Abstract
Using relatively primitive tools in the 1920s, Otto Warburg demonstrated that tumor cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not. High rates of glucose uptake, fueling glycolysis, are now used clinically to identify cancer cells. However, the Warburg effect does not account for the metabolic diversity that has been observed amongst cancer cells nor the influences that might direct such diversity. Modern tools have shown that the oncogenes, variable hypoxia levels, and the utilization of different carbon sources affect tumor evolution. These influences may produce metabolic symbiosis, in which lactate from a hypoxic, glycolytic tumor cell population fuels ATP production in the oxygenated region of a tumor. Lactate, once considered a waste product of glycolysis, is an important metabolite for oxidative phosphorylation in many tissues. While much is known about how muscle and the brain use lactate in oxidative phosphorylation, the contribution of lactate in tumor bioenergetics is less defined. A refocused perspective of cancer metabolism that recognizes metabolic diversity within a tumor offers novel therapeutic targets by which cancer cells may be starved from their fuel sources, and thereby become more sensitive to traditional cancer treatments.
Keywords: glycolysis, lactate, metabolism, metabolic symbiosis, mitochondria, Warburg
THE WARBURG EFFECT: DIRECTING DECADES OF THOUGHT ON CANCER METABOLISM
Using a new invention created in his laboratory, the manometer, Professor Otto Warburg compared the oxygen consumption of normal or tumor tissue slices and he identified a shift in the energy production ratio between fermentation and respiration, stating, “cancer cells can obtain approximately the same amount of energy from fermentation as from respiration, whereas in the normal body cells obtain much more energy from respiration than from fermentation [1].” Without direct evidence, Warburg claimed that the respiratory machinery of cancer cells, mitochondria, must be damaged, thereby causing an increased reliance upon fermentation. In Warburg’s perspective, cancer arose in two phases. First, the respiratory system was damaged irreversibly. The second phase took place as injured cells struggled to obtain sufficient energy from fermentation. Cells that survived this metabolic transition were eventually detected as cancer [1].
While the Warburg effect has been widely embraced [2-4], it has been misinterpreted from its original meaning during the past decade. The Warburg effect is often referred to as aerobic glycolysis, without mention of the respiratory defect that Warburg suspected to cause this phenomenon [5,6]. Another misrepresentation of the Warburg effect attributes increased glycolysis to hypoxia alone [7]. As reviewed by Zu and Guppy [8], a simplified view of the Warburg effect has misguided opportunities to refine our understanding of cancer metabolism.
The role of oxidative phosphorylation in cancer metabolism has been under appreciated, despite Warburg’s acknowledgement that this process was occurring in cancer cells [1]. The emphasis upon cancer cell’s increased glycolytic rate has established a myth that cancer cells subsist upon glucose alone. Lactate in the tumor microenvironment has been overlooked as a potential fuel, and instead identified as an acidic promoter of metastasis [9,10]. Yet Jain and coworkers have shown in vivo that tumor cells with defective glycolytic ability acidify their environment at a rate similar to that of the parental tumor cell line with functional glycolysis, suggesting that lactate production is not the predominant source of tumor acidification [11]. Increased expression of proton pumps, such as the vacuolar-ATPase, at the cell membrane are significant contributors to extracellular acidification in tumors [12,13]. A limited perspective of lactate metabolism ignores its normal physiologic role that cancer cells may also utilize. Lactate catabolism occurs in normal tissues, such as myocardium [14,15] and the brain [16], where astrocytes perform glycolysis despite sufficient oxygenation and feed lactate to neurons [17]. The role of lactate and other metabolites in the tumor microenvironment must be reevaluated.
A resurgence in the Warburg effect has also been fueled by the findings that many human tumors harbor deleterious mitochondrial DNA mutations (mtDNA) [18-20], and mutations in oncogenes and tumor suppressor genes affect metabolism [21-23]. Many of these mtDNA mutations cause nonsynonymous changes, which could affect the function of one of the 13 polypeptides encoded by this genome. This mitochondrial alteration reinforces Warburg’s early contention that the mitochondrial function in tumor cells was compromised. Viewing cancer metabolism through a strict Warburg lens, however, gives poor resolution to a complex process. The Warburg effect established a single “abnormal” metabolic phenotype of elevated glycolysis for all cancer cells. In the 1950s, an early detractor of the Warburg effect, Sidney Weinhouse insisted that knowledge of normal cellular metabolism was insufficient, thereby suggesting that understanding of cancer metabolism was similarly incomplete [24]. Warburg’s perspective also neglects the tumor microenvironment as a determinant of cancer metabolism, and numerous experiments have been performed in conditions that do not replicate faithfully the influence of metabolite concentrations, stromal cells, or hypoxia upon tumors [8]. It is clear that cancer cells demonstrate the Warburg effect of elevated glycolysis [25], however, the goal of this review is to emphasize that cancer metabolism is more complicated than can be explained by a single metabolic phenotype. Recent investigations are refining our understanding of the metabolic traits of cancer cells, and simultaneously offering novel strategies to impair a cancer’s ability to acquire energy.
