez-Cuesta M.C., Santos C.N. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites--a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015;59:1307–1323. doi: 10.1002/mnfr.201400745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C.C., Lucey A., Doyle L. Flavonoid containing polyphenol consumption and recovery from exercise-induced muscle damage: a systematic review and meta-analysis. Sports Med. 2021;51:1293–1316. doi: 10.1007/s40279-021-01440-x. [DOI] [PubMed] [Google Scholar]
- Chaput J.P., Wong S.L., Michaud I. Duration and quality of sleep among Canadians aged 18 to 79. Health Rep. 2017;28:28–33. [PubMed] [Google Scholar]
- Chimento A., De Amicis F., Sirianni R., Sinicropi M.S., Puoci F., Casaburi I., Saturnino C., Pezzi V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004;70:1103–1114. doi: 10.1055/s-2004-835835. [DOI] [PubMed] [Google Scholar]
- Crowley K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Archivio M., Filesi C., Di Benedetto R., Gargiulo R., Giovannini C., Masella R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- Di Meo F., Valentino A., Petillo O., Peluso G., Filosa S., Crispi S. Bioactive polyphenols and neuromodulation: molecular mechanisms in neurodegeneration. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi A., Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res. Rev. 2012;11:329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Elbourne D.R., Altman D.G., Higgins J.P., Curtin F., Worthington H.V., Vail A. Meta-analyses involving cross-over trials: methodological issues. Int. J. Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Folmer R.L., Smith C.J., Boudreau E.A., Hickok A.W., Totten A.M., Kaul B., Stepnowsky C.J., Whooley M.A., Sarmiento K.F. Prevalence and management of sleep disorders in the veterans health administration. Sleep Med. Rev. 2020;54 doi: 10.1016/j.smrv.2020.101358. [DOI] [PubMed] [Google Scholar]
- Gardener S.L., Rainey-Smith S.R., Weinborn M., Bondonno C.P., Martins R.N. Intake of products containing anthocyanins, flavanols, and flavanones, and cognitive function: a narrative review. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.640381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicki J.M., Nauser T. Fast antioxidant reaction of polyphenols and their metabolites. Antioxidants. 2021;10 doi: 10.3390/antiox10081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachul H., Brandao L.C., D'Almeida V., Bittencourt L.R., Baracat E.C., Tufik S. Isoflavones decrease insomnia in postmenopause. Menopause. 2011;18:178–184. doi: 10.1097/gme.0b013e3181ecf9b9. [DOI] [PubMed] [Google Scholar]
- Harrington Y.A., Parisi J.M., Duan D., Rojo-Wissar D.M., Holingue C., Spira A.P. Sex hormones, sleep, and memory: interrelationships across the adult female lifespan. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.800278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hirose A., Terauchi M., Akiyoshi M., Owa Y., Kato K., Kubota T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: a randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016;293:609–615. doi: 10.1007/s00404-015-3849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Duffy J.F. Circadian rhythm sleep-wake disorders in older adults. Sleep Med Clin. 2018;13:39–50. doi: 10.1016/j.jsmc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Kuratsune H., Umigai N., Takeno R., Kajimoto Y., Nakano T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: a pilot study. Phytomedicine. 2010;17:840–843. doi: 10.1016/j.phymed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Laffleur F., Bauer B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021;607 doi: 10.1016/j.ijpharm.2021.120994. [DOI] [PubMed] [Google Scholar]
- Lalkovicova M., Danielisova V. Neuroprotection and antioxidants. Neural Regen Res. 2016;11:865–874. doi: 10.4103/1673-5374.184447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach M.J., Page A.T. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med. Rev. 2015;24:1–12. doi: 10.1016/j.smrv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Li H.Y., Gan R.Y., Shang A., Mao Q.Q., Sun Q.C., Wu D.T., Geng F., He X.Q., Li H.B. Plant-based foods and their bioactive compounds on fatty liver disease: effects, mechanisms, and clinical application. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/6621644. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Jiang S., Han M., Yang Z., Lv J., Deng C., Reiter R.J., Yang Y. Exogenous melatonin as a treatment for secondary sleep disorders: a systematic review and meta-analysis. Front. Neuroendocrinol. 2019;52:22–28. doi: 10.1016/j.yfrne.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Liu Z.M., Ho S.C., Woo J., Chen Y.M., Wong C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause. 2014;21:653–660. doi: 10.1097/GME.0000000000000102. [DOI] [PubMed] [Google Scholar]
