Abstract
Objective
Albeit with no disease-modifying effects, intra-articular steroid injections (IASIs) are still widely used to relieve symptoms of knee osteoarthritis. Previous literature has reported conflicting results regarding the safety of IASI in terms of periprosthetic joint infection (PJI) in total knee arthroplasty (TKA). This study tried to determine whether preoperative IASIs increased the risk of PJI, with different time intervals between the injections and surgery.
Methods
A computerized search of MEDLINE, EMBASE, and Cochrane Library was conducted for studies published before October 2022, which investigated the PJI rates of patients who received IASIs before TKA and patients who did not. The primary outcome was the association between preoperative IASI and PJI in TKA. The time point from which IASIs could be applied without risking PJI was also assessed.
Results
Fourteen studies, with 113,032 patients in the IASI group and 256,987 patients in the control group, were included. The pooled odds ratio of PJI was 1.13 (95% confidence interval [CI] 1.00–1.27, p = 0.05), indicating no increased risk of PJI. With the time interval < 6 months, the pooled odds ratio was 1.19 (95% CI 0.99–1.43, p = 0.06). However, with the time interval < 3 months, the pooled odds ratio was 1.26 (95% CI 1.06–1.50, p < 0.01).
Conclusion
IASI is not a safe procedure for patients who are expected to undergo TKA. The time interval between the injections and surgery was an important factor in assessing the safety of IASI. Preoperative injections that were applied within 3 months increased the risk of PJI in TKA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-023-03637-4.
Keywords: Total knee arthroplasty, Injection, Steroid, Periprosthetic joint infection
Introduction
Osteoarthritis (OA) of the knee is one of the most common causes of musculoskeletal disability worldwide. The life time risk of symptomatic knee OA has been reported to be about 40% [1–3]. However, there are no available disease-modifying treatments [4] and instead, the current guidelines relied on traditional analgesics and non-pharmacologic interventions for pain relief [5, 6]. Nearly 30% of patients received intra-articular steroid injections (IASIs) before undergoing total knee arthroplasty (TKA) [7].
Several medications are used in intra-articular injection, including corticosteroid, hyaluronic acid, platelet-rich plasma, and stem cells [8, 9]. Although widely applied, the medications have showed limited evidence for the efficacy in treating OA of the knee [10, 11]. The risk benefit of each medication should be evaluated carefully, considering the inherent risk of intra-articular injection such as periprosthetic joint infection (PJI).
PJI is a devastating complication in TKA, occurring at a rate of 0.5%–1.8% [12, 13]. Unfortunately, a controversy remains unresolved regarding whether preoperative IASIs increase the risk of PJI [4, 14–16] or not [17–19]. The low incidence of PJI has been an obstacle in designing a robust study. Some studies included patients who underwent knee joint arthroplasty and patients who underwent hip joint arthroplasty together in assessing the risk of PJI [20–22]. Some authors did not specify medications injected into the joint (corticosteroid, hyaluronic acid, or others) [23, 24]. The previous studies have investigated the risk of PJI in the setting of various time intervals (from 4 weeks to 1 year) between preoperative injections and arthroplasty [25–27]. These inconsistencies compromised the reliability of the conclusions.
The American Academy of Orthopedic Surgeons (AAOS) has recently downgraded its recommendation on IASI [5]. If the aforementioned controversy is resolved, the role of IASI can be established in a spectrum of treatments for knee osteoarthritis. A meta-analysis is expected to compensate the inconsistencies of each primary study. This study tried to determine whether preoperative IASIs increased the risk of PJI in TKA, with different time intervals between the injections and surgery.
Methods
The present meta-analysis was written according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses guidelines [28]. Ethical approval and acquisition of informed consent from participants were not required because all data were based on already published studies and were anonymously analyzed without any potential harm to the participants.
Literature search
A computerized search of MEDLINE, EMBASE, and Cochrane Library was conducted for studies published before October 2022, which investigated the PJI rates of patients who received IASIs before TKA and patients who did not. The search query included synonyms for total knee arthroplasty, steroid, injection, infection, and complication as follows: ([arthroplasty, replacement, knee] OR [total knee arthroplasty]) AND ([steroids] OR [adrenal cortex hormones] OR [corticosteroid]) AND [injections] AND ([safety] OR [infection] OR [complication]). The search was confined to studies on “humans” in the “English” language. Bibliographies of the studies were checked to identify additional relevant studies.