RECENT STUDIES OF CANCER METABOLISM PROVIDE INSIGHT ON SHORTCOMINGS OF THE WARBURG EFFECT
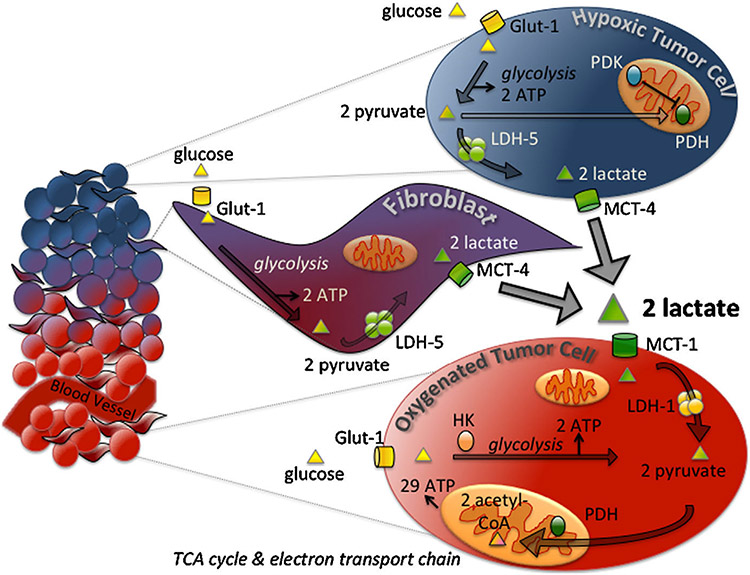
Recent experiments have highlighted limitations of the Warburg effect and offered compelling evidence for greater complexity within tumor metabolism. Dewhirst’s group has proposed that as the tumor grows, cancer cells establish metabolic symbiosis: a relationship between two populations of metabolically distinct cancer cells in which one population’s end product of glycolysis, lactate, serves as a metabolite for oxidative phosphorylation for the second cell population [26]. Lisanti and coworkers describe a variation of metabolic symbiosis, “the Reverse Warburg effect,” in which cancer-associated fibroblasts perform aerobic glycolysis and provide cancer cells with metabolites for oxidative phosphorylation [27,28]. A combined diagram of metabolic symbiosis and the Reverse Warburg phenomenon is shown in Figure 1.
Figure 1.
Metabolic symbiosis in the tumor microenvironment. During tumor growth, inefficient formation of blood vessels produces areas of normoxia and hypoxia within the tumor. Hypoxic cells generate two ATP per glucose via glycolysis, while oxygenated cells can produce a greater number of ATP from oxidative phosphorylation (29 ATP per two acetyl-CoA). Hypoxic cells import glucose via glucose transporters (Glut-1), and generate two molecules of pyruvate from one molecule of glucose. Hexokinase (HK) is the first rate-limiting enzyme of glycolysis. Under normoxic conditions, pyruvate is metabolized to acetyl-CoA by pyruvate dehydrogenase (PDH) and acetyl-CoA enters the TCA cycle. In hypoxic conditions, PDH is inhibited by PDK and lactate dehydrogenase-5 (LDH-5) converts pyruvate to lactate. Lactate from hypoxic cells is exported through monocarboxylate transporter 4 (MCT-4). Oxygenated cells import lactate via MCT-1, and convert the lactate back to pyruvate via LDH-1. Though not shown in the figure, cancer cells demonstrating the Warburg phenotype of high glycolysis, perhaps those harboring mitochondrial DNA mutations, may be found throughout the hypoxic and oxygenated regions of the tumor.
To illustrate the complexity of cancer metabolism and examine supporting evidence for metabolic symbiosis, this review will investigate three important drivers of metabolic diversity within tumors. First, what is the role of hypoxia in shaping metabolic diversity? Second, how do specific oncogene mutations promote the evolution of metabolic diversity within tumors? Third, how does tumor evolution develop the capacity to utilize lactate as a major energy source? We will examine how these factors influence the development of metabolic symbiosis. Finally, we will discuss nodes of metabolism that are potential therapeutic targets and may be used to break metabolic symbiosis.
THE ROLE OF HYPOXIA IN THE DEVELOPMENT OF TUMOR METABOLISM: LIMITED OXYGEN DIRECTS THE FUEL SOURCE
Hypoxic stress responses are mediated in part by Hif-1α, which triggers a series of events including increased expression of glucose transporter 1 (Glut-1), increased glycolytic enzyme activity, and tumor metastasis, and is associated with therapy resistance [25,29-31]. Hif-1α simultaneously limits OXPHOS and controls the expression of pyruvate dehydrogenase kinase (PDK), an enzyme that phosphorylates and inactivates pyruvate dehydrogenase, thus limiting pyruvate utilization for oxidative phosphorylation [32]. Recently, Semenza and coworkers have shown that an important enzyme in glycolysis, an isoform of pyruvate kinase (PKM2) is transcriptionally regulated by Hif-1. PKM2 interacts with prolyl-hydroxylase and Hif-1α to provide stimulation of HIF-1 target genes in a positive feedback loop [33].
