Abstract
Aims
Data standards are consensual specifications for the representation of data arising from different sources. If provided with internationally harmonized variables, permissible values, and clinical definitions, they have the potential to enable reliable between- and within-country analysis of care and outcomes. The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart) is a European Society of Cardiology project that allows participating countries to collect patient data to undertake quality improvement, observational studies, drug and device surveillance, and registry-based randomized controlled trials for cardiovascular conditions. This paper describes the methodology for development of harmonized data standards for EuroHeart.
Methods and results
We adopted a five-step process for the development of harmonized data standards. The process includes (i) identification of clinical domains for data standard development by evaluating specific cardiovascular conditions with high prevalence and opportunities for quality improvement; (ii) construction of data standard specifications by systematic review of the literature; (iii) selection of variables by a domain-specific Working Group using a modified Delphi method; (iv) validation of data standards by a domain-specific Reference Group; and (v) implementation of the developed data standards into an IT platform.
Conclusion
This paper describes the approach adopted by EuroHeart for the development of clinical data standards for cardiovascular disease. The methodology has been developed and is used by EuroHeart to create a suite of international data standards for cardiovascular diseases. The EuroHeart data standards may be used to systematically capture individual patient data about clinical care and for research.
Keywords: EuroHeart, Methodology, Data standards, Data variables, Data definitions
Graphical Abstract
Graphical Abstract.
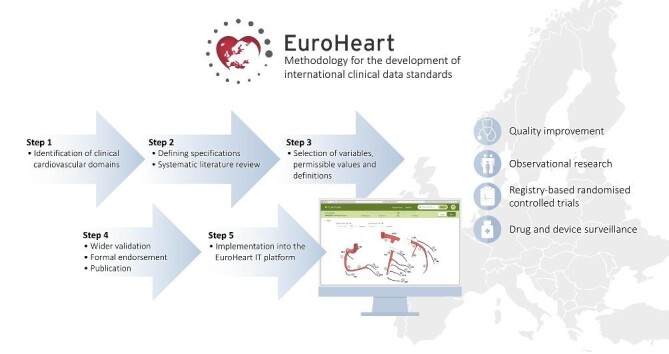
The EuroHeart methodology for the development of international clinical data standards for cardiovascular conditions.
Introduction
Advances in cardiovascular innovations and technologies have led to improvement in patient outcomes.1 Alongside these developments, vast quantities of heterogeneous patient data have been collected in clinical trials, registries, and electronic healthcare records (EHRs).2–6 Standardization of data definitions across various clinical and research settings allows the seamless transfer of data,7 as such enhancing the efficiency and the cost-effectiveness of initiatives that aim to improve care and outcomes.8,9
Defining data standards for a cardiovascular disease involves the identification and definition of variables pertinent to the individual, the disease, and its diagnosis, treatment, and outcomes. While data standards for several cardiovascular diseases have been established, there are variations in the methodology by which the data standards are developed.10–12 The American College of Cardiology (ACC) and the American Heart Association (AHA) have established a Task Force for data standards, which in addition to creating high-quality data standards for a number of cardiovascular condition has laid out a structured approach for data standard development.10 Such recommendations, however, are designed to meet the specifications of the American healthcare system.
The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart) initiative, supported by the European Society of Cardiology (ESC), aims to facilitate the continuous collection of patient data across Europe to improve the quality of care and outcomes of people with cardiovascular disease.13 To achieve this mission, EuroHeart defines data variables for cardiovascular conditions and integrates these into a bespoke IT platform to enable real-time data collection. This will enable the online analysis and direct reporting of patient characteristics, processes of care, and pre-defined quality indicators, as well as observational research, registry-based randomized controlled trials (R-RCTs), and post-marketing drug and device monitoring.13
This paper outlines the methodology for the development of the EuroHeart data standards for cardiovascular disease.
Methods
Herein, we use the term data standards as consensual specifications for the representation of data from different sources or settings.14 They include the specifications for data variables, permissible values, and definitions (Table1). In this paper, the term data is reserved for individual observations (e.g. 180 cm) and the term variables for data items (e.g. height).9 Permissible values are the type of information captured by the variables, which may, for example, be numeric, binary (no, yes), dates, or free text for qualitative variables. Data variables may also be classified according to how critical their collection is for the meaningful interpretation of the dataset. Definitions are the explicit description of the factual meaning of the information captured by the variable (e.g. height on admission in centimetres).9
Table 1.