Inclusion and exclusion criteria
The included studies fulfilled the following criteria: (1) patients diagnosed with PJI after TKA; (2) patients who had a history of preoperative IASI; (3) follow-up duration more than six months to determine PJI and sufficient data to tabulate 2 × 2 contingency tables for odds ratios; and (4) publication type of original articles. We excluded studies with the following criteria: (1) patients who had arthroplasty other than primary TKA, such as revision TKA, unicompartmental knee arthroplasty, and total hip arthroplasty; (2) intra-articular injection applied perioperatively for pain control purpose; (3) outcomes that did not include PJI rates; and (4) review articles, editorials, letters, and single case studies. The above process was independently performed by two reviewers with consultation from a third reviewer for reaching a consensus when any disagreements were present.
Data extraction and quality assessment
Two reviewers independently extracted data from each study using a standardized data extraction form: patient characteristics such as the size of study population, the number of patients with PJI, and the time points of preoperative IASIs; and study characteristics including authors, institutions, publication year, study design, and follow-up duration.
The risk of bias was assessed using the Cochrane-recommended Risk of Bias in Non-randomized Studies of Intervention (ROBINS-I) tool because all included studies were non-randomized. The ROBINS-I tool provides signaling questions for reviewers to determine low, moderate, serious, or critical risk of bias among evaluated studies [29].
Data synthesis and analyses
The primary outcome of this study was the association between IASI and PJI. The time point from which IASIs could be applied without risking PJI was also assessed. Two by two tables were made for the odds ratio of PJI in association with IASI. As most primary studies reported time points of IASIs with 3-month intervals, the odds ratios were assessed with the time interval < 3 months and the time interval < 6 months. Although a few studies investigated both superficial infection and deep infection, only the latter was counted in the present study because diagnostic standard for superficial infection was unclear; many factors could affect the incidence of superficial infection; and it was intra-articular infection that led to devastating outcomes.
Summary estimates of odds ratios were calculated with a random-effects model to avoid overestimation of the study results. Heterogeneity was evaluated with forest plots and was quantified by Higgins I2 test, in which 25%, 50%, and 75% were considered as low, moderate, and high heterogeneities, respectively [30]. All statistical analyses were performed using the Review Manager (RevMan) program Version 5.4.1 (The Nordic Cochrane Center, The Cochrane Collaboration, 2014; Copenhagen Denmark).
Results
The electronic search query applied in MEDLINE, EMBASE, and Cochrane Library is summarized in Additional file 1.
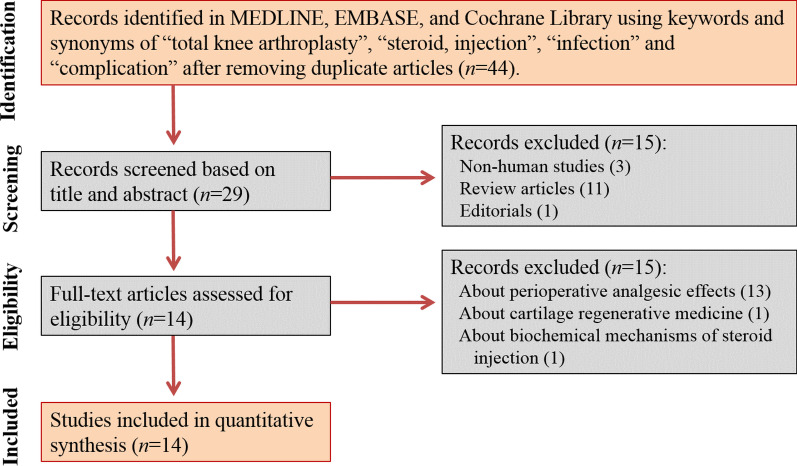