A general suppression of metabolic demand occurs under hypoxia; cells with low oxygen supply downregulate the Na-K-ATPase and protein synthesis [34]. Cellular respiration is affected by low, but physiological levels of oxygen, indicating that mitochondria are tissue “oxygen sensors” [35,36]. In support of this idea, recent experiments have shown that a decrease in mitochondrial oxygen consumption has been recorded at oxygen perfusion levels of 1–3%, well before oxygen is truly limited at 0.3% [34]. Thus, hypoxic regions of a tumor are likely to be metabolically dormant, another aspect that may promote refractoriness to anti-cancer therapeutics. The entirety of a tumor is not hypoxic, however, as tumors construct their own, albeit inefficient, vasculature [37]. Additionally, hypoxia levels can fluctuate significantly in tumors within time scales of an hour [38]. Hypoxic fluctuation could promote the development of an adaptive response within cancer cells to varying oxygen levels and could also generate metabolic diversity amongst the cells of a tumor [39]. Hypoxia would drive glycolysis and lactate production, while periods of oxygenation could permit cancer cells to utilize lactate according to its high concentration within the tumor microenvironment.
KEY GENETIC CHANGES IN THE REPROGRAMMING OF METABOLISM
Other key findings that have led to increased acceptance of the Warburg effect are data indicating that specific genetic alterations in key regulatory proteins can alter drive changes in metabolism [40]. For example, Myc, Ras, AKT, and p53 are four key genes often altered in cancers [23,25,41,42]. Mutations that activate Ras [43], Akt [44], and inactivate p53 [45] are associated with increased glycolysis.
The tumor suppressor gene, p53, has been shown to control a number of important metabolic functions [46]. Nonmutated p53 stimulates OXPHOS through expression of a synthesis of cytochrome c oxidase 2 (SCO2), a chaperone protein for the assembly of cytochrome oxidase [45]. Glycolysis is suppressed in cells with wild-type p53, which down regulates glucose transporters. In addition, p53 controls TIGAR [47] (TP53-induced glycolysis and apoptosis regulator), a protein that lowers glycolysis by limiting the activity of phosphofructokinase-1 (PFK-1) and through a direct dephosphorylation of fructose-2,6 bisphosphate, [48].
Myc overexpression is found in 10–20% of all tumors and has been shown to increase glycolysis [25]. At the same time, Myc is also involved in mitochondrial biogenesis [49-51]. We have recently shown that Myc overexpression also increases the cellular capacity to perform both glycolysis and oxidative phosphorylation, and thus provides the cell with metabolic flexibility [52]. Through its regulation of mitochondrial biogenesis, it appears that Myc helps to direct cellular metabolic flexibility depending on the carbon sources and oxygen levels available to cancer cells during tumor development.
Complete genome sequencing of tumors has revealed a remarkable diversity of specific mutations in genes [53-56]. This strategy has revealed an unexpected mutation in isocitrate dehydrogenase [57] in gliomas [58-60] that changed cell growth through the production of a novel metabolite, 2-hydroxyglutarate [61,62]. This unique metabolite has been shown to help drive genome wide epigenetic changes by altering histone lysine demethylases [63,64]. Just as genetic alterations can drive metabolism, a provocative study by Papadopoulos and coworkers has linked glucose deprivation with K-Ras mutations [65], indicating that humble metabolites can change the genetic fingerprint of a cancer cell. Furthermore, it has been recently demonstrated that oncogenic K-Ras mutations reprogram metabolism in cancer cells, increasing glycolysis and lowering glucose utilization through the TCA cycle [66]. This study also showed that a large portion of glutamine, which has been identified as essential for tumor growth in other cancer cell systems [67], is being used to drive the biosynthesis of key amino acids using transamination reactions. Finally, it would appear that despite an increase in glycolysis, some mitochondrial function is essential for K-Ras driven tumors, in that loss of the mitochondrial transcription factor, TFAM, reduces K-Ras-driven mouse lung tumors [68]. Perhaps increased demand for glutamine in K-Ras mutated cells is due to its conversion to glutamate and its use in the TCA cycle to help resupply carbons lost by the conversion of glucose to lactate rather than pyruvate. Together these studies indicate that genetic alterations promote metabolic flexibility through the consumption of available carbon sources.
FUEL SOURCES FOR CANCER: EATING YOUR CAKE AND HAVING IT TOO
Despite its historical emphasis, glucose consumption cannot be the sole carbon source by which cancer metabolism is fueled. PET-FDG imaging is used to visualize tumors, yet the documented variability of FDG uptake in cancers [69,70] suggests that tumors are utilizing fuel sources other than glucose. Capaldi and coworkers have demonstrated that HeLa cells are capable of obtaining their ATP requirement almost entirely from glutamine and pyruvate [71]. Thompson has suggested that certain cancer cells become addicted to glutamine in order to utilize glucose-derived substrates for fatty acid synthesis [72]. Fatty acids offer yet another energy source, and there is evidence that prostate cancer, which is characterized by slow glycolysis and poor FDG uptake, demonstrates a preference for fatty acid oxidation despite the presence of abundant glucose [73,74].