Terminologies and definitions
| Terminology | Definition |
|---|---|
| Data standards | Consensual specifications for the representation of data arising from different sources. |
| Cardiovascular domain | A distinct category of cardiovascular disease or treatment. |
| Variable | Data field that is to be collected. |
| Candidate variable | Variable that has been extracted from the literature but that has not been agreed upon. |
| Permissible value | Format and structure of the information that is allowed to be captured within a variable. |
| Variable definition | Explicit description of the factual meaning of the information captured by a variable. |
Operational framework
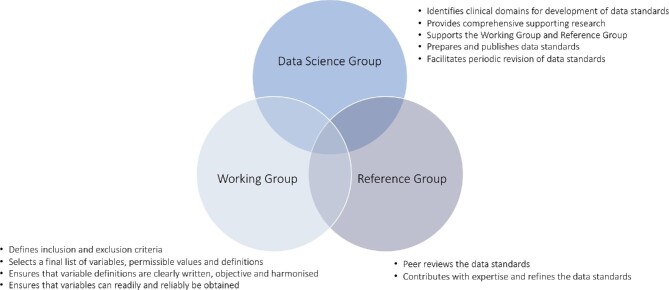
Data Science Group
Under the auspice of EuroHeart, the Data Science Group comprises a chair, medical experts, and project managers (Figure 1). The Data Science Group is responsible for
Figure 1.
Operational framework during the development of the EuroHeart data standards.
Developing a standardized methodology for the construction of data standards.
Identifying potential domain areas for data standard development. Potential clinical domains for creation of data standards are based on the importance of the cardiovascular condition/procedure and the purpose of the data standards. The identified clinical areas may include, but are not limited to, the ESC Clinical Practice Guidelines.
Ensuring that the developed methodology is applied across all domains and according to the agreed timelines with other stakeholders.
Providing supporting research, such as systematic literature reviews, and the evaluation of any ongoing national data efforts.
Translating the research findings into a candidate set of variables, permissible values, and definitions.
Supporting the consistent development and refinement of different cardiovascular data standards together with the Working Group and the Reference Group.
Co-ordinating with national registry leaders of countries participating in the EuroHeart project to facilitate the transition to, or the harmonization with, the developed data standards.
Supporting the transparent publication of the developed data standards in scientific documents alongside their development process.
Undertaking the periodic evaluation, revision, and update of the EuroHeart data standards.
Working Group
A Working Group is established for each cardiovascular domain (Figure 1). The nomination of members for the Working Group is solicited by relevant ESC Associations and Working Groups, and other ESC member country National Cardiac Societies. Ideally, the Working Group should include approximately 10–20 cardiovascular domain experts and members with experience in developing and maintaining national quality registries. This group forms the ‘core’ team for the data standard development and aims to
Define the inclusion and exclusion criteria for the data standards in development.
Identify the clinical setting(s) for which the data standards are applicable.
Specify the data standard characteristics and anticipated number of variables.
Develop a proposal of the subcategories within the data standards by constructing a conceptual framework of the patient journey.
Provide a final list of variables, permissible values, and definitions to be included in the data standards.
Ensure that variable definitions are clearly written, objective, and harmonized against current Clinical Practice Guidelines. Close attention is paid to definitions regarding the timing of events and procedures, device and drug names, and consistency with respect to other variables.
Ensure that variables may be readily and reliably obtained in real-life clinical settings.
Reference Group
The Reference Group defines a team whose members are nominated by the relevant ESC Associations and Working Groups (Figure 1). It may also include representatives from the ESC National Cardiac Societies, the ESC Patient Forum, the ESC Association of Cardiovascular Nursing and Allied Professions, and the ESC Committee for Young Cardiovascular Professionals. The involvement of these professional bodies provides broader insights and a more generalizable perspective. Ideally, the Reference Group should include approximately 20–30 representatives from as many ESC member countries as possible to increase the acceptance and uptake of the developed standards. The objective of the Reference Group is to
Provide feedback on the data standard characteristics and inclusion and exclusion criteria.
Review and provide feedback on the proposed data standards.
