Abstract
Background & Aims
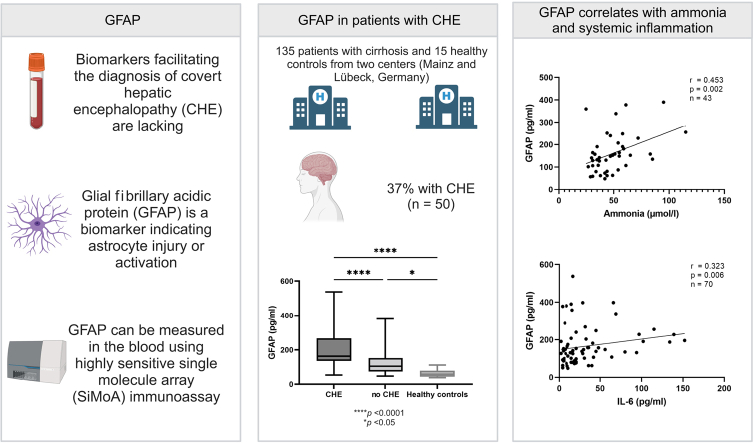
Blood biomarkers facilitating the diagnosis of covert hepatic encephalopathy (CHE) in patients with cirrhosis are lacking. Astrocyte swelling is a major component of hepatic encephalopathy. Thus, we hypothesised that glial fibrillary acidic protein (GFAP), the major intermediate filament of astrocytes, might facilitate early diagnosis and management. This study aimed to investigate the utility of serum GFAP (sGFAP) levels as a biomarker of CHE.
Methods
In this bicentric study, 135 patients with cirrhosis, 21 patients with ongoing harmful alcohol use and cirrhosis, and 15 healthy controls were recruited. CHE was diagnosed using psychometric hepatic encephalopathy score. sGFAP levels were measured using a highly sensitive single-molecule array (SiMoA) immunoassay.
Results
In total, 50 (37%) people presented with CHE at study inclusion. Participants with CHE displayed significantly higher sGFAP levels than those without CHE (median sGFAP, 163 pg/ml [IQR 136; 268] vs. 106 pg/ml [IQR 75; 153]; p <0.001) or healthy controls (p <0.001). sGFAP correlated with results in psychometric hepatic encephalopathy score (Spearman's ρ = −0.326, p <0.001), model for end-stage liver disease score (Spearman's ρ = 0.253, p = 0.003), ammonia (Spearman’s ρ = 0.453, p = 0.002), and IL-6 serum levels (Spearman's ρ = 0.323, p = 0.006). Additionally, sGFAP levels were independently associated with the presence of CHE in multivariable logistic regression analysis (odds ratio 1.009; 95% CI 1.004–1.015; p <0.001). sGFAP levels did not differ between patients with alcohol-related cirrhosis vs. patients with non-alcohol-related cirrhosis or between patients with ongoing alcohol use vs. patients with discontinued alcohol use.
Conclusions: sGFAP levels are associated with CHE in patients with cirrhosis. These results suggest that astrocyte injury may already occur in patients with cirrhosis and subclinical cognitive deficits and that sGFAP could be explored as a novel biomarker.
Impact and implications
Blood biomarkers facilitating the diagnosis of covert hepatic encephalopathy (CHE) in patients with cirrhosis are lacking. In this study, we were able to demonstrate that sGFAP levels are associated with CHE in patients with cirrhosis. These results suggest that astrocyte injury may already occur in patients with cirrhosis and subclinical cognitive deficits and that sGFAP could be explored as a novel biomarker.
Keywords: CHE, HE, GFAP, Biomarkers, Complications of cirrhosis, Psychometric hepatic encephalopathy score, Cognitive deficit
Abbreviations: CHE, covert hepatic encephalopathy; GFAP, glial fibrillary acidic protein; HE, hepatic encephalopathy; HE2, grade 2 hepatic encephalopathy; MELD, model for end-stage liver disease; MHE, minimal hepatic encephalopathy; OHE, overt hepatic encephalopathy; OR, odds ratio; PHES, psychometric hepatic encephalopathy score; ROC, receiver operating characteristic; sGFAP, serum glial fibrillary acidic protein; SiMoA, single-molecule array; WBC, white blood cell
Graphical abstract
Highlights
-
•
Blood biomarkers for covert hepatic encephalopathy in patients with cirrhosis are lacking.
-
•
Patients with covert hepatic encephalopathy had significantly higher serum GFAP levels.
-
•
Serum GFAP correlated with ammonia and IL-6 serum levels.
-
•
Serum GFAP levels did not differ based on alcohol use or alcohol-/non-alcohol-related aetiology of liver disease.