As described below, there is increasing evidence that lactate is consumed within tumors. Lactate dehydrogenase (LDH) is a key enzyme required for lactate utilization as it interconverts pyruvate and lactate. In relation to clinical practice, elevated LDH serum levels are correlated with a poorer prognosis in prostate [75], colorectal cancer [76], melanoma and head and neck cancers [77-80]. LDH is a tetramer that exists in multiple isozymes based upon its composition from two subunits, M and H, which are encoded by LDHA and LDHB genes, respectively. Most relevant to metabolic symbiosis are the isozymes, LDH-1 and LDH-5, homotetramers of H and M, respectively. There are two key unresolved issues about the interconversion of pyruvate and lactate. First, it has been suggested that LDH-H is more sensitive than LHD-M to substrate inhibition by pyruvate, leading to the well-published theory that LDH-H participates in aerobic metabolism of pyruvate, while LDH-M is involved in the anaerobic process of lactate production from elevated pyruvate concentrations that develop during periods of increased glycolysis [81,82]. Yet this notion has been challenged since the physiologic concentrations of lactate and pyruvate in vivo never reach the level where inhibition would occur [83]. Secondly, the subsequent removal of either lactate or pyruvate from the cytoplasm would promote the reaction in one direction or the other. To this end, Brooks has proposed specific intracellular shuttles that transport lactate-derived pyruvate into the mitochondria, where it is converted to acetyl-CoA and enters the TCA cycle [15,84].
The tissue distribution of LDH isozymes provides additional information in regards to their metabolic roles. In normal tissue, LDH-1 has the greatest expression in the heart, brain, and kidney, while LDH-5 predominates more glycolytic tissues, such as the skeletal muscle and liver [85]. Elevated expression of LDH-1 in tumor cells could indicate the occurrence of oxidative metabolism, while LDH-5 expression is likely to correlate with glycolytic metabolism. The association between LDH-5 and anaerobic metabolism is further supported by the fact that Hif-1α promotes the transcription of the LDH-A gene [86]. The importance of LDH-A and glycolysis under oxygen poor conditions is underscored by investigations demonstrating that silencing LDH-A expression promotes mitochondrial respiration and limits the ability of tumor cells to proliferate under hypoxic conditions [87]. However, the expression levels of LDH subunits in specific tumors remain a significant gap in our knowledge. Future examinations of LDH expression in tumors should note the expression of LDH subunits to determine the potential for the conversion of lactate to pyruvate.
To appreciate the potential role of lactate in tumor metabolism, it is important to understand lactate utilization in normal tissues. The brain, skeletal and cardiac muscle consume lactate regularly, indicating a mechanism by which tissues can use this “waste” fuel of glycolysis [88]. Evidence suggests that lactate production and catabolism occur by distinct pathways [89], supporting the lactate exchange aspect of metabolic symbiosis in tumors. Monocarboxylate transporters (MCTs) are bidirectional channels that facilitate the movement of lactate and a proton down their concentration gradient. Distinct MCT isoforms have been identified in muscle fibers according to their metabolic phenotype [90,91]. MCT4 is found primarily in cells with high rates of glycolysis, such as white muscle cells, and expression of this transporter is increased under hypoxic conditions. MCT1 is associated with oxidative red fibers [26]. Increased muscle training and lactate clearance has been associated with increased expression of MCT1 in muscle sarcolemma. MCT1 has also been associated with lactate export in T cells [58]. Yet even within normal tissue, many unknowns persist regarding lactate metabolism. In particular, the precise location of LDH and the transport of lactate from the cytoplasm into mitochondria are debated [83]. Several investigators claim to have purified LDH from isolated mitochondria, while others insist that this “localization” is only contamination of cytoplasmic LDH [83]. After finding that isolated mitochondria could oxidize lactate, Brooks has proposed an intracellular lactate shuttle with MCT1 as the lactate transporter [15,92].
Metabolic symbiosis offers an efficient system in which well-oxygenated cancer cells consume lactate produced by hypoxic, glycolytic cancer cells. Dewhirst and coworkers demonstrated that SiHa cells, a human cervical squamous cancer line, metabolized lactate preferentially over glucose in vitro. Within SiHa xenograph tumors, two subpopulations were identified. One population, localized to the well-vascularized portion of the tumor, expressed high levels of MCT1 at the plasma membrane, suggesting that the cells were importing lactate for oxidative phosphorylation. MCT4 expression in this population was barely detectable in the cytoplasm. The second cell population was found at hypoxic tumor regions and lacked MCT1 expression. These populations, with their unique protein signatures, suggest a relationship between tumor oxygenation and the metabolic phenotype of a cancer cell. MCT1 inhibition with α-cyano-4-hydroxycinnamate (CHC) induced a metabolic switch from lactate to glucose consumption in oxygenated SiHa cells, thereby revealing the metabolic flexibility within this cell type. In vivo, CHC administration in a mouse model of lung cancer and xenographic human colorectal adenocarcinoma reduced hypoxia at the tumor periphery, induced tumor core necrosis, and slowed overall tumor growth. Necrosis of the hypoxic tumor core and improved oxygenation at the tumor periphery indicate that the previously oxidative SiHa cells switched to a glycolytic metabolism, thereby reducing their oxygen demand and depriving the tumor core of glucose. Further studies are required to characterize the metabolism of these cells such as identifying an increase in lactate levels upon CHC administration and a simultaneous decrease of oxygen consumption. WiDr human colorectal adenocarcinoma cells showed high levels of glycolysis in culture, yet they expressed MCT1 and were susceptible to growth-inhibition by CHC as xenograft tumors, suggesting that the metabolic phenotype of a cancer cell may differ in vitro and in vivo. New methods should be developed to demonstrate metabolic flexibility in culture models of cancer cells. Ideally, methods correlating expression levels of metabolic proteins favoring either glycolysis or oxidative phosphorylation must be correlated with these two ATP generating processes.