Of those 44 articles, we removed three non-human studies and additionally ruled out twelve studies after screening titles and abstracts, which left 29 original articles. Based on full text reviews, fifteen studies were not found to be in the field of interest: thirteen studies about perioperative injection for analgesic effects; one study about the injection of cartilage regenerative medicine; and one study about cytokine-related mechanisms following steroid injection. Accordingly, fourteen studies were included in the final analysis (Fig. 1).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart
Study characteristics
A total of 370,019 patients were analyzed. Of these, there were 113,032 patients in the IASI group and 256,987 patients in the control group. Nine studies investigated the time intervals between IASIs and TKA [4, 15–17, 19, 25, 27, 31, 32]. In making the diagnosis of PJI, seven studies were based on microbiologic culture results and the other seven studies relied on the relevant disease codes, such as the Current Procedural Terminology code and the International Classification of Diseases 9th Revision code regarding postoperative infections and operative procedures to address them [4]. The characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Authors | Year | Mean age | Sex (M/F) | Sample size | PJI rate (n/N) | Criteria for PJI | |
|---|---|---|---|---|---|---|---|
| IASI group | Control group | ||||||
| Amin et al. [19] | 2015 | 63.82 | N/A | 1,628 | 4/783 | 12/845 | MSIS criteria |
| Bedard et al. [4] | 2017 | N/A | 30,162/53,522 | 83,684 | 403/29,603 | 563/54,081 | Disease codes |
| Bhattacharjee et al. [25] | 2021 | N/A | 28,081/48,789 | 76,970 | 63/8,226 | 696/68,744 | Disease codes |
| Cancienne et al. [15] | 2015 | N/A | 11,340/24,550 | 35,890 | 949/22,240 | 500/13,650 | Disease codes |
| Desai et al. [33] | 2009 | 68 | 100/150 | 270 | 0/90 | 0/180 | Microbiologic culture |
| Grondin et al. [34] | 2021 | 71.8 | 84/220 | 180 | 4/83 | 2/97 | Microbiologic culture |
| Khan et al. [35] | 2022 | 69.5 | 645/675 | 1,169 | 7/521 | 15/648 | Disease codes |
| Khanuja et al. [17] | 2016 | 66 | 164/440 | 604 | 10/302 | 12/302 | CDC criteria |
| Kurtz et al. [31] | 2021 | N/A | 32,678/57,159 | 89,837 | 273/33,331 | 424/56,506 | Disease codes |
| Papavasiliou et al. [14] | 2006 | N/A | N/A | 144 | 3/54 | 0/90 | Microbiologic culture |
| Rhode et al. [32] | 2021 | 61.33 | 104/313 | 367 | 24/189 | 15/178 | Microbiologic culture |
| Richardson et al. [16] | 2019 | N/A | 17,244/28,487 | 58,337 | 532/16,656 | 1,117/41,681 | Disease codes |
| Tang et al. [36] | 2021 | 63.9 | 751/1,018 | 1,429 | 0/51 | 7/1,378 | Medical records |
| Turcotte et al. [27] | 2020 | 66.5 | N/A | 19,510 | 28/903 | 416/18,607 | Disease codes |
PJI, periprosthetic joint infection; IASI, intra-articular steroid injection; N/A, not available; MSIS, musculoskeletal infection society; CDC, centers for disease control and prevention
Risk of bias assessment
The overall risk of bias was assessed to be low in twelve studies based on ROBINS-I assessment [4, 14–17, 25, 27, 31–33, 35, 36]. Moderate risk of bias was assigned to two studies [19, 34]. However, no studies were determined to be at serious risk of bias (Fig. 2).
Fig. 2.