Introduction
Hepatic encephalopathy (HE) is a severe complication of cirrhosis.1 The two lowest HE grades – namely, minimal HE (MHE) and grade 1 HE – are collectively depicted as covert HE (CHE).2 Although signs and symptoms of CHE are commonly subclinical and only detectable with specialised tests, CHE impairs health-related quality of life and is associated with an increased risk for the development of overt HE (OHE), hospitalisation, and death.[3], [4], [5] In consequence, CHE often remains undiagnosed in routine clinical practice, which prevents the initiation of medical therapy.6 Therefore, easy-to-use and reliable testing strategies would be beneficial to support the diagnosis. In this context, diagnostic serum biomarkers facilitating the diagnosis of CHE are of particular relevance. Moreover, they may contribute to a deeper understanding of the underlying pathophysiological processes. A recent study showed the diagnostic value of IL-6 for the diagnosis of CHE as well as for risk prediction of OHE development in patients with cirrhosis.7 In addition, another recent study indicated the prognostic impact of serum ammonia on OHE development and mortality.8 However, these markers have the disadvantage of responding unspecifically to inflammatory processes (IL-6) or have susceptible pre-analytics (ammonia).
From a pathophysiological perspective, hyperammonaemia is a hallmark of HE. High ammonia levels induce astrocyte swelling, cerebral oedema, and neuronal cell death.9,10 Thus, markers of neuronal or astrocyte cell injury may be valuable for the diagnosis of HE. In this context, glial fibrillary acidic protein (GFAP), the most abundant intermediate filament protein in the cytoskeleton of astrocytes,11 might be of particular relevance. In general, GFAP is a biomarker indicating astrocyte injury or activation.11 GFAP is a critical cell structural protein and plays a role in various physiological processes such as the maintenance of the blood–brain barrier, and astrocyte migration and proliferation.12,13 In addition, various diseases such as traumatic brain injuries or neurodegeneration result in reactive (astro)gliosis and an increased expression of intermediate filament proteins such as GFAP.12 Today, even very low GFAP levels in the blood can be detected using high-sensitivity assays.14
In this proof-of-concept study, we hypothesised that ammonia-driven astrocyte swelling and mild neuronal cell death with consecutive reactive gliosis in patients with CHE may lead to elevated serum GFAP (sGFAP) levels, which could be used as a novel biomarker for CHE in patients with cirrhosis.
Patients and methods
Study cohort
In this proof-of-concept study, the data of prospectively recruited individuals (between July 2019 and July 2021) from two German tertiary care centres (University of Mainz and University of Lübeck) were retrospectively analysed. Participants from Mainz were recruited during outpatient visits or during elective measurement of the hepatic venous pressure gradient. Participants from Lübeck were recruited during outpatient visits or during hospital admission caused by reasons other than HE. Diagnosis of cirrhosis was based on histology, conclusive appearance in ultrasound or radiological imaging, endoscopic features of portal hypertension, and medical history. Patients with cirrhosis with a previous episode of OHE during the last 6 weeks, preterminal comorbidities, severe neurological comorbidities, a history of or ongoing chemotherapy with paclitaxel and carboplatin, or ongoing chronic harmful alcohol consumption were not approached for this study. In addition to the main cohort, a second cohort, including 21 patients with ongoing chronic harmful alcohol use, was recruited in Lübeck. Additionally, we obtained blood from 10 patients with grade 2 HE (HE2) according to the West Haven criteria (Lübeck, n = 8; Mainz, n = 2). Furthermore, 15 healthy individuals, who did not meet the criteria for neurological or psychiatric diseases, were included.
Blood was collected from all patients for biochemical analysis immediately after study inclusion (between 9 a.m. and 2 p.m.). Specifically, blood samples for the determination of venous ammonia levels were immediately cooled on ice and analysed without delay.
Diagnosis of CHE
First, all patients were screened for clinical signs of OHE according to the West Haven criteria.2 Next, detailed medical history, physical examination, and, if indicated, additional examinations were performed to rule out active infections. If OHE and active infections were excluded, the portosystemic encephalopathy syndrome test (gold standard) was used to screen for CHE. Owing to the bicentric character of the study and uncertainty of diagnosis of grade 1 HE between different centres, we decided to use the term CHE and not to distinguish between grade 1 HE and MHE.15 CHE was diagnosed in individuals with a psychometric hepatic encephalopathy score (PHES) below −4 according to the age-adjusted German norms published by Weissenborn et al. (Hannover, Germany).3 Both centres used the same test manual, which is commercially available with German norms, from Weissenborn et al.16 The PHES consists of the number connection test A, the number connection test B, the digit symbol test, the serial dotting test, and the line tracing test. To minimise distraction and other confounding factors, patients performed the test between 9 a.m. and 2 p.m. in a quiet, separate room.
Determination of sGFAP levels
Blood samples were spun at 2,000×g at room temperature for 10 min within 60 min after withdrawal at the day of study inclusion and stored in polypropylene tubes at −80 °C until batched sGFAP analysis. sGFAP levels were determined using the highly sensitive single-molecule array (SiMoA) technology. Samples were measured in duplicate in several rounds by SiMoA HD-1 (Quanterix, Billerica, MA, USA) using the GFAP Discovery kits according to the manufacturer’s instructions. Resorufin-β-d-galactopyranoside was incubated at 33 °C for 60 min before running the assay. The coefficient of variation (as a percentage) of the two replicates was obtained by dividing the SD of both replicates by the mean of both replicates multiplied by 100. Coefficients of variation above 20% (or missing replicate result) were measured twice. Measurements were performed in a blinded fashion without information about clinical data.