Stromal fibroblasts offer another potential source of lactate. The role of stromatogenesis in promoting tumor growth and angiogenesis [93] makes fibroblasts strong candidates for supporting tumor metabolism. Lisanti’s group describes a symbiotic relationship in which epithelial breast cancer cells manipulate nearby stromal fibroblasts to perform aerobic glycolysis. The stromal cells then “feed” the cancer cells lactate generated by glycolysis [28,94]. The stromal cells used in Lisanti’s experiments have lost expression of caveolin-1 (Cav-1), an integral membrane protein that is involved in lipid transport, membrane trafficking, and signal transduction [95]. The investigators propose that oxidative stress and mitochondrial dysfunction in Cav-1 deficient fibroblasts drives stromal cells’ glycolytic phenotype and the symbiosis between cancer and stromal cells. Indeed, the loss of Cav-1 expression in stromal cells of breast cancer is associated with early disease recurrence, advanced tumor stage, and lymph node metastasis [96,97]. Most recently, Lisanti and coworkers have identified MCT4 expression on tumor-associated fibroblasts and MCT1 expression on breast cancer cells from patient biopsy slices, yet both transporters were not revealed in the same tissue sample [98]. Interestingly, Hussein and Brooks [91] were unable to detect significant expression of MCT1 in pure cultures of MDA-MB-231 breast cancer cells. Lisanti’s group has also identified changes in the gene expression profile of MCF7 breast cancer cells after culturing them in lactate. These changes in gene expression are similar to gene expression profiles of ER+ luminal breast cancers, and are associated with increased metastasis, tumor recurrence, and reduced survival [99]. This model of tumor metabolism highlights the ability of cancer cells to manipulate their microenvironment in order to increase their fuel supply and promote proliferation and metastasis [27].
While a metabolic cooperation may exist between cancer and stromal cells, it could also occur in the reverse of what Lisanti has described. Sivridis et al. [93] have proposed a “metabolic co-operation” between oxidative stromal cells and glycolytic colorectal adenocarcinoma cells based upon their differential expression of metabolite transporters and metabolic enzymes. This collective evidence suggests the metabolic roles adopted by stromal and cancer cells may differ between cancer types. Other cell populations in the tumor microenvironment might also supply tumor cells with metabolites. Macrophages infiltrating tumors accumulate 2-FDG in vivo, and could act as lactate suppliers [100-102]. Similarly, T lymphocytes undergo a metabolic switch upon activation, relying primarily upon glycolysis to meet their ATP needs, even in the presence of sufficient oxygen levels [103].
Thus, it would appear that the evolving neoplasm can eat glucose, and consume the remaining carbons as lactate in a different region of the tumor. Appreciating this cancer cell ecology presents a finer lens in which to view the growing tumor mass. Further investigation is needed regarding lactate’s role in tumor metabolism including the determination of factors that direct lactate utilization in cancer cells, MCT1 as a prognostic indicator, and the trafficking of lactate within cancer cells of specific tumor types. It also remains to be determined as to whether the ATP generated from lactate oxidation contributes to tumor growth or is immediately consumed in order to export the protons that accompany lactate into a cell during import/maintain cellular redox state [85].
PASSING THE TEST OF METABOLIC SYMBIOSIS
Tumor types beyond those examined by Dewhirst demonstrate traits of metabolic symbiosis. Elevated expression of MCT1 has been identified in the rim of C6 gliomas in a rat brain [104]. Both gliomas and cervical cancers exist in environments that naturally have high lactate levels [89,105], suggesting that cancers growing in such conditions are predisposed to develop metabolic symbiosis. Further investigations are required to establish metabolic symbiosis as a general phenomenon of cancer metabolism. Tumors should be examined with high-resolution 3D reconstructive imaging to identify consumption of different metabolites in particular areas of the tumor. Despite the wide spread use of FDG/PET imaging to follow tumor growth and metastasis, this method lacks sufficient resolution to observe metabolic diversity within most tumors. Additional metabolite analogue dyes are also required to detect utilization of fuel sources other than glucose in vivo.