Assess the applicability of the data standards in different patient groups and across different countries.
Critically appraise the proposed data standards.
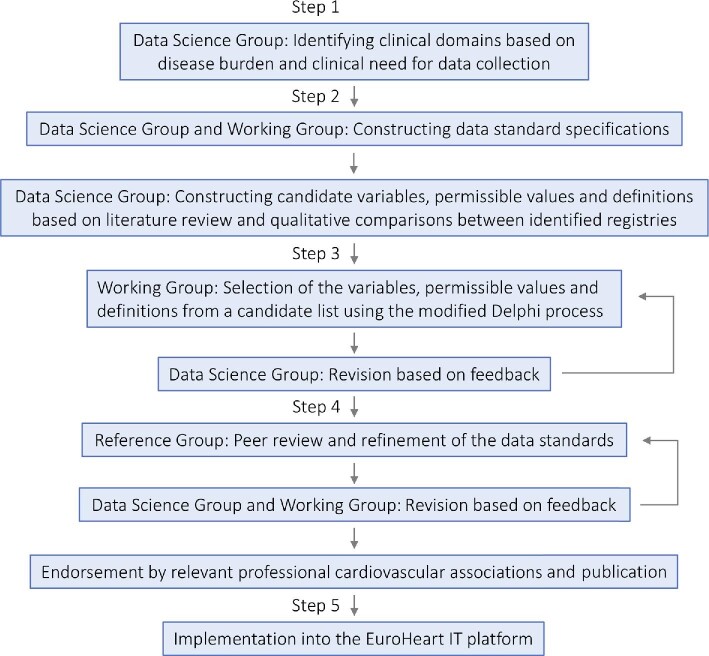
The five-step process
The EuroHeart data standards are developed through a five-step process (Figure 2): (i) identification of clinical domains for data standard development by evaluating specific cardiovascular conditions with high prevalence and opportunities for quality improvement; (ii) construction of data standard specifications by systematic review of the literature; (iii) selection of variables by a domain-specific Working Group using a modified Delphi method; (iv) validation of data standards by a domain-specific Reference Group; and (v) implementation of the developed data standards into an online IT platform.
Figure 2.
Process for development of the EuroHeart data standards.
Step 1: identifying the clinical domains
Potential clinical domains for which data standards are to be developed are identified by the Data Science Group in collaboration with, and on approval by, the EuroHeart Executive Committee.13 The identified domains are based on the disease burden and clinical need for data collection. The latter point may be driven by paucity of registries, heterogeneity of existing registries, recognized gaps, or variation in care and outcomes. During the pilot phase of EuroHeart, four cardiovascular conditions were selected: acute coronary syndrome and percutaneous coronary intervention; heart failure; atrial fibrillation; and valvular heart disease.13
Step 2: evidence synthesis and constructing data standard specifications
The specifications of the data standards are determined by the inclusion and exclusion criteria and the clinical setting(s) for which the data standards are applicable. Such specifications are defined by the Working Group members and developed from a conceptual framework of the patient journey. This facilitates the selection of variables and ensures that the registry captures information relevant to the continuum of the patient care. This step is achieved by close working between the Data Science Group and members of the Working Group through the following steps:
Identifying the target population, which is the cohort of patients for whom the data standards are intended to be used (e.g. patients with acute coronary syndrome).
Determining the clinical setting(s) for the data standards in development (e.g. in-hospital care for patients with acute coronary syndrome).
Conducting a systematic literature review to identify existing registries and data standard documents pertinent to the clinical area.
A systematic review of the literature, required for the construction of the candidate data variables, is undertaken by the Data Science Group. The review aims to identify data variables relevant to the proposed clinical domain and assess their importance, evidence base, validity, reliability, feasibility, and applicability in relation to contemporary knowledge (Table2).9,15 Data variables may be adopted from clinical trials, registries, or published data standard documents. The search strategy involves the use of medical online databases including, but not limited to, PubMed®, MEDLINE®, and Embase®, using MeSH (medical subject headings) terms. In addition, Clinical Practice Guidelines from the ESC and other professional organizations, as well as other statements such as consensus documents and quality indicators, are important sources for the candidate data variables.16 The latter provide tools for measuring processes of care that can be captured in registries and thus form an essential source for candidate variables. Of note, the ESC quality indicators applicable to the domain in development are automatically selected as candidate variables.