Determination of IL-6 and ammonia serum levels
IL-6 serum levels were determined in a subset of patients at the day of study inclusion immediately after testing with PHES using a commercially available chemiluminescence immunoassay (Cobas e 411 Analyser, F. Hoffmann-La Roche AG, Basel, Swiss).17
Venous ammonia levels were measured according to a standard operating procedure that involved rapid sample transport on ice to the central laboratory within 5 min, and the upper limit of normal was 72 μmol/L.
Ethics
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (sixth revision, 2008). The studies for both cohorts (Mainz and Lübeck) were approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz and of the University of Lübeck. Written informed consent was obtained from all participants.
Statistical analysis
Categorical data are expressed as frequencies and percentages, and metric data as medians with IQRs. Differences between two independent groups (e.g. CHE vs. no CHE) with metric data (e.g. sGFAP) were evaluated using an unpaired t test or a Mann–Whitney U test depending on data distribution. Differences between three or more groups with metric data were evaluated using an ordinary one-way ANOVA with Tukey’s multiple-comparisons test. For comparison of two or more patient groups with categorical variables, a chi-square test was applied. For correlation analyses, two-tailed Spearman’s rank correlation coefficient (Spearman’s ρ) was used. To identify predictors for the presence of CHE, multivariable logistic regression models were used. Multivariable linear regression models were used to determine associations of variables with continuous dependent variables (PHES or sGFAP). The discriminate ability of sGFAP for the identification of patients with CHE was analysed with help of the area under the curve of receiver operating characteristic (ROC) curves and its 95% CI. Values of p <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics (version 27, IBM Corp, Armonk, NY, USA) and GraphPad Prism (version 9.4, GraphPad Software, San Diego, CA, USA).
Results
Demographics and baseline characteristics
In total, 135 patients were included in this study (Mainz, n = 85; Lübeck, n = 50). Baseline characteristics of the study cohort are displayed in Table 1. At study inclusion, 50 individuals (37%) were diagnosed with CHE by PHES. The characteristics of the participants stratified by study site (Mainz and Lübeck) are shown in Table S1.
Table 1.
Baseline characteristics of the study cohort.
| Variable | All patients (N = 135) | Patients with CHE (n = 50) | Patients without CHE (n = 85) | p value | |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 60 (53; 66) | 61 (54; 68) | 60 (53; 66) | 0.356 | |
| Male sex, n (%) | 67 (50) | 28 (56) | 39 (46) | 0.256 | |
| Aetiology |
Alcohol, n (%) Other, n (%) |
80 (59) 55 (41) |
37 (74) 13 (26) |
43 (51) 42 (49) |
0.008 |
| MELD score, median (IQR) | 11 (9; 16) | 14 (11; 18) | 10 (8; 15) | <0.001 | |
| Child–Pugh A/B/C, n (%) | 64/60/11 (47/44/8) | 11/32/7 (22/64/14) | 53/28/4 (62/33/5) | <0.001 | |
| History of OHE, n (%) | 17 (13) | 12 (24) | 5 (6) | 0.002 | |
| History of ascites, n (%) | 84 (62) | 40 (80) | 44 (52) | 0.001 | |
| Sodium (mmol/L), median (IQR) | 138 (135; 140) | 137 (133; 140) | 138 (137; 140) | 0.016 | |
| Albumin (g/L), median (IQR) | 32 (27; 39) | 28 (24; 35) | 35 (29; 43) | <0.001 | |
| WBC count (/nl), median (IQR) | 5.6 (3.8; 7.6) | 5.3 (3.8; 7.5) | 5.8 (3.9; 8.0) | 0.693 | |
| IL-6 (pg/ml), median (IQR)∗ | 19 (9; 40) | 33 (16; 66) | 13 (5; 30) | 0.001 | |
| Ammonia (μmol/L), median (IQR)† | 44 (35; 58) | 55 (44; 65) | 38 (31; 47) | <0.001 | |
| GFAP (pg/ml), median (IQR) | 138 (87; 196) | 163 (136; 268) | 106 (75; 153) | <0.001 | |
| CHE, n (%) | 50 (37) | 50 (100) | 0 (0) | ||
| PHES, median (IQR) | −3 (−8; 0) | −10 (−12; −7) | −1 (−3; 1) | <0.001 | |
Data are expressed as medians and IQRs or as frequencies and percentages. Differences between individuals with CHE and those without CHE with metric data were evaluated using an unpaired t test or a Mann–Whitney U test depending on data distribution. Regarding categorical variables, a chi-square test was applied. p values < 0.05 are highlighted in bold font.
CHE, covert hepatic encephalopathy; GFAP, glial fibrillary acidic protein; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score; WBC, white blood cell.
Measured in 70 patients.
Measured in 43 patients.