To define a tumor as metabolically symbiotic, several criteria must be met. It is expected that metabolically distinct populations will have unique protein signatures showing different expression levels of pertinent enzymes and carbohydrate transporters (Table 1). For example, the glycolytic cell population in a tumor, whether cancer or stromal cell, would be expected in hypoxic areas of the tumor and to express high levels of Hif-1α, HK, Glut-1, MCT-4, and LDH-5. The cell population performing OXPHOS, on the other hand, would have decreased expression of the aforementioned molecules and elevated expression of PDH, MCT-1, and LDH-1. Administration of radioactively labeled lactate should accumulate within the oxidizing cell population. While these two cell populations must be identified in vivo, promising candidates for metabolic symbiosis should demonstrate metabolic flexibility in vitro when fed lactate or glucose as the predominant metabolite. Additionally, tumor cells may require other cells types, such as immune or stromal cells, to promote and participate in metabolic symbiosis.
Table 1.
Predicted Expression Levels of the Enzymes and Metabolite Transporters Most Relevant to Metabolic Symbiosis
| Protein | Expression in hypoxic cells | Expression in oxygenated cells |
|---|---|---|
| Glucose transporter 1 (Glut-1) | High | Low |
| Hexokinase (HK) | High | Low |
| Monocarboxylate transporter 1 (MCT-1) | Low | High |
| Monocarboxylate transporter 1 (MCT-4) | High | Low |
| Lactate dehydrogenase subunit M (LDH-M) (four subunits of LDH-M produce LDH-5) | High | Low |
| Lactate dehydrogenase subunit H (LDH-H) (four subunits of LDH-M produce LDH-1) | Low | High |
| Pyruvate dehydrogenase (PDH) | Low | High |
| Pyruvate dehydrogenase kinase (PDK) | High | Low |
Most significantly for clinical intervention, this new view of metabolism in cancer biology suggests an important means for fighting tumor growth: employing compounds that destroy the symbiosis and reduce a tumor’s ability to utilize essential substrates. In addition to limiting a cancer cell’s fuel sources, inhibiting OXPHOS may promote general oxygenation within a tumor, thereby reducing hypoxia-induced resistance to chemo- or radiation therapy [26]. Combining metabolic inhibitors with standard forms of cancer therapy will provide a more powerful treatment approach. This one-two punch would diminish cancer cells’ bioenergetic potential to adapt to other treatment strategies.
CONCLUSION
The tumor must be recognized as an evolving ecosystem, adapting constantly to oxygen and nutrient availability. Viewing cancer metabolism as a singular phenotype of increased glycolysis due to mitochondrial damage neglects the dynamic nature of cancer growth, and overlooks opportunities for novel therapeutic targets. The model of metabolic symbiosis provides a system by which cancer cells may adapt to or maximize the use of available resources, thereby increasing their capacity to grow and metastasize. Components of metabolic symbiosis, such as metabolic transporters and enzymes, may serve clinically as prognostic indicators in the future.
Potential therapeutic targets within metabolic symbiosis may be divided into two categories, carbon source related or pathway related. Inhibition of MCT-1 and Glut-1 could starve cancer cells of their preferred carbon source, while inhibition of pyruvate dehydrogenase or LDH would destroy the pathways by which cancer cells process fuel sources. Investigation of inhibitory compounds and safe delivery systems to reduce the tumor cells’ ability to acquire and process nutrients are promising avenues by which to expand our arsenal against cancer.
ACKNOWLEDGMENTS
We greatly appreciate the insightful and stimulating conversations with Dr. Stergios Moschos who helped shape our thinking on this topic. We appreciate helpful discussions with Drs. Michelle Barbi de Moura, Doug Green, Michael Lotze, and Richard Steinman. This work was funded, in part, under a grant with the Pennsylvania Department of Health, PA CURE. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This work was also supported by funding from P30CA047904, P50CA097190, and P50CA121973. ECN is supported by the University of Pittsburgh Physician Scientist Training Program.
Abbreviations:
- LDH
lactate dehydrogenase
- MCTs
monocarboxylate transporters
- CHC
α-cyano-4-hydroxycinnamate
- Cav-1
caveolin-1
- PDH
pyruvate dehydrogenase.