Table 2.
Criteria for the selection of the EuroHeart data variables
| Domain | Criteria |
|---|---|
| Importance | Variables related to quality indicators that are important for monitoring and benchmarking of quality of care. |
| Variables related to areas where there are disparities or suboptimal care. | |
| Variables addressing appropriateness of medical interventions. | |
| Evidence base | Variables based on evidence consistent with current medical knowledge and the ESC Clinical Practice Guidelines. |
| Validity | Variables that can correctly assess what they are designed to measure. |
| Reliability | Variables that can be collected and assessed in a reproducible manner, including when collected by different people. |
| Feasibility | Variables can be collected and assessed readily and easily within acceptable time frames. |
| Applicability | Variables that support the purpose of the registry, e.g. quality improvement, observational and randomized research, and drug and device monitoring. |
ESC, European Society of Cardiology.
In addition to systematic reviews, qualitative comparisons between identified registries help evaluate the feasibility of the candidate data variables within their respective registries. Case report forms and published articles from the identified registries are reviewed, and information mapped to a single tabular form and qualitatively assessed in relation to the quality of data reported.
Step 3: selection of variables, permissible values, and definitions
The third step aims to build consensus on the candidate variables extracted from the systematic literature review. When selecting the variables, careful attention is paid towards balancing completeness vs. complexity, so that variables may be readily and reliably obtained in naturalistic clinical settings. The main goal is to focus on variables that capture the patient, treatment, and outcome characteristics.
The selection of variables from a pool of candidate variables is determined using a modified Delphi process. As such, the Data Science Group presents the results of the systematic literature review to the Working Group members who are also informed with the voting criteria.17,18 Each variable is voted upon by each member of the Working Group. This process is anonymous, iterative, and interposed with a series of web conference meetings, along with extensive correspondence by e-mail. To facilitate the selection process, preliminary permissible values and definitions may be provided for each variable before the Delphi voting. Variable definitions include a concise description of the component of care being captured with all relevant information. For instance, the collection of data about the measurement of cardiac troponin in an acute coronary syndrome registry requires the specification of the time of the measurement (e.g. within 24 h from hospital admission), the type of assay used (e.g. high-sensitivity troponin T), the units of measurement (e.g. ng/L), and the permissible value data type and format [e.g. numerical value vs. binary (elevated, non-elevated)].
Based on the voting results, the EuroHeart variables may be classified into three levels (Table3). Level 1 variables are considered essential and mandatory by the Working Groups and are consequently both defined and pre-programmed into the EuroHeart IT platform. Many of the level 1 variables include key patient and disease characteristics, guideline recommended treatments, pre-defined quality indicators, and other variables pertinent to accountability and public reporting of quality of care. Level 2 variables are optional but relevant to clinical practice. Standardized definitions are provided for level 2 variables, but they are not pre-programmed into the EuroHeart IT platform. Country-specific level 3 variables, which may address regulatory or administrative requirements, can be integrated into the EuroHeart IT platform locally.
Table 3.
Level of variables in the EuroHeart data standards
| Level | Definition |
|---|---|
| Level 1 | Variables that are mandatory to collect and that are clinically defined and pre-programmed into the EuroHeart IT platform. |
| Level 2 | Variables for which standardized definitions are provided, but the collection of these variables is not mandatory, and the variables are not pre-programmed into the EuroHeart IT platform. |
| Level 3 | Variables that are locally defined and ‘country-specific’ and that, for example, address local regulatory or administrative requirements. These variables are not provided in the data standards and are not pre-programmed into the EuroHeart IT platform. |
Following the selection of variables, the permissible values and definitions for variables are finalized based on the data available from the literature review as well as the comments and feedback obtained during the modified Delphi process by members of the Working Group. The proceedings of the Working Group are then assembled by the Data Science Group and a draft of the data standards is compiled.