Correlation of sGFAP with MELD, PHES, IL-6, and ammonia levels
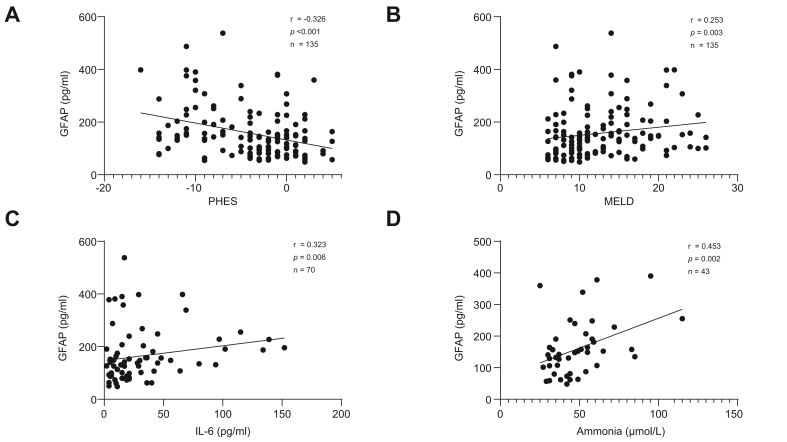
sGFAP levels were inversely correlated with PHES (Spearman’s ρ = −0.326, p <0.001, n = 135). In addition, sGFAP levels were positively correlated with the MELD score (Spearman’s ρ = 0.253, p = 0.003, n = 135), IL-6 serum levels (Spearman’s ρ = 0.323, p = 0.006, n = 70), and ammonia levels (Spearman’s ρ = 0.453, p = 0.002, n = 43) (Fig. 1).
Fig. 1.
Correlation between GFAP and PHES, MELD, IL-6, or ammonia. (A) Correlation between serum levels of GFAP and PHES. (B) Correlation between serum levels of GFAP and MELD. (C) Correlation between serum levels of GFAP and IL-6. (D) Correlation between serum levels of GFAP and ammonia. A two-tailed Spearman’s rank correlation coefficient (Spearman’s ρ) was used. GFAP, glial fibrillary acidic protein; MELD, model for end-stage liver disease; PHES, psychometric hepatic encephalopathy score; r, Spearman's ρ.
Association of sGFAP with CHE
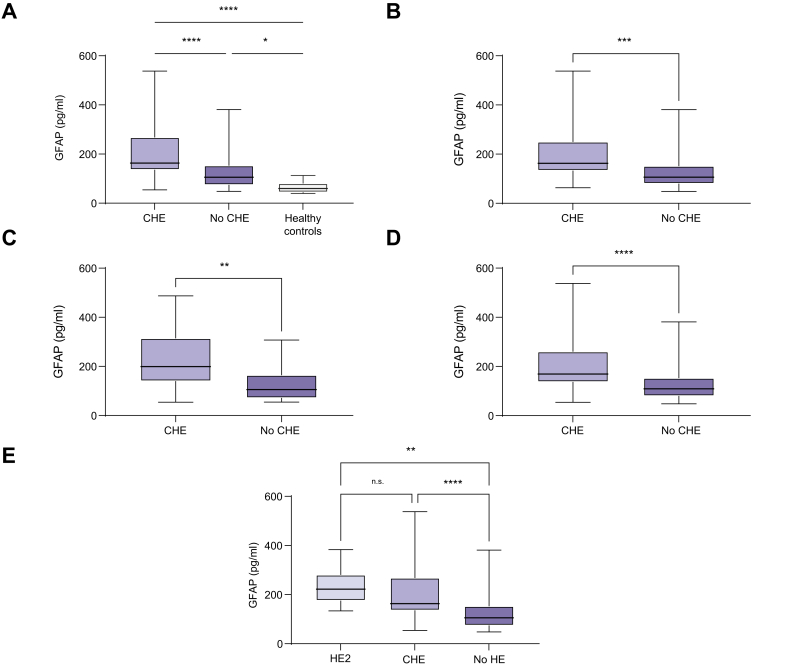
Participants diagnosed with CHE according to the PHES had significantly higher sGFAP levels than those without CHE (163 pg/ml [IQR 136; 268] vs. 106 pg/ml [IQR 75; 153], p <0.001; Fig. 2A). This finding was confirmed in sensitivity analyses for both centres (Fig. 2B and C). In addition, participants without CHE had higher sGFAP levels compared with healthy controls (median sGFAP levels, 106 pg/ml vs. 60 pg/ml; p <0.05) (Fig. 2A). sGFAP levels were also significantly elevated in participants with CHE compared with those without CHE, when all individuals with a history of OHE were excluded (median sGFAP levels, 169 pg/ml vs. 109 pg/ml; p <0.001) (Fig. 2D). Participants with HE2 had numerically higher sGFAP levels compared with those with CHE (222 pg/ml [IQR 176; 280] vs. 163 pg/ml [IQR 136; 268]; p = 0.744) (Fig. 2E).
Fig. 2.