- Losso J.N., Finley J.W., Karki N., Liu A.G., Prudente A., Tipton R., Yu Y., Greenway F.L. Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Therapeut. 2018;25:e194–e201. doi: 10.1097/MJT.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens-Talcott S.U., Rios J., Jilma-Stohlawetz P., Pacheco-Palencia L.A., Meibohm B., Talcott S.T., Derendorf H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008;56:7796–7802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- Miner B., Stone K.L., Zeitzer J.M., Han L., Doyle M., Blackwell T., Gill T.M., Redeker N.S., Hajduk A., Yaggi H.K. Self-reported and actigraphic short sleep duration in older adults. J. Clin. Sleep Med. 2022;18:403–413. doi: 10.5664/jcsm.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Shergis J.L., Guo X., Zhang A.L., Li Y., Lu C., Xue C.C. Updated clinical evidence of Chinese herbal medicine for insomnia: a systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2015;16:1462–1481. doi: 10.1016/j.sleep.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Noorwali E., Hardie L., Cade J. Bridging the reciprocal gap between sleep and fruit and vegetable consumption: a review of the evidence, potential mechanisms, implications, and directions for future work. Nutrients. 2019;11 doi: 10.3390/nu11061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hrobjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahande S.B., Maghbooli Z., Hossein-Nezhad A., Qorbani M., Moeini-Nodeh S., Haghi-Aminjan H., Hosseini S. Effects of French maritime pine bark extract (Oligopin®) supplementation on bone remodeling markers in postmenopausal osteopenic women: a randomized clinical trial. Phytother Res. 2019;33:1233–1240. doi: 10.1002/ptr.6320. [DOI] [PubMed] [Google Scholar]
- Park I., Ochiai R., Ogata H., Kayaba M., Hari S., Hibi M., Katsuragi Y., Satoh M., Tokuyama K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: a randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017;117:979–984. doi: 10.1017/S0007114517000587. [DOI] [PubMed] [Google Scholar]
- Patel R., Zhu L., Sohal D., Lenkova E., Koshki N., Woelfel J., Ranson C., Valle-Oseguera C.S., Rogan E.L. Use of 2015 beers criteria medications by older medicare beneficiaries. Consult. Pharm. 2018;33:48–54. doi: 10.4140/TCP.n.2018.48. [DOI] [PubMed] [Google Scholar]
- Peng R.M., Lin G.R., Ting Y., Hu J.Y. Oral delivery system enhanced the bioavailability of stilbenes: resveratrol and pterostilbene. Biofactors. 2018;44:5–15. doi: 10.1002/biof.1405. [DOI] [PubMed] [Google Scholar]
- Pennisi M., Bertino G., Gagliano C., Malaguarnera M., Bella R., Borzi A.M., Madeddu R., Drago F., Malaguarnera G. Resveratrol in hepatitis C patients treated with pegylated-interferon-alpha-2b and ribavirin reduces sleep disturbance. Nutrients. 2017;9 doi: 10.3390/nu9080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon W.R., Carr M., Gorman C., Perlis M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J. Med. Food. 2010;13:579–583. doi: 10.1089/jmf.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop P., Bronskill S.E., Piggott K.L., Stall N.M., Savage R.D., Visentin J.D., McCarthy L.M., Giannakes V., Wu W., Gruneir A., Gatley J.M., Rochon P.A. Management of sleep disorders in community-dwelling older women and men at the time of diagnosis. J. Am. Geriatr. Soc. 2019;67:2094–2101. doi: 10.1111/jgs.16038. [DOI] [PubMed] [Google Scholar]
- Preville M., Bosse C., Vasiliadis H.M., Voyer P., Laurier C., Berbiche D., Perodeau G., Grenier S., Beland S.G., Dionne P.A., Gentil L., Moride Y. Correlates of potentially inappropriate prescriptions of benzodiazepines among older adults: results from the ESA study. Can. J. Aging. 2012;31:313–322. doi: 10.1017/S0714980812000232. [DOI] [PubMed] [Google Scholar]
- Rahimifard M., Maqbool F., Moeini-Nodeh S., Niaz K., Abdollahi M., Braidy N., Nabavi S.M., Nabavi S.F. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017;36:11–19. doi: 10.1016/j.arr.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Riemann D. Epidemiology of sleep disorders, sleep deprivation, dreaming and spindles in sleep. J. Sleep Res. 2019;28 doi: 10.1111/jsr.12822. [DOI] [PubMed] [Google Scholar]
- Saccomano S.J. Sleep disorders in older adults. J. Gerontol. Nurs. 2014;40:38–45. doi: 10.3928/00989134-20131029-06. [DOI] [PubMed] [Google Scholar]
- Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25 doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuto M., Trovato Salinaro A., Caligiuri I., Ontario M.L., Greco V., Sciuto N., Crea R., Calabrese E.J., Rizzolio F., Canzonieri V., Calabrese V. Redox modulation of vitagenes via plant polyphenols and vitamin D: novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021;199 doi: 10.1016/j.mad.2021.111551. [DOI] [PubMed] [Google Scholar]