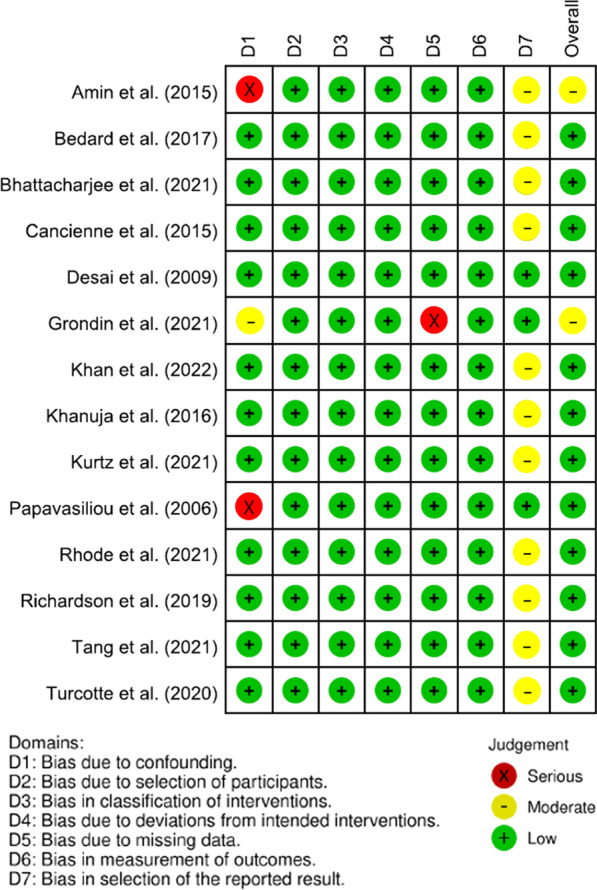
Traffic light plot regarding risk of bias scores for the included studies
Risk of PJI with different time intervals between IASIs and TKA
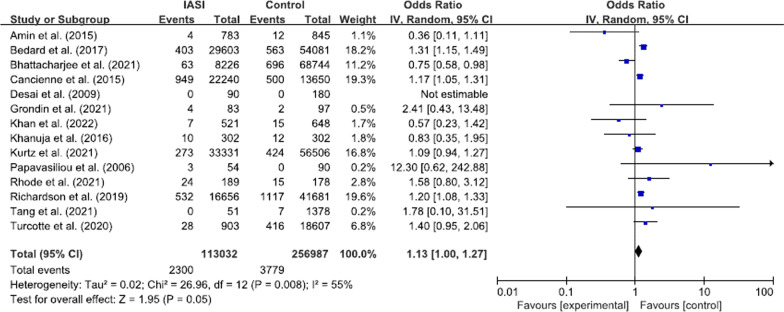
Overall, 2,300 (2.0%) PJIs were noted in the IASI group and 3,779 (1.5%) PJIs were reported in the control group. The pooled odds ratio of PJI was 1.13 (95% confidence interval [CI] 1.00–1.27, p = 0.05, I2 = 55%; Fig. 3), which indicated no increased risk of PJI.
Fig. 3.
Forest plot regarding the overall effect of IASI on the risk of PJI. IASI, intra-articular steroid injection; PJI, periprosthetic joint infection
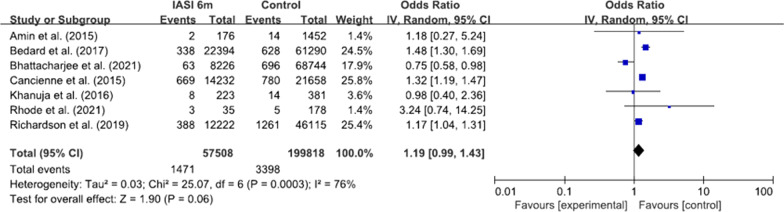
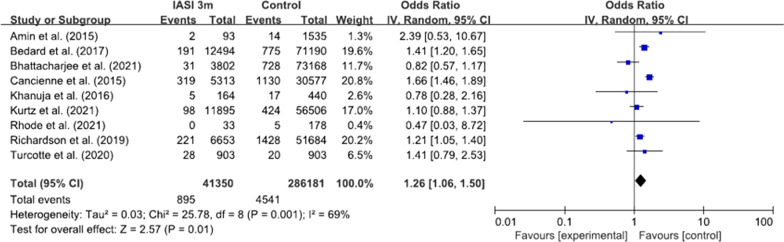
Nine studies reported regarding the time intervals between preoperative injections and surgery [4, 15–17, 19, 25, 27, 31, 32]. With the time interval < 6 months, the pooled odds ratio was 1.19 (95% CI 0.99–1.43, p = 0.06, I2 = 76%; Fig. 4). However, with the time interval < 3 months, the pooled odds ratio was 1.26 (95% CI 1.06–1.50, p < 0.01, I2 = 69%; Fig. 5).