Serum levels of GFAP in different patient cohorts. (A) GFAP serum levels in patients with cirrhosis with (n = 50) or without CHE (n = 85) and healthy individuals (n = 15). (B) GFAP serum levels in patients from the Mainz cohort with cirrhosis with or without CHE. (C) GFAP serum levels in patients from the Lübeck cohort with cirrhosis with or without CHE. (D) GFAP serum levels in patients without a history of OHE with or without CHE. (E) GFAP serum levels in patients with HE2 and CHE, and without HE. Data are presented as boxplots with median, IQR, and range. Differences between two independent groups (e.g. CHE vs. no CHE) with metric data were evaluated using a Mann–Whitney U test. Differences between three or more groups with metric data were evaluated using an ordinary one-way ANOVA with Tukey’s multiple-comparisons test. In (E), HE2 vs. CHE, p = 0.744 (ns). ∗ p <0.05, ∗∗p <0.01, ∗∗∗p <0.001. ∗∗∗∗p <0.0001. CHE, covert hepatic encephalopathy; GFAP, glial fibrillary acidic protein; HE2, grade 2 hepatic encephalopathy; OHE, overt hepatic encephalopathy.
To investigate whether sGFAP is independently associated with the presence of CHE in patients with cirrhosis, a multivariable logistic regression model was built. In addition to sGFAP, variables that are known to be potentially associated with CHE were included into the regression model (MELD, age, albumin serum levels, and history of OHE). In this model, sGFAP levels remained significantly associated with the presence of CHE, with an odds ratio (OR) of 1.009 (95% CI 1.004–1.015; p <0.001) (Table 2). Moreover, we repeated this analysis after exclusion of all individuals with a history of OHE (n = 118 patients included in the model). Again, sGFAP levels remained significantly associated with the presence of CHE (OR 1.009; 95% CI 1.004–1.014; p <0.001) (Table 2).
Table 2.
Multivariable logistic regression models to analyse the association of variables with CHE.
| Variable | OR (95% CI) | p value |
|---|---|---|
| Total cohort (N= 135) | ||
| GFAP | 1.009 (1.004–1.015) | <0.001 |
| MELD | 1.038 (0.945–1.140) | 0.433 |
| Male sex | 1.482 (0.642–3.422) | 0.357 |
| Albumin | 0.933 (0.877–0.992) | 0.028 |
| History of OHE | 4.662 (1.297–16.755) | 0.018 |
| Only patients without a history of OHE (n = 118) | ||
| GFAP | 1.009 (1.004–1.014) | <0.001 |
| MELD | 1.035 (0.939–1.140) | 0.489 |
| Male sex | 1.385 (0.573–3.346) | 0.470 |
| Albumin | 0.934 (0.876–0.995) | 0.035 |
p values < 0.05 are highlighted in bold font. CHE, covert hepatic encephalopathy; GFAP, glial fibrillary acidic protein; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy; OR, odds ratio.
Next, we investigated whether sGFAP is independently associated with poorer results in PHES in patients with cirrhosis. Here, we conducted a linear regression analysis, including the same variables as previously mentioned. In this regression analysis, higher sGFAP levels remained significantly associated with poorer results in PHES (β = -0.251, p <0.001) (Table 3).
Table 3.
Multivariable linear regression model to analyse the association of variables with PHES.
| Variable | Regression coefficient | 95 % CI of regression coefficient | β | p value |
|---|---|---|---|---|
| GFAP | −0.013 | −0.021 to −0.006 | −0.251 | <0.001 |
| MELD | −0.131 | −0.297 to 0.034 | −0.134 | 0.118 |
| Sex | −0.782 | −2.202 to 0.639 | −0.078 | 0.278 |
| Albumin | 0.165 | 0.059 to 0.271 | 0.266 | 0.002 |
| History of OHE | 4.090 | 1.921 to 6.258 | 0.270 | <0.001 |
R2 = 0.357. p values < 0.05 are highlighted in bold font. GFAP, glial fibrillary acidic protein; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score.
Finally, we sought to detect variables independently associated with higher sGFAP levels by using linear regression analysis. The only factors associated with higher sGFAP levels were higher age (β = 0.281, p <0.001) and the presence of CHE (β = 0.336, p <0.001) (Table 4).
Table 4.
Multiple linear regression model to analyse the association of variables with sGFAP levels.
| Variable | Regression coefficient | 95 % CI of regression coefficient | β | p value |
|---|---|---|---|---|
| CHE | 67.028 | 33.041 to 101.014 | 0.336 | <0.001 |
| MELD | 1.126 | −2.364 to 4.616 | 0.060 | 0.524 |
| Sex | −0.531 | −30.750 to 29.688 | −0.035 | 0.972 |
| Albumin | −1.000 | −3.313 to 1.313 | −0.084 | 0.394 |
| History of OHE | 17.494 | −29.352 to 64.340 | 0.060 | 0.461 |
| Age | 2.663 | 1.148 to 4.177 | 0.281 | <0.001 |
R2 = 0.228. p values < 0.05 are highlighted in bold font. CHE, covert hepatic encephalopathy; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy; sGFAP, glial fibrillary acidic protein serum.
