Tofacitinib is a small molecule JAK inhibitor FDA approved for the treatment of adult patients with moderately to severely active UC who have an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers. Use of tofacitinib in combination with biologic therapies for UC or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.1 Ustekinumab is a monoclonal antibody that binds to the p40 protein subunit shared by both the interleukin (IL)-12 and IL-23 cytokines. Ustekinumab is FDA approved for the treatment of adult patients with moderately to severely active UC.2
The efficacy of induction and maintenance therapy with tofacitinib and ustekinumab vs placebo in patients with UC has been demonstrated in phase 3 trials.3,4 However, these agents have not been directly compared in head-to-head trials. A recent metaanalysis positioned both of these agents as equivalent after failure with anti-TNF inhibitor therapies.5 Further, a recent real-world comparative effectiveness analysis conducted in patients with both anti-TNF and vedolizumab failure demonstrated no difference in steroid-free remission rates between tofacitinib and ustekinumab at 12 to 16 weeks.6
This retrospective cohort study was a comparative efficacy analysis that evaluated real-world outcomes reported with tofacitinib vs ustekinumab up to 52 weeks after treatment initiation in patients with UC who had failed anti-TNF agents.7 At baseline, patients treated with tofacitinib had a higher median C-reactive protein level than ustekinumab-treated patients (5.1 mg/L vs 2.8 mg/L, respectively). Also at baseline, tofacitinib-treated patients more commonly had a Mayo endoscopic subscore of 2 or 3 (54% and 28%) vs ustekinumab-treated patients (33% and 36%).
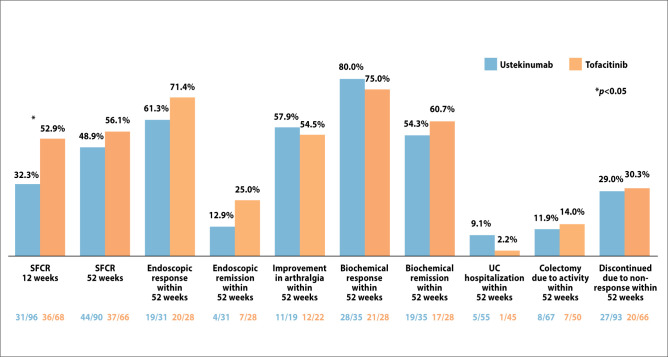
Both ustekinumab and tofacitinib were effective in achieving a steroid-free clinical remission at 12 weeks (32.3% and 52.9%, respectively) and 52 weeks (56.1% and 48.9%, respectively). After adjustment for confounding, there were no significant differences in the rates of steroid-free clinical remission with tofacitinib vs ustekinumab at either 12 weeks (odds ratio, 1.94; 95% CI, 0.96-3.92; P=.064) or 52 weeks (odds ratio, 1.16; 95% CI, 0.58-2.31; P=.681). There was also no significant difference in the rate of drug survival with tofacitinib vs ustekinumab (hazard ratio, 1.26; 95% CI, 0.74-2.15; P=.399).
Week 52 rates of endoscopic response were higher than rates of endoscopic remission but were similar between the 2 agents. Several other outcomes were reported, all of which showed similar rates between ustekinumab and tofacitinib (Figure 6).
Figure 6.
Outcomes among patients with ulcerative colitis and more than 1 prior anti–tumor necrosis factor failure who initiated tofacitinib or ustekinumab. SFCR, steroid-free clinical remission; UC, ulcerative colitis. Adapted from Dalal RS, et al. ACG abstract 42. Am J Gastroenterology. 2022;117(suppl 105).7
The rate of treatment discontinuation because of adverse events were low and similar between tofacitinib (3.3%) and ustekinumab (2.6%). One patient discontinued ustekinumab owing to nausea and arthralgia, and another patient discontinued tofacitinib owing to elevated liver enzymes.
References
- New York, NY: Pfizer Labs; 2022. Xeljanz [package insert]. [Google Scholar]
- Horsham, PA: Janssen Biotech, Inc.; 2022. Stelara [package insert]. [Google Scholar]
- Sandborn WJ, Su C, Sands BE et al. OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- Sands BE, Sandborn WJ, Panaccione R et al. UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–1214. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First-and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18(10):2179–2191.e6.. doi: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal RS, Mitri J, Goodrick H, Allegretti JR. Realworld comparison of tofacitinib vs ustekinumab among bio-exposed patients with ulcerative colitis: a propensity score analysis. Inflamm Bowel Dis. 2021;27(10):1694–1697. doi: 10.1093/ibd/izab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal RS, Sharma PP, Bains K et al. One-year comparative effectiveness of ustekinumab versus tofacitinib for ulcerative colitis after anti-tumor necrosis factor failure [ACG abstract 42]. Am J Gastroenterology. 2022;117(suppl 105) [PMC free article] [PubMed] [Google Scholar]