Fig. 4.
Forest plot regarding the effect of IASI within 6 months on the risk of PJI. IASI, intra-articular steroid injection; PJI, periprosthetic joint infection
Fig. 5.
Forest plot regarding the effect of IASI within 3 months on the risk of PJI. IASI, intra-articular steroid injection; PJI, periprosthetic joint infection
Discussion
The most important finding of this study was that preoperative IASIs had a significant association with PJI when the time interval between the injections and surgery was considered. IASIs that were applied within 3 months increased the risk of PJI in TKA. Because the time interval of 3–6 months has not been investigated enough in the literature, it is safe to keep 6-month interval between IASIs and TKA.
Previous studies have reported inconsistent results regarding the association between preoperative IASI and TKA. Primary studies that could not find the association had a small sample size [14, 17, 19, 32–34, 36]. Due to the low incidence of PJI, those studies were likely to be underpowered and bore the risk of type 2 error. Studies that were based on large database [4, 15, 25, 31] or collected an enough study population [16] proved the increased risk of PJI when IASIs were applied preoperatively. A large database study by Cancienne et al., however, had a limitation in that the database was confined to a Medicare-only population [15], making it difficult to apply their results to the younger population [4]. Recent meta-analyses did not distinguish injected medications in assessing the risk of PJI [23, 24].
Specification of injected medications is crucial in establishing practical guidelines of osteoarthritis treatments. Current guidelines present recommendations separately for each medication. The intra-articular corticosteroid recommendation has been downgraded because of potential risk in accelerating osteoarthritis [5]. Despite its short-term effect for symptomatic knee osteoarthritis, intra-articular corticosteroid injection was associated with subchondral insufficiency fracture, osteonecrosis, and rapid bone loss [37]. Knee osteoarthritis is a progressive disease, and unfortunately no disease-modifying treatments are available to date [38, 39]. Because non-surgical managements and TKA should be considered together in treating knee osteoarthritis, the safety time point from which IASI could be performed without risking PJI needs to be determined.
Nine studies have investigated the safety time point, reporting different results [4, 15–17, 19, 25, 27, 31, 32]. A recent national database study by Bhattacharjee et al. stratified the time interval between IASIs and TKA into biweekly cohorts. It concluded that TKA performed within 4 weeks of IASI was associated with a higher risk of PJI [25]. Bedard et al. classified the injection cohort by monthly intervals and found that the odds of PJI remained higher for the injection cohort out to a duration 6 months between injections and TKA [4]. Other two studies also reported that the safety time point was 6 months [15, 16]. The present meta-analysis investigated the time point with 3-month interval. The pooled analysis did not show the increased risk of PJI within 6 months; however, the odds of PJI were significantly increased within 3 months of IASI.
Several limitations should be noted. First, the primary studies included in this meta-analysis were retrospective studies. Because of its low incidence, the risk of PJI has not been investigated in randomized controlled trials. Second, there was moderate heterogeneity that could not be fully addressed although a random-effects model was adopted. Six studies utilized large insurance-related database. Accordingly, the diagnosis of PJI was made using the relevant disease codes, whereas the diagnosis was based on microbiologic culture results in the other studies. Third, other potential risk factors of PJI could not be investigated. The number of intra-injections [26] and patient’s demographics might also be important factors. Further research is required to fully understand the association between IASI and PJI. Fourth, the mechanism of how IASIs lead to PJI could not be elucidated. It might be the result of mechanical inoculation or the chemical influence of intra-articular steroid. However, this study could not investigate the mechanism, which is an inherent limitation of meta-analyses.
Conclusions
IASI is not a safe procedure for patients who are expected to undergo TKA. The time interval between the injections and surgery was an important factor in assessing the safety of IASI. Preoperative injections that were applied within 3 months increased the risk of PJI in TKA.
Supplementary Information
Additional file 1. The electronic search query applied in MEDLINE, EMBASE, and Cochrane Library.
Acknowledgements
Not applicable.