To examine the diagnostic accuracy of sGFAP in detecting CHE, ROC curve analyses were performed. The area under the ROC curve for sGFAP was 0.736 (95% CI 0.649–0.823; p <0.001) in the total cohort.
Association of harmful alcohol use with sGFAP levels
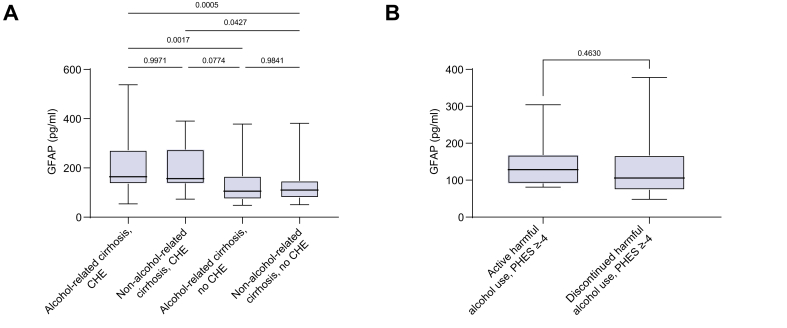
To investigate the association of a history of harmful alcohol use with sGFAP levels, we conducted a sensitivity analysis between individuals with and without an alcohol-related aetiology of cirrhosis. As displayed in Fig. 3A, individuals with an alcohol-related aetiology and CHE did not differ in terms of sGFAP levels compared with those with a non-alcohol-related aetiology and CHE. The same was true for individuals without CHE and an alcohol-related aetiology compared with those with a non-alcohol-related aetiology (Fig. 3A).
Fig. 3.
Serum levels of GFAP in patients with alcohol-related cirrhosis and those without alcohol-related cirrhosis. (A) GFAP serum levels in individuals with cirrhosis with or without CHE stratified by alcohol-related aetiology or non-alcohol-related aetiology of cirrhosis. (B) GFAP serum levels in individuals with active or discontinued harmful alcohol use and PHES ≥−4. Data are presented as boxplots with median, IQR, and range. Differences between two independent groups with metric data were evaluated using a Mann–Whitney U test. Differences between three or more groups with metric data were evaluated using an ordinary one-way ANOVA with Tukey’s multiple-comparisons test. Values of p are shown in the figure. HE, covert hepatic encephalopathy; GFAP, glial fibrillary acidic protein; PHES, psychometric hepatic encephalopathy score.
Additionally, we investigated the impact of ongoing harmful alcohol use on sGFAP levels in individuals with and without CHE. For this purpose, we compared the data of the main cohort with 21 additionally recruited patients with ongoing harmful alcohol use. Of these, 12 (57%) were classified as having ‘CHE’ according to PHES. Although those patients were not tested in a state of acute intoxication, it has to be acknowledged in these cases that CHE cannot be sufficiently diagnosed owing to the confounding effect of alcohol. As displayed in Fig. 3B, sGFAP levels did not differ between individuals with active harmful alcohol use and those with discontinued harmful alcohol use in the subcohorts of individuals with a PHES ≥ −4. This finding was also validated in a comparison of sGFAP levels between individuals with ongoing harmful alcohol use and those with discontinued harmful alcohol use and a PHES <−4 (p = 0.072).
Discussion
Identification of novel blood biomarkers to facilitate CHE diagnosis and to elucidate underlying pathophysiological processes accompanying CHE development is urgently required. In this bicentric study, we demonstrate that sGFAP levels are significantly increased in patients with cirrhosis and CHE compared with patients without CHE. In addition, sGFAP levels correlate with PHES, ammonia, and systemic inflammation, as reflected by IL-6 serum levels. Our data may therefore provide indirect evidence of a potential astrocyte injury and/or activation with subsequent release of GFAP into the serum in patients with cirrhosis and CHE.
As the major intermediate filament protein in astrocytes, GFAP is a biomarker of astrocyte injury and/or activation.11 High-sensitivity assays allow the detection of even low GFAP concentrations in the blood.14 In addition, point-of-care tests that could determine sGFAP levels within no more than 15 min are currently under investigation.11,18 However, it must be acknowledged that the determination of GFAP is currently very costly. Recently, sGFAP levels were found to be associated with disease severity in patients with chronic inflammatory central nervous system diseases such as progressive multiple sclerosis.14 Furthermore, normal sGFAP levels after out-of-hospital cardiac arrest were associated with favourable neurological outcomes after 6 months.19 Importantly, sGFAP levels are more stable in the context of preanalytics and showed a higher association with the pathology of cerebral amyloid beta compared with GFAP levels in the cerebrospinal fluid.20 Thus, sGFAP is a fast, readily accessible and reliable biomarker. Our current study expands this growing body of evidence on the potential clinical usefulness of measuring sGFAP beyond neurodegenerative applications by demonstrating higher sGFAP levels in patients with cirrhosis and CHE than in patients with cirrhosis and no CHE or healthy controls. Nevertheless, the aforementioned evidence clearly suggests that sGFAP levels are not specific for HE in patients with cirrhosis. Consequently, sGFAP levels must be interpreted under careful consideration of potential comorbidities. One of the most important confounders for interpreting sGFAP levels in patients with cirrhosis, especially in the Western world, may be harmful alcohol use.21 Of note, GFAP is also expressed in Schwann cells, and alcohol-related peripheral neuropathy may also lead to increased sGFAP levels.22 Surprisingly, despite the potential impact on astrocyte integrity and peripheral nerves, we did not find a significant difference in sGFAP levels between patients with alcohol-related cirrhosis and those with non-alcohol-related cirrhosis or between patients with ongoing harmful alcohol use and those with discontinued harmful alcohol use. Importantly, our results indicate that alcohol may not confound the interpretation of sGFAP levels and, thus, could be used in patients with cirrhosis in the context of HE, irrespective of the most common aetiologies. Consequently, elevated sGFAP levels may, thus, be related to HE and not to alcohol-associated cerebral injury in these individuals.