REFERENCES
- 1.Warburg O. Origin of cancer cells. Science 1956;123:309–314. [DOI] [PubMed] [Google Scholar]
- 2.Godinot C, de Laplanche E, Hervouet E, Simonnet H. Actuality of Warburg’s views in our understanding of renal cancer metabolism. J Bioenerg Biomembr 2007;39:235–241. [DOI] [PubMed] [Google Scholar]
- 3.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–707. [DOI] [PubMed] [Google Scholar]
- 4.Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the Warburg effect: Molecular basis for the reprogramming of cancer cell metabolism. Cancer Res 2009;69:2163–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun QA, Chen XX, Ma JH, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA 2011;108:4129–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 2011;208:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minchenko A, Leshchinsky I, Opentanova I, et al. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene—Its possible role in the Warburg effect. J Biol Chem 2002;277:6183–6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zu XL, Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem Biophys Res Commun 2004;313:459–465. [DOI] [PubMed] [Google Scholar]
- 9.Stern R, Shuster S, Neudecker BA, Fromby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: The Warburg effect revisited. Exp Cell Res 2002; 276:24–31. [DOI] [PubMed] [Google Scholar]
- 10.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–899. [DOI] [PubMed] [Google Scholar]
- 11.Helmlinger G, Schell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res 2002;8:1284–1291. [PubMed] [Google Scholar]
- 12.De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol 2005;1:779–786. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Sayans M, Manuel Somoza-Martin J, Barros-Angueira F, Gandara Rey JM, Garcia-Garcia A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev 2009;35:707–713. [DOI] [PubMed] [Google Scholar]
- 14.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans—dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 1988;82:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans 2002;30:258–264. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: Demonstration of an essential component of brain lactate shuttles. PLoS ONE 2008;3: e2915, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magistretti PJ. Neuron-glia metabolic coupling plasticity. J Exp Biol 2006;209:2304–2311. [DOI] [PubMed] [Google Scholar]
- 18.Fliss MS, Usadel H, Caballero OL, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 2000;287:2017–2019. [DOI] [PubMed] [Google Scholar]
- 19.Polyak K, Li Y, Zhu H, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet 1998;20:291–293. [DOI] [PubMed] [Google Scholar]
- 20.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene 2006;25:4647–4662. [DOI] [PubMed] [Google Scholar]
- 21.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 22.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev 2008;18:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010;330:1340–1344. [DOI] [PubMed] [Google Scholar]
- 24.Weinhouse S. Respiratory impairment in cancer cells. Science 1956;124:267–269. [DOI] [PubMed] [Google Scholar]
- 25.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85–95. [DOI] [PubMed] [Google Scholar]
- 26.Sonveaux P, Vegran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008;118:3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate “fuel” tumor growth and metastasis Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010;9:3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migneco G, Whitaker-Menezes D, Chiavarina B, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis Evidence for stromal-epithelial metabolic coupling. Cell Cycle 2010;9:2412–2422. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Kang YB. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 2010;16:5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vleugel MM, Greijer AE, Shvarts A, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1 alpha expression in invasive breast cancer. J Clin Pathol 2005;58:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 2009;37:4587–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006;3:177–185. [DOI] [PubMed] [Google Scholar]
- 33.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011;145:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 2011;300:C385–C393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson DF, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. Am J Physiol 1977;233: C135–C140. [DOI] [PubMed] [Google Scholar]
- 36.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 1988;263:2712–2718. [PubMed] [Google Scholar]
- 37.Yu JL, Rak JW, Carmeliet P, Nagy A, Kerbel RS, Coomber BL. Heterogeneous vascular dependence of tumor cell populations. Am J Pathol 2001;158:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pigott KH, Hill SA, Chaplin DJ, Saunders MI. Microregional fluctuations in perfusion within human tumours detected using laser Doppler flowmetry. Radiother Oncol 1996;40:45–50. [DOI] [PubMed] [Google Scholar]
- 39.Mayer A, Hockel M, Horn LC, Schmidberger H, Vaupel P. GLUT-1 staining of squamous cell carcinomas of the uterine cervix identifies a novel element of invasion. Int J Oncol 2011;38:145–150. [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 41.de Moura MB, dos Santos LS, Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen 2010;51:391–405. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer 2005;5:857–866. [DOI] [PubMed] [Google Scholar]
- 43.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci USA 2005;102:5992–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell 2007;129:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science 2006;312:1650–1653. [DOI] [PubMed] [Google Scholar]
- 46.Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harb Perspect Biol 2010;2:a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006;126:107–120. [DOI] [PubMed] [Google Scholar]
- 48.Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: Old friends and recent additions. Cold Spring Harb Symp Quant Biol 2011; LXXVI (in press). [DOI] [PubMed] [Google Scholar]
- 49.Dang CV. MYC, microRNAs and glutamine addiction in cancers. Cell Cycle (Georgetown, TX) 2009;8:3243–3245. [DOI] [PubMed] [Google Scholar]
- 50.Samper E, Morgado L, Estrada JC, et al. Increase in mitochondrial biogenesis, oxidative stress, and glycolysis in murine lymphomas. Free Radic Biol Med 2009;46:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, Wang Y, Zeller KI, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 2005;25:6225–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graves JA, Rothermund K, Wang T, Qian W, Van Houten B, Prochownik EV. Point mutations in c-Myc uncouple neoplastic transformation from multiple other phenotypes in rat fibroblasts. PLoS ONE 2010;5:e13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature 2011;470:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010;463:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 2010;464:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011;475:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray CM, Hutchinson R, Bantick JR, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chemi Biol 2005;1:371–376. [DOI] [PubMed] [Google Scholar]