Author contributions
J-HS helped in conception and design and collection and assembly of data. YMK contributed to administrative support. YBJ performed provision of study materials or patients. YMK and J-HS helped in data analysis and interpretation. All authors contributed to manuscript writing and final approval of manuscript.
Funding
No funding was received for this study.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51:230–236. doi: 10.1038/s41588-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 4.Bedard NA, Pugely AJ, Elkins JM, Duchman KR, Westermann RW, Liu SS, et al. The John N. Insall award: do intraarticular injections increase the risk of infection after TKA? Clin Orthop Relat Res. 2017;475:45–52. doi: 10.1007/s11999-016-4757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty) J Am Acad Orthop Surg. 2022;30:721–729. doi: 10.5435/JAAOS-D-21-01233. [DOI] [PubMed] [Google Scholar]
- 6.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. Knee. 2014;21:6–11. doi: 10.1016/j.knee.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag. 2020;2020:3873098. doi: 10.1155/2020/3873098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradelli L, Sinigaglia T, Migliore A, Checchia GA, Franceschi F, Frediani B, et al. Non-surgical treatment of knee osteoarthritis: multidisciplinary Italian consensus on best practice. Ther Clin Risk Manag. 2021;17:507–530. doi: 10.2147/TCRM.S288196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jüni P, Hari R, Rutjes AWS, Fischer R, Silletta MG, Reichenbach S, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;2015:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijn SRW, Rovers MM, van Tienen TG, Hannink G. Intra-articular corticosteroid injections increase the risk of requiring knee arthroplasty. Bone Joint J. 2020;102-B:586–592. doi: 10.1302/0301-620X.102B5.BJJ-2019-1376.R1. [DOI] [PubMed] [Google Scholar]
- 12.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387:386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 13.Crowe B, Payne A, Evangelista PJ, Stachel A, Phillips MS, Slover JD, et al. Risk factors for infection following total knee arthroplasty: a series of 3836 cases from one institution. J Arthroplasty. 2015;30:2275–2278. doi: 10.1016/j.arth.2015.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Papavasiliou AV, Isaac DL, Marimuthu R, Skyrme A, Armitage A. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2006;88:321–323. doi: 10.1302/0301-620X.88B3.17136. [DOI] [PubMed] [Google Scholar]
- 15.Cancienne JM, Werner BC, Luetkemeyer LM, Browne JA. Does timing of previous intra-articular steroid injection affect the post-operative rate of infection in total knee arthroplasty? J Arthroplasty. 2015;30:1879–1882. doi: 10.1016/j.arth.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Richardson SS, Schairer WW, Sculco TP, Sculco PK. Comparison of infection risk with corticosteroid or hyaluronic acid injection prior to total knee arthroplasty. J Bone Joint Surg Am. 2019;101:112–118. doi: 10.2106/JBJS.18.00454. [DOI] [PubMed] [Google Scholar]
- 17.Khanuja HS, Banerjee S, Sodhi GS, Mont MA. Do prior intra-articular corticosteroid injections or time of administration increase the risks of subsequent periprosthetic joint infections after total knee arthroplasty? J Long Term Eff Med Implants. 2016;26:191–197. doi: 10.1615/JLongTermEffMedImplants.2016014045. [DOI] [PubMed] [Google Scholar]
- 18.Joshy S, Thomas B, Gogi N, Modi A, Singh BK. Effect of intra-articular steroids on deep infections following total knee arthroplasty. Int Orthop. 2006;30:91–93. doi: 10.1007/s00264-005-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin NH, Omiyi D, Kuczynski B, Cushner FD, Scuderi GR. The risk of a deep infection associated with intraarticular injections before a total knee arthroplasty. J Arthroplasty. 2016;31:240–244. doi: 10.1016/j.arth.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Charalambous CP, Prodromidis AD, Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplasty. 2014;29:2175–2180. doi: 10.1016/j.arth.