Step 4: wider validation of the developed data standards
The developed data standards are reviewed independently by the members of the Reference Group, by online surveys, web conference meetings, or e-mail correspondence. This validation process aims to assess the suitability of the proposed variable for application in various registries and across different countries. Furthermore, this step aims to assess the external generalizability of the data standards and their suitability to be used for different purposes such as benchmarking, quality improvement, observational and randomized clinical trials, and drug and device safety surveillance. The Data Science Group collates input from members of the Reference Group, and prepares a document with the final data variables, permissible values, and definitions that is then circulated among the members of the Working Group for final approval. Once approved, the data standard document is sent to relevant professional cardiovascular associations for formal endorsement before being submitted for publication. Revised data standards are periodically published online as a supplement and on the EuroHeart website (www.escardio.org/euroheart).
Step 5: implementation of the developed data standards into the EuroHeart IT platform
The EuroHeart data standards are pre-programmed into the EuroHeart IT platform that is delivered to interested countries based on their existing infrastructure and their willingness to adopt the EuroHeart IT platform that is periodically updated. In addition, this platform collects and automatically calculates and reports many of the ESC quality indicators for the respective clinical domain area with a comparison between the centre's performance and the national average being presented. For instance, the EuroHeart IT platform for acute coronary syndrome and percutaneous coronary intervention allows the automatic calculation and feedback on the majority of the ESC quality indicators for acute myocardial infarction.19 Alternatively, countries may implement the EuroHeart data standards into their existing data collection platforms, or use the data standards without an IT infrastructure.
Discussion
This paper describes the EuroHeart methodology for the development of data standards for cardiovascular disease. During recent years, the adoption of clinical registries, administrative databases, and EHRs has opened up major opportunities for cost-efficient observational and randomized clinical studies.20–23 However, comparison and collaboration between different data sources remain complex, mostly due to varying data variables and definitions with non-standardized vocabulary for presenting clinical concepts. Standardized data variables, permissible values, and definitions would provide opportunities to overcome this ambiguity and enable collaboration between various data sources and facilitate efficient exchange of data and delivery of international observational and randomized research and quality improvement. The framework in this paper provides a structured methodology for developing clinical data standards, underpinned by an approach that encompasses scientific evidence and expert opinion.
Today, cardiovascular disease accounts for a substantial health and economic burden in Europe and globally, with an increasing burden especially in developing countries.24 Data from national registries, health surveys, and administrative records show persisting geographic and social variation in cardiovascular morbidity, mortality, and treatment.24,25 By implementing a common lexicon with data standards into national registries in Europe, pooled data from multiple geographical locations might be used for quality improvement, benchmarking of care providers, and research. Existing national cardiovascular registries, clinical trial case report forms, and EHRs are distinct entities with varying data variables, permissible values, and definitions. This limits the possibilities of linkage between large datasets and collaborative initiatives. To address these limitations, initiatives such as the Cardiology Audit and Registration Data Standards (CARDS) and the ACC/AHA have established Task Forces for developing data standards.10,12 However, the data standards presented by CARDS were established in 2004 and are now outdated.12 In contrast, data standards presented by ACC/AHA have recently been updated using a similar methodology to the one presented in this paper, but are designed for the American healthcare system and are not implemented into a bespoke IT platform.10,26,27 In addition, the ACC/AHA data standards often include over 300 variables that are challenging to capture in real-life clinical settings.26,27
EuroHeart is an international collaboration that aims to improve the quality of cardiovascular care and facilitate observation and randomized research through continuous and longitudinal capture of individual patient data.13 To achieve this aim, a purpose-built IT platform enabling real-time data collection and monitoring of standards of care is delivered in parallel with cardiovascular data standards. Once fully adopted, the IT infrastructure will facilitate pragmatic R-RCTs, surveillance of device therapies, and observation research with pooled data from several European countries.13 Nonetheless, the success of this type of research using linked datasets from several geographical locations is dependent on the harmonization of clinical data variables, permissible values, and definitions.
We believe the methodology described in this paper provides a transparent and organized approach for the development of clinical data standards. Not only does this ensure consistency across the various cardiovascular domains that EuroHeart is planning to capture, but it also provides a scientific base, validity, and hopefully wide acceptance of the developed data standards. The completion of a systematic review of the literature enables the collection of data variables that are contemporary and relevant to current practice. In addition, the use of a modified Delphi method to build consensus and the obtaining of feedback and endorsement from various stakeholders provide a wide representation and perspective to the developed variables. The proposed methodology has now been, and is being, used for the development of data standards for several cardiovascular domains, including acute coronary syndrome, heart failure, atrial fibrillation, and valvular heart disease.