Several HE-related processes have the potential to contribute to the observed association between HE and elevated sGFAP levels. First and foremost, astrocyte injury caused by cell swelling and neuronal cell death with reactive gliosis and astrocyte activation are two mechanisms that may lead to increased sGFAP levels. In line with this, Angelova et al.10 recently found a significant neuronal cell death with a consecutive rise in GFAP-labelled astrocytes in bile duct-ligated rats, which represent an established animal model of MHE. Moreover, it is well known that leakage through the blood–brain barrier, allowing GFAP to pass into the blood stream, is a prerequisite for detectable levels in the peripheral blood.23 However, the detailed mechanism on how astrocyte activation leads to elevated sGFAP levels is currently not fully understood. Interestingly, GFAP is expressed not only exclusively in astrocytes but also in peripheral cells such as enteric glia or hepatic stellate cells,11 and the proportion of GFAP-positive glia cells in the gut increases when treated with cytokines.24 As low-grade systemic inflammation is associated with the development of HE, and blood levels of the pro-inflammatory cytokine IL-6 are associated with the presence of MHE,7 the elevation of sGFAP levels in patients with CHE might partly be cytokine/inflammation-mediated. However, the design of the current study does not allow us to assess to which extent each of the potential GFAP sources actually contributes to the observed elevation.
It is an interesting finding that even patients without CHE according to PHES had significantly higher sGFAP levels compared with healthy controls. On the one hand, this could be explained by the fact that HE is not a dichotomous disease, as possibly indicated by cut-offs of tests such as the PHES, but a disorder that encompasses a continuum of cognitive deficits. Therefore, even patients with cirrhosis but without CHE might already have mild cerebral injury, as reflected by higher sGFAP levels, caused by the complex interplay of ammonia and systemic inflammation. On the other hand, it has to be acknowledged that sGFAP levels are age-dependent, and this may have contributed to lower sGFAP levels in the control groups.11,25
One strength of this study is its bicentric design, including a total of 181 individuals with cirrhosis or healthy controls. However, several limitations have to be mentioned. First, the sample size of some subgroups, leading to a potential lack of power, as well as the fact that sGFAP levels appear to be age-dependent, prevents us from establishing potential cut-offs for sGFAP for distinguishing between patients with CHE and without CHE in the present study. However, our results clearly indicate that larger and prospective multicentre studies are warranted. Second, owing to our cross-sectional study design, we are only able to reveal associations and are unable to prove causality. Therefore, future studies should focus on longitudinal assessments of CHE and associated changes in sGFAP levels. Third, the ideal time point for sGFAP measurement remains unknown. This question should be addressed in a future longitudinal study to elucidate the dynamics of sGFAP levels in patients with cirrhosis. Fourth, sGFAP assays are currently not available in standard hospital laboratories and are still expensive.
In conclusion, sGFAP levels are independently associated with the presence of CHE in patients with cirrhosis. This finding may add valuable insights into the pathophysiological processes of HE, even at early stages. In addition, sGFAP might be a future candidate biomarker for CHE. Our results clearly indicate that large prospective trials are warranted to elucidate the prognostic value of sGFAP for predicting higher grades of HE and the longitudinal changes under therapy. In addition, larger studies may develop prediction models including sGFAP for diagnosing CHE.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Performed research: SJG, CQ, FL, JUM, CL. Contributed to acquisition of data: SJG, SD, LK, MN, EMS, CQ, SE, SB., JMS, M-AW, FL, JUM, CL. Designed the experiments and analysed the data: SJG, CL. Contributed reagents/materials/analysis tools: SJG, SB, PRG, JMS, FL, SE, SB, JUM, CL. Wrote the paper: SJG, CL. Statistical analysis: SJG, CL. Guarantor of the article: CL. Approved the final version of the manuscript and the authorship list: all authors.