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Lai Q, Cai K, Lin T, Zhou C, Chen Z, Zhang Q. Prior intra-articular corticosteroid injection within 3 months may increase the risk of deep infection in subsequent joint arthroplasty: a meta-analysis. Clin Orthop Relat Res. 2022;480:971–979. doi: 10.1097/CORR.0000000000002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing D, Yang Y, Ma X, Ma J, Ma B, Chen Y. Dose intraarticular steroid injection increase the rate of infection in subsequent arthroplasty: grading the evidence through a meta-analysis. J Orthop Surg Res. 2014;9:107. doi: 10.1186/s13018-014-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Li L, Ren X, Nie L. Do preoperative intra-articular injections of corticosteroids or hyaluronic acid increase the risk of infection after total knee arthroplasty? A meta-analysis. Bone Joint Res. 2022;11:171–179. doi: 10.1302/2046-3758.113.BJR-2021-0350.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avila A, Acuña AJ, Do MT, Samuel LT, Kamath AF. Intra-articular injection receipt within 3 months prior to primary total knee arthroplasty is associated with increased periprosthetic joint infection risk. Knee Surg Sports Traumatol Arthrosc. 2022;30:4088–4097. doi: 10.1007/s00167-022-06942-3. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharjee S, Wallace S, Luu HH, Shi LL, Lee MJ, Chen AF. Do we need to wait 3 months after corticosteroid injections to reduce the risk of infection after total knee arthroplasty? J Am Acad Orthop Surg. 2021;29:e714–e721. doi: 10.5435/JAAOS-D-20-00850. [DOI] [PubMed] [Google Scholar]
- 26.Kokubun BA, Manista GC, Courtney PM, Kearns SM, Levine BR. Intra-articular knee injections before total knee arthroplasty: outcomes and complication rates. J Arthroplasty. 2017;32:1798–1802. doi: 10.1016/j.arth.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Turcotte J, Aja J, Menon N, MacDonald J, King P. Impact of preoperative intra-articular injection on infection rates following total knee arthroplasty an analysis of over 19,000 patients. J Orthop Sports Med. 2020 doi: 10.26502/josm.511500029. [DOI] [Google Scholar]
- 28.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt FL, Oh I-S, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62:97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz SM, Mont MA, Chen AF, Valle CD, Sodhi N, Lau E, et al. Intra-articular corticosteroid or hyaluronic acid injections are not associated with periprosthetic joint infection risk following total knee arthroplasty. J Knee Surg. 2022;35:983–996. doi: 10.1055/s-0040-1721128. [DOI] [PubMed] [Google Scholar]
- 32.Rhode DT, Siegel MA, Volchenko E, Patetta MJ, Schwarzman GR, Chmell SJ, et al. Do intra-articular corticosteroid injections prior to total knee arthroplasty increase postoperative complication rates: a retrospective review. J Knee Surg. 2021 [DOI] [PubMed]
- 33.Desai A, Ramankutty S, Board T, Raut V. Does intraarticular steroid infiltration increase the rate of infection in subsequent total knee replacements? Knee. 2009;16:262–264. doi: 10.1016/j.knee.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Grondin J, Menu P, Métayer B, Crenn V, Dauty M, Fouasson-Chailloux A. Intra-articular injections prior to total knee arthroplasty do not increase the risk of periprosthetic joint infection: a prospective cohort study. Antibiotics (Basel) 2021;10:330. doi: 10.3390/antibiotics10030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan IA, Small I, Sutton RM, Goh GS, Sherman MB, Mazur DW, et al. Cumulative intraarticular injections are not a risk factor for periprosthetic joint infection following total knee arthroplasty. J Arthroplasty. 2022;37:1059–1063.e1. doi: 10.1016/j.arth.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Tang A, Almetwali O, Zak SG, Bernstein JA, Schwarzkopf R, Aggarwal VK. Do preoperative intra-articular corticosteroid and hyaluronic acid injections affect time to total joint arthroplasty? J Clin Orthop Trauma. 2021;16:49–57. doi: 10.1016/j.jcot.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293:656–663. doi: 10.1148/radiol.2019190341. [DOI] [PubMed] [Google Scholar]
- 38.Arden NK, Perry TA, Bannuru RR, Bruyère O, Cooper C, Haugen IK, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17:59–66. doi: 10.1038/s41584-020-00523-9. [DOI] [PubMed] [Google Scholar]
- 39.Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12:92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The electronic search query applied in MEDLINE, EMBASE, and Cochrane Library.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.