The methodology for development of clinical data standards is inclusive of clinical ‘content’ and ‘patient’ experts from a range of geographic, experiential, and specialist backgrounds. Still, the method is not without limitations. Given the nature of the topic, the selection of data variables by content experts may be prone to biases, subjectivities, and/or conflicts of interest.9,28 Members of the Data Science Group and Working Groups are required to disclose all relevant relationships with industry; however, as the data standards do not include any recommendations for clinical care, the potential for conflict of interest is likely to be negligible. Furthermore, the proposed methodology encompasses scientific evidence (e.g. systematic literature review, qualitative comparison between existing registries) and the use of the modified Delphi process and involves a Reference Group including patients, young cardiologists, and representatives from the nursing and allied healthcare professional community. However, we recognize that there may have been pressure for experts to provide results within a timeline and this may have ‘forced decisions’. Despite efforts to select variables based on pre-specified criteria (Table2), future updates will have to re-evaluate the selected variables based on accumulated data on their reliability and feasibility. Translation of these data standards into computational phenotypes to enable syntactic interoperability (i.e. the ability for systems to communicate and exchange data) and semantic interoperability (i.e. the ability for systems to communicate, effectively exchange, interpret, and use data) is also relevant but beyond the scope of the EuroHeart project at present.29
Conclusions
This paper provides a methodology for development of clinical data standards based on scientific evidence and expert consensus. It is anticipated that data standards developed using the proposed framework will have a wide applicability in various settings, including registries, clinical trials, EHRs, and public reporting programmes. As a part of the EuroHeart project, the developed data standards, and their implementation into a functioning IT platform, will facilitate standardized pan-European data collection, reporting of quality indicators, observational and registry-based randomized research, and post-marketing surveillance of devices and pharmacotherapies. The anticipation is that the proposed methodology may also be adopted by other initiatives when developing clinical data standards.
Acknowledgements
We thank Catherine Reynolds (EuroHeart Project Manager, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK), and Ebba Bergman and Lan Vu Thi (EuroHeart Project Managers, Uppsala Clinical Research Center, Uppsala, Sweden) for their support and contribution to the EuroHeart project. We thank Ida Björkgren (Uppsala Clinical Research Center, Uppsala, Sweden) for the editorial support.
Contributor Information
Gorav Batra, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala Science Park, Hubben, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden.
Suleman Aktaa, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds Institute for Data Analytics, University of Leeds, and Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Lars Wallentin, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala Science Park, Hubben, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden.
Aldo P Maggioni, Italian Association of Hospital Cardiologists Research Center (ANMCO), Florence, Italy.
Chris Wilkinson, Population Health Sciences Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
Barbara Casadei, Division of Cardiovascular Medicine, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, UK.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds Institute for Data Analytics, University of Leeds, and Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Funding
European Society of Cardiology.
Conflict of interest
G.B. reports honoraria for lectures and scientific advice from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, outside this work. S.A. has nothing to declare. L.W. reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co., and Roche Diagnostics, and consulting fees from Abbott, outside this work. A.P.M. has received personal fees for participation in committees of studies sponsored by AstraZeneca, Bayer, Fresenius, and Novartis, outside this work. C.W received research funding from Bristol Myers Squibb, outside this work. B.C. has received in kind support from Roche Diagnostics and iRhythm for clinical studies of atrial fibrillation, outside this work. C.P.G. reports personal fees from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Menarini, Oxford University Press, Raisio Group, Vifor Pharma, and Wondr Medical, and grants from Abbott, BMS, British Heart Foundation, Horizon 2020, and NIHR, outside this work.