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of interest
The authors disclose no potential financial or non-financial conflict of interests regarding this study.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Paula Kämper for excellent technical assistance. This study contains parts of the medical thesis of CQ and SD. SJG and EMS are supported by the Clinician Scientist Fellowship ‘Else Kröner Research College: 2018_Kolleg.05’.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100671.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Rose C.F., Amodio P., Bajaj J.S., Dhiman R.K., Montagnese S., Taylor-Robinson S.D., et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526–1547. doi: 10.1016/j.jhep.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D., et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of the liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 3.Patidar K.R., Bajaj J.S. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol. 2015;13:2048–2061. doi: 10.1016/j.cgh.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patidar K.R., Thacker L.R., Wade J.B., Sterling R.K., Sanyal A.J., Siddiqui M.S., et al. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014;109:1757–1763. doi: 10.1038/ajg.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labenz C., Baron J.S., Toenges G., Schattenberg J.M., Nagel M., Sprinzl M.F., et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment Pharmacol Ther. 2018;48:313–321. doi: 10.1111/apt.14824. [DOI] [PubMed] [Google Scholar]
- 6.Labenz C., Adarkwah C.C., Wörns M.-A., Miehlke S., Hofmann W.P., Buggisch P., et al. Management der hepatischen Enzephalopathie in Deutschland: eine Umfrage unter Gastroenterologen und Allgemeinmedizinern. Z Gastroenterol. 2020;58:49–56. doi: 10.1055/a-1010-6974. [DOI] [PubMed] [Google Scholar]
- 7.Gairing S.J., Anders J., Kaps L., Nagel M., Michel M., Kremer W.M., et al. Evaluation of IL-6 for stepwise diagnosis of minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Commun. 2022;6:1113–1122. doi: 10.1002/hep4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tranah T.H., Ballester M.-P., Carbonell-Asins J.A., Ampuero J., Alexandrino G., Caracostea A., et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J Hepatol. 2022;77:1554–1563. doi: 10.1016/j.jhep.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Sepehrinezhad A., Zarifkar A., Namvar G., Shahbazi A., Williams R. Astrocyte swelling in hepatic encephalopathy: molecular perspective of cytotoxic edema. Metab Brain Dis. 2020;35:559–578. doi: 10.1007/s11011-020-00549-8. [DOI] [PubMed] [Google Scholar]
- 10.Angelova P.R., Kerbert A.J.C., Habtesion A., Hall A., Abramov A.Y., Jalan R. Hyperammonaemia induces mitochondrial dysfunction and neuronal cell death. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelhak A., Foschi M., Abu-Rumeileh S., Yue J.K., D'Anna L., Huss A., et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18:158–172. doi: 10.1038/s41582-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 12.Middeldorp J., Hol E.M. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Butterworth R.F. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J Clin Exp Hepatol. 2015;5(Suppl. 1):S96–S103. doi: 10.1016/j.jceh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelhak A., Huss A., Kassubek J., Tumani H., Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter B., Walter K., Bissonnette J., Leise M.D., Lai J., Tandon P., et al. Assessment of the spectrum of hepatic encephalopathy: a multicenter study. Liver Transpl. 2018;24:587–594. doi: 10.1002/lt.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001 May;34(5):768-73. doi:10.1016/s0168-8278(01)00026-5. PMID: 11434627. [DOI] [PubMed]
- 17.Labenz C., Nagel M., Kämper P., Engel S., Bittner S., Kaps L., et al. Association between serum levels of neurofilament light chains and minimal hepatic encephalopathy in patients with liver cirrhosis. Clin Transl Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue J.K., Yuh E.L., Korley F.K., Winkler E.A., Sun X., Puffer R.C., et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol. 2019;18:953–961. doi: 10.1016/S1474-4422(19)30282-0. [DOI] [PubMed] [Google Scholar]
- 19.Moseby-Knappe M., Mattsson-Carlgren N., Stammet P., Backman S., Blennow K., Dankiewicz J., et al. Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensive Care Med. 2021;47:984–994. doi: 10.1007/s00134-021-06481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simrén J., Weninger H., Brum W.S., Khalil S., Benedet A.L., Blennow K., et al. Differences between blood and cerebrospinal fluid glial fibrillary Acidic protein levels: the effect of sample stability. Alzheimers Dement. 2022;18:1988–1992. doi: 10.1002/alz.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalçik H., Yardimoglu M., Filiz S., Gonca S., Dalçik C., Erden B.F. Chronic ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity: an immunocytochemical observation in various regions of adult rat brain. Int J Neurosci. 2009;119:1303–1318. doi: 10.1080/00207450802333672. [DOI] [PubMed] [Google Scholar]
- 22.Notturno F., Capasso M., DeLauretis A., Carpo M., Uncini A. Glial fibrillary acidic protein as a marker of axonal damage in chronic neuropathies. Muscle Nerve. 2009;40:50–54. doi: 10.1002/mus.21323. [DOI] [PubMed] [Google Scholar]
- 23.Brunkhorst R., Pfeilschifter W., Foerch C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl Stroke Res. 2010;1:246–251. doi: 10.1007/s12975-010-0040-6. [DOI] [PubMed] [Google Scholar]
- 24.von Boyen G.B.T., Steinkamp M., Reinshagen M., Schäfer K.-H., Adler G., Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols N.R., Day J.R., Laping N.J., Johnson S.A., Finch C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.