- 58.Basanta D, Scott JG, Rockne R, Swanson KR, Anderson AR. The role of IDH1 mutated tumour cells in secondary glioblastomas: An evolutionary game theoretical view. Phys Biol 2011;8:015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reitman ZJ, Jin G, Karoly ED, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA 2011;108:3270–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res 2009;69:9157–9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010;17:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 2011;12:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat Med 2011;17:291–293. [DOI] [PubMed] [Google Scholar]
- 65.Yun JY, Rago C, Cheong I, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 2009;325:1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaglio D, Metallo CM, Gameiro PA, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 2011;7:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci 2010;35:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 2010;107:8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avril N, Menzel M, Dose J, et al. Glucose metabolism of breast cancer assessed by F-18-FDG PET: Histologic and immunohistochemical tissue analysis. J Nucl Med 2001;42:9–16. [PubMed] [Google Scholar]
- 70.Westerterp M, Sloof GW, Hoekstra OS, et al. (18)FDG uptake in oesophageal adenocarcinoma: Linking biology and outcome. J Cancer Res Clin Oncol 2008;134:227–236. [DOI] [PubMed] [Google Scholar]
- 71.Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 2004;64:985–993. [DOI] [PubMed] [Google Scholar]
- 72.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 2007;104:19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu YY, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res 2010;30:369–374. [PubMed] [Google Scholar]
- 74.Liu Y Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis 2006;9:230–234. [DOI] [PubMed] [Google Scholar]
- 75.Yamada Y, Nakamura K, Aoki S, et al. Lactate dehydrogenase, Gleason score and HER-2 overexpression are significant prognostic factors for M1b prostate cancer. Oncol Rep 2011;25:937–944. [DOI] [PubMed] [Google Scholar]
- 76.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Lactate dehydrogenase 5 expression in operable colorectal cancer: Strong association with survival and activated vascular endothelial growth factor pathway—A report of the tumour angiogenesis research group. J Clin Oncol 2006;24:4301–4308. [DOI] [PubMed] [Google Scholar]
- 77.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The oblimersen melanoma study group. J Clin Oncol 2006;24:4738–4745. [DOI] [PubMed] [Google Scholar]
- 78.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: A randomized phase II study evaluating the activity of BEvacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2011; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Danner BC, Didilis VN, Wiemeyer S, et al. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer Res 2010;30:1347–1351. [PubMed] [Google Scholar]
- 80.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51:349–353. [DOI] [PubMed] [Google Scholar]
- 81.Quistorff B, Grunnet N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging US 2011;3:457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gladden LB. 200th anniversary of lactate research in muscle. Exerc Sport Sci Rev 2008;36:109–115. [DOI] [PubMed] [Google Scholar]
- 83.Van Hall G Lactate as a fuel for mitochondrial respiration. Acta Physiol Scand 2000;168:643–656. [DOI] [PubMed] [Google Scholar]
- 84.Brooks GA. Cell-cell intracellular lactate shuttles. J Physiol 2009;587:5591–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem 2010;17:672–697. [DOI] [PubMed] [Google Scholar]
- 86.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic-enzymes by hypoxia-inducible factor-1. J Biol Chem 1994;269:23757–23763. [PubMed] [Google Scholar]
- 87.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006;9:425–434. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: The influence and therapeutic potential for MCT and CD147 regulation. Future Oncol 2010;6:127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol Endocrinol Metab 2001;281:E794–E802. [DOI] [PubMed] [Google Scholar]
- 90.Bonen A Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc 2000;32:778–789. [DOI] [PubMed] [Google Scholar]
- 91.Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics 2011;43:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 1999;96:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sivridis E, Giatromanolaki A, Koukourakis MI. Proliferating fibroblasts at the invading tumour edge of colorectal adenocarcinomas are associated with endogenous markers of hypoxia, acidity, and oxidative stress. J Clin Pathol 2005;58:1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 95.Liu PS, Rudick M, Anderson RGW. Multiple functions of caveolin-1. J Biol Chem 2002;277:41295–41298. [DOI] [PubMed] [Google Scholar]
- 96.Witkiewicz AK, Dasgupta A, Sotgia F, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol 2009;174:2023–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sloan EK, Ciocca DR, Pouliot N, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol 2009;174:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011;10:1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell “stemness”, driving recurrence, metastasis and poor clinical outcome in breast cancer. Cell Cycle 2011;10:1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluoro-deoxyglucose invivo—High accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–1980. [PubMed] [Google Scholar]
- 101.Poettgen C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology 2007;73:316–323. [DOI] [PubMed] [Google Scholar]
- 102.Roiniotis J, Dinh H, Masendycz P, et al. Hypoxia prolongs monocyte/macrophage survival and enhanced glycolysis is associated with their maturation under aerobic conditions. J Immunol 2009;182:7974–7981. [DOI] [PubMed] [Google Scholar]
- 103.Jones RG, Thompson CB. Revving the engine: Signal transduction fuels T cell activation. Immunity 2007;27: 173–178. [DOI] [PubMed] [Google Scholar]
- 104.Grillon E, Farion R, Fablet K, et al. The spatial organization of proton and lactate transport in a rat brain tumor. PLoS ONE 2011;6: e17416, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuchiiwa T, Nio-Kobayashi J, Takahashi-Iwanaga H, Yajima T, Iwanaga T. Cellular expression of monocarboxylate transporters in the female reproductive organ of mice: Implications for the genital lactate shuttle. Histochem Cell Biol 2011;135:351–360. [DOI] [PubMed] [Google Scholar]