Data availability
The data underlying this article are available in the article.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo Ret al. . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung S-C, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe Cet al. . Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 2014;383:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held Cet al. . Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J 2017;38:3056–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held Cet al. . Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J 2018;39:3766–3776. [DOI] [PubMed] [Google Scholar]
- 5. Bebb O, Hall M, Fox KAA, Dondo TB, Timmis A, Bueno Het al. . Performance of hospitals according to the ESC ACCA quality indicators and 30-day mortality for acute myocardial infarction: national cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J 2017;38:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson Aet al. . The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 7. Dudeck J. Aspects of implementing and harmonizing healthcare communication standards. Int J Med Inform 1998;48:163–171. [DOI] [PubMed] [Google Scholar]
- 8. Gregson J, Stone GW, Ben-Yehuda O, Redfors B, Kandzari DE, Morice M-Cet al. . Implications of alternative definitions of peri-procedural myocardial infarction after coronary revascularization. J Am Coll Cardiol 2020;76:1609–1621. [DOI] [PubMed] [Google Scholar]
- 9. Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User's Guide. 3rd ed.Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 10. Hendel RC, Bozkurt B, Fonarow GC, Jacobs JP, Lichtman JH, Smith EEet al. . ACC/AHA 2013 methodology for developing clinical data standards: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol 2014;63:2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez-Pereira S, Crespo-Leiro MG . et al. International Consortium for Health Outcomes Measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Heart Fail 2020;8:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flynn MR, Barrett C, Cosío FG, Gitt AK, Wallentin L, Kearney Pet al. . The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J 2005;26:308–313. [DOI] [PubMed] [Google Scholar]
- 13. Wallentin L, Gale CP, Maggioni A, Bardinet I, Casadei B. EuroHeart: European Unified Registries on Heart Care Evaluation and Randomized Trials—an ESC project to develop a new IT registry system which will encompass multiple features of cardiovascular medicine. Eur Heart J 2019;40:2745–2749. [DOI] [PubMed] [Google Scholar]
- 14. Richesson RL, Krischer J. Data standards in clinical research: gaps, overlaps, challenges and future directions. J Am Med Inform Assoc 2007;14:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacLean CH, Kerr EA, Qaseem A. Time out—charting a path for improving performance measurement. N Engl J Med 2018;378:1757–1761. [DOI] [PubMed] [Google Scholar]
- 16. Aktaa S, Batra G, Wallentin L, Baigent C, Erlinge D, James Set al. . European Society of Cardiology methodology for the development of quality indicators for the quantification of cardiovascular care and outcomes. Eur Heart J Qual Care Clin Outcomes 2020; 10.1093/ehjqcco/qcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones J, Hunter D. Qualitative research: consensus methods for medical and health services research. BMJ 1995;311:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Normand S-LT, McNeil BJ, Peterson LE, Palmer RH. Eliciting expert opinion using the Delphi technique: identifying performance indicators for cardiovascular disease. Int J Qual Health Care 1998;10:247–260. [DOI] [PubMed] [Google Scholar]
- 19. Schiele F, Aktaa S, Rossello X, Ahrens I, Claeys MJ, Collet J-Pet al. . 2020 Update of the quality indicators for acute myocardial infarction: a position paper of the Association for Acute Cardiovascular Care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care 2021;10:224–233. [DOI] [PubMed] [Google Scholar]
- 20. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng Met al. . Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 21. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt Net al. . Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 2017;377:1240–1249. [DOI] [PubMed] [Google Scholar]
- 22. Erlinge D, Omerovic E, Fröbert O, Linder R, Danielewicz M, Hamid Met al. . Bivalirudin versus heparin monotherapy in myocardial infarction. N Engl J Med 2017;377:1132–1142. [DOI] [PubMed] [Google Scholar]
- 23. Bowman L, Baras A, Bombien R, Califf RM, Chen Z, Gale CPet al. . Understanding the use of observational and randomized data in cardiovascular medicine. Eur Heart J 2020;41:2571–2578. [DOI] [PubMed] [Google Scholar]
- 24. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SEet al. . European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 25. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter Aet al. . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dehmer GJ, Badhwar V, Bermudez EA, Cleveland JC, Cohen MG, D'Agostino RSet al. . 2020 AHA/ACC key data elements and definitions for coronary revascularization: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Corona). Circ Cardiovasc Qual Outcomes 2020;13:e000059. [DOI] [PubMed] [Google Scholar]
- 27. Bozkurt B, Hershberger RE, Butler J, Grady KL, Heidenreich PA, Isler MLet al. . 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circ Cardiovasc Qual Outcomes 2021;14:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ 2011;343:d5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liyanage H, Krause P, de Lusignan, S. Using ontologies to improve semantic interoperability in health data. BMJ Health Care Informatics 2015;22:309–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.