- Sewell K.R., Erickson K.I., Rainey-Smith S.R., Peiffer J.J., Sohrabi H.R., Brown B.M. Relationships between physical activity, sleep and cognitive function: a narrative review. Neurosci. Biobehav. Rev. 2021;130:369–378. doi: 10.1016/j.neubiorev.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Shi G., Hua M., Xu Q., Ren T. Resveratrol improves treatment outcome and laboratory parameters in patients with Takayasu arteritis: a randomized double-blind and placebo-controlled trial. Immunobiology. 2017;222:164–168. doi: 10.1016/j.imbio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Siegel J.M. Sleep function: an evolutionary perspective. Lancet Neurol. 2022;21:937–946. doi: 10.1016/S1474-4422(22)00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Yadav S.S. A review on health benefits of phenolics derived from dietary spices. Curr Res Food Sci. 2022;5:1508–1523. doi: 10.1016/j.crfs.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings D.T., Lach H.W., Lorenz R.A. Applied nursing research : ANR; 2021. Circadian Rhythm and Quality of Life in Older Adults. [DOI] [PubMed] [Google Scholar]
- Svensson T., Saito E., Svensson A.K., Melander O., Orho-Melander M., Mimura M., Rahman S., Sawada N., Koh W.P., Shu X.O., Tsuji I., Kanemura S., Park S.K., Nagata C., Tsugane S., Cai H., Yuan J.M., Matsuyama S., Sugawara Y., Wada K., Yoo K.Y., Chia K.S., Boffetta P., Ahsan H., Zheng W., Kang D., Potter J.D., Inoue M. Association of sleep duration with all- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman A., Putland S., Lack L., McEvoy R.D., Adams R., Grunstein R., Stocks N., Kaambwa B., Van Ryswyk E., Gordon C., Vakulin A., Lovato N. The effect of cognitive behavioural therapy for insomnia on sedative-hypnotic use: a narrative review. Sleep Med. Rev. 2021;56 doi: 10.1016/j.smrv.2020.101404. [DOI] [PubMed] [Google Scholar]
- Taherzadeh Z., Khaluyan H., Iranshahy M., Rezaeitalab F., Eshaghi Ghalibaf M.H., Javadi B. Evaluation of sedative effects of an intranasal dosage form containing saffron, lettuce seeds and sweet violet in primary chronic insomnia: a randomized, double-dummy, double-blind placebo controlled clinical trial. J. Ethnopharmacol. 2020;262 doi: 10.1016/j.jep.2020.113116. [DOI] [PubMed] [Google Scholar]
- Thompson S.G. Why sources of heterogeneity in meta-analysis should be investigated. BMJ (Clinical research ed.) 1994;309:1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs A.S., Kennedy K.E.R., Alfonso-Miller P., Wills C.C.A., Grandner M.A. A randomized, double-blind, placebo-controlled trial of a polyphenol botanical blend on sleep and daytime functioning. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph18063044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um M.Y., Kim J.Y., Han J.K., Kim J., Yang H., Yoon M., Kim J., Kang S.W., Cho S. Phlorotannin supplement decreases wake after sleep onset in adults with self-reported sleep disturbance: a randomized, controlled, double-blind clinical and polysomnographic study. Phytother Res. 2018;32:698–704. doi: 10.1002/ptr.6019. [DOI] [PubMed] [Google Scholar]
- Umigai N., Takeda R., Mori A. Effect of crocetin on quality of sleep: a randomized, double-blind, placebo-controlled, crossover study. Compl. Ther. Med. 2018;41:47–51. doi: 10.1016/j.ctim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Wang W., Yang L., Liu T., Ma Y., Huang S., He M., Wang J., Wen A., Ding Y. Corilagin ameliorates sleep deprivation-induced memory impairments by inhibiting NOX2 and activating Nrf2. Brain Res. Bull. 2020;160:141–149. doi: 10.1016/j.brainresbull.2020.03.010. [DOI] [PubMed] [Google Scholar]
- Wilkerson A.K., McRae-Clark A.L. A review of sleep disturbance in adults prescribed medications for opioid use disorder: potential treatment targets for a highly prevalent, chronic problem. Sleep Med. 2021;84:142–153. doi: 10.1016/j.sleep.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10 doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M., Cho S. Triphlorethol A, a dietary polyphenol from seaweed, decreases sleep latency and increases non-rapid eye movement sleep in mice. Mar. Drugs. 2018;16 doi: 10.3390/md16050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Tuo H., Wang S., Zhao L. The effects of dietary nutrition on sleep and sleep disorders. Mediat. Inflamm. 2020 doi: 10.1155/2020/3142874. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Sandhu A., Edirisinghe I., Burton-Freeman B. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.